Abstract
Purpose
To report experience designing and establishing a reproductive registry and sample biorepository and to describe initial subject characteristics and biospecimens.
Methods
Beginning in December 2017, patients presenting for reproductive care at the University of Michigan were approached for study enrollment. Following consent, subjects completed detailed reproductive and health questionnaires. A variety of reproductive specimens and tissues were collected and processed for multiple downstream applications.
Results
Subject enrollment began in December of 2017. There are currently 1798 subjects enrolled. Female participants report a variety of reproductive disorders. Available samples include semen, sperm, follicular fluid, granulosa cells, immature oocytes, ovarian and uterine tissue, and blood samples.
Conclusion
We report the successful establishment of a reproductive registry and sample biorepository. Furthermore, we describe methods for collection and storage of a variety of reproductive tissue processed for multiple downstream translational applications.
Keywords: Biorepository, Reproductive research, Precision medicine, Translational research, IVF
Introduction
Over the past decade, increasing attention has been paid to optimizing and applying precision medicine. The significance of this individualized approach to healthcare was recently highlighted by the 2015 announcement of the precision medicine initiative [1]. Defined by the NIH as a healthcare paradigm which relies on an understanding of the contribution of each person’s unique genetic makeup, environment, and lifestyle to their health, precision medicine recognizes that these individual differences have a tremendous impact on the ability to understand and treat disease [2]. Research into novel individualized treatments has rapidly expanded through the use of proteomics, genomics, metabolomics, and large-scale computational analysis. However, these emerging fields require massive amounts of biomedical data for analysis and have increased the need for high-quality specimens with complementary reliable and accurate clinical data [3, 4]. Biobanks have thus become an invaluable resource and critical link for conducting large population-based research in an effort to better understand complex disease pathophysiology [5]. Biological repositories not only provide an inventory of specimens for use by independent investigators, but also enhance collaboration between clinicians, basic scientists, and translational researchers. There are currently many human tissue banking initiatives active across the world, some with millions of samples. Examples of these biorepositories include the UK Biobank, the Kaiser Permanente Research Bank, the NIH’s “All of Us” biobank, and the Central Biorepository at the University of Michigan [6–9].
While there are numerous examples of medical and oncologic biorepositories, there are very few reports of reproductive-specific biorepositories [10–15]. Research in the field of gynecology, and particularly reproductive endocrinology and infertility (REI) depends on the acquisition of human tissues to understand the pathophysiology of reproductive health disorders and to develop personalized treatments. While REI clinics already utilize personalized techniques such as preimplantation genetic testing (PGT), endometrial receptivity assays (ERAs), and karyotype analyses, much remains unknown about the pathological processes contributing to infertility. Indeed, clinical testing fails to identify a cause for infertility in 15–30% of cases, resulting in a diagnosis of unexplained infertility [16]. Thus, there is clearly a need for improved research into the mechanisms and treatment of reproductive disease in both men and women.
In 2017, the Department of Obstetrics and Gynecology at the University of Michigan initiated the Reproductive Subjects Registry and Sample Repository (RSRSR). The purpose of this initiative is to create a streamlined process for the acquisition, processing, and storage of biological samples and clinical data obtained from patients seen in a variety of Michigan Medicine reproductive clinics to be used for research in the field of reproductive medicine. Herein, we discuss the design and implementation of the RSRSR biorepository and database. Additionally, we describe the specimens collected to date as well as the current demographic makeup of biobank participants.
Material and methods
The protocols and procedures of RSRSR have been approved by the Institutional Review Board of the University of Michigan Medical School (IRBMED), registered under ID HUM00125627.
Patient recruitment and consent
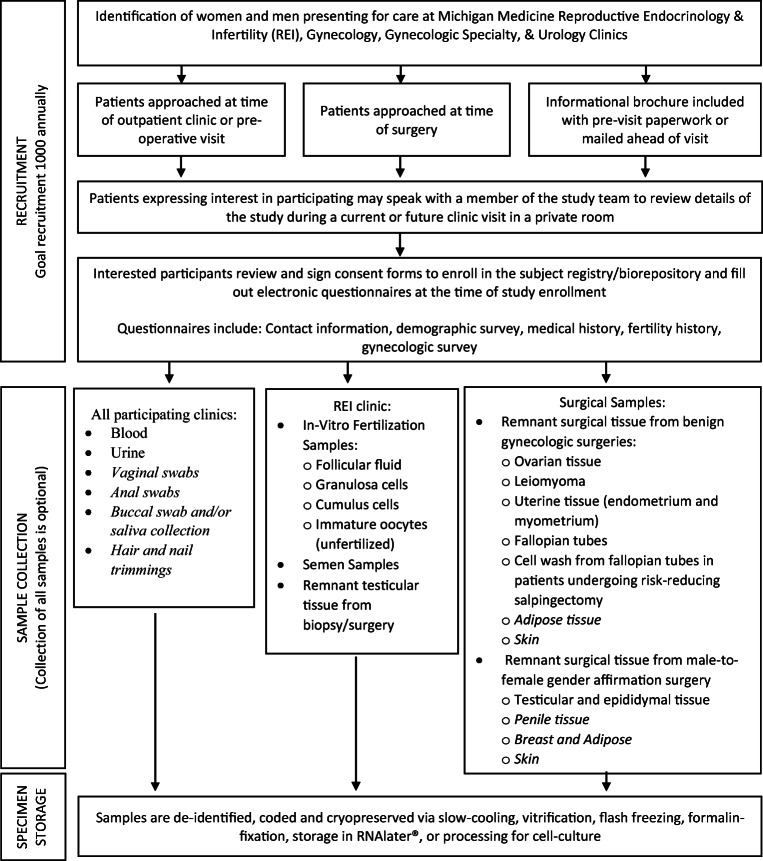
Patient recruitment, data collection, specimen collection, and storage are summarized in Fig. 1. All patients (male and female) receiving reproductive health care (including both outpatient visits and surgery for benign gynecologic/urologic conditions) at the University of Michigan who are greater than 18 years of age and can read and understand an English language consent are eligible for study enrollment.
Fig. 1.
Recruitment, collection, and storage protocol. Schematic of patient recruitment strategy, tissue collection, and storage methodology. Note, italicized items indicate categories for which RSRSR has IRB permission to obtain; however, no samples have yet been collected
The University of Michigan has a number of reproductive health care clinics from which participants may be enrolled including reproductive endocrinology and infertility, reproductive urology, general gynecology, transgender medicine, urogynecology, minimally invasive gynecologic surgery, and gynecologic care for patients at high risk of breast cancer. Patients may be approached at the time of an outpatient clinic visit, a pre-operative visit, or at the time of surgery. Before their clinic visit, patients are provided with an informational brochure regarding the biorepository. Before or after their health care visit, patients are approached by a study team member and asked if they would be interested in participating. If interest is confirmed, RSRSR is described in detail (either at the current visit or a future visit if preferred) and all elements of the consent form are explained with time for questions.
Clinical data collection and storage
Subjects are asked to complete several electronic questionnaires at the time of study enrollment. All answers to study questions are directly imported into a Research Electronic Data Capture (REDCap) database hosted at the University of Michigan. REDCap is a secure, web-based software platform designed to support data capture for research studies [17, 18].
Demographic data collected includes information on sex assigned at birth, gender identity, age, race, ethnicity, sexual orientation, partner status, education history, religious beliefs, and annual household income. Patients are asked to provide medical information regarding current medical problems, height, weight, substance use, smoking history, and caffeine use. Additionally, patients are questioned about exposure to possible environmental toxicants including regular exposure to fertilizers, paint, cleaning supplies, nail products, insecticides, gasoline, tar, and soft plastics. A comprehensive obstetric and gynecologic questionnaire collects information on menstrual history, contraceptive use, prior pregnancies or miscarriages, terminations, sexually transmitted infections, or past gynecologic surgeries. Additionally, a more focused fertility history gathers data on difficulties becoming pregnant and prior fertility evaluations and treatments. Treatments captured include oral or injectable ovulation induction, intra-uterine inseminations (IUIs), and in vitro fertilization (IVF) cycles. Participants also provide consent for approved researchers to access their medical records so that fertility treatment data and outcomes can be collected after study enrollment. Capture of fertility diagnoses and outcomes can later be used to identify relevant biologic specimens.
Biological sample collection and storage
Blood
Blood samples from male outpatient participants are collected by a member of the study team at the time of semen analysis. Blood may also be collected by the University Phlebotomy team when a patient presents for any routine blood draw. For female outpatient participants, blood is collected by the University Phlebotomy team when a participant presents to have blood drawn for any clinical indication. For those participants undergoing surgery, blood is collected at the time of surgery. Blood samples are aliquoted and stored as serum, plasma, white blood cells, and whole blood. Serum and plasma are separated by centrifuging tubes at for 10 min at 2000g, 4 °C. Aliquots of 0.5 mL are stored at − 80 °C. Following aliquoting of plasma, white blood cells are placed in a cryovial with RNAlater® (Invitrogen, AM7021) and stored at 4 °C overnight and then transferred to − 80 °C. A separate tube of whole blood is stored in 1-mL aliquots at − 80 °C.
Urine
Urine samples are collected at the time of study enrollment and then transported to the laboratory for processing. Urine is aliquoted to fill 5-mL vials and placed at − 20 °C overnight and then placed at − 80 °C the following day for long-term storage. Of note, patients may opt out of blood and urine collection.
Semen samples
Discarded semen samples are obtained following routine semen analysis ordered for clinical evaluation. Following collection of semen for semen analysis, samples are allowed to liquefy at 37 °C for at least 30 min. A formal semen analysis is performed by trained Andrology staff according to the WHO, 5th edition [19]. Following analysis, samples are transported from the fertility clinic to the research laboratory for processing on the same day. During transport, samples are maintained at 25°–37 °C. Upon arrival, the patient label is removed and the sample labeled with the corresponding study ID. An aliquot of whole semen (0.5 mL) is mixed with an equal volume of freezing medium (Origio, ART-8022) and cryopreserved by first cooling at 4 °C followed by freezing in liquid nitrogen vapors and ultimately, long-term storage in liquid nitrogen. The remaining sample is centrifuged at room temperature for 20 min at 300g to pellet the sperm and the seminal fluid set is aside. The sperm pellet is resuspended in modified sperm washing media (Origio, ART-1006) with 5% human serum albumin (HSA) (Origio, ART-3001). An equal volume of freezing media is added and the sample stored in 0.5-mL aliquots in cryovials. It is then cryopreserved as described above and placed in liquid nitrogen for long-term storage the following day. The seminal fluid is centrifuged at 4 °C for 40 min at 7197g and the supernatant stored at − 80 °C in 0.5-mL aliquots.
Follicular fluid
At the time of transvaginal oocyte retrieval, follicular fluid from the 2nd and/or 3rd follicle from each ovary is collected if there was no visible blood contamination during aspiration. Fluid is pooled and placed on ice during transport. Upon arrival to the laboratory, the follicular fluid is then centrifuged at 4 °C for 40 min at 7197g. The supernatant is then stored in 1-mL aliquots at − 80 °C.
Cumulus cells
After all cumulus oocyte complexes (COCs) are recovered from follicular fluid, they are first rinsed in HEPES-buffered human tubal fluid (HEPES-HTF) (Cooper Surgical, ART-1023). The outer layer of cumulus cells is carefully trimmed away by using two 28-gauge insulin needles. The cumulus cells are then transferred into a 1.5-mL microcentrifuge tube and kept on the ice while transported for processing from the embryology laboratory to the research laboratory. The cumulus cells are then incubated in pre-warmed hyaluronidase (100 IU/mL) for 10 min at 37 °C. Cells are gently pipetted at 5-min intervals to facilitate enzymatic digestion. Next, cells are centrifuged at 300g, 4 °C for 10 min and the supernatant removed. Cells are then washed with 1.5 mL of Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, 14190250) before being snap frozen in liquid nitrogen and stored at − 80 °C.
Granulosa cells
During the retrieval, the mural granulosa cell clumps are directly picked up with a 200-μL tip from the dish after oocyte collection. After gently rinsing the cell clump in HEPES-HTF with 5mg/mL HSA, the cells are pooled and transferred into a microcentrifuge tube and kept on ice for transport from the embryology lab. Cells are then centrifuged at 300g, 4 °C for 10 min and the supernatant removed. The granulosa cells are then snap frozen in liquid nitrogen and stored at − 80 °C.
Immature and unfertilized oocytes
In subjects undergoing intracytoplasmic sperm injection (ICSI), at 39 h after human chorionic gonadotropin (HCG) trigger, all COCs are briefly treated with hyaluronidase (80 mIU/mL) in HEPES-HTF with 5 mg/mL HSA. The cumulus cells are then mechanically removed by gently pipetting. All oocytes are cultured in Quinn’s Advantage Protein Plus Fertilization Medium (Cooper Surgical, ART-1520) for an additional 3–4 h at 37 °C in 5% CO2/5% O2 incubator. Developmental status of the oocyte is assessed right before ICSI. Oocytes lacking the first polar body are determined to be immature. The immature oocytes are cultured overnight in the same condition, and if oocytes do not progress to maturity, they are cryopreserved by vitrification the following morning. Of note, immature oocytes are typically discarded during the clinical embryo culture process.
For patients undergoing conventional insemination, at 40 h after HCG, all of COCs are inseminated with 1 million/mL motile sperm. A fertilization check is carried out 17–18 h after insemination. Oocytes that do not display any pronuclear structure(s) are separated and then cryopreserved by vitrification on the same day. Oocyte vitrification is carried out using the Irvine Vit Kit (90133-SO) according to the manufacture protocol. Generally, no more than 2 same developmental stage oocytes are first equilibrated in equilibration solution (ES) for 12 min, then transferred to vitrification solution (VS) for 30–45 s, and finally loaded to a Vitroguard straw with minimal media. The straw is then plunged into liquid nitrogen within 80 s after exposure to VS.
Surgical samples
A small section of tissue is resected using sterile instruments and placed into 50-mL conical tubes containing sterile Hanks’ balanced salt solution (HBSS) (Gibco, 24-020-117). Specimens resected include endometrium, cervix, myometrium, leiomyoma, fallopian tube, ovarian wedge, epididymis, and testicle wedge. Specimens are then transported to the laboratory and divided for storage. Sample aliquots are flash frozen, paraffin embedded, stored in RNAlater® (Invitrogen, AM7021), or prepared for cell culture as previously described and as outlined below [20, 21].
Paraffin embedding
Surgical specimens are first rinsed in cold PBS and then dissected to fit in a 3 × 2.5-cm cassette. The tissue is fixed in 10% formalin (Fisher, 23-032059) at 4 °C overnight and then placed in 70% ethanol until processed by the University of Michigan histology core for immunohistochemistry.
Endometrial stromal cell isolation and culture
Endometrium is dissected from the fresh specimen and minced in Ca and Mg free HBSS (Gibco, 24-020-117). Following sedimentation for 5 min, the supernatant is set aside at 4 °C. The tissue is then centrifuged for 5 min at 4 °C, 500g, and the supernatant discarded. The tissue is then digested as previously described to obtain endometrial stromal cells [22]. The cells are then placed in 1 mL of complete medium (DMEM/F12 (Gibco, 11320033), 10% FBS (Gibco, 10082147), 1% Pen/Strep, (Gibco, 15140122)) and counted. The remaining cells are diluted in complete medium and placed at 37 °C with 5% CO2. Cells are passaged to expand the cell population and then cryopreserved once cell density reaches 1 × 106–7 cells/mL by resuspending in freezing medium (Gibco, 12648010), aliquoting into 1.2–2-mL cryovials and then gradient cooling overnight in a − 80 °C freezer. Samples are then transferred to liquid nitrogen for long-term storage.
Leiomyoma and myometrium cell isolation and culture
Leiomyoma and myometrium cells are isolated and cultured as previously described [20]. Briefly, specimens are placed into 50-mL conical tubes and washed with DPBS (Gibco, 14190250) × 3. Tissue is then transferred to HBBS and minced into 1-mm3 pieces. Tissue is then digested in HBBS with 150 μg/mL DNAse (Sigma, D5025) and 1.5 mg/mL collagenase (Sigma, C0130) and rotated at 37 °C for 4–12 h. The sample is then filtered through a 100-μM cell strainer and centrifuged at 250g for 8 min. Cells are then resuspended in 10 mL of Smooth Muscle Cell Growth Medium-2 Bulletkit (Lonza, CC-3182) with 1% Pen/Strep and centrifuged at 250g for 8 min. The supernatant is discarded and the cells resuspended in 1 mL of Smooth Muscle Cell Growth Medium-2 Bulletkit (Lonza) with 1% Pen/Strep and counted. Cells are then passaged and cryopreserved as above in freezing medium (Gibco, 12648010). Samples are stored in liquid nitrogen long term.
Determination of biorepository use
The Biorepository Committee is made up of 2 reproductive endocrinology physicians-scientists, a basic scientist and a research administration specialist. Researchers (both internal and external) requesting to use stored samples are asked to submit a research proposal to the Biorepository Committee. If the committee determines that the research proposal is an appropriate use of the collected samples, the researcher is given permission to submit an IRB request to conduct their research. Factors assessed by the committee include the significance of the proposed study, the soundness of the research methodology, the total amount of specimen required to answer the research question, the involvement of OBGYN learners/trainees/junior faculty, and how the proposed research will add to the scientific literature. Following IRB approval (or exemption), the samples will be released to the researcher in a de-identified state per the protocol of the individual study. Of note, all tissue/gametes must be studied in accordance with federal and state regulations.
Results
Subject recruitment and enrollment
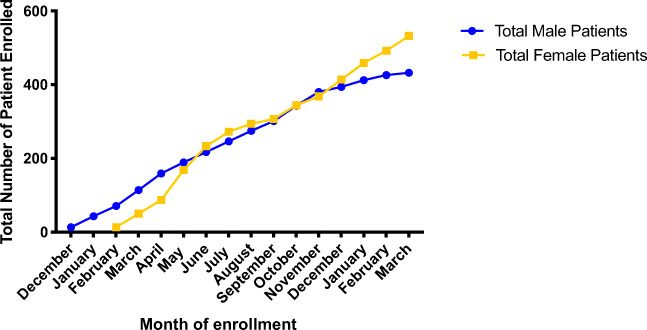
RSRSR began enrolling patients in December of 2017. Subject enrollment over the first study year is demonstrated in Fig. 2. Males presenting for semen analysis were first targeted for recruitment as a means of obtaining biologic samples at the time of enrollment. On average, 32 males were enrolled per month during the first study year with an enrollment rate of 46.28%. There are currently 663 males enrolled in RSRSR. Female patients were first approached for study enrollment in February of 2018. On average, 38 females were enrolled per month during the first study year, with an enrollment rate of 46.27%. There are currently 1135 female subjects enrolled in RSRSR.
Fig. 2.
Subject enrollment during first year of recruitment. Total number of male and female participants enrolled by month during the first year of study recruitment
Subject demographics and baseline characteristics
Female subjects
Baseline demographics and gynecologic history for female subjects are described in Table 1. The average age of female participants is 42.8 years with a range of 18.5–80.6 years at time of enrollment. The mean BMI is 29.4 kg/m2 with a range of 15.1–67.9 kg/m2. Participants are predominantly Caucasian (80.5%) and Non-Hispanic (89.7%). They are also highly educated, with over 65% reporting a Bachelor’s degree or higher and over 46% reporting an annual household income greater than $100,000. 21.4% of female participants reported a history of infertility and a range of gynecologic disorders were also reported including menorrhagia (21.5%), metrorrhagia (15.1%), fibroids (21.5%), PCOS (13.5%), and endometriosis (9.9%).
Table 1.
Demographic and baseline characteristics for female participants (n = 1135)
| Mean age (range) | 42.8 (18.5–80.6) |
| Mean body mass index (range) | 29.4 (15.1–67.9) |
| Race | |
| American Indian/Alaska Native | 0.5% |
| Asian American | 4.9% |
| Black or African-American | 8.5% |
| White/Caucasian | 80.5% |
| Unknown | 0.5% |
| Other | 2.6% |
| No response | 2.4% |
| Ethnicity | |
| Hispanic/Latino/Latina/Latinx | 3.4% |
| Non-Hispanic/Non-Latino | 89.7% |
| No response | 6.9% |
| Highest level of education completed | |
| Less than high school | 1.1% |
| High school diploma or GED | 7.1% |
| Some college, no degree | 14.5% |
| Associate or technical degree | 9.8% |
| Bachelor’s degree | 29.3% |
| Master’s degree (MA, MS, etc.) | 24.8% |
| Doctoral degree (MD, PhD, JD, etc.) | 10.9% |
| No response | 2.4% |
| Annual household income | |
| Less than $50,000 | 18.9% |
| $50,000–$74,999 | 15.3% |
| $75,000–$99,999 | 13.5% |
| $100,000–$149,999 | 22.5% |
| $150,000–$200,000 | 12.4% |
| More than $200,000 | 11.4% |
| No Response | 6.0% |
| Current Smoker | 4.4% |
| History of infertility | 21.4% |
| Gynecologic history | |
| Menorrhagia | 21.5% |
| Metrorrhagia | 16.1% |
| History of fibroids | 21.5% |
| History of PCOS | 13.5% |
| History of endometriosis | 9.9% |
Male subjects
Baseline and demongraphic data for male subjects are described in Table 2. The average age of male participants is 35.1 years with a range of 18.3–69.6 years. Mean BMI is 28.7 kg/m2 with a range of 17.1–61.3 kg/m2. Similar to female subjects, male subjects are predominantly Caucasian (80.7%) and non-Hispanic (89.6%). Male subjects are also highly educated with 66% reporting a Bachelor’s degree or higher and over 54% reporting an annual household income greater than $100,000. A total of 12.7% of males reported history of a previous pregnancy and 5.1% reported use of testosterone.
Table 2.
Demographic and baseline characteristics for male participants (n = 663)
| Mean age (range) | 35.1 (18.3–69.6) |
| Mean body mass index (range) | 28.7 (17.1–61.3) |
| Race | |
| American Indian/Alaska Native | 0.3% |
| Asian American | 7.1% |
| Black or African-American | 5.1% |
| Native Hawaiian or Other Pacific Islander | 0.2% |
| White/Caucasian | 80.7% |
| Unknown | 0.5% |
| Other | 4.1% |
| No response | 2.1% |
| Ethnicity | |
| Hispanic/Latino/Latina/Latinx | 5.0% |
| Non-Hispanic/Non-Latino | 89.6% |
| No response | 5.4% |
| Highest level of education completed | |
| Less than high school | 0.5% |
| High school diploma or GED | 6.8% |
| Some college, no degree | 16.0% |
| Associate or technical degree | 9.4% |
| Bachelor’s degree | 35.1% |
| Master’s degree (MA, MS, etc.) | 15.8% |
| Doctoral degree (MD, PhD, JD, etc.) | 15.1% |
| No response | 1.4% |
| Annual household income | |
| Less than $50,000 | 13.4% |
| $50,000–$74,999 | 14.6% |
| $75,000–$99,999 | 14.2% |
| $100,000– $149,999 | 25.9% |
| $150,000–$200,000 | 13.6% |
| More than $200,000 | 15.1% |
| No response | 3.2% |
| Current smoker | 11.0% |
| Prior paternity | 12.7% |
| Testosterone use | 5.1% |
Sample collection
A gradual and step-wise approach to sample collection began in December of 2017. Semen samples were the first specimens obtained following completion of routine semen analyses (Table 3). Once all procedures for collection, transport, and storage were streamlined, urine collection began in April of 2018 followed by IVF samples in June of 2018. Surgical samples and blood were first obtained in December of 2018. A description of the number of samples collected to date by both participant number and total available aliquots is demonstrated in Table 3. A variety of specimens have been collected to date. This includes blood samples from 255 participants (114 collected at time of surgery and 141 not related to surgery) separated into plasma, serum, white blood cells, and separate collections of whole blood and blood for heavy metal testing. Semen samples have been collected from 482 participants cryopreserved as sperm and seminal fluid with aliquots of whole sperm. IVF samples have been collected from 78 participants and include follicular fluid, granulosa cells, cumulus cells, and immature/unfertilized oocytes. A range of surgical tissues have been collected and include ovarian tissue, fibroids, endometrium, myometrium, tissue from all three portions of the fallopian tube, and cell washes from the fimbriated ends of the fallopian tubes in patients at risk for malignancy. Additionally, testicular and epididymal tissue is also available.
Table 3.
Samples collected to date
| Blood samples | Number of samples (by participant) |
|---|---|
| Blood collected at time of surgery | |
| White blood cells | 94 |
| Heavy metals/serum | 98 |
| Plasma | 114 |
| Serum | 114 |
| Blood collected at all other times | |
| Serum | 126 |
| Plasma | 114 |
| whole blood | 141 |
| Urine | 86 |
| IVF samples | |
| Follicular fluid | 77 |
| Granulosa cells | 78 |
| Immature oocytes | 20 |
| Cumulus cells | 77 |
| Gynecologic surgery tissue samples | |
| Ovarian tissue | 53 |
| Fibroid/leiomyoma tissue | 41 |
| Myometrium | 78 |
| Endometrium | 13 |
| Endometrioma | 43 |
| Fallopian tube | 84 |
| Fimbriated ends | 82 |
| Cervical tissue | 77 |
| Male samples | |
| Semen | 482 |
| Testicular tissue | 1 |
| Epididymis | 1 |
| Number of samples (by aliquot) | |
| Blood | |
| Blood collected at time of surgery | |
| White blood cells | 108 |
| Heavy metals/serum | 604 |
| Plasma | 838 |
| Serum | 949 |
| Blood collected at all other times | |
| Serum | 739 |
| Plasma | 944 |
| Whole blood | 874 |
| Urine | 770 |
| Semen | |
| Whole semen | 2039 |
| Sperm | 4664 |
| Seminal fluid | 2288 |
| IVF samples | |
| Cumulus cells | 94 |
| Granulosa cells | 95 |
| Follicular fluid | 1082 |
| Immature oocytes | 67 |
| Surgical tissue flash frozen | |
| Endometrium | 147 |
| Myometrium | 330 |
| Leiomyoma | 412 |
| Cervix | 473 |
| Fallopian tube | 401 |
| Fimbriated end | 215 |
| Ovary | 119 |
| Testicle | 16 |
| Epididymis | 3 |
| Surgical tissue stored in RNAlater | |
| Endometrium | 117 |
| Myometrium | 314 |
| Leiomyoma | 403 |
| Cervix | 481 |
| Fallopian tube | 329 |
| Fimbriated end | 184 |
| Ovary | 104 |
| Testicle | 10 |
| Epididymis | 3 |
| Surgical tissue stored as cell lines | |
| Endometrium | 138 |
| Myometrium | 526 |
| Leiomyoma | 418 |
| Fimbriated end | 4 |
| Number of samples (cassettes) | |
| Surgical tissue paraffin embedded | |
| Endometrium | 37 |
| Myometrium | 85 |
| Leiomyoma | 81 |
| Cervix | 83 |
| Fallopian tube | 146 |
| Fimbriated end | 79 |
| Ovary | 27 |
| Testicle | 6 |
| Epididymis | 1 |
Discussion
Here, we report our experience successfully establishing a reproductive biorepository. We have implemented a streamlined process for collecting both clinical data and reproductive tissue from both female and male participants. Clinical data includes both relevant medical and fertility history as well as fertility treatments and outcomes. Thus far, we have collected blood, urine, sperm/seminal fluid, testicular and epididymal tissue, ovarian tissue, and uterine tissue and cells. Furthermore, we are also collecting samples from patients undergoing IVF such as follicular fluid, granulosa cells, and immature/unfertilized oocytes. Importantly, samples from each participant are processed and stored by a variety of methods (i.e., cryopreservation, flash frozen, paraffin embedded, stored in RNAlater®) allowing for multiple downstream evaluations of the same specimen/patient. This large collection of samples and corresponding fertility phenotyping will allow for a comprehensive translational assessment that includes the ability to perform complementary evaluations of reproductive tissues. Indeed, RSRSR has already resulted in multiple internal and external collaborations utilizing advanced analytic techniques such as proteomic and RNA analysis of sperm and seminal fluid, single-cell RNA-sequencing of reproductive tissues, and novel markers of fertility. Additionally, we plan to expand sample collection and currently have IRB approval to collect additional samples such as vaginal and anal swabs, buccal swabs/saliva, and hair and nail trimmings.
Major strengths of our biorepository include the variety of specimens included (from female, male, transgender, and nonbinary participants), with corresponding comprehensive clinical data. Furthermore, we are also collecting data regarding fertility treatment cycles and outcomes, thus allowing for a comprehensive picture of infertility patients undergoing treatment with linked biologic specimens. Additional strengths include recruitment from a variety of clinics such as general gynecology, reproductive endocrinology, urogynecology, transgender medicine, and reproductive urology. This allows for inclusion of a spectrum of patients and pathophysiologic processes. Finally, streamlined protocols for collection, processing, and storage of tissues allow for consistent quality of specimens.
A weakness of RSRSR is the fairly homogeneous patient population. The predominantly Caucasian population is fairly reflective of the infertility population seen at the University of Michigan and likely reflects initial heavy recruitment from this site. With growth and additional recruitment, a more diverse population should be captured. Additional limitations include small sample sizes of some specimens such as testicular tissue. As the biorepository continues to grow, we expect an increase of all sample types.
Despite these weaknesses, we believe our biorepository offers tremendous potential for innovative research in the fields of gynecology, urology, and reproductive medicine. As previously mentioned, approximately 15–30% of infertility is unexplained [16]. Furthermore, it has been suggested that many diagnoses in reproductive endocrinology, such as diminished ovarian reserve (DOR) and recurrent pregnancy loss (RPL), are simply descriptive explanations, as the mechanisms underpinning these phenomena remain unclear [23]. These gaps in knowledge are persistent among both female and male infertility. For example, in up to 45% of male patients diagnosed with male factor infertility, the underlying etiology of altered semen parameters is unknown [24]. These knowledge gaps in reproductive medicine have profound impacts on patients relying on infertility treatments. CDC data suggests that IVF treatments fail over 65% of the time on a per-cycle basis [25]. Furthermore, the average cycle costs $14,000–18,000 when medications are included [26, 27]. Thus, despite our advances in treatment, it is clear that much work still remains to be done. Our hope is through utilization of well-phenotyped samples; our current understanding of male and female reproductive medicine will be expanded, ultimately leading to improved and more individualized treatment options for patients.
Funding
This work was supported by the University of Michigan Department of Obstetrics & Gynecology. This work was also funded by the National Institutes of Health Grants 5K12HD065257-10 (DF & SBS), 5R01HD088638-03(EEM) & 5R01MD011570-05(EEM).
Declarations
Ethics approval
The protocols and procedures reported have been approved by the Institutional Review Board of the University of Michigan Medical School (IRBMED), registered under ID HUM00125627.
Conflict of interest
EEM is a consultant for Myovant Sciences. The other authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIH. The Promise of Precision Medicine. 2020; Available from: https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/promise-precision-medicine.
- 3.De Souza YG, Greenspan JS. Biobanking past, present and future: responsibilities and benefits. AIDS. 2013;27(3):303–312. doi: 10.1097/QAD.0b013e32835c1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaught J. Biobanking comes of age: the transition to biospecimen science. Annu Rev Pharmacol Toxicol. 2016;56:211–228. doi: 10.1146/annurev-pharmtox-010715-103246. [DOI] [PubMed] [Google Scholar]
- 5.Carrick DM, Mette E, Hoyle B, Rogers SD, Gillanders EM, Schully SD, Mechanic LE. The use of biospecimens in population-based research: a review of the National Cancer Institute's Division of Cancer Control and Population Sciences grant portfolio. Biopreserv Biobank. 2014;12(4):240–245. doi: 10.1089/bio.2014.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer LJ. UK Biobank: bank on it. Lancet. 2007;369(9578):1980–1982. doi: 10.1016/S0140-6736(07)60924-6. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser Permanente Research Bank. Available from: https://researchbank.kaiserpermanente.org/.
- 8.All of Us Research Program, I., et al., The "All of Us" Research Program. N Engl J Med, 2019. 381(7): p. 668-676. [DOI] [PMC free article] [PubMed]
- 9.University of Michigan Central Biorepository. Available from: https://research.medicine.umich.edu/our-units/central-biorepository.
- 10.Schenk M, Huppertz B, Obermayer-Pietsch B, Kastelic D, Hörmann-Kröpfl M, Weiss G. Biobanking of different body fluids within the frame of IVF-a standard operating procedure to improve reproductive biology research. J Assist Reprod Genet. 2017;34(2):283–290. doi: 10.1007/s10815-016-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santillan MK, Leslie KK, Hamilton WS, Boese BJ, Ahuja M, Hunter SK, Santillan DA. Collection of a lifetime: a practical approach to developing a longitudinal collection of women's healthcare biological samples. Eur J Obstet Gynecol Reprod Biol. 2014;179:94–99. doi: 10.1016/j.ejogrb.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krawetz SA, Casson PR, Diamond MP, Zhang H, Legro RS, Schlaff WD, Coutifaris C, Brzyski RG, Christman GM, Santoro N, Eisenberg E, Reproductive Medicine Network Establishing a biologic specimens repository for reproductive clinical trials: technical aspects. Syst Biol Reprod Med. 2011;57(5):222–227. doi: 10.3109/19396368.2011.604819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruth KS, Perry JRB, Henley WE, Melzer D, Weedon MN, Murray A. Events in early life are associated with female reproductive ageing: a UK Biobank Study. Sci Rep. 2016;6:24710. doi: 10.1038/srep24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheldon E, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 2011;95(6):2120–2122. doi: 10.1016/j.fertnstert.2011.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capps B. Models of biobanks and implications for reproductive health innovation. Monash Bioeth Rev. 2015;33(4):238–257. doi: 10.1007/s40592-015-0042-y. [DOI] [PubMed] [Google Scholar]
- 16.Gelbaya TA, Potdar N, Jeve YB, Nardo LG. Definition and epidemiology of unexplained infertility. Obstet Gynecol Surv. 2014;69(2):109–115. doi: 10.1097/OGX.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 20.Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril. 2016;106(3):766–772. doi: 10.1016/j.fertnstert.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh EE, Chibber S, Wu J, Siegersma K, Kim J, Bulun S. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 expression and regulation in uterine leiomyoma. Fertil Steril. 2016;105(4):1070–1075. doi: 10.1016/j.fertnstert.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy AR, et al. Generation of multicellular human primary endometrial organoids. J Vis Exp. 2019;152. [DOI] [PMC free article] [PubMed]
- 23.Zhang PY, Yu Y. Precise personalized medicine in gynecology cancer and infertility. Front Cell Dev Biol. 2019;7:382. doi: 10.3389/fcell.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C, European Association of Urology Working Group on Male Infertility European Association of Urology guidelines on Male Infertility: the 2012 update. Eur Urol. 2012;62(2):324–332. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 25.Sunderam S, Kissin DM, Zhang Y, Folger SG, Boulet SL, Warner L, Callaghan WM, Barfield WD. Assisted reproductive technology surveillance - United States, 2016. MMWR Surveill Summ. 2019;68(4):1–23. doi: 10.15585/mmwr.ss6804a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain T, Grainger DA, Ball GD, Gibbons WE, Rebar RW, Robins JC, Leach RE. 30 years of data: impact of the United States in vitro fertilization data registry on advancing fertility care. Fertil Steril. 2019;111(3):477–488. doi: 10.1016/j.fertnstert.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Wu AK, Odisho AY, Washington SL, Katz PP, Smith JF. Out-of-pocket fertility patient expense: data from a multicenter prospective infertility cohort. J Urol. 2014;191(2):427–432. doi: 10.1016/j.juro.2013.08.083. [DOI] [PubMed] [Google Scholar]