Abstract
We previously demonstrated that angiopoietin-like protein 8 (ANGPTL8) forms ANGPTL3/8 and ANGPTL4/8 complexes that increase with feeding to direct fatty acids (FA) toward adipose tissue through differential modulation of lipoprotein lipase (LPL) activity. Each complex correlated inversely with high density lipoprotein cholesterol (HDL) in control subjects. We thus investigated ANGPTL3/8 and ANGPTL4/8 levels in type 2 diabetes patients, who can present with decreased HDL. While ANGPTL3/8 levels in type 2 diabetes patients were similar to those previously observed in normal controls, ANGPTL4/8 levels were roughly twice as high as those in control subjects. Concentrations of ANGPTL3/8 and ANGPTL4/8 in type 2 diabetes patients were inversely correlated with HDL, with the correlation being significant for ANGPTL4/8. We therefore measured the ability of the various ANGPTL proteins and complexes to inhibit endothelial lipase (EL), the enzyme which hydrolyzes phospholipids (PL) in HDL. While confirming ANGPTL3 as an EL inhibitor, we found that ANGPTL4 was a more potent EL inhibitor than ANGPTL3. Interestingly, we observed that while ANGPTL3/8 had increased EL-inhibitory activity compared to ANGPTL3 alone, ANGPTL4/8 exhibited decreased potency in inhibiting EL compared to ANGPTL4 alone. Together, these results show for the first time that ANGPTL4 is a more potent EL inhibitor than ANGPTL3 and suggest a possible reason for why ANGPTL4/8 levels are correlated inversely with HDL.
Keywords: Endothelial lipase, HDL, Triglycerides, Angiopoietin-like protein, Phospholipids, Postprandial state
Highlights
-
•
ANGPTL4/8 levels are increased in patients with type 2 diabetes.
-
•
ANGPTL4/8 levels are inversely correlated with HDL in type 2 diabetes patients.
-
•
ANGPTL4 is an inhibitor of endothelial lipase (EL).
-
•
ANGPTL4 inhibits EL more potently than ANGPTL3 inhibits EL.
-
•
ANGPTL4/8 inhibits EL less potently than ANGPTL4 inhibits EL.
Endothelial lipase; HDL; triglycerides; angiopoietin-like protein; phospholipids; postprandial state
1. Introduction
Triglyceride (TG) and high-density lipoprotein (HDL) metabolism are interwoven in a complex process that has yet to be completely elucidated. Their levels are often inversely correlated with each other, both during fasting conditions as well as postprandially [1]. Patients with type 2 diabetes in particular often present with increased TG and decreased HDL [2]. The reasons for the decreased HDL levels are not fully known, however, one factor proposed to be involved is cholesteryl ester transfer protein (CETP), which catalyzes the transfer of TG from apolipoprotein B (ApoB)-containing lipoprotein particles to HDL in exchange for the transfer of cholesteryl esters from HDL to ApoB-containing particles [3]. As a result of CETP activity, increases in TG are thought to lead to increased transfer of TG to HDL, resulting in more TG-rich HDL, which undergoes a higher rate of catabolism, thus leading to decreased HDL.
Several other factors, however, are clearly important in regulating HDL levels. The enzyme endothelial lipase (EL) is a phospholipase that hydrolyzes phospholipids (PL) in HDL [4]. Decreased EL activity occurring with EL gene knockout has been associated with increased HDL, while increased EL activity is thought to cause decreased HDL due to the removal of PL from HDL which results in PL-poor HDL particles that are more readily cleared from the circulation [5, 6]. Similarly, decreased levels of the EL inhibitor angiopoietin-like protein 3 (ANGPTL3) that occur with ANGPTL3 knockout mutations are associated with decreased HDL [7]. In addition, administration of a neutralizing anti-ANGPTL3 antibody has been reported to cause decreased HDL [8]. Because ANGPTL3 can form a complex with ANGPTL8 to potently inhibit lipoprotein lipase (LPL), the enzyme that hydrolyzes TG to generate fatty acids (FA), decreased HDL is also accompanied by decreased TG [9, 10]. Thus, ANGPTL3 inhibition provides an example in which TG and HDL do not move in opposite directions, as both decrease at the same time.
In a recent study, we examined the mechanisms by which the atypical ANGPTL protein ANGPTL8 [11, 12, 13, 14] acts to increase TG. We demonstrated that ANGPTL8 acts as a nutrient sensor to form complexes with ANGPTL3 and ANGPTL4 to increase and decrease respectively their LPL-inhibitory activities to direct FA storage in adipose tissue [15]. ANGPTL3/8 functions primarily in an endocrine manner by inhibiting LPL in skeletal muscle so that circulating TG that can be routed to the fat [15, 16]. In the adipose tissue, LPL inhibition is simultaneously reduced by increased localized ANGPTL4/8 [15]. Interestingly, we found that in control subjects, circulating levels of ANGPTL3/8 and ANGPTL4/8 increased significantly after feeding and that levels of both complexes were inversely correlated with HDL [15].
In light of these findings and because increased TG and decreased HDL are often seen in type 2 diabetes [2], we investigated circulating levels of ANGPTL3/8 and ANGPTL4/8 in type 2 diabetes patients. We found that ANGPTL3/8 and ANGPTL4/8 were both directly correlated with triglycerides (TG) and inversely correlated with HDL, with the inverse HDL correlation being significant for ANGPTL4/8. We therefore considered whether ANGPTL4 and/or ANGPTL4/8 might inhibit EL and measured the ability of each of the respective ANGPTL proteins and complexes to inhibit EL activity. In so doing, we confirmed that ANGPTL3 inhibits EL, but also found that ANGPTL4 was a more potent EL inhibitor than ANGPTL3. Interestingly, we also observed that ANGPTL4/8 was a much less potent EL inhibitor than ANGPTL4. Together, these results suggest that as ANGPTL4/8 levels increase there is decreased inhibition of EL, leading to more EL-mediated hydrolysis of PL in HDL, which in turn leads to decreased HDL. Our data thus show for the first time that ANGPTL4 is a more potent EL inhibitor than ANGPTL3 and suggest a possible explanation for why ANGPTL4/8 concentrations correlate inversely with HDL levels.
2. Materials and methods
2.1. Lipid measurements and serum samples from type 2 diabetes patients and normal subjects
After obtaining proper informed consent for exploratory analyses, baseline serum samples were collected from 93 patients with type 2 diabetes enrolled in a clinical trial. These samples were drawn under fasting morning conditions using serum separator tubes and stored at -80 °C prior to subsequent analyses. Serum samples were also obtained from 99 normal subjects for the Eli Lilly Research Blood Donor Program after the anonymized subjects gave their informed consent for sample collection. These samples were also drawn under fasting morning conditions using serum separator tubes and stored at -80 °C prior to subsequent analyses. All lipid markers and ApoB were measured using a Roche Cobas instrument. The ratio of ApoB to low density lipoprotein cholesterol (LDL-C) was used as a surrogate marker for small dense LDL-C. Non-HDL-C was calculated by subtracting HDL-C from total cholesterol (TC). Levels of TG and HDL-C were used to calculate the TG/HDL ratio.
2.2. Recombinant proteins and complexes
Human ANGPTL sequences were ANGPTL8: NP_061157.3, ANGPTL3: NP_055310.1, and ANGPTL4: NP_647475.1. C-terminal HIS-tagged ANGPTL4 and ANGPTL3 were produced stably in CHO cells and transiently in HEK293 cells, respectively. Both were purified through nickel-nitrilotriacetic acid (Ni-NTA) affinity, followed by size exclusion chromatography (SEC). ANGPTL3/8 complex was produced in HEK293 cells through transient co-transfection. Nucleotide sequences encoding mouse IgG kappa signal peptide-HIS tag-mature human serum albumin (HSA)-PreScission cleavage site-mature ANGPTL8 were inserted into a mammalian expression vector containing a cytomegalovirus (CMV) promoter, as were the nucleotide sequences encoding C-terminal Flag-tagged ANGPTL3. Protein expression was performed through transient co-transfection of both expression constructs in HEK293 cells cultured in serum-free media. Culture media were harvested 5 days post transfection and stored at 4 °C for subsequent protein purification at 4 °C. Four liters of culture media were supplemented with 1 M Tris-HCl (pH 8.0) and 5 M NaCl to final concentrations of 25 mM and 150 mM, respectively. The media were incubated with 150 ml of Ni-NTA resin (Qiagen) overnight. The resin was then packed into a column and washed with buffer A (50 mM Tris-HCl, pH 8.0, 0.3 M NaCl). Elution was performed with a 0–300 mM imidazole gradient in buffer A. Fractions containing HIS-HSA-ANGPTL3/8 complexes were pooled, concentrated, loaded onto a HiLoad Superdex 200 column (GE Healthcare), and eluted with buffer A. Fractions containing HIS-HSA-ANGPTL3/8 complexes were again pooled, concentrated, and digested with PreScission protease to remove HSA from the fusion protein. The PreScission digested protein sample was loaded onto another HiLoad Superdex 200 column and eluted with storage buffer (20 mM HEPES pH 8.0, 150 mM NaCl). Fractions containing ANGPTL3/8 were pooled and concentrated.
To ensure purity during the ANGPTL3/8 purification process, the initial Ni-NTA affinity purification first removed free ANGPTL3. After SEC, purified HIS-HSA-ANGPLT3/8 complex and free HIS-HSA-ANGPLT8 were obtained. PreScission digestion (which cleaved between HSA and ANGPTL8) resulted in ANGPTL3/8 complex, HIS-HSA, and free ANGPTL8. Free ANGPTL8 was precipitated out, leaving only ANGPTL3/8 complex and HIS-HSA. ANGPTL3/8 complex and HIS-HSA were separated with a second SEC step, resulting in highly purified ANGPTL3/8 complex without HIS-HSA contamination. This ensured that very pure ANGPTL3/8 complex was produced. The exact same approach was used for expression and purification of the ANGPTL4/8 complex. All proteins and complexes were maintained at a <0.01 EU/ug of endotoxin. Protein concentrations were determined using a bicinchoninic acid (BCA) assay. One μg of each recombinant ANGPTL protein or complex was characterized using gradient gel electrophoresis with Bio-Rad 4–20% Mini-Protean Tris-glycine gels, followed by Coomassie Blue staining to verify the purity of the respective proteins and complexes, which were all stored at -80 °C. For purposes of molar conversions, a molecular weight of 179 kD was used for ANGPTL3/8 (3:1 ratio), while a molecular weight of 64 kD was used for ANGPTL4/8 (1:1 ratio).
The nucleotide sequence encoding C-terminal HIS-tagged human EL (NP_006024.1) was inserted into a mammalian expression vector containing a cytomegalovirus (CMV) promoter. EL protein expression was performed through transient transfection in HEK293 cells cultured in serum-free media. Culture media were harvested 5 days post transfection and stored at 4 °C for subsequent protein purification. Two liters of culture media were supplemented with 1 M Tris-HCl (pH 7.5) and 5 M NaCl to final concentrations of 25 mM and 150 mM, respectively. The media were incubated with 25 ml of HisPur Ni-NTA resin (ThermoFisher) for 3.5 h. The resin was then packed into a column and washed with buffer A (50 mM Tris-HCl, pH 7.5, 0.3 M NaCl). Elution was performed with a 0–300 mM imidazole gradient in buffer A. Fractions containing EL-His were pooled, concentrated, loaded onto a HiLoad Superdex 200 column (GE Healthcare), and eluted with PBS. The fractions were pooled, concentrated, aliquoted and stored at -80 °C. The recombinant EL protein concentration was determined using a bicinchoninic acid (BCA) assay.
2.3. ANGPTL antibodies and serum assays
An anti-human ANGPTL8 antibody (residues 22–198) was generated using hybridoma techniques by Precision Antibody Sciences. An anti-human ANGPTL4 antibody was generated by immunization with recombinant ANGPTL4 (residues 26–161). An anti-human ANGPTL3 antibody was generated after immunization with recombinant ANGPTL3 (residues 17–220). Clones of interest were screened for non-overlapping epitopes, and antigen-specific variable heavy (VH) and light (VL) gene sequences were determined from extracted RNA using a mouse Ig primer set (EMD Millipore). Variable domains were transferred into separate murine constant region expression vectors for antibody production, transfected into CHO cells, and purified using protein A chromatography. Antibodies were biotinylated using a Pierce kit and ruthenium-labeled using a MesoScale Discovery (MSD) kit, with MALDI-TOF performed to verify that appropriate labeling had occurred. Antibodies were diluted in 50% glycerol and stored at -20 °C.
Dedicated immunoassays were used to measure ANGPTL3/8 and ANGPTL4/8 complexes in human serum. For the ANGPTL3/8 assay, the capture antibody recognized ANGPTL8, and the detection antibody recognized ANGPTL3. For the ANGPTL4/8 assay, the capture antibody recognized ANGPTL4, and the detection antibody recognized ANGPTL8. For each assay, MesoScale Discovery (MSD) streptavidin plates were washed three times with TBST (Tris buffered saline containing 10 mmol/L Tris pH 7.40, 150 mmol/L NaCl, and 1 mL/L Tween 20). Plates were blocked with TBS plus 1% bovine serum albumin (BSA) for 1 h at room temperature (RT). After aspiration and washing, wells were incubated with biotinylated capture antibody for 1 h. Following aspiration and washing, 50 μL of recombinant ANGPTL3/8 or ANGPTL4/8 complex (serially diluted to form a standard curve) were added to the wells in assay buffer (50 mmol/L HEPES, pH 7.40, 150 mmol/L NaCl, 10 mL/L Triton X-100, 5 mmol/L EDTA, and 5 mmol/L EGTA). Serum samples were diluted in assay buffer and added to their respective wells for a 2-hour incubation at RT. After aspiration, wells were washed three times, and 50 μL of ruthenium-labeled detection antibody were added for a 1-hour incubation at RT. Following aspiration, wells were washed three times, and 150 μL of MSD read buffer were added. Electrochemiluminescence from electrical excitation of ruthenium in the wells was detected using an MSD plate reader.
2.4. Endothelial lipase (EL) cell-based activity assay
The nucleotide sequence encoding human EL (NP_006024.1) was inserted into pLenti6.3 vector (Invitrogen) to generate lentivirus, which was used to create an EL-stable expression HEK293T cell line, which was confirmed by qPCR and enzymatic activities. The cell line was grown and maintained in DMEM/F12 (3:1) (Invitrogen), 10% FBS (Hyclone), and 5 μg/ml blasticidin (Invitrogen). Human EL-stable expression cells were seeded at density of 50,000 cells/well in tissue culture-treated polystyrene 96-well plates (Costar) in growth medium (3:1 DMEM/F12, 10% FBS, and 5 μg/ml blasticidin). After overnight incubation at 37 °C, medium was replaced with 80 μL of medium (OptiMEM, Invitrogen) containing serially diluted ANGPTL proteins or complexes. Cells were incubated for 1 h before adding 20 μL of 5X working solution that was freshly prepared with reaction buffer (50 mM Tris-HCl, 140 mM NaCl, 2 mM CaCl2) containing the EnzChek phospholipase A1 (PLA1) selective substrate PED-A1 (N-((6-(2,4-DNP)amino)hexanoyl)-1-BODIPYTM-FL-C5)-2-hexyl-sn-glycero-3-phosphoethanolamine) (Invitrogen), DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and DOPG (1,2-dioleoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt) (Sigma) to achieve final concentrations of 1, 10, and 10 μM respectively. The 5X working solution was prepared by mixing one part of 1 mM PED-A1, one part of 10 mM DOPC, and one part of 10 mM DOPG and adding the mixture drop by drop into 197 parts of reaction buffer while mixing in a glass tube. This EL activity assay method (which follows the recommendations from the Invitrogen Fluorogenic Phospholipase A Substrates Manual) is optimized to allow measurement of the PLA1 activity of EL (which preferentially hydrolyzes PL at the sn-1 position). Cells were then incubated at either 22 °C or 37 °C, and fluorescence was monitored with a Synergy Neo2 plate reader with an excitation wavelength of 485 nm and an emission wavelength of 516 nm. Readings were taken at 1 and 30 min, with the 1-minute reading subtracted from the 30-minute reading in order to correct for background fluorescence.
2.5. Recombinant EL activity assay
To confirm the effects of ANGPTL4 and ANGPTL4/8 on endothelial lipase, a recombinant EL activity assay was performed by first pre-incubating 80 μL of human recombinant EL protein at a final concentration of 400 nM in reaction buffer (50 mM Tris-HCl, 140 mM NaCl, 2 mM CaCl2) containing increasing concentrations of ANGPTL4 or ANGPTL4/8. The pre-incubation was allowed to proceed for 1-hour at 37 °C before adding 20 μL of 5X working solution, the same solution as described for the EL cell-based activity assay. The incubation was continued at 37 °C, and fluorescence was monitored with a Synergy Neo2 plate reader with an excitation wavelength of 485 nm and an emission wavelength of 516 nm. Readings were taken at 1 and 30 min, with the 1-minute reading subtracted from the 30-minute reading in order to correct for background fluorescence.
2.6. Data analysis
A four-parameter logistic non-linear regression model was used to fit curves for EL activity assays and to estimate IC50 values, which were determined using the sigmoidal 4-PL fittings. For ANGPTL3/8 and ANGPTL4/8 immunoassays, MSD software was used for fitting of the individual calibration curves using a 5-parameter fit with 1/y2 weighting. For the correlations of ANGPTL3/8 and ANGPTL4/8 complexes with serum lipid parameters, associations between the complexes and selected markers were assessed using Spearman rank correlation coefficients.
3. Results
3.1. ANGPTL3/8 and ANGPTL4/8 correlations with lipid markers in type 2 diabetes
We previously showed in control subjects that ANGPTL3/8 and ANGPTL4/8 complexes increased with feeding to direct FA toward the adipose tissue for storage as TG [15]. Interestingly, we also demonstrated in the same study that levels of both complexes were inversely correlated with HDL while being directly correlated with TG and all other metabolic syndrome markers. Because type 2 diabetes patients often present with increased TG and decreased HDL, we examined circulating levels of ANGPTL3/8 and ANGPTL4/8 in type 2 diabetes patients to determine how these complexes correlated with multiple, different lipid parameters.
ANGPTL3/8 levels in type 2 diabetes patients (mean = 18 ng/mL) were similar to those of normal subjects (mean = 18 ng/mL) while ANGPTL4/8 levels in type 2 diabetes patients (mean = 48 ng/mL) were roughly 2-fold higher than those observed in normal subjects (mean = 22 ng/mL). These results were consistent with our previously reported observations [15]. Because normal subjects were anonymized, however, it was not possible to control or correct for factors such as BMI, which might be altered in type 2 diabetes patients and could thus affect ANGPTL4/8 levels.
As Table 1 demonstrates, in type 2 diabetes patients ANGPTL3/8 and ANGPTL4/8 complexes showed correlations with multiple different lipid markers that were similar to the correlations we had previously observed in normal subjects [15]. ANGPTL3/8 (but not ANGPTL4/8) was directly correlated with TC, LDL-C, non-HDL-C, and ApoB, likely due to the ability of ANGPTL3/8 (but not ANGPTL4/8) to block hepatic uptake of ApoB-containing particles [15]. Interestingly, however, ANGPTL3/8 was not correlated with the ApoB/LDL ratio (a surrogate for small dense LDL), while ANGPTL4/8 levels were directly correlated with the ApoB/LDL ratio.
Table 1.
Circulating ANGPTL3/8 and ANGPTL4/8 complex levels are correlated with many lipid markers in patients with type 2 diabetes. ANGPTL3/8 and ANGPTL4/8 were measured in fasting serum samples from type 2 diabetes patients, and associations with various lipid parameters were assessed. The only relationship to show a significant inverse correlation was that of ANGPTL4/8 compared to HDL-C.
| Lipid Marker | ANGPTL3/8 (mean level = 18 ng/mL) |
ANGPTL4/8 (mean level = 48 ng/mL) |
||
|---|---|---|---|---|
| R-value | p-value | R-value | p-value | |
| Triglyceride (TG) | 0.51 | <0.0001 | 0.30 | 0.003 |
| HDL-cholesterol (HDL-C) | - 0.16 | 0.12 (NS) | - 0.27 | 0.008 |
| TG/HDL-C ratio (TG/HDL) | 0.43 | <0.0001 | 0.32 | 0.001 |
| Total cholesterol (TC) | 0.34 | 0.0006 | 0.07 | 0.50 (NS) |
| LDL cholesterol (LDL-C) | 0.19 | 0.05 | - 0.03 | 0.77 (NS) |
| Non-HDL-C (nHDL-C) | 0.35 | 0.0003 | 0.15 | 0.15 (NS) |
| Apolipoprotein B (ApoB) | 0.38 | 0.0002 | 0.17 | 0.13 (NS) |
| ApoB/LDL (small dense LDL) | 0.12 | 0.25 (NS) | 0.26 | 0.01 |
We also observed that in the type 2 diabetes patients both ANGPTL3/8 and ANGPTL4/8 showed correlations with TG, HDL, and the TG/HDL ratio that were consistent with correlations previously observed in control subjects [15]. Both complexes were directly correlated with TG and the TG/HDL ratio, while being inversely correlated with HDL-C. In the case of ANGPTL3/8, the inverse correlation with HDL did not achieve significance, while the correlation of ANGPTL4/8 with HDL was statistically significant.
3.2. ANGPTL3 and ANGPTL3/8 inhibition of EL activity
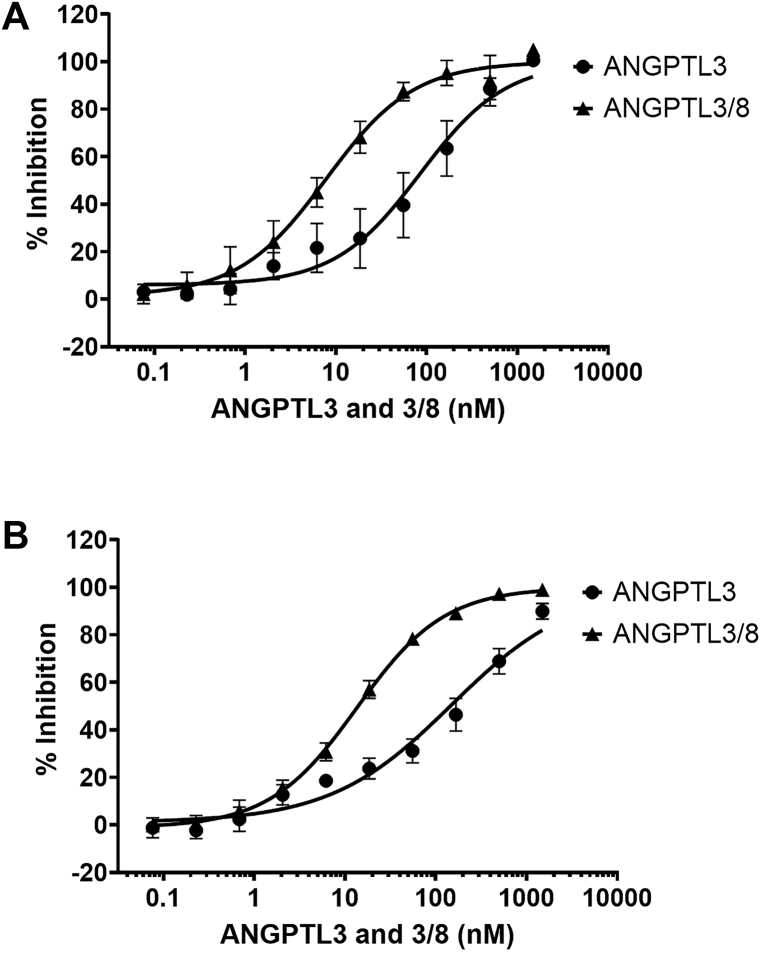
In light of these results, we investigated possible reasons for the inverse correlations of ANGPTL3/8 and ANGPTL4/8 with HDL by studying the ability of these complexes, as well as ANGPTL3 and ANGPTL4, to inhibit EL, the enzyme that hydrolyzes PL in HDL. ANGPTL3 is a well-known inhibitor of EL, and it is thought that the decreased HDL concentrations observed in human subjects with ANGPTL3 knockout mutations or in patients treated with anti-ANGPTL3 monoclonal antibodies are due to increases in EL activity [7, 8]. We first examined the effect of ANGPTL3 on EL activity. As Figure 1A shows, at 37 °C, ANGPTL3 alone demonstrated inhibition of EL with an IC50 of 85.4 nM. This was a similar IC50 compared to what we had previously observed ANGPTL3-mediated LPL inhibition [15]. Interestingly, for ANGPTL3/8, the observed IC50 was 7.9 nM, thus indicating a 10.8-fold increase in EL inhibition for the ANGPTL3/8 complex compared to ANGPTL3 alone. This was in contrast to the much greater increase in LPL inhibition that we previously described for ANGPTL3/8 versus ANGPTL3 [15]. Similar results with regard to EL inhibition by ANGPTL3 and ANGPTL3/8 were obtained when the experiments in Figure 1A were repeated at 22 °C (Figure 1B).
Figure 1.
ANGPTL3 and ANGPTL3/8 inhibition of EL. (A) The ability of ANGPTL3 or ANGPTL3/8 to inhibit EL at 37 °C was assessed using an EL stable expression cell line and phospholipase A1 selective substrate. Fluorescence was monitored with an excitation wavelength of 485 nm and an emission wavelength of 516 nm. Readings were taken at 1 and 30 min, with the 1-minute reading subtracted from the 30-minute reading to correct for background fluorescence. Results are shown as the mean ± SD (n = 6 from 3 independent experiments). (B) The ability of ANGPTL3 or ANGPTL3/8 to inhibit EL was assessed as in Figure 1A except at 22 °C. Results are shown as the mean ± SD (n = 6 from 3 independent experiments).
3.3. ANGPTL4 and ANGPTL4/8 inhibition of EL activity
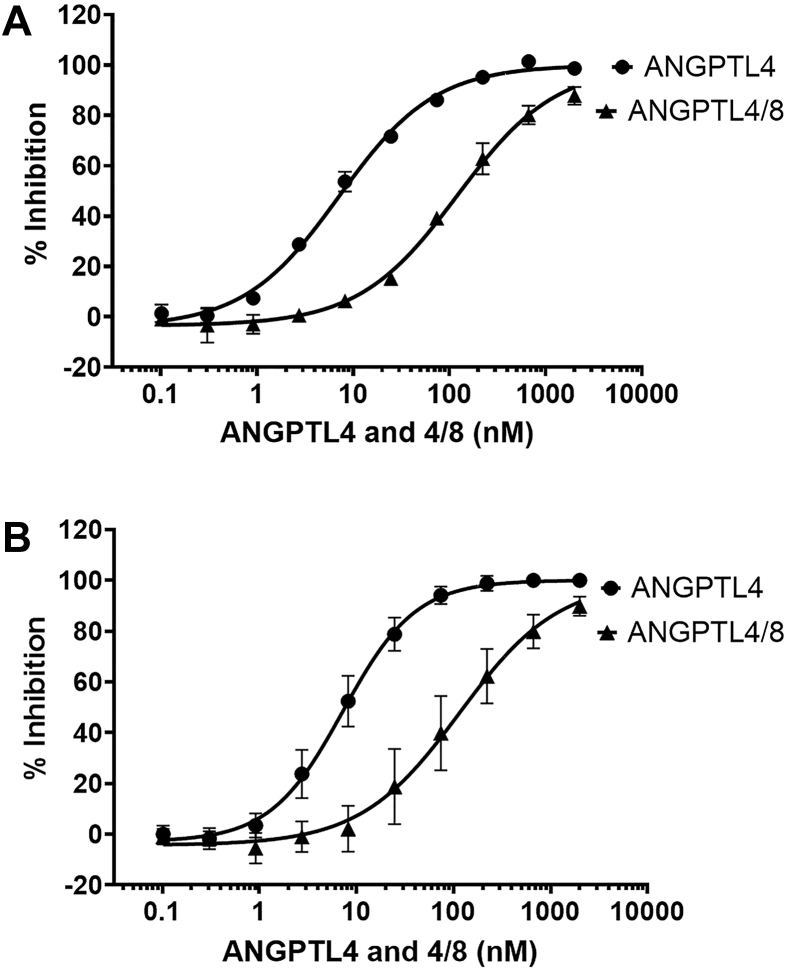
We next performed experiments to examine the ability of ANGPTL4 to inhibit EL at 37 °C (Figure 2A). To the best of our knowledge, ANGPTL4 has not been previously described to be an inhibitor of EL. We evaluated ANGPTL4 in the same human EL cell-based activity assay used to characterize ANGPTL3 and ANGPTL3/8 and observed that ANGPTL4 inhibited EL activity with an IC50 of 7.2 nM. Remarkably, this was a lower IC50 for EL inhibition than that of ANGPTL3, indicating that ANGPTL4 may be a more potent EL inhibitor than ANGPTL3. Interestingly, in contrast to the increase in EL inhibition observed with ANGPTL3/8 versus ANGPTL3, ANGPTL4/8 manifested decreased EL inhibitory activity, with an IC50 of 121 nM. Thus, ANGPTL4/8 exhibited a 16.8-fold decreased potency for EL inhibition compared to ANGPTL4 alone. This decreased potency for EL inhibition of ANGPTL4/8 versus ANGPTL4 paralleled the trend we had seen for their respective effects on LPL inhibition, although the absolute magnitude of the decreased potency was again less than what we had observed for LPL [15]. When these experiments were repeated at 22 °C (Figure 2B), similar results were again observed for the EL inhibition of ANGPTL4/8 versus ANGPTL4. This was in dramatic contrast to what we had previously observed for ANGPTL4/8-mediated inhibition of LPL, which was markedly decreased at 22 °C versus 37 °C [17].
Figure 2.
ANGPTL4 and ANGPTL4/8 inhibition of EL. (A) The ability of ANGPTL4 or ANGPTL4/8 to inhibit EL at 37 °C was assessed using an EL stable expression cell line and phospholipase A1 selective substrate. Fluorescence was monitored with an excitation wavelength of 485 nm and an emission wavelength of 516 nm. Readings were taken at 1 and 30 min, with the 1-minute reading subtracted from the 30-minute reading to correct for background fluorescence. Results are shown as the mean ± SD (n = 4 from 2 independent experiments). (B) The ability of ANGPTL4 or ANGPTL4/8 to inhibit EL was assessed as in Figure 2A except at 22 °C. Results are shown as the mean ± SD (n = 8 from 4 independent experiments).
Table 2 summarizes the results from the EL activity experiments performed with ANGPTL3, ANGPTL3/8, ANGPTL4, and ANGPTL4/8 at both 37 °C and 22 °C. Together, these results show for the first time that ANGPTL4 inhibits EL activity more potently than ANGPTL3. In addition, similar to what we have previously described for LPL, the individual ANGPTL proteins and their respective complexes appeared to exist in an almost symmetrically modifiable system for modulating EL activity. The IC50 values for EL inhibition by ANGPTL3 and ANGPTL4/8 were very similar, as were the IC50 values for inhibition of EL by ANGPTL4 and ANGPTL3/8. Interestingly, the dramatic temperature-dependent decrease in ANGPTL4/8-mediated LPL inhibitory activity that we previously described [17] was not observed for ANGPTL4/8-mediated inhibition of EL activity.
Table 2.
EL inhibition summary for ANGPTL3 versus ANGPTL3/8 and ANGPTL4 versus ANGPTL4/8. Individual IC50 concentrations were determined for ANGPTL3 versus ANGPTL3/8 and ANGPTL4 versus ANGPTL4/8 for the EL activity assays shown in Figures 1 and 2.
| Temperature | ANGPTL Protein | IC50 (nM) | ANGPTL Complex | IC50 (nM) | Change Direction | Fold Change |
|---|---|---|---|---|---|---|
| 37 °C | ANGPTL3 | 85.4 | ANGPTL3/8 | 7.9 | Increase | (+) 10.8 |
| ANGPTL4 | 7.2 | ANGPTL4/8 | 121 | Decrease | (-) 16.8 | |
| 22 °C | ANGPTL3 | 149 | ANGPTL3/8 | 13.6 | Increase | (+) 11.0 |
| ANGPTL4 | 7.5 | ANGPTL4/8 | 120 | Decrease | (-) 16.0 |
3.4. ANGPTL4 and ANGPTL4/8 inhibition of recombinant EL protein activity
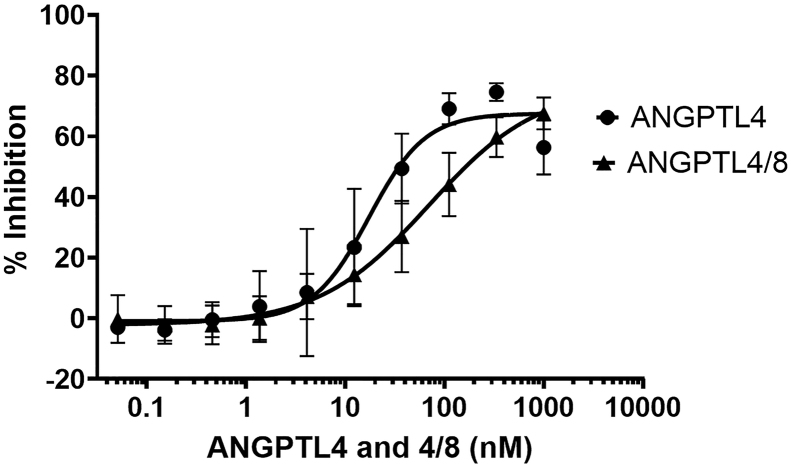
To confirm further the inhibition of EL by ANGPTL4 and ANGPTL4/8, we performed recombinant EL activity assays after first pre-incubating the human recombinant EL protein with increasing concentrations of either ANGPTL4 or ANGPTL4/8 complex. Figure 3 shows the results of these experiments, in which ANGPTL4 was confirmed to be a human recombinant EL inhibitor, consistent with what was observed in the cell-based EL activity assay (IC50 = 17.3 nM). Also similar to what was observed in the cell-based EL activity assay, ANGPTL4/8 had reduced EL inhibitory activity compared to ANGPTL4 (IC50 = 69.4 nM). Overall, these results confirmed that ANGPTL4 and ANGPTL4/8 directly inhibited EL activity, with ANGPTL4/8-mediated EL inhibition again being less than that of ANGPTL4.
Figure 3.
ANGPTL4 and ANGPTL4/8 inhibition of recombinant EL protein activity. The ability of ANGPTL4 or the ANGPTL4/8 complex to inhibit human recombinant EL protein activity was assessed using phospholipase A1 selective substrate. Fluorescence was monitored with an excitation wavelength of 485 nm and an emission wavelength of 516 nm. Readings were taken at 1 and 30 min, with the 1-minute reading subtracted from the 30-minute reading to correct for background fluorescence. Results are shown as the mean ± SD (n = 4 from 2 independent experiments).
4. Discussion
The major findings in this study are 1) ANGPTL4 is a more potent inhibitor of the enzyme EL than ANGPTL3, 2) formation of the ANGPTL4/8 complex results in reduced EL-inhibitory activity compared to ANGPTL4 alone, and 3) ANGPTL4/8 levels are increased in patients with type 2 diabetes and are inversely correlated with HDL levels. To our knowledge, this study is the first to demonstrate that ANGPTL4 is an inhibitor of EL. With an IC50 of 7.2 nM for EL inhibition, ANGPTL4 is actually a several-fold more potent EL inhibitor than ANGPTL3, based on our own observations for ANGPTL3 (IC50 = 71 nM), as well as those previously reported for ANGPTL3 [18]. In addition, formation of the ANGPTL4/8 complex (IC50 = 121 nM) decreased the ability of ANGPTL4 to inhibit EL by approximately 17-fold. This reduction in ANGPTL4/8-mediated EL inhibition compared to ANGPTL4 would be expected to lead to overall increased EL activity, and therefore increased hydrolysis of PL present in HDL, which would in turn result in decreased HDL.
These observations may provide an additional reason for why HDL decreases in the postprandial state [19, 20, 21], since the increased ANGPTL4/8 levels that occur after feeding would be predicted to result in decreased EL inhibition. The resulting increase in EL activity would thus result in decreased postprandial circulating HDL. Our findings with regard to ANGPTL-mediated EL inhibition might also help explain why HDL levels are inversely correlated with ANGPTL4/8 concentrations in patients with type 2 diabetes, as the increased ANGPTL4/8 levels in these patients would result in decreased EL inhibition, resulting in increased hydrolysis of PL present in HDL and thus decreased HDL. Interestingly, we once again observed that ANGPTL4/8 levels in type 2 diabetes patients were about twice as high as those of normal controls, while ANGPTL3/8 levels were similar to those of normal subjects [15]. Clearly, additional work will be required to understand the reasons for the increased ANGPTL4/8 levels in type 2 diabetes.
Our observations may also shed light on the relatively modest decreases in HDL observed after administration of an anti-ANGPTL3 monoclonal antibody [7]. If ANGPTL3 were the most important inhibitor of EL activity, then administration of a high dose of a therapeutic anti-ANGPTL3 neutralizing antibody would be expected to cause a more precipitous drop in circulating HDL levels than the 20–30% decreases that have so far been reported [7]. Another possibility might be that an anti-ANGPTL3 antibody could also neutralize ANGPTL3 present in the circulating ANGPTL3/8 complex. Since ANGPTL3/8 is roughly as potent an EL inhibitor as ANGPTL4, this could result in decreased ANGPTL3/8-mediated inhibition of EL, thus resulting in increased EL activity, which would in turn be expected to cause HDL levels to decrease. Interestingly, ANGPTL3 inactivation (but not ANGPTL4 inactivation) has also been associated with a cholesterol-reducing effect [8]. One possible explanation for this, which does not involve EL inhibition, may be that the ANGPTL3/8 complex blocks the ability of LPL to facilitate hepatic cholesterol-containing lipoprotein particle uptake, while the ANGPTL4/8 complex does not [15].
Similar to what we previously reported for LPL activity, the individual ANGPTL3 and ANGPTL4 proteins and their respective complexes appear to comprise an almost symmetrically modifiable system for the inhibition of EL activity. Although not identical, ANGPTL3 and ANGPTL4/8 share similar IC50 values for inhibition of EL, as do ANGPTL4 and ANGPTL3/8. A major, unanswered question, however, is why the IC50 values for EL inhibition for each of the individual ANGPTL proteins and complexes are so much greater than the corresponding IC50 values for the same ANGPTL proteins and complexes with regard to their inhibition of LPL. For instance, the most potent LPL inhibitors ANGPTL4 and ANGPTL3/8 have IC50 values for LPL inhibition of 0.29 and 0.14 nM respectively [15], while their IC50 values for EL inhibition are 7.2 nM and 7.9 nM respectively. More investigation will certainly be required in order to understand the reasons for these large differences in the absolute IC50 values for EL versus LPL inhibition.
In the case of the human ANGPTL8 heterozygous knockout mutations (121X and 131X), decreased circulating ANGPTL3/8 complex would be anticipated to result in reduced ANGPTL3/8-mediated EL inhibition, with the expected result being increased EL activity and reduced HDL [22, 23]. In fact, however, the opposite has been reported. Individuals carrying these mutations actually have HDL increases ranging from 6-10 mg/dL [22, 23]. Therefore, these mutations cannot be directly driving the HDL increases through ANGPTL3/8–mediated EL inhibition. Rather, our data suggest that what might be occurring is that ANGPTL8 knockout causes decreased levels of ANGPTL4/8 (and correspondingly increased levels of ANGPTL4 alone). This would result in increased EL inhibition by ANGPTL4 leading to increased HDL. Of course, this possibility will have to be tested by measuring ANGPTL3/8 and ANGPTL4/8 levels in ANGPTL8-knockout subjects.
Taken together, our data show that the ANGPTL8-4-3 system of proteins regulates EL activity in a manner somewhat similar to its regulation of LPL activity. In particular, just as ANGPTL4 is a more potent LPL inhibitor than ANGPTL3, so too is ANGPTL4 a more potent EL inhibitor than ANGPTL3. In the case of both LPL and EL, when ANGPTL8 combines with ANGPTL3 to form an ANGPTL3/8 complex, the potency of ANGPTL3-mediated inhibition is greatly increased, while when ANGPTL8 combines with ANGPTL4 to form an ANGPTL4/8 complex, the potency of ANGPTL4-mediated inhibition is greatly reduced. Although the mechanisms of ANGPTL4 and ANGPTL3/8 inhibition of LPL are reasonably well described [24, 25, 26, 27, 28, 29, 30], further study will certainly be required to elucidate the biochemical mechanisms governing EL inhibition by ANGPTL4 and ANGPTL4/8.
5. Conclusions
By demonstrating that ANGPTL4 is an inhibitor of EL while ANGPTL4/8 has decreased EL-inhibitory activity, we show that the ANGPTL8-4-3 system of proteins regulates EL activity in a symmetrically modifiable manner analogous to its regulation of LPL activity. In the case of each enzyme, ANGPTL8 forms complexes with ANGPTL3 and ANGPTL4 to increase or decrease markedly their respective abilities to inhibit the activities of both lipases.
Declarations
Author contribution statement
Yan Q. Chen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Thomas G. Pottanat: Performed the experiments; Analyzed and interpreted the data.
Robert W. Siegel, Mariam Ehsani, Yue-Wei Qian: Contributed reagents, materials, analysis tools or data.
Robert J. Konrad: Conceived and designed the experiments; Wrote the paper.
Funding statement
Robert J. Konrad was supported by Eli Lilly and Company.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Melissa Bellinger for assistance with cell lines and EL activity assays.
References
- 1.Rashid S., Uffelman K.D., Lewis G.F. The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J. Diabet. Complicat. 2002;16:24–28. doi: 10.1016/s1056-8727(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu L., Parhofer K.G. Diabetic dyslipidemia. Metabolism. 2014;63:1469–1479. doi: 10.1016/j.metabol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha S., Wu B.J., Guiney L., Barter P.J., Rye K.A. Cholesteryl ester transfer protein and its inhibitors. J. Lipid Res. 2018;59:772–783. doi: 10.1194/jlr.R082735. Epub 2018 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantis P., Coleman T., Schiekofer S., Nawroth P.P., Schlimmer P., Schneider J.G. Endothelial Lipase: a key player in HDL metabolism modulates inflammation and atherosclerotic risk. Mini Rev. Med. Chem. 2008;8:619–627. doi: 10.2174/138955708784534427. [DOI] [PubMed] [Google Scholar]
- 5.Lamarche B., Paradis M.E. Endothelial lipase and the metabolic syndrome. Curr. Opin. Lipidol. 2007;18:298–303. doi: 10.1097/MOL.0b013e328133857f. [DOI] [PubMed] [Google Scholar]
- 6.Badellino K.O., Rader D.J. The role of endothelial lipase in high-density lipoprotein metabolism. Curr. Opin. Cardiol. 2004;19:392–395. doi: 10.1097/01.hco.0000130161.89169.02. [DOI] [PubMed] [Google Scholar]
- 7.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 8.Dewey F.E., Gusarova V., Dunbar R.L., O'Dushlaine C., Schurmann C., Gottesman O. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovrov O., Kristensen K.K., Larsson E., Ploug M., Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J. Lipid Res. 2019;60:783–793. doi: 10.1194/jlr.M088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. Open Biol. 2016;6:150272. doi: 10.1098/rsob.150272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E., Valenzuela D.M., Murphy A.J., Cohen J.C., Hobbs H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren G., Kim J.Y., Smas C.M. Identification of rifl, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2012;303:E334–351. doi: 10.1152/ajpendo.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R. Lipasin, a novel nutritionally regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012;424:786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D.M., Cohen J.C., Hobbs H.H. Mice lacking ANGPTL8 (betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 2013;110:16109–16114. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y.Q., Pottanat T.G., Siegel R.W., Ehsani M., Qian Y.-W., Zhen E.Y., Regmi A., Roell W.C., Guo H., Luo M.J., Gimeno R.E., Van't Hooft F., Konrad R.J. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J. Lipid Res. 2020;61:1203–1220. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldoni F., Cheng H., Banfi S., Gusarova V., Cohen J.C., Hobbs H.H. ANGPTL8 has both endocrine and autocrine effects on substrate utilization. JCI Insight. 2020;5:138777. doi: 10.1172/jci.insight.138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y.Q., Pottanat T.G., Siegel R.W., Ehsani M., Qian Y.W., Roell W.C., Konrad R.J. Angiopoietin-like protein 4(E40K) and ANGPTL4/8 complex have reduced, temperature-dependent LPL-inhibitory activity compared to ANGPTL4. Biochem. Biophys. Res. Commun. 2021;534:498–503. doi: 10.1016/j.bbrc.2020.11.053. [DOI] [PubMed] [Google Scholar]
- 18.Gusarova V., Alexa C.A., Wang Y., Rafique A., Kim J.H., Buckler J.D. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 2015;56:1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Bruin T.W., Brouwer C.B., Gimpel J.A., Erkelens D.W. Postprandial decrease in HDL cholesterol and HDL apo A-I in normal subjects in relation to triglyceride metabolism. Am. J. Physiol. 1991;260:E492–498. doi: 10.1152/ajpendo.1991.260.3.E492. [DOI] [PubMed] [Google Scholar]
- 20.Cohn J.S., McNamara J.R., Cohn S.D., Ordovas J.M., Schaefer E.J. Postprandial plasma lipoprotein changes in human subjects of different ages. J. Lipid Res. 1988;29:469–479. PMID 3392464. [PubMed] [Google Scholar]
- 21.Westerveld H.T., Meijer E., Erkelens D.W., de Bruin T.W. Postprandial reduction in high-density lipoprotein cholesterol concentrations in postmenopausal women: improvement by 17beta-estradiol. Metabolism. 1996;45:827–832. doi: 10.1016/s0026-0495(96)90154-7. [DOI] [PubMed] [Google Scholar]
- 22.Peloso G.M., Auer P.L., Bis J.C., Voorman A., Morrison A.C., Stitziel N.O., Brody J.A. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am. J. Hum. Genet. 2014;94:223–232. doi: 10.1016/j.ajhg.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helkkula P., Kiiskinen T., Havulinna A.S., Karjalainen J., Koskinen S., Salomaa V., Daly M.J., Palotie A., Surakka I., Ripatti S. ANGPTL8 protein-truncating variant associated with lower serum triglycerides and risk of coronary disease. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin W., Romeo S., Chang S., Grishin N.V., Hobbs H.H., Cohen J.C. Genetic variation in ANGPTL4 provides insights into protein processing and function. J. Biol. Chem. 2009;284:13213–13222. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dijk W., Kersten S. Regulation of lipoprotein lipase by ANGPTL4. Trends Endocrinol. Metabol. 2014;25:146–155. doi: 10.1016/j.tem.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Gutgsell A.R., Ghodge S.V., Bowers A.A., Neher S.B. Mapping the sites of the lipoprotein lipase (LPL)-angiopoietin-like protein 4 (ANGPTL4) interaction provides mechanistic insight into LPL inhibition. J. Biol. Chem. 2019;294:2678–2689. doi: 10.1074/jbc.RA118.005932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mysling S., Kristensen K.K., Larsson M., Kovrov O., Bensadouen A., Jørgensen T.J., Olivecrona G., Young S.G., Ploug M. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. Elife. 2016;5 doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen K.K., Leth-Espensen K.Z., Mertens H.D.T., Birrane G., Meiyappan M., Olivecrona G., Jørgensen T.J.D., Young S.G., Ploug M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: insight into the regulation of plasma triglyceride metabolism. Proc. Natl. Acad. Sci. USA. 2020;117:4337–4346. doi: 10.1073/pnas.1920202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leth-Espensen K.Z., Kristensen K.K., Kumari A., Winther A.L., Young S.G., Jørgensen T.J.D., Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2026650118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.Q., Pottanat T.G., Zhen E.Y., Siegel R.W., Ehsani M., Qian Y.W., Konrad R.J. Apolipoprotein A5 lowers triglyceride levels via suppression of ANGPTL3/8-mediated LPL inhibition. J. Lipid Res. 2021;62:100068. doi: 10.1016/j.jlr.2021.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.