Abstract
Purpose
The aim of this study was to analyze the metabolic profiles of blastocoel fluid (BF) obtained from bovine embryos produced in vivo and in vitro.
Methods
Expanded blastocysts (20/group) that were in vitro and in vivo derived at day 7 were used. BF was collected and analyzed under direct infusion conditions using a microTOF-Q® mass spectrometer with electrospray ionization and a mass range of 50–650 m/z.
Results
The spectrometry showed an evident difference in the metabolic profiles of BF from in vivo and in vitro produced embryos. These differences were very consistent between the samples of each group suggesting that embryo fluids can be used to identify the origin of the embryo. Ions 453.15 m/z, 437.18 m/z, and 398.06 m/z were identified as biomarkers for the embryo’s origin with 100% sensitivity and specificity. Although it was not possible to unveil the molecular identity of the differential ions, the resulting spectrometric profiles provide a phenotype capable of differentiating embryos and hence constitute a potential parameter for embryo selection.
Conclusion
To the best of our knowledge, our results showed, for the first time, an evident difference between the spectrometric profiles of the BF from bovine embryos produced in vivo and in vitro.
Keywords: Biomarker, Blastocyst, Electrospray, In vivo, In vitro
Introduction
In vitro embryo production (IVP) is a well-established biotechnology commercially used to accelerate the multiplication of animals with the desired zootechnical characteristics. Moreover, it has also been used as a tool for animal preservation, genomic selection, and gene editing [1]. Currently, the use of IVP in cattle has grown worldwide, being responsible for more than 70% of the total embryos transferred.
Despite the advancements in the last decades, the efficiency of this biotechnology for calf production is far from optimal. The most limiting factor is not the quantity, but the quality of the embryos produced in vitro, which impairs its development, implantation, and birth rates. In fact, 60% of transferred IVP bovine embryos fail to establish and maintain pregnancy until birth [2, 3]. This high embryonic and fetal loss occurs, in part, due to the lower quality of these embryos associated with the inability to select blastocysts with the greatest potential to develop until their birth. To improve the success rate during embryo transference, an efficient strategy is essential to select blastocysts with the greatest potential to develop until their birth. It has been extensively reported that in vitro embryos are different from their in vivo counterparts, in terms of aspects such as lower number of total cells, higher number of apoptotic cells [4], lower tolerance to cryopreservation [5–7], increased intracellular accumulation of lipids [7], and differences in gene expression [4, 8, 9], attributed to the artificial culture conditions that IVP embryos are exposed to.
The current selection method for embryos is based on the morphological appearance, which is not accurate for the prediction of embryo quality [10, 11]. Hence, the use of non-invasive methodologies that are capable of indicating which embryo should be transferred has become a subject of great interest.
Among the current and very promising embryo, non-invasive methodologies available are those that evaluate metabolites, microRNAs, and cell-free DNA (cfDNA) in the embryo culture medium [12–15]. However, to date, no marker that could be used to indicate embryo quality has been identified in the culture medium. In humans, an alternative that has been evaluated as a potential source of biomarkers is blastocoel fluid (BF). The interest in this new source gained traction when Palini et al. (2013) detected the presence of genetic material in the BF of human embryos [16]. Currently, several authors argue that although embryos are subjected to some manipulation, the removal of BF is still less invasive than biopsy of cells, and it is being considered as a new alternative for predicting embryo quality [17–20].
The BF is predominantly composed of water and relies on the ion transport system of the nonpolar cells of the trophectoderm (TE), which are responsible for regulating the initial cavitation events through the expression of genes that facilitate the transport and retention of blastocoel fluid [21]. The components from the inner cell mass (ICM) and TE cells, including several types of proteins [22] and other molecules [23], are transported into the fluid. It is known that the BF plays a fundamental role during pre-implantation, and its components are used in the formation of the yolk sac. Hence, its composition can be directly related to the initial blastocyst development and may reflect embryo health [22, 24]. In fact, studies in humans have reported that the DNA present in the BF can be amplified and quantified, displaying a potential approach for performing genetic pre-implantation testing [24–28]. Nevertheless, further studies are still needed to recommend BF for genetic testing [29–31]. In domestic animals, the majority of studies on BF are related to cryopreservation processes, since the presence of fluid in the blastocoel can be associated with ice crystal formation and poorer post-warming survival [32]. The removal of BF prior to cryopreservation has already been successfully reported in bovine embryos [33], horses [34], murine [35] and feline [36]. In the case of cryopreservation, the BF is removed and discarded without any investigation or usage of the content.
Despite few studies focusing on the BF composition in cattle, quantifying glucose, pyruvate, lactate, and amino acid concentrations [18] and some proteins [22], there is a lack of reports in this area. The lack of interest in studying BF in cattle is probably due to the small volume obtained from each embryo and the difficulty of handling and analyzing such a small volume [19, 22, 37]. Nevertheless, with the availability of equipment with higher sensitivity and specificity, such as mass spectrometry, a small volume of BF is sufficient to perform analysis without the need for pooling samples. Therefore, the use of BF to evaluate embryo quality becomes viable and its use can also be associated with other assisted reproduction techniques such as cryopreservation and genomic selection, increasing the advantages of using IVP for animal production. In addition, the information obtained here can also be valuable for studies in human, since bovine and human preimplantation embryos seem to be more similar in terms of biochemical and regulatory processes, than mice and humans [38–40].
Considering that the BF plays an important role during embryo development, we hypothesized that the compounds present in BF may reflect embryo quality. To assess this hypothesis, we analyze the metabolic profile of blastocoel fluid obtained from bovine embryos produced in vivo and in vitro.
Material and methods
Unless otherwise specified, all reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
In vitro embryo production
The ovaries were collected from slaughterhouses and transported to the laboratory in saline solution (0.9% NaCl) supplemented with amikacin (250 μg/mL) at 35 °C. Follicles ranging from 3 to 8 mm in diameter were aspirated using a 10 mL syringe and an 18G gauge needle. Only cumulus-oocyte complexes (COCs) presenting a homogenous cytoplasm and at least three layers of cumulus cells were used (n = 316).
After selection, groups of 25 to 30 COCs were transferred to 150 μL drops of maturation medium consisting of TCM-199 with Earl’s salts (Gibco BRL, Burlington, Canada) supplemented with 0.075 mg/mL amikacin, 10% SFB (Gibco®), 0.1 mg/mL L-glutamine, 1 μM pyruvate, 1 μM/mL cysteamine, and 0.01 UI/mL FSH and were incubated for 22–24 h at 38.5 °C and 5% CO2 in air.
After maturation, the COCs were transferred to a fertilization medium (FEC) composed of Tyrode’s albumin lactate pyruvate [TALP] [41] supplemented with 0.5 mM penicillamine, 0.25 mM hypotaurine, 25 μM epinephrine, and 10 μg/mL heparin. Semen from a Nellore bull whose fertility was known was used. After thawing in a water bath at 37 °C for 30 s, the sperm cells were selected using a Percoll® gradient as described by Machado et al. [42]. The sperm cells were added to the fertilization drop at a final concentration of 1 × 106 sperm cells/mL. Matured oocytes were co-incubated with sperm cells for 18 h, and the day of insemination was considered as day 0 (D0).
After co-incubation, the presumptive zygotes were gently pipetted for partial removal of the cumulus cells and transferred to 150 μL drops of SOFaa medium [43] supplemented with 0.0293 mg/mL L-glutamine, 0.35 mM sodium tris-citrate, 2.8 mM myo-inositol, 8 mg/mL pyruvate, 0.075 mg/mL amikacin, essential amino acid solution (BME 50×), solution of non-essential amino acids (MEM 100×), 0.5 μg/mL ITS (insulin 10 mg/L, transferrin 5.5 mg/L, and selenium 5 μg/L), and 0.4% of bovine serum albumin (BSA) and were incubated during 7 days at 38.5 °C and 5% CO2 in air.
The embryos were evaluated on D2 (48 h post-insemination) for cleavage, and on D6 (144 h post-insemination) and D7 (168 h post-insemination) for stage of development. Embryos that were in the expanded blastocyst stage on D7 (168 h post-insemination) were used for collecting the BF.
In vivo embryo production
For in vivo embryo production, Nellore breed (Bos Taurus Indicus) donors (n = 12) were subjected to an ovarian superstimulation protocol, and it was obtained 4.66 embryos per cow (56/12) with a ratio of 2.58 expanded blastocyst per cow (31/12). On day 0 (D0) of synchronization, the animals received an intravaginal progesterone device (1 g; Sincrogest® Ourofino Saúde Animal, Cravinhos, Brazil) and 2 mg of estradiol benzoate (RIC-BE®, Tecnopec Ltda, São Paulo, Brazil). Four days later (D4), the animals were subjected to superstimulated treatment with 100 mg FSH (Folltropin-V®; Vetoquinol N.-A. Inc, QC, Canada) in decreasing doses, receiving two applications per day over 4 days (12/12 h). Along with the fifth application of FSH, luteolysis was induced with 500 μg of PGF2α (500 μg of Cloprostenol; Estron®, União Química Farmacêutica Nacional S/A, Embu-Guaçu, São Paulo, Brazil). In the sixth application of FSH, the intravaginal progesterone device was removed, and 12 h later, 50 μg of GnRH analog (Lecirelin; Gestran®, ARSA S.R.L., Buenos Aires, Argentina) was administered (i.m.). All animals were inseminated, with the same semen batch used in IVP, at 12 and 24 h after GnRH injection. Seven days after insemination, the embryos were collected through uterine washing, and only those that were at the EB stage were used for the experiment.
Blastocoel fluid collection
Only expanded blastocysts from both groups were used to collect BF. To avoid sample contamination, each embryo (in vitro and in vivo) was washed and micromanipulated in individual drops of 30 μL of phosphate-buffered saline (PBS) and covered with mineral oil. The entire process was performed in a 100-mm Petri dish. For BF collection, two glass pipettes coupled to a micromanipulator were used: one to fix the embryo (holding pipette) and the other, with an internal diameter of approximately 5 μm, to aspirate the fluid. The aspiration pipette was gently pressed against the zona pellucid area until it reached the embryo's cavity, and then, the liquid was totally aspirated (Fig. 1). Then, the liquid was placed in a drop containing 3 μL of deionized water previously prepared on the micromanipulation plate. Finally, the drop containing the BF was collected and stored individually in 200 μL microtubes at − 80 °C until analysis.
Fig. 1.
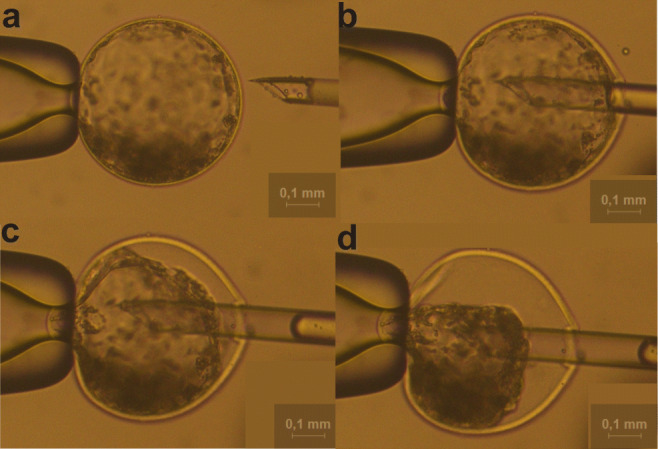
Blastocoel fluid collection of D7 expanded blastocyst (20×). a Intact expanded bovine blastocyst before collection. b The micropipette is introduced in the blastocoel for fluid collection. c Approximately 30% of the fluid collection. d Complete collection of the blastocoel fluid
Mass spectrometry (ESI-MS)
The stored samples of BF collected from in vivo (n = 20) and in vitro (n = 20) embryos were thawed and 50 μL of acetonitrile/H2O solution (50:50) with 0.1% formic acid was added. The analyses were performed by the direct infusion of samples in a micrOTOF-Q® mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with an electrospray ionization source operating in positive mode under the following conditions: injection flow of 180 μL/h, 8 V of collision energy, end plate off set 500 V, 4500 capillarity, 0.6 bar pressure, and 5 L/min of gas at 180 °C. The spectra were acquired in the range of 50–650 m/z. The spectra were acquired using oTOFcontrol® and DataAnalyis® was used to analyze the BF profiles of the in vivo and in vitro embryos, using both software (Bruker®). Fragmentation (MS/MS) of the sections showing differences between the spectra of in vivo and in vitro groups was performed in multiple reaction monitoring (MRM) mode with a 2 Da isolation window, initial collision energy of 5 V, with variation until all the precursors were fragmented.
Statistical analysis
The raw data generated from the ESI-MS [mass/charge (m/z) and signal intensity (I)] was analyzed using software Data Analysis (Bruker®) and MetaboAnalyst 4.0 version (https://www.metaboanalyst.ca/). Initially, all spectra of the in vitro and in vivo groups were processed with a mass tolerance of 0.05 m/z to get the archive data processed. After, the data was normalized by sum and cub root transformation. In order to provide a preliminary overview of the data, Volcano plot with threshold 4 was performed. Subsequently, a principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) multivariate regression model for variable importance in projection (VIP) was applied. The ions were selected for analysis through logistic regression, and the receiver operating characteristic (ROC) curves were used to illustrate their ability to discern BF in vivo or BF in vitro. For all analyses, p ≤ 0.05 were considered significant.
Results
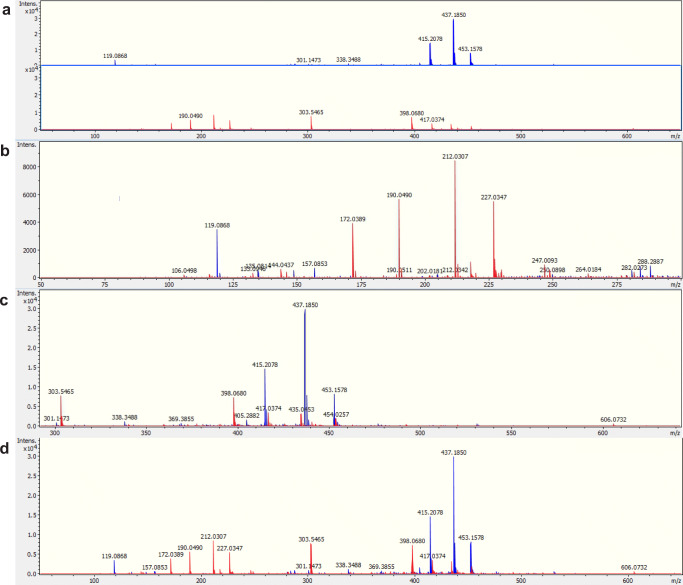
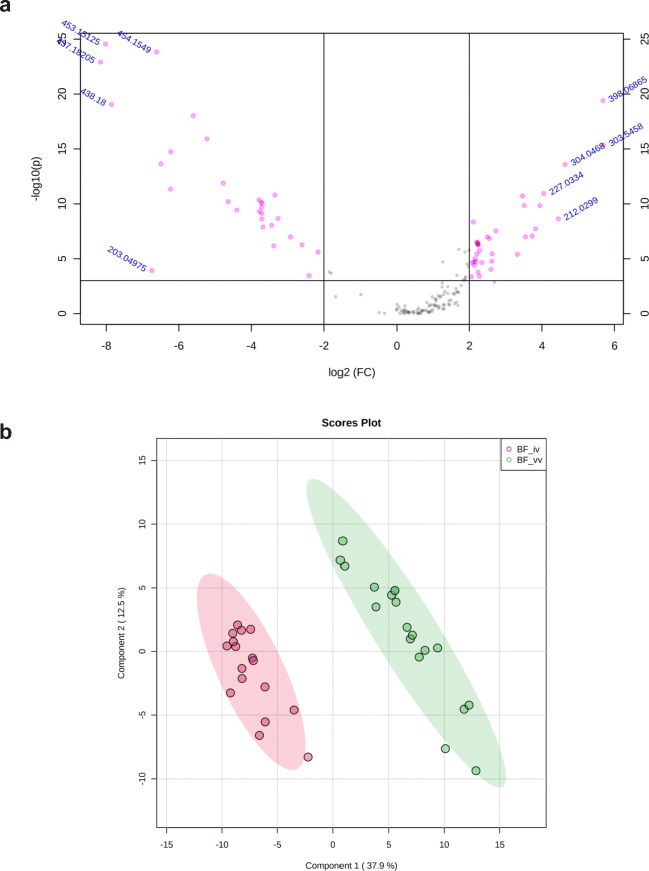
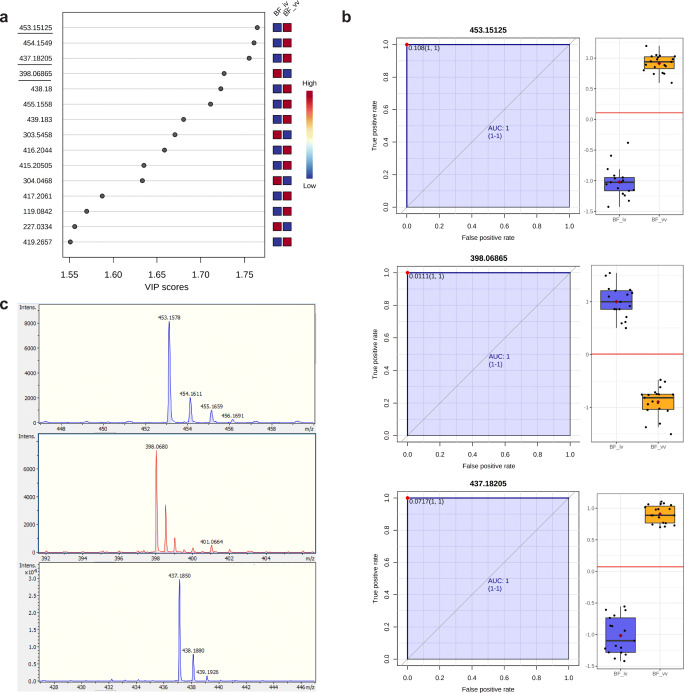
ESI-MS analysis revealed a distinctive spectrometric profile between BF from embryos produced in vivo and those obtained by in vitro production in range mass 50–650 m/z (Fig. 2). Considering a mass tolerance of 0.05 m/z, a total of 6119 peaks were obtained between the range of 50 and 650 m/z. After peak matching and alignment, a total of 162 peak groups were obtained. Volcano plot was performed an overview of the data identifies the principal ions (Fig. 3a). The principal component analysis (PCA) was able to distinguish the groups with formation of cluster (Fig. 3b). After the PLS-DA was performed and the list the principal ions were identified, they showed a VIP score > 1.55 (Fig. 4a). These results constitute a panel of ions capable of differentiating BF in vivo or in vitro. The ions 453.15 Da [M+H]+, 437.18 Da [M+H]+, and 398.06 Da [M+H]+ were selected for the ROC curve of individual biomarker analyses. The results of the ROC curve showed 100% specificity, indicating that these ions are biomarkers of embryo origin (Fig. 4b). Figure 4 c shows the spectrometric profile of the biomarkers in detail. To identify these specific ions, fragmentations (MS/MS) were performed, however, without success.
Fig. 2.
Representative spectrometric profile of the blastocoel fluid (BF) from expanded blastocysts of cattle used in vivo (blue) and in vitro (red) on day 7 (D7) of embryo development. a In vivo (above) and in vitro (below). b In vivo and in vitro with mass range 50–300 m/z; c in vivo and in vitro with mass range 300–650 m/z; d in vivo and in vitro with mass range 50–650 m/z
Fig. 3.
a Volcano plot of the ions of the blastocoel fluid in vivo and in vitro identified with fold change (FC) > 4 and p ≤ 0.05 (pink dots). The ten identified ions, five ions in the left (in vivo group), and five ions in the right (in vitro group) represent those with higher FC and minor p value. b Partial least square discriminatory analysis (PLS-DA) shows the 2D score plot between fluid blastocoels in vivo (red) and in vitro (green). Ellipses represent 95% confidence interval
Fig. 4.
General analysis of the blastocoel fluid in vivo and in vitro. a VIP score of the most important ions identified by partial least square discriminatory analysis (PLS-DA). The ions selected for the next analyses are underlined. b Univariate receiver operating characteristic—ROC curve and box plot of the ion selection by VIP score. AUC: area under curve (blue). c Representative spectrum of the selected ions in vivo and in vitro groups
Discussion
Considering that the BF plays an important role during embryo development, participating in cell differentiation and self-renewal processes, we hypothesized that the compounds present in BF may reflect embryo quality. To assess this hypothesis, we used embryos produced in vivo, which were considered to be of standard quality, and embryos produced in vitro, to be of inferior quality. Then, BF was removed from the embryos on the same day and at the same stage of development of the two embryo categories, and their spectrometric profile was analyzed.
Our results showed that the spectrometric profiles were clearly distinct between the BF of embryos produced in vitro and in vivo, indicating that there are physiological differences between them. These different profiles were very consistent, since samples from each of the groups were very similar. This high repeatability reinforces the idea that embryo fluids in vitro and in vivo follow a certain pattern of the mass spectrometric data. At the same time, the identity of the compounds related to the differential ions could be very enlightening; however, their identification was not possible as no matches for the MS/MS spectra were found in the compound spectrum databases. Assuming that the composition of the BF is different between the groups, the osmotic pressure would also vary and blastocyst expansion and hatching times would be probably affected. In this regard, using embryos at the initial blastocyst stage would be probable better than using expanded blastocyst. However, due to the difficulty to obtain large amount of the BF, it was not possible to use that type of embryos. Indeed, other studies that analyzed the blastocoel fluid also used only the expanded blastocyst stage [18, 22] to overcome the limitation of blastocoel fluid volume in other stages of development. Nevertheless, because we use for both groups embryos at the same stage of development and the different profiles were very consistent among samples, we can assume that spectrometric profiles can be differentiated in vitro and in vivo embryos.
Though, our results constitute a panel of differential ions that enable one to distinguish the in vivo BF from in vitro BF. The ions 453.15 Da [M+H]+, 437.18 Da [M+H]+, and 398.06 Da [M+H]+ can be considered as biomarkers for the embryo’s origin, with 100% sensitivity and specificity through ROC analysis. It is also important to highlight that the ion 437.18 Da [M+H]+, present only in the BF of embryos produced in vivo, was also detected in another study by our group, in culture medium of embryos IVP (submitted manuscripts). This could indicate that the in vitro embryos are able to produce the compound 437.18 Da but are not able to internalize it in their BF as in vivo embryo would do. Therefore, understanding the importance and the reason why this ion is present only in the BF of in vivo embryos, which is routinely used as the standard for embryo quality, can be crucial to improve the quality of the embryos produced in vitro. It is important to mention that in vivo embryos were obtained after ovarian stimulation, which has been reported as having some impact in embryo quality. Nevertheless, due to difficulties in obtaining large numbers of embryo produced in vivo, it was necessary to use stimulatory treatment to make the experiment feasible.
Studies with BF from human embryos represent an alternative for pre-implantation tests [24, 26, 44]. In particular, BF aspiration is considered less invasive to the embryo and appears to have a high predictive value in ploidy conditions and greater conformity with chromosomal status ploidies when compared to the polar body, blastomeres, and trophectoderm cells [24, 45]. However, only recently, it has been reported as an attempt to correlate the presence of DNA in the BF with the implantation potential of human embryos [30, 44]. Moreover, in studies involving proteomic analysis of humans and bovine BF, it was possible to identify a total of 286 and 23 proteins, respectively [20, 22]. Similarly, a study analyzing lactate in the culture media and BF of bovine embryos found a higher lactate concentration in the BF than in the medium [18]. All these results which showed the presence of genetic material and proteins in BF, as well as ours, suggested that BF is a potential source, in seeking biochemical markers for embryo quality.
Although it was not possible to unveil the molecular identity of the differential ions, our results, to the best of our knowledge, for the first time showed evident difference between the spectrometric profiles of the BF from bovine embryos produced in vivo and in vitro. These profiles provide a phenotype that can be used as a starting point for a new series of studies using BF to provide information about embryo characteristics.
Further studies should be carried out in an attempt to discover the identity of compounds relative to the differential ions detected in this study, or perhaps to associate different embryo evaluation techniques simultaneously, such as the presence of metabolites, cell-free DNA, and microRNAs in the culture medium as well as in the BF. This knowledge would provide important information not only to indicate specific markers of embryonic quality, but also to propose changes in the culture systems to produce an in vitro embryo as similar as possible to the in vivo and, thereby, increase the efficiency of in vitro production of bovine embryos.
Acknowledgements
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) for the scholarship granted to Fernandes GO. This research was only possible due to the assistance of the research team of the Mass Spectrometry Laboratory at Embrapa Genetic Resource and Dr. Marcelo Henrique Soller Ramada, University Catholica de Brasília. The authors thank Dr. José de Lima Cardozo Filho for his valuable advice regarding the analysis and his support during the laboratory work. The authors would also like to thank Dr. Carlos Frederico Martins and Dr. Andrei Fidelis (DVM).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Gabriela de Oliveira Fernandes, Otávio Augusto Costa de Faria, Daniel Nogoceke Sifuentes, and Maurício Machaim Franco. The first draft of the manuscript was written by Gabriela de Oliveira Fernandes and Margot Alves Nunes Dode and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil for the scholarship of Fernandes GO. The Embrapa Recursos Genéticos e Biotecnologia, Brazil. The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Declarations
Ethics approval
The experiment was approved by the Animal Use Ethics Committee of Embrapa Genetic Resources and Biotechnology (CEUA/CENARGEN) protocol number 004/2017.
Consent to participate
All authors agreed to participate in this work.
Consent for publication
All authors included in this study agree with its publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petersen B. Basics of genome editing technology and its application in livestock species. Reproduction in Domestic Animals. 2017;52(S3):4–13. doi: 10.1111/rda.13012. [DOI] [PubMed] [Google Scholar]
- 2.Viana JH. 2018 Statistics of embryo production and transfer in domestic farm animals. Embryo Technology Newsletter. 2019;36(4):14. [Google Scholar]
- 3.Ealy AD, Wooldridge LK, SR MC. BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. Journal of Animal Science. 2019;97(6):2555–2568. doi: 10.1093/jas/skz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corcoran D, Fair T, Park S, Rizos D, Patel OV, Smith GW, Coussens PM, Ireland JJ, Boland MP, Evans ACO, Lonergan P. Suppressed expression of genes involved in transcription and translation in in vitro compared with in vivo cultured bovine embryos. Reproduction. 2006;131(4):651–660. doi: 10.1530/rep.1.01015. [DOI] [PubMed] [Google Scholar]
- 5.Fair T, Lonergan P, Dinnyes A, Cottell DC, Hyttel P, Ward FA, Boland MP. Ultrastructure of bovine blastocysts following cryopreservation: effect of method of blastocyst production. Molecular Reproduction and Development. 2001;58(2):186–195. doi: 10.1002/1098-2795(200102)58:2<186::aid-mrd8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Rizos D, Fair T, Papadopoulos S, Boland MP, Lonergan P. Developmental, qualitative, and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Molecular Reproduction and Development. 2002;62(3):320–327. doi: 10.1002/mrd.10138. [DOI] [PubMed] [Google Scholar]
- 7.Sudano MJ, Santos VG, Tata A, Ferreira CR, Paschoal DM, Machado R, et al. Phosphatidylcholine and sphingomyelin profiles vary in Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced Blastocysts1. Biology of Reproduction. 2012;87(130):1–11. doi: 10.1095/biolreprod.112.102897. [DOI] [PubMed] [Google Scholar]
- 8.Machado GM, Ferreira AR, Pivato I, Fidelis A, Spricigo JF, Paulini F, Lucci CM, Franco MM, Dode MA. Post-hatching development of in vitro bovine embryos from day 7 to 14 in vivo versus in vitro. Molecular Reproduction and Development. 2013;80(11):936–947. doi: 10.1002/mrd.22230. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi T, Aizawa T, Munakata Y, Iwata H. Comparison of gene expression and mitochondria number between bovine blastocysts obtained in vitro and in vivo. J Reprod Dev. 2020;66(1):35–39. doi: 10.1262/jrd.2019-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson AJ, Lee RSF. Improving successful pregnancies after embryo transfer. Theriogenology. 2003;59(2):687–697. doi: 10.1016/S0093-691X(02)01248-7. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, Uyar A, Correia E, Díez C, Fernandez-Gonzalez A, Caamaño JN, Martínez-Bello D, Trigal B, Humblot P, Ponsart C, Guyader-Joly C, Carrocera S, Martin D, Marquant le Guienne B, Seli E, Gomez E. Prediction of pregnancy viability in bovine in vitro-produced embryos and recipient plasma with Fourier transform infrared spectroscopy. Journal of Dairy Science. 2014;97(9):5497–5507. doi: 10.3168/jds.2014-8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seli E, Vergouw CG, Morita H, Botros L, Roos P, Lambalk CB, Yamashita N, Kato O, Sakkas D. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertility and Sterility. 2010;94(2):535–542. doi: 10.1016/j.fertnstert.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 13.Leese HJ. Metabolism of the preimplantation embryo. 40 years on. 2012;143(4):417. doi: 10.1530/rep-11-0484. [DOI] [PubMed] [Google Scholar]
- 14.Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, Peinado V, Mercader A, Meseguer M, Blesa D, Moreno I, Valbuena D, Rubio C, Simon C. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Human Reproduction. 2018;33(4):745–756. doi: 10.1093/humrep/dey028. [DOI] [PubMed] [Google Scholar]
- 15.Juliano CS, Ana Clara FCMÁ, Hannah LG, Jason EB, Quinton AW, Gerrit JB. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. Journal of Endocrinology. 2018;236(1):R15–R27. doi: 10.1530/joe-17-0200. [DOI] [PubMed] [Google Scholar]
- 16.Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, Magnani M, et al. Genomic DNA in human blastocoele fluid. Reproductive BioMedicine Online. 2013;26(6):603–610. doi: 10.1016/j.rbmo.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Leaver M, Wells D. Non-invasive preimplantation genetic testing (niPGT): the next revolution in reproductive genetics? Human Reproduction Update. 2020;26(1):16–42. doi: 10.1093/humupd/dmz033. [DOI] [PubMed] [Google Scholar]
- 18.Gopichandran N, Leese H. Metabolic characterization of the bovine blastocyst, inner cell mass, trophectoderm and blastocoel fluid. Reproduction (Cambridge, England) 2003;126:299–308. doi: 10.1530/reprod/126.3.299. [DOI] [PubMed] [Google Scholar]
- 19.D'Alessandro A, Federica G, Palini S, Bulletti C, Zolla L. A mass spectrometry-based targeted metabolomics strategy of human blastocoele fluid: a promising tool in fertility research. Molecular BioSystems. 2012;8(4):953–958. doi: 10.1039/c1mb05358b. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PL, Beck HC, Petersen J, Hreinsson J, Wånggren K, Laursen SB, Sørensen PD, Christensen ST, Andersen CY. Proteomic analysis of human blastocoel fluid and blastocyst cells. Stem Cells and Development. 2012;22(7):1126–1135. doi: 10.1089/scd.2012.0239. [DOI] [PubMed] [Google Scholar]
- 21.Watson AJ, Barcroft LC. Regulation of blastocyst formation. Front Biosci. 2001;6:d708. doi: 10.2741/watson. [DOI] [PubMed] [Google Scholar]
- 22.Jensen PL, Grøndahl ML, Beck HC, Petersen J, Stroebech L, Christensen ST, Yding Andersen C. Proteomic analysis of bovine blastocoel fluid and blastocyst cells. Systems Biology in Reproductive Medicine. 2014;60(3):127–135. doi: 10.3109/19396368.2014.894152. [DOI] [PubMed] [Google Scholar]
- 23.Farra C, Choucair F, Awwad J. Non-invasive pre-implantation genetic testing of human embryos: an emerging concept. Human Reproduction. 2018;33(12):2162–2167. doi: 10.1093/humrep/dey314. [DOI] [PubMed] [Google Scholar]
- 24.Magli MC, Pomante A, Cafueri G, Valerio M, Crippa A, Ferraretti AP, et al. Preimplantation genetic testing: polar bodies, blastomeres, trophectoderm cells, or blastocoelic fluid? Fertility and Sterility. 2016;105(3):676–83.e5. doi: 10.1016/j.fertnstert.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Tobler KJ, Zhao Y, Ross R, Benner AT, Xu X, Du L, et al. Blastocoel fluid from differentiated blastocysts harbors embryonic genomic material capable of a whole-genome deoxyribonucleic acid amplification and comprehensive chromosome microarray analysis. Fertility and Sterility. 2015;104(2):418–425. doi: 10.1016/j.fertnstert.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Hammond ER, Shelling AN, Cree LM. Nuclear and mitochondrial DNA in blastocoele fluid and embryo culture medium: evidence and potential clinical use. Human Reproduction. 2016;31(8):1653–1661. doi: 10.1093/humrep/dew132. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Li N, Wang L, Sun H, Ma M, Wang H, Xu X, Zhang W, Liu Y, Cram DS, Sun B, Yao Y. Molecular analysis of DNA in blastocoele fluid using next-generation sequencing. Journal of Assisted Reproduction and Genetics. 2016;33(5):637–645. doi: 10.1007/s10815-016-0667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rule K, Chosed RJ, Arthur Chang T, David Wininger J, Roudebush WE. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. Journal of Assisted Reproduction and Genetics. 2018;35(8):1497–1501. doi: 10.1007/s10815-018-1223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuznyetsov V, Madjunkova S, Antes R, Abramov R, Motamedi G, Ibarrientos Z, et al. Evaluation of a novel non-invasive preimplantation genetic screening approach. PLoS One. 2018;13(5):e0197262–e019726e. doi: 10.1371/journal.pone.0197262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Song Z, Yao Y, Huang T, Mao R, Huang J, Ma Y, Dong X, Huang W, Huang J, Chen T, Qu T, Li L, Zhong Y, Gu J. Preimplantation genetic screening with spent culture medium/blastocoel fluid for in vitro fertilization. Scientific Reports. 2018;8(1):9275. doi: 10.1038/s41598-018-27367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tšuiko O, Zhigalina DI, Jatsenko T, Skryabin NA, Kanbekova OR, Artyukhova VG, et al. Karyotype of the blastocoel fluid demonstrates low concordance with both trophectoderm and inner cell mass. Fertility and Sterility. 2018;109(6):1127–34.e1. doi: 10.1016/j.fertnstert.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65(1):236–244. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Min SH, Kim JW, Lee YH, Park SY, Jeong PS, Yeon JY, Park H, Chang KT, Koo DB. Forced collapse of the blastocoel cavity improves developmental potential in cryopreserved bovine blastocysts by slow-rate freezing and vitrification. Reproduction in Domestic Animals. 2014;49(4):684–692. doi: 10.1111/rda.12354. [DOI] [PubMed] [Google Scholar]
- 34.Diaz F, Bondiolli K, Paccamonti D, Gentry GT. Cryopreservation of Day 8 equine embryos after blastocyst micromanipulation and vitrification. Theriogenology. 2016;85(5):894–903. doi: 10.1016/j.theriogenology.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Kazemi P, Dashtizad M, Shamsara M, Mahdavinezhad F, Hashemi E, Fayazi S, Hajarian H. Effect of blastocoel fluid reduction before vitrification on gene expression in mouse blastocysts. Molecular Reproduction and Development. 2016;83(8):735–742. doi: 10.1002/mrd.22681. [DOI] [PubMed] [Google Scholar]
- 36.Ochota M, Wojtasik B, Niżański W. Survival rate after vitrification of various stages of cat embryos and blastocyst with and without artificially collapsed blastocoel cavity. Reproduction in Domestic Animals. 2017;52(S2):281–287. doi: 10.1111/rda.12826. [DOI] [PubMed] [Google Scholar]
- 37.Tedeschi G, Albani E, Borroni EM, Parini V, Brucculeri AM, Maffioli E, Negri A, Nonnis S, Maccarrone M, Levi-Setti PE. Proteomic profile of maternal-aged blastocoel fluid suggests a novel role for ubiquitin system in blastocyst quality. Journal of Assisted Reproduction and Genetics. 2017;34(2):225–238. doi: 10.1007/s10815-016-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggeri RR, Watanabe Y, Meirelles F, Bressan FF, Frantz N, Bos-Mikich A. The use of parthenotegenetic and IVF bovine blastocysts as a model for the creation of human embryonic stem cells under defined conditions. Journal of Assisted Reproduction and Genetics. 2012;29(10):1039–1043. doi: 10.1007/s10815-012-9866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos RR, Schoevers EJ, Roelen BAJ. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol. 2014;12:117. doi: 10.1186/1477-7827-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marei WFA, Van den Bosch L, Pintelon I, Mohey-Elsaeed O, Bols PEJ, Leroy JLMR. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model. Human Reproduction. 2019;34(10):1984–1998. doi: 10.1093/humrep/dez161. [DOI] [PubMed] [Google Scholar]
- 41.Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995;44(6):859–869. doi: 10.1016/0093-691X(95)00271-9. [DOI] [PubMed] [Google Scholar]
- 42.Machado GM, Carvalho JO, Filho ES, Caixeta ES, Franco MM, Rumpf R, Dode MAN. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009;71(8):1289–1297. doi: 10.1016/j.theriogenology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using sofaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology. 1999;52(4):683–700. doi: 10.1016/S0093-691X(99)00162-4. [DOI] [PubMed] [Google Scholar]
- 44.Magli MC, Albanese C, Crippa A, Tabanelli C, Ferraretti AP, Gianaroli L. Deoxyribonucleic acid detection in blastocoelic fluid: a new predictor of embryo ploidy and viable pregnancy. Fertility and Sterility. 2019;111((1)):77–85. doi: 10.1016/j.fertnstert.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Gianaroli L, Magli MC, Pomante A, Crivello AM, Cafueri G, Valerio M, et al. Blastocentesis: a source of DNA for preimplantation genetic testing. Results from a pilot study. Fertility and Sterility. 2014;102(6):1692–9.e6. doi: 10.1016/j.fertnstert.2014.08.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.