Abstract
Purpose
Wide controversy is still ongoing regarding efficiency of preimplantation genetic testing for aneuploidy (PGT-A). This systematic review and meta-analysis, aims to identify the patient age group that benefits from PGT-A and the best day to biopsy.
Methods
A systematic search of the literature was performed on MEDLINE/PubMed, Embase and Cochrane Central Library up to May 2020. Eleven randomized controlled trials employing PGT-A with comprehensive chromosomal screening (CCS) on Day-3 or Day-5 were eligible.
Results
PGT-A did not improve live-birth rates (LBR) per patient in the general population (RR:1.11; 95%CI:0.87-1.42; n=1513; I2=75%). However, PGT-A lowered miscarriage rate in the general population (RR:0.45; 95%CI:0.25-0.80; n=912; I2=49%). Interestingly, the cumulative LBR per patient was improved by PGT-A (RR:1.36; 95%CI:1.13-1.64; n=580; I2=12%). When performing an age-subgroup analysis PGT-A improved LBR in women over the age of 35 (RR:1.29; 95%CI:1.05-1.60; n=692; I2=0%), whereas it appeared to be ineffective in younger women (RR:0.92; 95%CI:0.62-1.39; n=666; I2=75%). Regarding optimal timing, only day-5 biopsy practice presented with improved LBR per ET (RR: 1.37; 95% CI: 1.03-1.82; I2=72%).
Conclusion
PGT-A did not improve clinical outcomes for the general population, however PGT-A improved live-birth rates strictly when performed on blastocyst stage embryos of women over the 35-year-old mark.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02227-9.
Keywords: Systematic Reviews, Randomized Controlled trials, Infertility, Assisted Conception, Genetics, Clinical Guidelines
Introduction
Preimplantation genetic testing for aneuploidy (PGT-A) is the definitive tool in embryo selection on the grounds of euploidy [1]. It is mainly recommended for the very distinctive cohort of patients characterized by advanced maternal age (AMA), recurrent implantation failure (RIF), recurrent pregnancy loss (RM), cases of severe male infertility, or elective single ET [2, 3].
Two schools of thought—if not more—are emerging with regard to “when” and “how” PGT-A should be employed. The factual complex nature of PGT-A encompassing the demanding procedures of biopsy and subsequent genetic analysis along with various options and combinations adds another level of complexity in reaching a consensus on optimal practice.
Trophectoderm biopsy represents the current trend; nonetheless, D3 biopsy clearly still holds a valid place in clinical practice [4, 5]. Performing biopsy at cleavage stage may address the risk of fewer embryos reaching blastocyst stage. On the other hand, it has been reported in literature that embryos which were classified as mosaic during cleavage stage biopsy were then evaluated as euploid in a blastocyst stage biopsy [6]. Furthermore, cleavage stage biopsy harbors the peril of inaccurate diagnosis, on the grounds of the single-cell analysis [7], while trophectoderm biopsy may hold remarkable promise considering it allows for an abundance of biopsied cells [7]. Misdiagnosis following trophectoderm biopsy may reflect cases of mosaicism. Despite the fact that discordance between trophectoderm and inner cell mass is a rare event [8, 9], it should not be overlooked in the limited number of cases it may be observed. . Regarding the biopsy technique, controversies are apparent [7, 10, 11]. The day 3 (D3) vs day 5 (D5) biopsy battle has been at the spotlight for years with more recent data supporting performing biopsy at blastocyst stage coupled by comprehensive chromosomal screening (CCS) [12].
Several meta-analyses investigated the benefit of PGT-A, with 2 out of 3 meta-analyses concluding that PGT-A, when employing the FISH technique, was accompanied with lower ongoing pregnancy and live-birth rates, when compared to conventional cycles [3, 13, 14]. Contrarily, it was observed that PGT-A based on CCS may improve clinical pregnancy and live-birth rates [15, 16]. The fact that lately PGT-A has been marketed and provided on a more horizontal level has led to well-informed patients viewing it as an “add-on” to improve clinical outcome of IVF, a scenario harboring risk [17, 18]. The present meta-analysis aims to provide updated evidence regarding the efficiency of PGT-A on clinical outcomes. The extensive employment of CCS as well as the vitrification method for embryo cryopreservation renders meta-analyses based on less recent studies outdated. In comparison to other meta-analyses analyzing strictly CCS, the present one reports on considerably higher volume of data.
This study includes both a per ET and a per patient analysis, with the latter representing the intention-to-treat (ITT) analysis. The rationale behind this is that deviations from the protocol—in this case performing ET—may be due to valid prognostic factors that lead to exclusion of a case from the analysis. To provide an example on this, if the study design dictates that only euploid embryos should be included for ET, and if PGT-A indicates that all embryos tested are mosaic and yet the patient decides to proceed with transferring mosaic embryos, then this case would be excluded from the analysis, unless an ITT approach is adopted. A major decision that is not commonly accounted for are mosaic embryos. Since mosaic embryos may ultimately report on a euploid or aneuploid embryo, it depends on the respective protocol of each study whether they proceed with an ET or not—irrespectively of whether genetic analysis has been successful regarding identification of the true chromosomal status of the biopsied material. In order to be able to include these embryos which do not fall into the binomial outcome (euploid or aneuploid), for which PGT-A with CCS has been reported to be extremely accurate (false results ≤1%), employing an ITT was regarded as a prerequisite by the authors. Employing an ITT analysis ensures that all cases that were intended to be treated—irrespectively of whether an ET was performed—are included and reported on presenting an all-inclusive analysis.
This work aims to uniquely bring to literature network meta-analytical data on PGT-A regarding the efficiency of performing biopsy at the cleavage or the blastocyst stage, considering the age group that this procedure may prove beneficial for.
Methods
Search strategy
A systematic search of the literature was performed in the databases of PubMed/Medline, Embase, and Cochrane Central Library, limited to articles published in peer-reviewed journals up to June 2019 (Online Resource 1) and updated until the 10th of May 2020. The search strategy included the following keywords and their respective combinations: “In Vitro Fertilization,” “IVF,” “Intracytoplasmic Sperm Injection,” “ICSI,” “Assisted Reproduction,” “Assisted Reproduction Technologies,” “Preimplantation Genetic Screening,” “PGS,” “chromosome abnormality,” “aneuploidies,” “disorders,” “Day 3,” “cleavage stage embryo,” “Day 5,” “Day6,” “Blastocyst,” “biopsy,” “Randomized controlled trials,” and “RCT.” The initial search yielded 1819 studies from the three databases (PubMed/Medline: n=1098, Embase: n=608, Cochrane Central Library: n=113). From the total yield, 215 studies were duplicates thus removed. Following initial screening regarding titles and abstracts of all records, 1504 were refined resulting in relevant articles. Thenceforth, full texts were thoroughly screened, and citation mining of selected relevant publications was conducted for final inclusion. Following this thorough screening, a total of 10 studies [4, 5, 19–26] were included in this meta-analysis. Following the update, a total of 28 were identified. Following screening, only one relevant study was identified and added in this meta-analysis [27]. The flowchart of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) is depicted in Online Resource 2. Screening and study selection were performed by three independent authors (MA, PT, KS). Any disagreements between the authors were resolved by an arbitration mediated by the senior author. No protocol was submitted to the PROSPERO: International Prospective Register of Systematic Reviews, providing details on conducting of this study.
Study selection
The authors concurred on including only RCTs in this meta-analysis conducted in humans and published in English review articles, retrospective studies, nonrandomized prospective cohort studies, case series or reports were not eligible. The initial selection performed included screening of titles and abstracts of the studies in order to exclude obviously irrelevant studies. Following the initial selection, full-text articles of the remaining studies were obtained and thoroughly screened. A forward and backward citation mining was performed on all relevant studies. The population involved women undergoing IVF/ICSI cycles with or without PGT-A prior to embryo transfer (ET), with the former corresponding to the study group and the latter corresponding to the control group. Polar body biopsy was excluded from this meta-analysis, along with PGT for monogenic disorders, structural chromosomal abnormalities, or translocations. Regarding the control group, only morphological assessment of D3 or D5 embryos was performed prior to ET. Authors concurred on including studies that involved both fresh and frozen PGT-A cycles, as frozen cycles employing vitrification are reported to be of equal efficiency with fresh cycles, concerning clinical outcomes [28]. In this metanalysis, studies that included patients that were not randomized regarding PGT-A efficiency on pregnancy outcomes were not eligible for inclusion. These were studies comparing different techniques on chromosomal analysis such as NGS, aCGH, or PCR and studies that compared ovarian stimulation protocols or different culture media. The current meta-analysis included only studies that conducted 24 chromosome aneuploidy screening (24chr-AS), excluding those that conducted the analysis on certain number of chromosomes. The study by Forman et al., 2013 was also excluded as the number of embryos transferred between the two groups differed by protocol—single vs double ET.
Data extraction
Data extraction was performed independently by four authors (AR, PG, AP, and KN) according to the selection criteria. Regarding studies that did not provide age-subgroup analysis, personal communication was attempted with the authors. The communication attempts included two e-mails to the corresponding authors, 2 weeks apart, and a total waiting for response time of 1 month. Only the authors of the study conducted by Sui et al. responded to this inquiry.
Outcome measures
The primary outcome measures for this meta-analysis are live-birth rate per patient and miscarriage rate per clinical pregnancy. The secondary outcome measures are ongoing pregnancy rate per patient, clinical pregnancy per patient, cumulative live-birth rate per patient, live-birth rate per ET, ongoing pregnancy rate per ET, clinical pregnancy rate per ET, and cumulative live-birth rate per ET. Ongoing pregnancy is defined as a viable pregnancy at 20 weeks of gestation and clinical pregnancy as the presence of a gestational sack at 4–5 weeks of gestation. The per patient analysis—with the exception of cumulative live-birth rate—includes the events of the first attempt for ET only, while the population includes both women that underwent ET and those that were included in the randomization but did not proceed with ET due to any reason representing the ITT approach. Cumulative live-birth rate is defined as the number of live-births following multiple ETs. Cumulative live-birth is assessed according to the data provided by studies; however, it is uncertain whether all available embryos were transferred in all studies.
Assessment of risk of bias
Assessment of risk of bias was performed in included studies regarding selection bias (randomization), allocation concealment, selective reporting, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data, and other possible sources of bias. Bias assessment was performed independently by two authors (EM and GK), based on “Cochrane Risk of Bias Tool for Randomized Controlled Trials.” Any disagreements between the authors were determined by a third author (MS).
Statistical analysis
Meta-analysis regarding the age groups was performed via the RevMan (v.5.3). The network was performed employing frequentist methods via the “netmeta” package of the R programming language for statistical purposes. Network meta-analysis is performed by comparing direct and indirect effects. The direct effect is estimated by the studies comparing the two different groups directly, while the indirect effect is estimated by comparing the two groups to another “reference” group. The reference group in the present study is the one where embryo selection is based solely on morphology, without performing biopsy. Thus, the reference group may coincide with the control group. Risk ratio with 95% confidence intervals was employed for the analyses of the included studies. Either the fixed effect or the random effects model was employed for results pooling according to heterogeneity. Heterogeneity of the exposure effect was evaluated through I2 statistic. An I2 value 80% or greater indicated high heterogeneity, and thus, the meta-analysis was not performed. The random effects model was employed if I2 value was greater than 0 and a significant sample size difference was observed between the studies according to the 6th edition of the Cochrane Handbook. A chi-squared test for heterogeneity was also performed and the p-values were provided. Since study sizes in this meta-analysis differed significantly, the fixed effects model was employed only if I2=0%. Funnel plots for potential publication bias were conducted.
Results
Characteristics for each study included in this meta-analysis are presented in Table 1.
Table 1.
Principal characteristics of studies included in this network meta-analysis
| RCTs | Patient’s age (years) | Fresh/frozen cycles | Biopsy day/embryo stage | ET day/embryo stage | Ploidy analysis technique | PGT-A group (no of patients) | Control group (no of patients) | Outcome measures |
|---|---|---|---|---|---|---|---|---|
| Fiorentino et al., 2013 [4] | 36–43 | Fresh | D3/Cleavage | D5/Blastocyst | aCGH | 34 | 31 | OPR, CLB |
| Munné et al., 2019 [19] | 25–40 | Frozen | D5,6/Blastocyst | D5,6/Blastocyst | NGS | 330 | 331 | CPR, OPR, MR |
| Ozgur et al., 2019 [20] | ≤35 | Frozen | D5/Blastocyst | D5/Blastocyst | NGS | 109 | 111 | CPR, OPR, MR |
| Rubio et al., 2017 [5] | 38–41 | Fresh | D3/Cleavage | D5/Blastocyst | aCGH | 100 | 105 | CPR, OPR, MR, CLB |
| Scott et al., 2010 [22] | <43 | Fresh | D5/Blastocyst | NP/Blastocyst | qPCR | 13 | 15 | CPR |
| Scott et al., 2013a [23] | 21–42 | Fresh | D5/Blastocyst | D6/Blastocyst | qPCR | 72 | 83 | CPR, OPR, MR |
| Scott et al., 2013b [11] | <35 | Fresh | D3/Cleavage and D5/Blastocyst | D3/Cleavage and D5/Blastocyst | Microarray analysis and SNP | 113 | 113 | OPR |
| Sui et al., 2020 [27] | NP | Frozen | D5/Blastocyst | D5/Blastocyst | SNP | 103 | 104 | CPR1, OPR1, MR1, CLB |
| Treff et al., 2011 [24] | <43 | Fresh | D5/Blastocyst | NP/Blastocyst | qPCR | 37 | 39 | CPR |
| Yang et al., 2013 [26] | <35 | Frozen | D5/Blastocyst | D5/Blastocyst | aCGH | 55 | 48 | CPR, OPR, MR, CLB |
| Yang et al., 2017 [25] | <39 | Frozen | D5/Blastocyst | D5/Blastocyst | NGS | 85 | 84 | CPR, OPR, MR |
*RCTs, randomized controlled trials; D3, day 3; D5, day 5; ET, embryo transfer; PGT-A, preimplantation genetic testing for aneuploidy; NP, not provided; no, number; aCGH, array comparative genomic hybridization; NGS, next-generation sequencing; qPCR, quantitative polymerase chain reaction; SNP, single-nucleotide polymorphism; CPR, clinical pregnancy rates; OPR, ongoing pregnancy rates; MR, miscarriage rates; CLB, cumulative live-birth rates.1Data obtained following personal communication
Bias
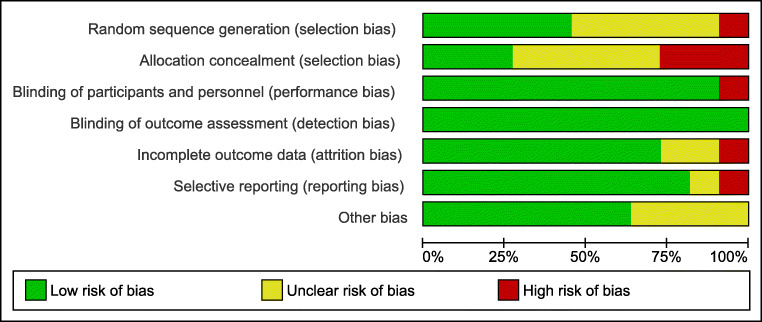
Assessment of bias for each study as well as for each item is presented in Figures 1 and 2. The performance and detection bias are considered low for all studies as the nature of the intervention and the outcomes do not allow for blinding of the personnel.
Fig. 1.
Assessment of risk of bias of studies included in the meta-analysis
Fig. 2.
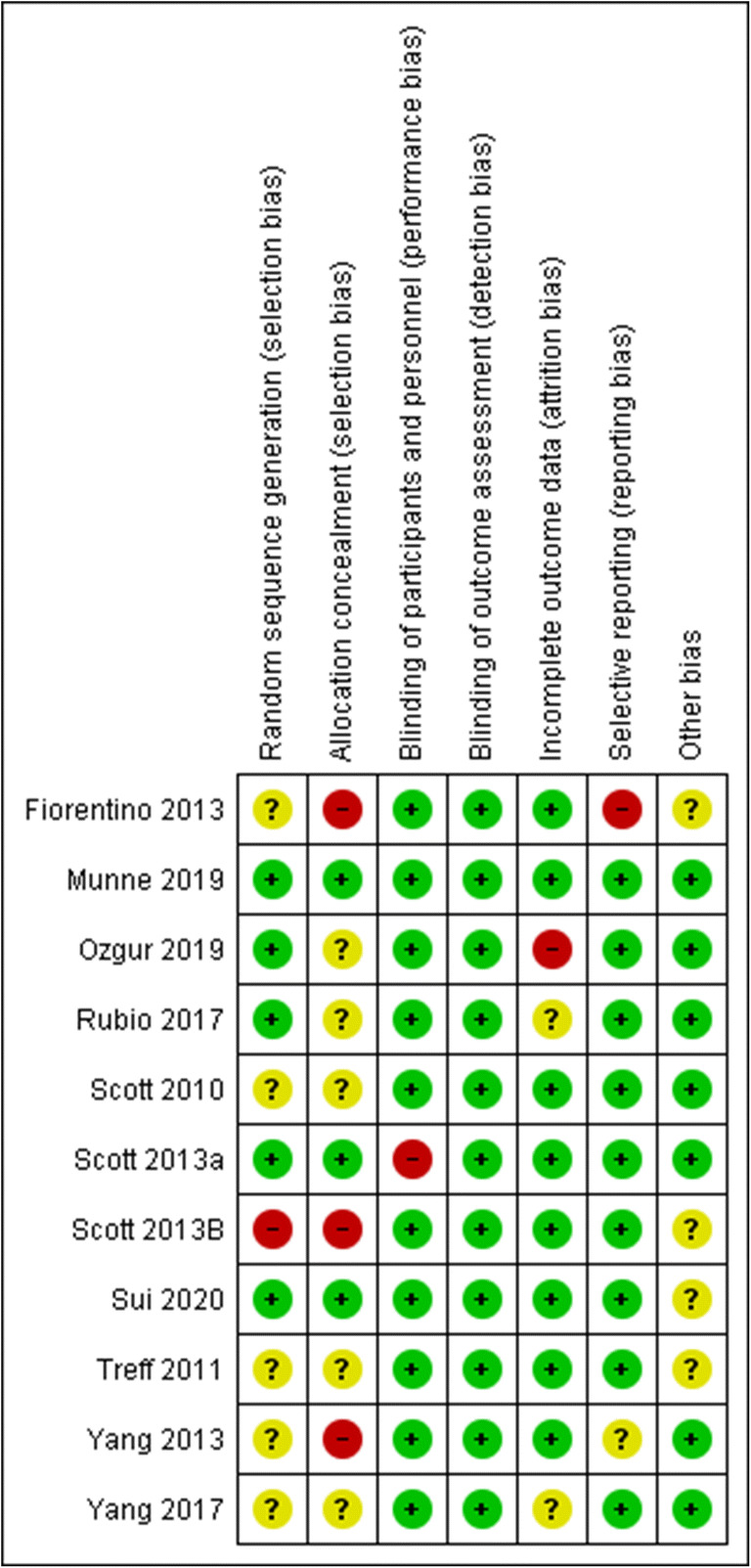
Summary of risk of bias assessment regarding each item for each study included in the meta-analysis
PGT-A in different age groups
A summary of findings regarding the primary outcomes as well as clinical pregnancy and cumulative clinical pregnancy per patient is presented in Table 2.
Table 2.
Summary of findings.
|
Patient or population: Couples undergoing IVF Settings: Assisted reproduction units Intervention: Preimplantation genetic testing for aneuploidy (PGT-A) Comparison: Morphological or morphokinetic evaluation | |||||
|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect(95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk for control group | Corresponding risk for the PGT-A group | ||||
| Live-Birth | 414 per 1000 | 431 per 1000 (360 to 588) | RR: 1.11 (0.87 to 1.42) | 1513 (6) | ⊕⊝⊝⊝very low a,b |
| `Live Birth - ≤35 years old | 481 per 1000 | 405 per 1000 (298 to 669) | RR: 0.92(0.62-1.39) | 666 (3) | ⊕⊝⊝⊝very low a,b |
| Live Birth - >35 years old | 290 per 1000 | 379 per 1000 (305 to 464) | RR: 1.29(1.05-1.60) | 692 (4) | ⊕⊕⊕⊝moderate c |
| Ongoing Pregnancy | 432 per 1000 | 474 per 1000 (389 to 825) | RR: 1.31 (0.90-1.91) | 933 (3) | ⊕⊝⊝⊝very low a,b |
| Miscarriage | 197 per 1000 | 101 per 1000 (49 to 158) | RR: 0.36 (0.17-0.73) | 912 (7) | ⊕⊕⊝⊝low a |
| Miscarriage - ≤35 years old | 161 per 1000 | 133 per 1000 (60 to 232) | RR: 0.73 (0.37 to 1.44) | 383 (3) | ⊕⊕⊝⊝low a |
| Miscarriage - >35 years old | 279 per 1000 | 104 per 1000 (33 to 326) | RR: 0.37 (0.12 to 1.17) | 221(2) | ⊕⊕⊕⊝moderate d |
| Clinical Pregnancy | 521 per 1000 | 546 per 1000 (495 to 714) | RR: 1.14 (0.95 to 1.37) | 1824 (9) | ⊕⊝⊝⊝very low a,b |
| Clinical Pregnancy - ≤35 years old | 570 per 1000 | 503 per 1000 (388 to 770) | RR 0.96 (0.68 to 1.35) | 679 (3) | ⊕⊝⊝⊝very low a,b |
| Clinical Pregnancy - >35 years old | 406 per 1000 | 434 per 1000 (361 to 520) | RR 1.07 (0.89 to 1.28) | 510 (2) | ⊕⊕⊕⊕high |
| Cumulative Live Birth | 368 per 1000 | 512 per 1000 (416 to 604) | RR 1.36 (1.13 to 1.64) | 580 (4) | ⊕⊝⊝⊝very low b,e |
*The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
CI, confidence interval; RR, risk ratio
Ongoing pregnancy/live birth, clinical pregnancy, and cumulative live-birth rates are measured per patient. Miscarriage rate is measured per clinical pregnancy
GRADE working group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate—the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited—the true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect
a: Downgraded two levels for serious risk of bias—More than one study presented with at least one item with high risk of bias or with at least two items with unclear risk of bias
b: Downgraded one level for inconsistency—Heterogeneity higher than 75%
c: Downgraded one level for risk of bias—One study presented with at least one item with high risk of bias or with at least two items with unclear risk of bias
d: Downgraded one level for imprecision—Wide confidence interval
e: At least half of included studies presented with two items with high risk of bias
Primary outcome measures
Live-birth rate per patient
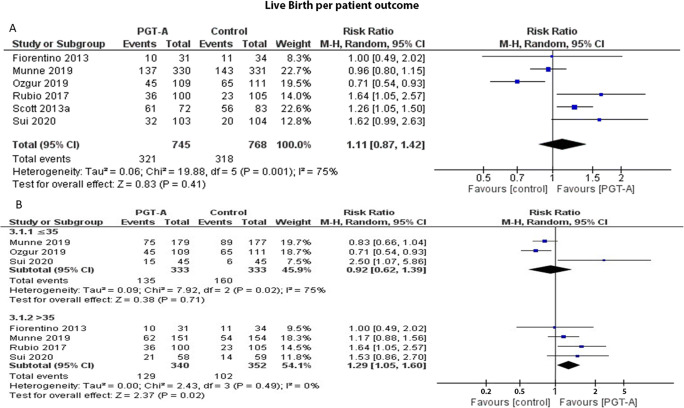
Six studies reported results on live-birth rates per patient. Heterogeneity among the studies was reported to be significantly high (I2=64%); thus, the random effects model was employed. The pooled results reported no statistically significant difference regarding live-birth rates following PGT-A (RR: 1.15; 95% CI: 0.94–1.41; n=1513) (Figure 3a). Following age-subgroup analysis for live-birth rates, when PGT-A was employed in women 35 years old or younger, no statistically significant difference was observed between the groups (RR: 0.97; 95% CI: 0.70–1.36; n=666). In the over 35-year-old age group, PGT-A improved live-birth rates (RR: 1.29; 95% CI: 1.05–1.60; n=629) (Figure 3b).
Fig. 3.
(A) Forest plot regarding the live-birth rate per patient outcome, comparing PGT-A to morphological evaluation (control), in the general population. (B) Forest plot regarding the live-birth rate per patient outcome, comparing PGT-A to morphological evaluation (control), in women of different age groups
Miscarriage rate per clinical pregnancy
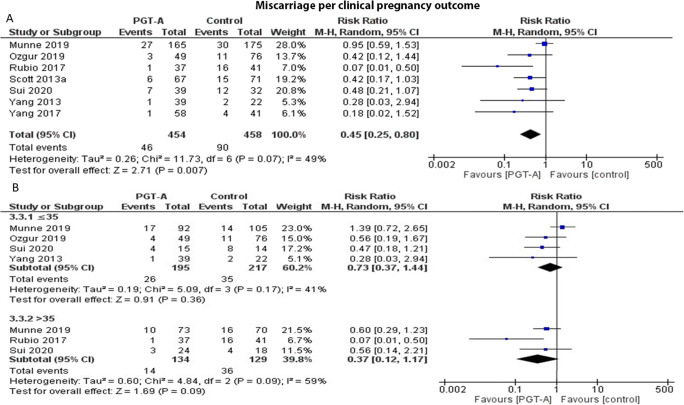
Seven studies reported results on miscarriage rates per clinical pregnancy. Heterogeneity among the studies was reported to be high (I2=57%); thus, the random effects model was employed. The pooled results reported a statistically significant lower miscarriage rate following PGT-A (RR: 0.36; 95% CI: 0.17–0.73; n=912) (Figure 4a). Following age-subgroup analysis for miscarriage rates, when PGT-A was employed in women 35 years old or younger, no statistically significant difference was observed between the groups (RR: 0.86; 95% CI: 0.38–1.95; n=412). Similarly, in the over 35-year-old age group, no statistically significant difference was observed (RR: 0.24; 95% CI: 0.12–1.37; n=263) (Figure 4b).
Fig. 4.
(A) Forest plot regarding the miscarriage rate per clinical pregnancy outcome, comparing PGT-A to morphological evaluation (control), in the general population. (B) Forest plot regarding the miscarriage rate per clinical pregnancy, comparing PGT-A to morphological evaluation (control), in women of different age groups
Secondary outcome measures
Ongoing pregnancy rate per patient
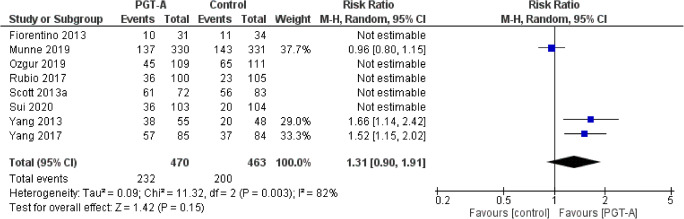
Three studies reported results on ongoing pregnancy rates per patient. Heterogeneity among the studies was reported to be significantly high (I2=82%); thus, the random effects model was employed. The pooled results reported no statistically significant difference regarding ongoing pregnancy rates following PGT-A (RR: 1.31; 95% CI: 0.90–1.91; n=933) (Figure 5).
Fig. 5.
Forest plot regarding the ongoing pregnancy rate per patient outcome, comparing PGT-A to morphological evaluation (control), in the general population
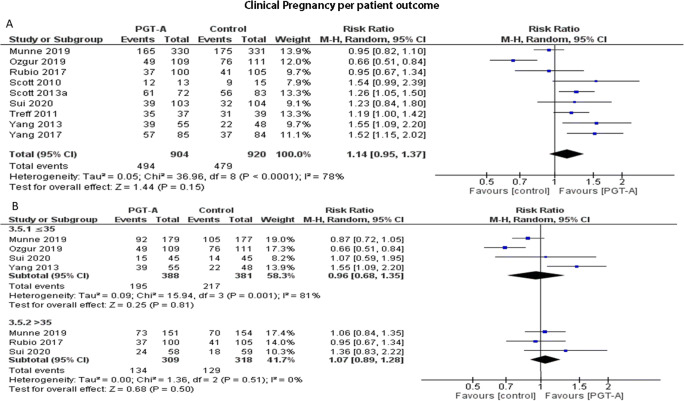
Clinical pregnancy rate per patient
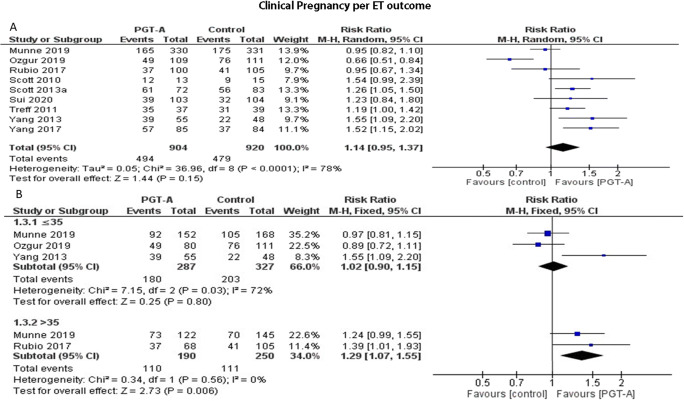
Nine studies reported results on clinical pregnancy rates per patient. Heterogeneity among the studies was reported to be significantly high (I2=79%); thus, the random effects model was employed. The pooled results reported no statistically significant difference regarding clinical pregnancy rates following PGT-A (RR: 1.14; 95% CI: 0.95–1.37; n=1824) (Figure 6a). Following age-subgroup analysis for clinical pregnancy rates per patient, when PGT-A was employed in women 35 years old or younger, no statistically significant difference was observed between the groups (RR: 0.96; 95% CI: 0.68–1.35; n=769). In the over 35-year-old age group, clinical pregnancy did not differ between the two groups (RR: 1.07; 95% CI: 0.89–1.28; n=627) (Figure 6b).
Fig. 6.
(A) Forest plot regarding the clinical pregnancy rate per patient outcome, comparing PGT-A to morphological evaluation (control), in the general population. (B) Forest plot regarding the clinical pregnancy rate per patient outcome, comparing PGT-A to morphological evaluation (control), in women of different age groups
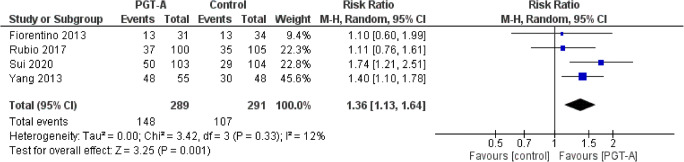
Cumulative live-birth rate per patient
Four studies reported results on cumulative live-birth rates per patient. Heterogeneity among the studies was reported to be insignificant (I2=12%), nonetheless higher than I2=0%; thus, the random effects model was employed. The cumulative live-birth rate per patient was improved following PGT-A compared to control (RR: 1.36; 95% CI: 1.13–1.64; n=580). No subgroup analysis was performed due to the limited number of studies (Figure 7).
Fig. 7.
Forest plot regarding the cumulative live-birth rate per patient outcome, comparing PGT-A to morphological evaluation (control), in the general population
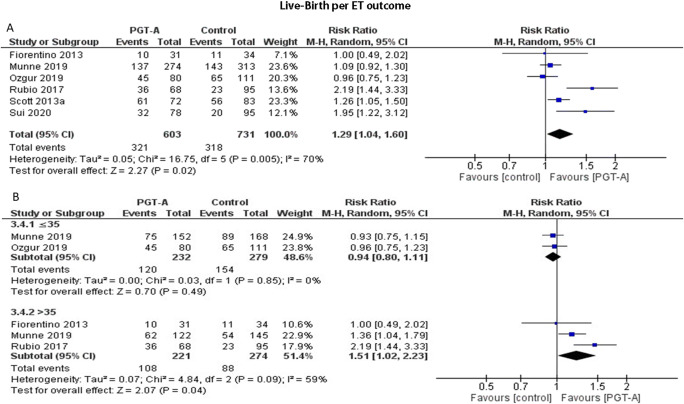
Live-birth rate per ET
Six studies reported results on live-birth rates per ET cycle. Heterogeneity among the studies was reported to be significantly high (I2=74%); thus, the random effects model was employed. The pooled results reported a statistically significant higher ongoing pregnancy following PGT-A (RR: 1.32; 95% CI: 1.04–1.66; n=1334) (Figure 8a). Following subgroup analysis, when PGT-A was employed in women 35 years old or younger, no statistically significant difference was observed between the groups (RR: 0.94; 95% CI: 0.80–1.11; n=511). In the over 35-year-old age group, PGT-A improved live-birth rates (RR: 1.51; 95% CI: 1.02–2.23; n=495) (Figure 8b).
Fig. 8.
(A) Forest plot regarding the live-birth rate per ET outcome, comparing PGT-A to morphological evaluation (control), in the general population. (B) Forest plot regarding the live-birth rate per ET outcome, comparing PGT-A to morphological evaluation (control), in women of different age groups
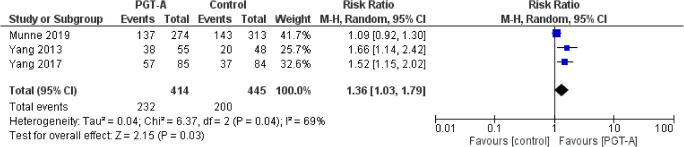
Ongoing pregnancy rate per ET
Three studies reported results on ongoing pregnancy rates per ET cycle. Heterogeneity among the studies was reported to be significantly high (I2=69%); thus, the random effects model was employed. The pooled results reported a statistically significant higher ongoing pregnancy following PGT-A (RR: 1.36; 95% CI: 1.03–1.79; n=859) (Figure 9).
Fig. 9.
Forest plot regarding the ongoing pregnancy rate per ET outcome, comparing PGT-A to morphological evaluation (control), in the general population
Clinical pregnancy rate per ET
Nine studies reported results on clinical pregnancy rates per ET cycle. Heterogeneity among the studies was reported to be high (I2=61%); thus, the random effects model was employed. The pooled results reported a statistically significant higher clinical pregnancy rates following PGT-A (RR: 1.28; 95% CI: 1.12–1.46; n=1673) (Figure 10a). Following subgroup analysis, when PGT-A was employed in women 35 years old or younger, no statistically significant difference was observed between the groups (RR: 1.02; 95% CI: 0.90–1.15; n=614). In the over 35-year-old age group, PGT-A improved clinical pregnancy rates (RR: 1.29; 95% CI: 1.07–1.55; n=440) (Figure 10b).
Fig. 10.
(A) Forest plot regarding the clinical pregnancy rate per ET outcome, comparing PGT-A to morphological evaluation (control), in the general population. (B) Forest plot regarding the clinical pregnancy rate per ET outcome, comparing PGT-A to morphological evaluation (control), in women of different age groups
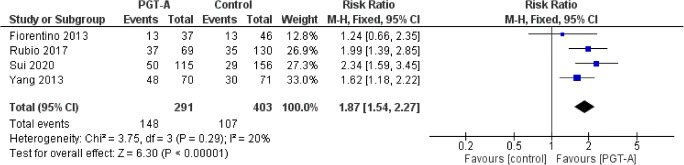
Cumulative live-birth rate per ET
Four studies reported results on cumulative live-birth rate per ET cycle. Heterogeneity among the studies was reported to be insignificant (I2=20%), nonetheless higher than I2=0%; thus, the random effects model was employed. The cumulative live-birth rate per ET cycle was statistically significantly improved following PGT-A compared to control (RR: 1.87; 95% CI: 1.54–2.27; n=694). No subgroup analysis was performed due to the limited number of studies (Figure 11).
Fig. 11.
Forest plot regarding the cumulative live-birth rate per ET outcome, comparing PGT-A to morphological evaluation (control), in the general population
PGT-A biopsy on different days
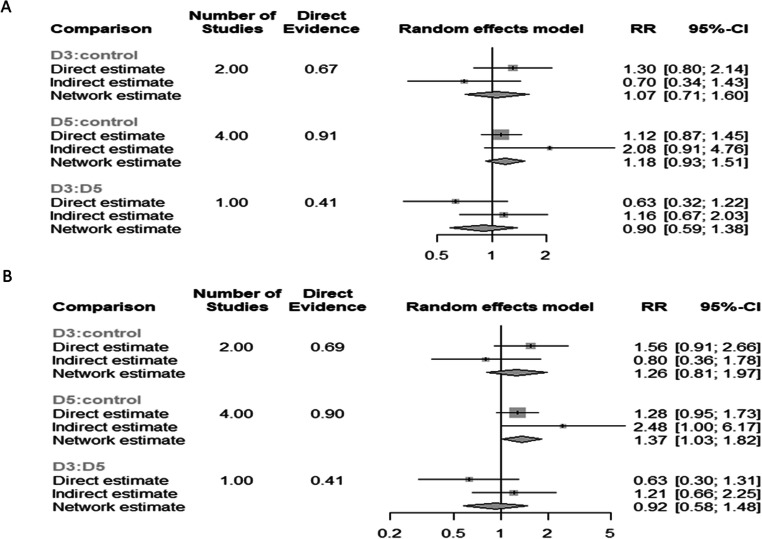
Live-birth rates per patient
Seven studies reported results on live-birth rates per patient. Three different study designs (D5 biopsy vs D3 biopsy; D5 biopsy vs control; D3 biopsy vs control) compared a total of two different biopsy days. The control group refers to embryo selection based solely on morphological evaluation without performing biopsy. A total 1629 patients were evaluated. Seven pairwise comparisons were evaluated. Heterogeneity among the studies was reported to be significantly high (I2=72.4%); thus, the random effects model was employed. No statistically significant difference was presented in the network estimate between the 2 days of biopsy (D3 vs D5) (RR: 0.90; 95% CI: 0.59–1.38). No statistically significant difference was observed regarding D3 biopsy (D3 vs control) (RR: 1.07; 95% CI: 0.71–1.60). PGT-A employing D5 biopsy did not present with a statistically significant higher live-birth rates compared to control (D5 vs control) (RR: 1.18; 95% CI: 0.93–1.51) (Figure 12a).
Fig. 12.
(A) Forest plot of the network regarding the live-birth rate per patient outcome, comparing PGT-A with day 5 biopsy to PGT-A with day 3 biopsy to morphological evaluation (control), in the general population. (B) Forest plot of the network regarding the live-birth rate per ET outcome, comparing PGT-A with day 5 biopsy to PGT-A with day 3 biopsy to morphological evaluation (control), in the general population
Live-birth rates per ET
Seven studies reported results on live-birth rates per ET cycle. Three different study designs (D5 biopsy vs D3 biopsy; D5 biopsy vs control; D3 biopsy vs control) compared a total of two different biopsy days. The control group refers to embryo selection based solely on morphological evaluation without performing biopsy. A total 1450 ET cycles were evaluated. Seven pairwise comparisons were evaluated. Heterogeneity among the studies was reported to be significantly high (I2=71.5%); thus, the random effects model was employed. No statistically significant difference was presented in the network estimate between the 2 days of biopsy (D3 vs D5) (RR: 0.92; 95% CI: 0.58–1.48). No statistically significant difference was observed regarding the D3 biopsy (D3 vs control) (RR: 1.26; 95% CI: 0.81–1.97). The D5 biopsy presented with a statistically significant higher live-birth rates when compared to control (RR: 1.37; 95% CI: 1.03–1.82) (Figure 12b).
Clinical pregnancy, miscarriage, and cumulative live-birth rates
Only one study reported ongoing pregnancy, clinical pregnancy, miscarriage, and cumulative live-birth rates following D3 biopsy PGT-A, and no study presented the D3 vs D5 design. Conducting a network meta-analysis with only indirect estimates and based on a single study for one of the two designs would result in very low-quality evidence that would be susceptible to bias. Thus, no network meta-analysis was performed regarding these outcomes.
Fresh vs frozen ET
In order to eliminate all possible confounders and additional biases, the comparison between fresh and frozen ET strategy following PGT-A was performed only on women aged 35 or older. Four studies met the inclusion criteria. Only when frozen ET was performed PGT-A improved live-birth rate (RR: 1.39; 95% CI: 1.09–1.78; n=384). On the other hand, no statistically significant difference was observed when comparing PGT-A followed by a fresh ET with control group (RR: 1.56; 95% CI: 0.73–3.34; n=228).
Discussion
Our results indicate that PGT-A did not improve live-birth rates per patient in the general population. This can be attributed mainly to the fact that no benefits were showcased when applied in younger women, rendering it ineffective. In the over 35-year-old group, PGT-A seems to improve live-birth rates. Ongoing pregnancy rate per patient was not improved following PGT-A. Due to the limited number of studies, no subgroup analysis was performed. Miscarriage rate was statistically significantly lower following PGT-A in the general population. When performing subgroup analysis for miscarriage rate, neither of the two age groups presented with any statistically significant difference. However, the results regarding the over 35-year-old group should be interpreted with caution as the confidence interval is very wide. Moreover, the lack of statistical difference regarding miscarriage rate in the over 35-year-old group could be partly attributed to the smaller sample size. This explanation may be supported by the wide confidence interval, as well as the fact that the sample size, namely 263 pregnancies, is the smallest employed in the present meta-analysis. Miscarriage is an event subject to multidimensional etiology, and even more so, it requires a larger population studied. Further to this, the fact that clinical pregnancy rates per patient were not improved following PGT-A coupled by the differences observed in ongoing pregnancy and live-birth rates indicates that the lack of statistical significance regarding miscarriage rate in women over 35 years old may be attributed to sample size. A clear conclusion of our study is that albeit PGT-A does not seem to improve clinical pregnancy rate irrespectively of age group, it improves ongoing pregnancy and live-birth rates in the over 35-year-old age group, as well as decreasing miscarriage rate. In light of this data, one could attempt the extrapolation that albeit PGT-A does not seem to affect chances of achieving a clinical pregnancy, by decreasing miscarriage rate, it improves chances of sustaining a pregnancy leading to live-birth for the over 35-year-old age group.
The cumulative live-birth rate was statistically significantly improved following PGT-A. The improved cumulative live-birth rate per patient for the PGT-A group may seem as a paradox. In theory, one would expect that if all patients—being subjected to PGT-A or not—received the total number of embryos, cumulative live-birth rate would be at least equal between the two groups in the per patient analysis especially taking into account biopsy application. However, perhaps not a true cumulative perspective can be exercised in true clinical practice. Discrepancies in clinical practice may lead to discrepant definitions of cumulative pregnancy in the studies published. This may be attributed to different study protocols or patient attrition and other factors involved. It should be mentioned that the majority of studies reported on cumulative live birth as a secondary outcome, with significant differences in strategy regarding the number of ETs. Results of this study,indicate that PGT-A may reduce the number of ETs required to achieve a live-birth by indentifying and excluding aneuploid embryos when employing the cumulative approach. Another point to consider is the fact that not all available embryos for transfer may be transferred; hence, meta-analysis may lead to a statistically significant difference. It should be highlighted that inter-study differences pertaining even to the definition of a cumulative pregnancy add another level of complexity to consider here and may well provide the basis for interpreting what may be perceived as unusual results. Taking into account these discrepancies, and reflecting on the pragmatic value of the cumulative live-birth rate, this outcome could also be described as a “combined-attempts live-birth rate”. This is due to the fact that it is uncertain whether all embryos were transferred, which would reflect the true cumulative approach. Only the study by Yang et al. (2013) provided information on the number of patients with cryopreserved embryos. This study employed a true cumulative approach as all patients without a clinical pregnancy proceeded with FET. In light of the high heterogeneity and the risk of bias identified, the authors of this meta-analysis have already graded the quality of evidence “very low,” as it can be clearly seen in Table 2. Information regarding the number of embryos cryopreserved and transferred in each group of the included studies are provided in Supplementary Table 1.
On the other hand, analyzing data on PGT-A cycles that reached and completed the ET procedure—indicating that PGT-A identified at least one euploid embryo—changes the perspective showcasing an improvement on live-birth rates. Following subgroup analysis PGT-A did not improve live-birth rates in younger women. In women over the 35-year-old mark, enhanced live-birth rates were reported when compared to the control group where ET was performed based on morphological evaluation. Ongoing pregnancy and cumulative pregnancy rates per ET were improved following PGT-A. Similarly, regarding clinical pregnancy rates, improved results were observed in the PGT-A group both in the general population and in women over the 35-year-old mark. In women 35 years old or younger, no statistically significant difference was observed.
The present meta-analysis is the only one comparing D3 and D5 biopsy for PGT-A. Although a Cochrane protocol regarding the day of biopsy has been published, the imminent study, according to title and protocol, is designed to compare different biopsy days for preimplantation genetic testing for monogenic diseases (PGT-M). Concerning the biopsy day, results of our study refer only on the outcome of live birth. No statistically significant difference was observed between D3 and D5 biopsy. However, only the D5 biopsy group presented with a statistically significantly increased live-birth rate per ET. It should be noted that only one of the studies included herein performed a direct comparison between D3 and D5 biopsy [21]. In this study, it was reported that patients allocated to receive D3 biopsy presented with a significantly reduced live-birth rate compared to patients allocated to D5 biopsy [21]. Results on ongoing pregnancy, clinical pregnancy, miscarriage, and cumulative live-birth rates could not be provided as there was only one study comparing D3 biopsy and PGT-A vs control—referring to the embryo selection based solely on morphological criteria, without performing biopsy—and no studies comparing D3 vs D5 biopsies. This may be attributed to most studies performing cleavage stage biopsy, employing FISH—not CCS.
Trophectoderm biopsy does not seem to reduce the implantation potential of the embryo. In a recent study, Tiegs and colleagues [29] attempted to assess the true effect of trophectoderm biopsy alone in live-birth rates for the first time. Design of this study entailed performing trophectoderm biopsy and proceeding to a frozen ET employing solely morphological criteria and not PGT-A results—which remained unknown and undisclosed at the time of ET. The authors reported that the biopsy and the no-biopsy group presented with similar live-birth rates. The aforementioned study also reported a negative predictive value of 100% when PGT-A was performed with a day 5 biopsy. Albeit it is widely acknowledged that RCTS should be relied upon to concur on safety and efficiency, nonetheless, not all RCTs are designed equal. Hence respective results even originating from an RCT study may be of high or low quality. Taking into consideration non-selection studies and their role in drawing conclusions, albeit as a study model, they are generally considered inferior to the weight conveyed by RCT data; nonetheless, their contribution should be accounted for. To elaborate on this, non-selection studies and RCTs present with different types of outcomes. While RCTs compare the employment of PGT-A regarding pregnancy and live-birth rates, they fail to provide information on the accuracy of PGT-A. In order to evaluate the diagnostic test accuracy, by assessing sensitivity, specificity, and positive and negative predictive value, solely non-selection studies should be evaluated as this information cannot be extracted from RCTs. This is especially true as PGT-A may not exactly fit into the RCT approach as it is not a single dimensional intervention, but rather presents as a multidimensional intervention when analyzing what is an efficient and successful outcome.
Undoubtably, factoring in the element of embryo morphology would provide further insight on how embryo quality at the time of ET affects outcome. Nonetheless, the majority of studies present a general classification of embryo quality. All studies included in the present meta-analysis employed what is described as “the higher quality embryos” available for transfer, according to morphological assessment. It should be mentioned that embryos that were not considered to be of “good quality” were not subjected to biopsy in the first place in the studies that provide information on embryo quality. All studies employing the strategy of frozen embryo transfer also employed luteal phase support.
Regarding the comparison between fresh and frozen ET strategy, an attempt to avoid additional bias and possible confounders was performed by only including one age group. The rationale behind opting for the over 35-year-old age group is that only this group of patients presented with a statistically significant difference, thus enabling a comparison. Moreover, women over the age of 35 are a more time-sensitive group; thus, investigating the optimal strategy should be prioritized for these patients. However, the comparison between fresh and frozen ET—albeit both report on D5 ETs—is susceptible to bias a priori as fresh ET studies performed a D3 biopsy, while frozen ET studies report on D5 biopsy. This is a major discrepancy that cannot be overlooked. The only study design that would not be subject to the confounder of the discrepant biopsy day when considering the comparison with the frozen ET group would be Scott’s study in 2013 reporting on D5 biopsy and a fresh D6 ET. However, in this case, the discrepant ET day, namely D5 vs D6, poses another confounder. Nonetheless, the authors did not provide age-subgroup analysis; thus, the study could not be included. In order to properly compare fresh ET to frozen ET following PGT-A, one would need to ascertain ideally same biopsy and same ET days. Nonetheless, this would not reflect clinical routine practice. Albeit the comparison between fresh and frozen is valid—despite the discrepancies—as it reports on the different strategies adopted, nonetheless, the superiority of the frozen ET strategy may well be attributed to two factors. Firstly, blastocyst biopsy being widely acknowledged as the biopsy day of choice is superior as results sourced herein indicate; secondly, the cryopreservation technique of vitrification enables excellent results, a statement that has been strongly supported and coupled by robust data [28]. When considering confounders pertaining to either PGT-A approach employing a fresh or a frozen embryo transfer, the performance of the thawed embryo along with endometrial receptivity should be accounted for. Despite the superiority of the vitrification process when compared to slow freezing, still, the survival rate of embryos following thawing has not yet reached 100% [30], resulting to a smaller number of embryos available for ET compared with the fresh transfer approach. On the other hand, the frozen ET approach, albeit it may seem to compromise the embryo’s implantation dynamic, allows for optimal endometrial receptivity and better synchronization [31].
The lack of improvement on live-birth rates following PGT-A in the pooled results is in concordance with previous recent systematic reviews and meta-analyses [3, 13, 14] that performed a per patient analysis, despite the fact that FISH studies were not included in the present meta-analysis. Similarly, our results are in agreement with meta-analyses that performed only per ET analysis [15, 16]. Albeit this work shares data with past meta-analyses, partly accounting for the concordant conclusions, the strength of the present meta-analysis is inclusion of the largest number of RCTs to date solely based on CCS. The statistically significant improvement in live birth in 35-year-old or older women is in discordance to a previous meta-analysis that demonstrated significantly lower ongoing pregnancy and live-birth rates [14]. This is attributed purely to RCTs reporting on FISH data being excluded herein, whereas Mastenbroek’s meta-analysis included mainly RCTs with FISH, employing principally cleavage stage biopsy—which at the time was common practice.
FISH did not improve pregnancy and live-birth rates, in contrast to CCS. This can be attributed to not all chromosomes being evaluated, along with FISH providing false-positive results [2, 32]. For this reason, studies involving FISH were excluded. The authors decided to include studies employing either PCR, aCGH, or NGS as a high level of agreement has been reported in the results acquired by these techniques [4, 5, 19–26]. The fact that only trophectoderm biopsy provided a statistically significant improvement in live-birth rates may originate from the fact that a smaller proportion of cells are removed. The enhanced results of trophectoderm biopsy are in agreement with numerous studies [33, 34]. PGT-A providing improved live-birth rates in older women may be explained by a “risk-benefit analysis.” In women younger than 35 years old, aneuploidy rates are lower [35]; thus, it may seem that there is no benefit in performing PGT-A. In older women, the rise in aneuploidy rates alternate the risk-benefit ratio favoring PGT-A. Previous meta-analyses have not reported results on women 35 years old or younger.
The enhanced results of PGT-A regarding cumulative live-birth rates may demonstrate the effectiveness of PGT-A in cycles with an abundance of embryos. It was observed that PGT-A led to significantly improved clinical outcomes when the analysis was performed per ET, an observation that was not confirmed in the per patient analysis. Since the population in the per patient analysis was higher than that in the per ET analysis if the sample size would lead to the discordant results, then one would expect the opposite outcome. Hence, this difference cannot be attributed to sample size. However, this difference may be explained as follows: PGT-A offers an additional screening, allowing practitioners to select the “best” embryo for transfer. Hence, when transferring “best” embryos that have been selected based on this additional criterion—with the prerequisite that biopsy does not exert a negative influence on the embryos’ implantation potential—it is expected that less ETs may be required to achieve a live birth. On the other hand, when transferring embryos not chromosomally examined, based purely on morphology and no other criterion, it is justified that perhaps more ETs may be required to reach the same clinical end point. This is true when comparing PGT-A to non-PGT-A cycles. However, concurrently and from a different perspective, when assessing PGT-A alone, it is understandable that the more embryos are available in a PGT-A cycle the higher the live-birth rate. This meta-analysis showed that cumulative live-birth rate is improved when PGT-A is performed. In reality, this statement is of no significance for patients that presented with a small number of embryos that did not allow for multiple ETs. The question is whether there is data to support that we should decide on a minimum adequate number of embryos as a cutoff point to ascertain optimal PGT-A application.
Moreover, the authors have observed that a number of studies have performed the randomization at the biopsy stage of the embryo and not at the oocyte retrieval stage. This may mean that patients that did not reach the required stage or the cutoff limit of the number of embryos that reach the required stage were excluded from the study. This may lead to the conclusion that the number of embryos available for biopsy may be of great significance when examining effectiveness of PGT-A. However, conduction of further and differently designed studies is a requirement before attempting to make a statement on the importance and the role that the number of embryos plays in deciding whether PGT-A is beneficial. It should be mentioned that when conducting a study which includes randomization prior to the biopsy stage, the number of patients without good quality embryos at the biopsy stage is expected to be similar between the PGT-A and the non-PGT-A groups. In an unbiased study, the number of patients not undergoing a biopsy or transfer due to poor developing embryos would be expected to be the same between the two groups. In contrast, the number of patients that do not proceed to embryo transfer due to a diagnosis of aneuploid embryos would be an additional number of patients besides the patients not proceeding to embryo biopsy on the grounds of poor embryo development.
When designing a study, it is paramount to ascertain conclusive results, providing the number of embryos per cycle along with the number of cells removed for the subsequent ploidy analysis. A computer-based randomization, employing true random algorithms, with age stratification—in case of employing more than one of the analyzed age groups—should be part of the design. Another important issue is the distinction between single-center and multi-center studies. It has been voiced that multi-center studies may be less biased regarding treatment effects when compared to single-center studies. On the other hand, less protocol variations are observed in single-center studies, at the cost of a smaller population. According to a recent analysis, it has been reported that the bias of larger treatment effects is only present in continuous outcomes and not in dichotomous [36]. Since clinical outcomes in the field of assisted reproduction are mainly dichotomous, the authors included both study types. When addressing only dichotomous outcomes, neither single-center nor multi-center studies present with an increased risk of bias. An ITT analysis and cycle cancellation rate should be included. Clinical outcomes with regard to clinical pregnancy, ongoing pregnancy, and live-birth, in line with clinical pregnancy loss, stand for integral end points. With regard to outcome measures, extra caution should be exercised to avoid discrepancies in definitions. From defining clinical and ongoing pregnancy to miscarriage and live-birth variations between studies harbors the risk of resulting to a domino effect and compromised results. It should be highlighted that ongoing pregnancy is not defined in the glossary proposed by ICMART. This may add confusion as the outcome definition may vary among studies leading to results’ discrepancies. It is of importance to clearly define ongoing pregnancy as it is an outcome widely reported on by studies in the field of assisted reproduction. Ideal design of a hypothetical RCT has been aptly described by Orvieto [37]. It should be mentioned that the ITT, which is an absolute necessity regarding RCTs, may in turn harbor bias when performed incorrectly. Not excluding the patients that opted to withdraw from the study for reasons that are irrelevant to the proposed intervention may add bias, by altering the effect estimate, to the ITT of the study and subsequently to future meta-analyses. When randomization is performed at the correct stage, the incorrect application of ITT is a rare event. Despite the numerous concerns voiced in literature [38, 39], the authors are more inclined to suggest that randomization performed at the biopsy stage harbors no additional bias, as it minimizes inclusion of patients that withdrew from the study for reasons irrelevant to the proposed intervention.
Concerning limitations of the current study, still the number of studies fitting inclusion criteria for this meta-analysis is small. However, it is the first meta-analysis that includes strictly RCTs employing complete chromosomal screening, as well as a comparison between the days of biopsy. The fact that only a single study reported on a direct comparison between cleavage and trophectoderm biopsy poses another limitation. No publication bias was detected as assessed by funnel plots. Two of the studies included in the present meta-analysis [5, 27] presented with more than one oocyte retrievals, in order to accumulate an adequate number of embryos. However, neither the number of oocyte retrievals performed nor the number of oocytes retrieved differed between the PGT-A and the non-PGT-A groups. This lack of statistically significant difference between the two groups means that the strategy of multiple retrievals did not lead to any difference which may had been a confounder considering the efficiency of PGT-A regarding clinical outcomes. Thus, the authors of this meta-analysis deemed that studies with multiple retrievals were suitable for inclusion. The data provided by Scott’s 2013 study could present as a confounder for the present meta-analysis, especially since a number of studies have reported lower live-birth rates following day 6 ET [40]. However, the authors have decided to include this study. A delayed ET on day 6 may negatively influence clinical outcomes of PGT-A presenting a compromised efficiency of PGT-A when we are discussing fresh ETs. Nonetheless, performing a delayed day 6 ET following PGT-A may better reflect the reality of clinical practice as without PGT-A there is a limited number of reasons to delay ET to day 6 which has been acknowledged to run the risk of missing the implantation window. When considering the results pertaining to efficiency of PGT-A in the general population, a limitation is the fact that the age for the majority of women analyzed in this study is younger than 35. The age of this population is not representative of the age of women commonly undergoing PGT-A. According to SART, 62% of women undergoing IVF cycles in 2020 in USA is older than 35, whereas in Europe, the respective population recorded for 2016 is 54%. However, in the present study, the majority of patients included were younger than 35. This fact may serve as a reason for caution when interpreting the results regarding the general population. Another reason for caution when interpreting the results of this study is the fact that the majority of studies employed a double embryo transfer. Double embryo transfer provides improved pregnancy and live-birth rates [41, 42], at the risk of leading to twin pregnancy which has been associated with poorer neonatal outcomes [43, 44]. Recent ASRM guidelines propose the employment of single ET in patients with at least 2 high-quality embryos, who are under the age of 38 years old [45]. In case of PGT-A, the guidelines of ASRM propose single embryo transfer regardless of patients’ age [45]. It has been voiced that elective single ET, following PGT-A, may provide similar live-birth rates to double ET without PGT-A [46]. Thus, in order to safely conclude on the efficiency of PGT-A, future RCTs should employ single embryo transfer in both PGT-A and control groups, as the majority of patients included present with at least two high-quality embryos.
This meta-analysis shows that PGT-A employing CCS, on blastocyst stage embryos, performed on women aged over the 35-year-old mark may improve clinical outcomes and live-birth rates. A valid interpretation of this metanalysis is that despite the fact that PGT-A may not appear to improve clinical pregnancy, it does however appear to decrease miscarriage rates. In leu of that, PGT-A may ultimately be viewed as means to ascertain higher chances that a pregnancy will lead to a live birth instead of a miscarriage in the over 35-year-old age group.
When considering the general population studied herein, results indicate that PGT-A did not improve live-birth rates per patient. The exact requirements justifying application of PGT-A in order for a beneficial effect to be ascertained should be further investigated in the context of future RCTs.
The limitation of failing to accurately define a successful PGT-A cycle
This meta-analysis did not showcase any detrimental effect of PGT-A application on outcomes examined. Interestingly results sourced herein support both opposing schools of thought depending on the perspective analyzed. The degree of conflicting conclusions may lie in the foolproof nature of statistical analysis. According to one scenario showcased herein, PGT-A did not ascertain a positive outcome, while according to another perspective, it was effective in improving outcomes. When asked the question “is PGT-A effective?” surprisingly—and relying our thesis on infallible statistical analysis—we can answer both “yes” and “no” and argue both sides of the story. More specifically, on one hand, clinical and live-birth rates were not improved when analyzed per patient. On the other hand, clinical and ongoing pregnancy and live-birth rates were improved when analyzed per ET. Having ascertained this as a prerequisite, our data showed that PGT-A improves outcomes when performed for women 35 years old and older and only when embryos are biopsied at the blastocyst stage, while analysis pertains to CCS. According to this, we may have to face up to the straightforward reality that in order for PGT-A to be effective, we need good quality euploid embryos to be transferred. Opting for this invasive procedure will make a difference only when the pool of embryos analyzed originates from intended mothers of advanced maternal age, where oocyte aneuploidy is anticipated to be increased.
Further to acknowledging this complexity, there are more issues to consider. The main purpose of PGT-A cycle is to perform an ET maximizing implantation potential while minimizing risk of pregnancy loss [47]. However, there are different levels of success in performing PGT-A. Could it be achieving a precise diagnosis and ensuring no false-positive or negative results? Could it be enabling ET of a balanced embryo, or the outcome of a successful clinical pregnancy, or a child of good pediatric follow-up? These scenarios pose as confounders in designing “a study of foolproof methodology.” The ideal case scenario could describe attainment of a diagnosis providing conclusive results indicating at least a euploid embryo for transfer. If results indicate aneuploidy for all embryos, ET may be cancelled [48]. Nonetheless, this may represent a successful PGT-A, especially when such results may provide closure for the couple [49] having undergone a course of futile IVF treatments. The possibility of lysed or degenerated embryos leads to ET cancellation, even though PGT-A may—in the meantime—provide results. Inconclusive results may highlight technical errors related to the embryo’s manipulation, the possibility of contamination during biopsy and subsequent tubing, as well as the efficiency of the molecular technique employed for ploidy [14, 15, 50]. Nevertheless, in PGT-A cycles, embryos with unidentified ploidy status due to inconclusive results may be eventually transferred, probably resulting in clinical pregnancy [20]. Could that be viewed as a success? The multifaceted nature of PGT-A success stands as a limitation when interpreting results. When reporting on PGT-A cycle success, we should clearly distinguish between efficiency of diagnosis and successful outcome regarding achieving a pregnancy leading to a healthy offspring. This may stand as a reason accounting for conflicting results and should be reported as a limitation in performing meta-analyses.
Considerations
Despite the fact that PGT-A application has been employed for almost 30 years, there is still an ongoing controversy regarding its effectiveness. In 2019, the Preimplantation Genetic Diagnosis International Community (PGDIS) published a position statement, concluding that PGT-A improved implantation, pregnancy, and live-birth rates[51]. This statement has been rebutted in literature [52]. It may be of value to consider that universal approaches or catholic applications may—to a certain extent—be outdated in the era of personalized medicine. According to the results of the present study, this seems to be the case with PGT-A. Data presented herein support that PGT-A performed on D5, employing CCS and accompanied with frozen D5 ET, enhances live-birth rates in women over the age of 35. Albeit this is a rather specific conclusion, another valid point to consider adding another level of complexity to evaluating PGT-A is the fact that effectiveness of PGT-A programs may differ and be subject to factors such as a poor or efficient biopsy directly affecting subsequent analysis and outcome [51]. In light of the numerous contradicting theses in literature on when, how, and for whom, the authors would like to respectfully suggest that perhaps relying solely on maternal age to draw the line and guide application of PGT-A may represent a rather coarse distinction criterion on who may benefit. This may be the underlying factor leading to the contradicting data, as patients may not be adequately profiled. To elaborate on that, indubitably maternal age should be the major criterion in decision-making, as aneuploidy rates increase when maternal age is over 35 years [35]; however, other characteristics may be of value that merit investigation and should be consulted. From the IVF cycle’s performance perspective, the number and quality of embryos available for biopsy can be a defining factor in deciding whether PGT-A is beneficial. Furthermore, it has been observed that women with diminished ovarian reserve [53], auto-immune disorders [54], the implication of certain causes of male infertility [55], and other lifestyle factors [56] have been associated with higher risk for embryo aneuploidy. More studies are required to investigate and evaluate the characteristics of the couples for whom PGT-A is beneficial. The development of an algorithm to assist in deciding the optimal population for PGT-A may be of clinical significance, and in the era of individualization, this may be what the future should hold. In the meantime, PGT-A may be of great significance—a necessity—and not an add-on for couples presenting with a higher risk for aneuploidies. However, this fact alone should not enable PGT-A’s status as another universally employed IVF add-on before the data is there to conclusively argue that.
Supplementary information
Search Strategy for the conduction of systematic search of literature in databases (DOCX 13 kb)
PRISMA flowchart regarding the search results (DOCX 41 kb)
(DOCX 12 kb)
Acknowledgements
We are very appreciative to all embryologists, clinicians, and scientists at the Department of Physiology of the National and Kapodistrian University of Athens Medical School and at the Centre for Human Reproduction, Genesis Athens Clinic.
Author contribution
Conceptualization, M.S. and K.S.; methodology, E.M.; software, E.M.; formal analysis, E.M. and S.G.; investigation, P.T., and M.A.; data curation, P.T., and M.A.; writing—original draft preparation, A.R., P.G, A.P., and K.N.; writing—review and editing, G.K., and N.V.; supervision, M.S. and K.P.
Declarations
Ethics approval
This is a systematic review of previously published data and therefore does not require ethical approval.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Penzias A, Bendikson K, Butts S, Coutifaris C, Falcone T, Fossum G, Gitlin S, Gracia C, Hansen K, la Barbera A, Mersereau J, Odem R, Paulson R, Pfeifer S, Pisarska M, Rebar R, Reindollar R, Rosen M, Sandlow J, Vernon M, Widra E. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429–436. doi: 10.1016/j.fertnstert.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Goossens V, Harton G, Moutou C, Traeger-Synodinos J, Van Rij M, Harper J. ESHRE PGD Consortium data collection IX: cycles from January to December 2006 with pregnancy follow-up to October 2007. Hum Reprod. 2009;24:1786–1810. doi: 10.1093/humrep/dep059. [DOI] [PubMed] [Google Scholar]
- 3.Twisk M, Mastenbroek S, van Wely M, Heineman MJ, Van der Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst Rev. 2006. [DOI] [PubMed]
- 4.Fiorentino FRL, Bono S, Capalbo A, Spizzichino L, Baroni E, Harton G, et al. Preimplantation genetic screening on day 3 embryos using array comparative genomic hybridization in patients with advanced maternal age: a prospective double blinded randomized controlled trial. Hum Reprod (Oxford, England). 2013.
- 5.Rubio CBJ, Rodrigo L, Castillon G, Guillen A, Vidal C, Giles J, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. (no pagination. 2017. [DOI] [PubMed]
- 6.van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17:620–627. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- 7.Cimadomo D, Capalbo A, Ubaldi FM, Scarica C, Palagiano A, Canipari R, Rienzi L. The impact of biopsy on human embryo developmental potential during preimplantation genetic diagnosis. Biomed Res Int. 2016;2016:1–10. doi: 10.1155/2016/7193075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachdev NM, McCulloh DH, Kramer Y, Keefe D, Grifo JA. The reproducibility of trophectoderm biopsies in euploid, aneuploid, and mosaic embryos using independently verified next-generation sequencing (NGS): a pilot study. J Assist Reprod Genet. 2020;37:559–571. doi: 10.1007/s10815-020-01720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victor AR, Griffin DK, Brake AJ, Tyndall JC, Murphy AE, Lepkowsky LT, Lal A, Zouves CG, Barnes FL, McCoy RC, Viotti M. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum Reprod. 2019;34:181–192. doi: 10.1093/humrep/dey327. [DOI] [PubMed] [Google Scholar]
- 10.Harton G, Magli M, Lundin K, Montag M, Lemmen J, Harper J. ESHRE PGD Consortium/Embryology Special Interest Group—best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS) Hum Reprod. 2010;26:41–46. doi: 10.1093/humrep/deq265. [DOI] [PubMed] [Google Scholar]
- 11.Scott KL, Hong KH, Scott RT., Jr Selecting the optimal time to perform biopsy for preimplantation genetic testing. Fertil Steril. 2013;100:608–614. doi: 10.1016/j.fertnstert.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Gleicher N, Orvieto R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review. J Ovarian Res. 2017;10:21. doi: 10.1186/s13048-017-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Checa MA, Alonso-Coello P, Sola I, Robles A, Carreras R, Balasch J. IVF/ICSI with or without preimplantation genetic screening for aneuploidy in couples without genetic disorders: a systematic review and meta-analysis. J Assist Reprod Genet. 2009;26:273–283. doi: 10.1007/s10815-009-9328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17:454–466. doi: 10.1093/humupd/dmr003. [DOI] [PubMed] [Google Scholar]
- 15.Dahdouh EM, Balayla J, Garcia-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod BioMed Online. 2015;30:281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Natsuaki MN, Dimler LM. Pregnancy and child developmental outcomes after preimplantation genetic screening: a meta-analytic and systematic review. World J Pediatr. 2018;14:555–569. doi: 10.1007/s12519-018-0172-4. [DOI] [PubMed] [Google Scholar]
- 17.Lawrenz B, El Khatib I, Liñán A, Bayram A, Arnanz A, Chopra R, et al. The clinicians´ dilemma with mosaicism—an insight from inner cell mass biopsies. Hum Reprod. 2019;34:998–1010. doi: 10.1093/humrep/dez055. [DOI] [PubMed] [Google Scholar]
- 18.Weissman A, Shoham G, Shoham Z, Fishel S, Leong M, Yaron Y. Chromosomal mosaicism detected during preimplantation genetic screening: results of a worldwide Web-based survey. Fertil Steril. 2017;107:1092–1097. doi: 10.1016/j.fertnstert.2017.02.119. [DOI] [PubMed] [Google Scholar]
- 19.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–1079. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 20.Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36:629–636. doi: 10.1007/s10815-018-01399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott RT, Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100:624–630. doi: 10.1016/j.fertnstert.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Scott R, Tao X, Taylor D, Ferry K, Treff N. A prospective randomized controlled trial demonstrating significantly increased clinical pregnancy rates following 24 chromosome aneuploidy screening: biopsy and analysis on day 5 with fresh transfer. Fertil Steril. 2010;94(suppl 1):S2. [Google Scholar]
- 23.Scott R, Upham K, Forman E, Hong K, Scott K, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 24.Treff NR, Tao X, Taylor D, Levy B, Ferry KM, Scott RT. P-427 Significantly increased implantation and clinical pregnancy rates following PGS: a prospective randomized controlled trial of 24 chromosome aneuploidy screening. Hum Reprod. 2011;26(suppl_1).
- 25.Yang Z, Liu J, Zhang S, Kuang Y, Lu S, Lin J. The combined use of time-lapse and next-generation sequencing improves clinical outcomes: results from a randomized pilot study. Fertility and sterility Conference: 73rd annual congress of the American society for reproductive medicine, ASRM 2017 United states. 2017;108:e242.
- 26.Yang Z, Salem S, Liu X, Kuang Y, Salem R, Liu J. Selection of euploid blastocysts for cryopreservation with array comparative genomic hybridization (aCGH) results in increased implantation rates in subsequent frozen and thawed embryo transfer cycles. Mol Cytogenet [Internet]. 2013;6. 10.1002/central/CN-00917859/full. [DOI] [PMC free article] [PubMed]
- 27.Sui Y-L, Lei C-X, Ye J-F, Fu J, Zhang S, Li L, et al. In vitro fertilization with single-nucleotide polymorphism microarray-based preimplantation genetic testing for aneuploidy significantly improves clinical outcomes in infertile women with recurrent pregnancy loss: a randomized controlled trial. Reprod Dev Med. 2020;4:32. [Google Scholar]
- 28.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25:2–14. doi: 10.1093/humupd/dmy033. [DOI] [PubMed] [Google Scholar]
- 29.Tiegs AW, Tao X, Zhan Y, Whitehead CV, Seli E, Patounakis G, et al. A multi-center, prospective, blinded, non-selection study evaluating the predictive value (PV) of an aneuploid diagnosis with PGT-A and the impact of biopsy. Fertil Steril. 2020;114:e30. doi: 10.1016/j.fertnstert.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 30.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boynukalin FK, Turgut NE, Gultomruk M, Ecemis S, Yarkiner Z, Findikli N, et al. Impact of elective frozen vs. fresh embryo transfer strategies on cumulative live birth: do deleterious effects still exist in normal & hyper responders? PLOS ONE Public Libr Sci. 2020;15:e0234481. doi: 10.1371/journal.pone.0234481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harper J, Coonen E, De Rycke M, Fiorentino F, Geraedts J, Goossens V, et al. What next for preimplantation genetic screening (PGS)? A position statement from the ESHRE PGD Consortium Steering Committee. Hum Reprod. 2010;25:821–823. doi: 10.1093/humrep/dep476. [DOI] [PubMed] [Google Scholar]
- 33.Kokkali G, Traeger-Synodinos J, Vrettou C, Stavrou D, Jones G, Cram D, et al. Blastocyst biopsy versus cleavage stage biopsy and blastocyst transfer for preimplantation genetic diagnosis of beta-thalassaemia: a pilot study. Hum Reprod (oxford, england) 2007;22:1443–1449. doi: 10.1093/humrep/del506. [DOI] [PubMed] [Google Scholar]
- 34.Prates R, Jordan A, Goodall N-N, Tortoriello D, Kiltz R, Jaroudi S. Multiple advantages of blastocyst versus cleavage stage biopsy for preimplantation genetic diagnosis (PGD) of single gene disorders. Fertil Steril. 2013;100:S84. [Google Scholar]
- 35.Kim YJ, Lee JE, Kim SH, Shim SS, Cha DH. Maternal age-specific rates of fetal chromosomal abnormalities in Korean pregnant women of advanced maternal age. Obstet Gynecol Sci. 2013;56:160–166. doi: 10.5468/ogs.2013.56.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander PE, Bonner AJ, Agarwal A, Li S-A, Hariharan A, Izhar Z, et al. Sensitivity subgroup analysis based on single-center vs multi-center trial status when interpreting meta-analyses pooled estimates: the logical way forward. J Clin Epidemiol. 2016;74:80–92. doi: 10.1016/j.jclinepi.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Orvieto R. Preimplantation genetic screening- the required RCT that has not yet been carried out. Reprod Biol Endocrinol. 2016;14:35. doi: 10.1186/s12958-016-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orvieto R, Gleicher N. Preimplantation genetic testing for aneuploidy (PGT-A)-finally revealed. J Assist Reprod Genet. 2020;37:669–672. doi: 10.1007/s10815-020-01705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schattman GL. Preimplantation genetic testing for aneuploidy: it’s déjà vu all over again! Fertil Steril. 2019;112:1046–1047. doi: 10.1016/j.fertnstert.2019.08.102. [DOI] [PubMed] [Google Scholar]
- 40.Bourdon M, Pocate-Cheriet K, Finet de Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, et al. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34:1948–1964. doi: 10.1093/humrep/dez163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JCM, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ [Internet]. 2010:341 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3006495/. [DOI] [PMC free article] [PubMed]
- 42.Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2020 [cited 2021 Apr 3]; Available from: 10.1002/14651858.CD003416.pub5/full [DOI] [PMC free article] [PubMed]
- 43.Ombelet W, De Sutter P, Van der Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction--the Belgian project. Hum Reprod Update. 2005;11:3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- 44.Multiple gestation pregnancy The ESHRE Capri Workshop Group. Hum Reprod. 2000;15:1856–1864. [PubMed] [Google Scholar]
- 45.Practice Committee of the American Society for Reproductive Medicine Electronic address: ASRM@asrm.org, Practice Committee of the Society for Assisted Reproductive Technology. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107:901–903. doi: 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 46.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;(100):100–107.e1. [DOI] [PubMed]
- 47.Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011–2012. Fertil Steril. 2016;105:394–400. doi: 10.1016/j.fertnstert.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murugappan G, Shahine LK, Perfetto CO, Hickok LR, Lathi RB. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum Reprod. 2016;31:1668–1674. doi: 10.1093/humrep/dew135. [DOI] [PubMed] [Google Scholar]
- 49.Goldman KN, Blakemore J, Kramer Y, McCulloh DH, Lawson A, Grifo JA. Beyond the biopsy: predictors of decision regret and anxiety following preimplantation genetic testing for aneuploidy. Hum Reprod. 2019;34:1260–1269. doi: 10.1093/humrep/dez080. [DOI] [PubMed] [Google Scholar]
- 50.Handyside AH. 24-chromosome copy number analysis: a comparison of available technologies. Fertil Steril. 2013;100:595–602. doi: 10.1016/j.fertnstert.2013.07.1965. [DOI] [PubMed] [Google Scholar]
- 51.Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grifo J, Rubio C, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS position statement on the transfer of mosaic embryos 2019. Reprod BioMed Online Elsevier. 2019;39:e1–e4. doi: 10.1016/j.rbmo.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Gleicher N, Albertini DF, Barad DH, Homer H, Modi D, Murtinger M, et al. The 2019 PGDIS position statement on transfer of mosaic embryos within a context of new information on PGT-A. Reprod Biol Endocrinol. 2020;18:57. doi: 10.1186/s12958-020-00616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahine LK, Marshall L, Lamb JD, Hickok LR. Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertil Steril. 2016;106:1124–1128. doi: 10.1016/j.fertnstert.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Dabi Y, Guterman S, Jani JC, Letourneau A, Demain A, Kleinfinger P, et al. Autoimmune disorders but not heparin are associated with cell-free fetal DNA test failure. J Transl Med [Internet]. 2018:16 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6276207/. [DOI] [PMC free article] [PubMed]
- 55.Vendrell X, Ferrer M, García-Mengual E, Muñoz P, Triviño JC, Calatayud C, Rawe VY, Ruiz-Jorro M. Correlation between aneuploidy, apoptotic markers and DNA fragmentation in spermatozoa from normozoospermic patients. Reprod BioMed Online. 2014;28:492–502. doi: 10.1016/j.rbmo.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Hawuła W, Ulańska A, Hanke W. Lifestyle factors and sperm aneuploidy. Reprod Biol. 2014;14:190–199. doi: 10.1016/j.repbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy for the conduction of systematic search of literature in databases (DOCX 13 kb)
PRISMA flowchart regarding the search results (DOCX 41 kb)
(DOCX 12 kb)