Abstract
Background
The cancer burden in adolescents and young adults (AYAs) deserves more attention. However, global cancer statistics for AYAs are often presented as aggregates, concealing important heterogeneity. This study aimed to describe the worldwide profile of cancer incidence, mortality, and corresponding trends from 1990 to 2019 among 15-39-year olds by focusing on the patterns by age, sex, sociodemographic index (SDI), and regions.
Patients and methods
Global, regional, and country data on the number of cancer cases and cancer-related deaths for 29 cancer types were collected from the 2019 Global Burden of Disease (GBD) Study. We also summarized the results using five levels of the SDI and 21 GBD regions.
Results
In 2019, an estimated 1 335 100 new cancer cases and 397 583 cancer-related deaths occurred among AYAs worldwide. While the incidence rate increased mildly, the death rate decreased significantly between 1990 and 2019, with an estimated annual percentage change of 0.38 (95% confidence interval 0.36-0.39) and −0.93 (95% confidence interval −0.95 to −0.92), respectively. The cancer burden was disproportionally greater among women than among men. The cancer profiles varied substantially across geographical regions, with the highest burden being in South Asia and East Asia. Besides, the cancer incidence in the high SDI regions was four times higher than that in the low SDI regions; however, the mortality burden in the high SDI region was lower than that in the low SDI region, which reflected the differences in cancer profiles across SDI regions and the inferior outcomes in the low SDI regions.
Conclusion
This study updates the previous epidemiological data of the cancer burden of AYAs. The cancer burden in AYAs varied substantially according to age, sex, SDI, and geographical regions. These findings highlight that the specific cancer profile of AYA patients requires targeted cancer control measures to reduce the cancer burden in this age group.
Key words: cancer burden, adolescents and young adults, incidence, death, trend
Highlights
-
•
The cancer burden in AYAs varied substantially according to age, sex, SDI, and geographical regions.
-
•
Cancer burden in AYAs was disproportionally greater among women than among men.
-
•
Cancer profiles of AYAs varied across different geographical regions and SDI regions.
-
•
Cancer burden in AYAs was still considerable in the low SDI regions.
Introduction
The burden of cancer is distributed unequally across age groups. Cancers in adolescents and young adults (AYAs) have been defined by the National Cancer Institute as diagnoses occurring between the ages of 15 and 39.1 A growing body of evidence shows that cancers in AYAs have distinct features, in terms of the distribution of cancer types, cancer biology, risk factors, prognosis, and survivorship, compared with that of cancers diagnosed in other age groups.2, 3, 4, 5 Although the overall incidence of cancer in AYAs is lower than that in older adults, the loss of healthy years of life is disproportionately higher in AYAs.6,7 Compared with older patients with cancer, AYAs with cancer have a higher risk of long-term effects, such as infertility, organ dysfunction, and secondary cancers.8, 9, 10 Thus the cancer burden of AYAs may lead to a decline in productivity growth and social structure. An accurate profile of the global cancer burden in AYAs is needed to direct health care policies and improve cancer-associated outcomes.
The global burden of cancer among AYAs is often ignored by cancer researchers and has rarely been studied in depth. Research on cancers in AYAs has mainly been conducted in high sociodemographic index (SDI) countries, especially the United States and several European countries, and seldom in countries with limited resources.2,11, 12, 13 Although Fidler et al.6 first described the global cancer profile of young adults in 2012 using data from GLOBOCAN 2012, the pattern of the cancer burden among AYAs has changed dramatically in the last decade. Subsequently, Trama et al.3 summarized the epidemiological data of the cancer burden of AYAs in 2018, but the data source, Population Based Cancer Registries, for this study covered only a small fraction of the populations living in low- and middle-income countries.
In this study, we collected epidemiological data, including incidence and mortality by sex, age, SDI, region, and country, on AYA cancers between 1990 and 2019 from the Global Burden of Disease (GBD) 2019 Study. Thereafter, we calculated the estimated annual percentage changes (EAPCs) to assess the trends of incident and death rates. An accurate evaluation of the worldwide burden and trends of AYA cancers will help to inform policy development and enhance service delivery.
Methods
Data source and collection
The GBD study was conducted by the Institute for Health Metrics and Evaluation (IHME), which provides a systematic scientific assessment of disease and injury incidence, prevalence, and mortality. The GBD 2019 Study provides a comprehensive epidemiologic estimation of the burden of 369 diseases and injuries in 204 countries and territories from 1990 to 2019.14 IHME’s general approach to the GBD 2019 Study and its major improvements over previous cycles have been explained in the previous publications.14
As the features of cancers in AYAs are distinct from those in the younger and older groups, different classification systems may uncover additional unique features. We investigated the incidence, mortality, and corresponding trends of the 29 cancer types that were included in the GBD 2019 Study: bladder cancer; brain and central nervous system (CNS) cancer; breast cancer; cervical cancer; colon and rectum cancer (referred to as colorectal cancer); esophageal cancer; gallbladder and biliary tract cancer; Hodgkin’s lymphoma; kidney cancer; larynx cancer; leukemia; lip and oral cavity cancer; liver cancer; tracheal, bronchus, and lung cancer (referred to as lung cancer); malignant skin melanoma; mesothelioma; multiple myeloma; nasopharynx cancer; non-Hodgkin’s lymphoma; nonmelanoma skin cancer; other pharynx cancers; ovarian cancer; pancreatic cancer; prostate cancer; stomach cancer; testicular cancer; thyroid cancer; uterine cancer; and other malignant neoplasms.
To assess the cancer burden of AYAs, global, regional, and country and territory estimates of the number of cancer cases and cancer-related deaths that occurred from 1990 to 2019 were collected from the GBD 2019 Study. To analyze the variations across age groups, the incidence and death rates among individuals aged 0-14 years, 15-39 years, 40 years and older were also extracted.
The SDI, as a composite indicator of a country’s lag-distributed income per capita, average years of schooling, and the fertility rate in females under the age of 25 years, is developed by GBD researchers. It is the geometric mean of 0 to 1 indices of total fertility rate under the age of 25, mean education for those aged 15 and older, and lag distributed income per capita. As a composite, a location with an SDI of 0 would have a theoretical minimum level of development relevant to health, while a location with an SDI of 1 would have a theoretical maximum level. According the value of SDI, all countries are classified into low SDI (0-0.454743), low–middle SDI (0.454743-0.607679), middle SDI (0.607679-0.689504), high–middle SDI (0.689504-0.805129), and high SDI (0.805129-1) categories (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2021.100255). According to a geographical hierarchy, the GBD 2019 Study data were grouped into 21 regions, and the variations in the cancer burden in these regions were analyzed. In addition, the incidence and death rates of all cancers in different SDI and geographical regions were described.
Statistical analysis
EAPCs were used to assess the trends in cancer incidence and death rates, which were calculated using a regression model. The whole process includes two steps. Step 1: linear regression of 30 years’ data; that is, y = α + βx + ε, where y = ln (rate), x = calendar year, and ε was the error term. Step 2: Calculation of linear regression parameters. EAPC = 100 × [exp(β) − 1].15,16 All the calculations were performed using R software (version 3.6.3). All the tests were two-tailed. A 95% confidence interval (CI) for each quantity was used for the analyses. The statistical significance was set at P < 0.05.
Ethics approval
This study was approved by an independent ethics committee of The First Affiliated Hospital, College of Medicine, Zhejiang University.
Consent for publication
Not applicable.
Results
Overview of the cancer burden
Worldwide, it was estimated that there were 1 335 100 [95% uncertainty interval (UI) 1 243 397-1 426 785] new cancer cases and 397 583 (95% UI 371 460-426 061) cancer-related deaths among AYAs in 2019, with a female : male ratio of 1.35 for incidence and 1.04 for mortality (Tables 1 and 2). The incidence rate was 44.99 and the death rate was 13.39 cancer-associated deaths per 100 000 people per year in 2019 (Tables 1 and 2). While the incidence rate increased mildly, the death rate decreased significantly from 1990 to 2019, with the EAPC being 0.38 (95% UI 0.36-0.39) and −0.93 (95% CI −0.95 to 0.92), respectively (Tables 1 and 2, Supplementary Data File S1, available at https://doi.org/10.1016/j.esmoop.2021.100255). Female predominance was a hallmark of the cancer burden in AYAs. The incidence rate of all cancers in females was 38.3% higher than that in males (52.32 versus 37.82 per 100 000). The cancers of the breast (11.51/100 000) and cervix (8.13/100 000) affected 37.6% of female patients (Table 1, Supplementary Data File S1, available at https://doi.org/10.1016/j.esmoop.2021.100255), while hematological malignancies, including leukemia (3.76/100 000), Hodgkin’s lymphoma (1.20/100 000), and non-Hodgkin’s lymphoma (2.21/100 000), accounted for the majority of cancers in males (Table 1, Supplementary Data File S1, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Table 1.
Worldwide incidence and rates by sex for the 29 cancer types in 2019 among 15-39-year olds and the change in the trends from 1990 to 2019
| Both sexes |
Female |
Male |
1990-2019 EAPC, No. (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Cases | Rate per 100 000 | Cases | Rate per 100 000 | Cases | Rate per 100 000 | ||
| Total cancers | 1 335 100 | 44.99 | 766 692 | 52.32 | 568 407 | 37.82 | 0.38 (0.36 to 0.39) |
| Breast | 169 859 | 5.72 | 168 775 | 11.51 | 1083 | 0.07 | 0.90 (0.87 to 0.93) |
| Nonmelanoma skin cancer | 140 718 | 4.74 | 81 053 | 5.53 | 59 664 | 3.97 | 0.83 (0.79 to 0.86) |
| Cervical cancer | 119 258 | 4.01 | 119 258 | 8.13 | — | — | 0.02 (0.01 to 0.05) |
| Leukemia | 101 206 | 3.41 | 44 636 | 3.04 | 56 570 | 3.76 | −0.49 (−0.53 to −0.46) |
| Colorectal cancer | 76 089 | 2.56 | 29 258 | 1.99 | 46 831 | 3.11 | 1.22 (1.17 to 1.27) |
| Brain and central nervous system cancer | 61 510 | 2.07 | 28 318 | 1.93 | 33 192 | 2.20 | 0.76 ( 0.76 to 0.80) |
| Testicular cancer | 57 400 | 1.93 | — | — | 57 400 | 3.81 | 1.28 (1.22 to 1.33) |
| Non-Hodgkin’s lymphoma | 52 426 | 1.76 | 19 154 | 1.30 | 33 272 | 2.21 | 0.50 (0.44 to 0.54) |
| Stomach | 49 007 | 1.65 | 21 125 | 1.44 | 27 882 | 1.85 | −1.21 (−1.25 to −1.16) |
| Thyroid | 46 832 | 1.57 | 34 545 | 2.35 | 12 285 | 0.81 | 1.77 (1.71 to 1.83) |
| Malignant skin melanoma | 37 265 | 1.25 | 21 251 | 1.45 | 16 014 | 1.06 | 0.57 (0.51 to 0.63) |
| Ovarian cancer | 35 831 | 1.20 | 35 831 | 2.44 | — | — | 0.89 (0.82 to 0.95) |
| Hodgkin’s lymphoma | 33 387 | 1.12 | 15 297 | 1.04 | 18 090 | 1.20 | −0.36 (−0.42 to −0.30) |
| Lung | 32 600 | 1.09 | 127 721 | 0.87 | 19 828 | 1.31 | −1.13 (−1.19 to −1.07) |
| Lip and oral cavity | 29 440 | 0.99 | 12 255 | 0.83 | 17 184 | 1.14 | 0.95 (0.88 to 1.02) |
| Nasopharynx | 28 562 | 0.96 | 8359 | 0.57 | 20 202 | 1.34 | 1.68 (1.60 to 1.76) |
| Liver | 25 429 | 0.85 | 6079 | 0.41 | 19 350 | 1.28 | −3.51 (−3.57 to −3.45) |
| Kidney | 21 135 | 0.71 | 8468 | 0.57 | 12 667 | 0.84 | 2.02 (1.93 to 2.11) |
| Uterine cancer | 19 415 | 0.65 | 19 415 | 1.32 | — | — | 0.64 (0.56 to 0.72) |
| Bladder | 14 095 | 0.47 | 3415 | 0.23 | 10 679 | 0.71 | 0.86 (0.75 to 0.96) |
| Pancreatic cancer | 9401 | 0.31 | 3279 | 0.22 | 6121 | 0.40 | 0.56 (0.44 to 0.69) |
| Esophageal cancer | 8087 | 0.27 | 2829 | 0.19 | 5258 | 0.34 | −0.99 (−1.11 to −0.88) |
| Other pharynx cancer | 7102 | 0.23 | 2622 | 0.17 | 4480 | 0.29 | 1.13 (0.98 to 1.28) |
| Prostate | 5471 | 0.18 | — | — | 5471 | 0.36 | 2.13 (1.94 to 2.31) |
| Larynx | 4214 | 0.14 | 1155 | 0.07 | 3059 | 0.20 | −0.63 (−0.80 to −0.45) |
| Gallbladder and biliary tract | 3841 | 0.12 | 1974 | 0.13 | 1866 | 0.12 | −0.21 (−0.39 to −0.03) |
| Multiple myeloma | 2930 | 0.09 | 1096 | 0.07 | 1834 | 0.12 | 0.95 (0.72 to 1.18) |
| Mesothelioma | 1466 | 0.04 | 710 | 0.05 | 756 | 0.05 | −0.90 (−1.18 to −0.61) |
| Other malignant neoplasms | 141 110 | 4.75 | 63 752 | 4.35 | 77 357 | 5.14 | 0.79 (0.76 to 0.82) |
CI, confidence interval; EAPC, estimated annual percentage change.
Table 2.
Worldwide deaths and rates by sex for the 29 cancer types in 2019 among 15–39-year olds and the change in the trends from 1990 to 2019
| Both sexes |
Female |
Male |
1990-2019 EAPC, No. (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Cases | Rate per 100 000 | Cases | Rate per 100 000 | Cases | Rate per 100 000 | ||
| Total cancers | 397 583 | 13.39 | 203 011 | 13.85 | 194 571 | 12.94 | −0.93 (−0.95 to −0.92) |
| Leukemia | 45 105 | 1.51 | 18 862 | 1.28 | 26 242 | 1.74 | −1.58 (−1.63 to −1.54) |
| Breast | 43 087 | 1.45 | 42 742 | 2.91 | 345 | 0.02 | −0.10 (−0.15 to −0.04) |
| Brain and central nervous system cancer | 29 105 | 0.98 | 11 947 | 0.81 | 17 158 | 1.14 | −0.29 (−0.35 to −0.22) |
| Colorectal cancer | 28 351 | 0.95 | 11 466 | 0.78 | 16 885 | 1.12 | −0.10 (−0.17 to −0.03) |
| Stomach | 27 895 | 0.93 | 12 923 | 0.88 | 14 971 | 0.99 | −2.12 (−2.18 to −2.06) |
| Cervical cancer | 27 168 | 0.91 | 27 168 | 1.85 | — | — | −0.81 (−0.87 to −0.74) |
| Lung | 24 771 | 0.83 | 9462 | 0.64 | 15 308 | 1.01 | −1.38 (−1.45 to −1.31) |
| Non-Hodgkin’s lymphoma | 20 797 | 0.70 | 7608 | 0.51 | 13 188 | 0.87 | −0.18 (−0.25 to −0.10) |
| Liver | 18 572 | 0.62 | 4381 | 0.29 | 14 190 | 0.94 | −3.98 (−4.05 to −3.91) |
| Lip and oral cavity | 10 043 | 0.33 | 3609 | 0.24 | 6433 | 0.42 | 0.68 (0.55 to 0.80) |
| Ovarian cancer | 8895 | 0.29 | 8895 | 0.60 | — | — | 0.39 (0.26 to 0.52) |
| Hodgkin’s lymphoma | 8093 | 0.27 | 3231 | 0.22 | 4861 | 0.32 | −1.58 (−1.69 to −1.46) |
| Pancreatic cancer | 7608 | 0.25 | 2593 | 0.17 | 5015 | 0.33 | 0.51(0.37 to 0.65) |
| Esophageal cancer | 6214 | 0.20 | 2059 | 0.14 | 4154 | 0.27 | −1.24 (−1.37 to −1.11) |
| Nasopharynx | 6079 | 0.20 | 1901 | 0.12 | 4178 | 0.27 | −2.15 (−2.28 to −2.02) |
| Testicular cancer | 5352 | 0.18 | — | — | 5352 | 0.35 | −0.04 (−0.19 to 0.12) |
| Other pharynx cancer | 4359 | 0.14 | 1537 | 0.10 | 2822 | 0.18 | 0.72 (0.54 to 0.91) |
| Malignant skin melanoma | 4248 | 0.14 | 1972 | 0.13 | 2275 | 0.15 | −0.92 (−1.09 to −0.76) |
| Kidney | 4016 | 0.13 | 1423 | 0.09 | 2592 | 0.17 | 0.90 (0.70 to 1.09) |
| Thyroid | 2852 | 0.09 | 1774 | 0.12 | 1078 | 0.07 | −0.10 (−0.31 to 0.11) |
| Gallbladder and biliary tract | 2389 | 0.08 | 1280 | 0.08 | 1109 | 0.07 | −0.46 (−0.68 to −0.23) |
| Larynx | 2249 | 0.07 | 630 | 0.04 | 1619 | 0.10 | −1.23 (−1.45 to −1.00) |
| Bladder | 2050 | 0.06 | 631 | 0.04 | 1418 | 0.09 | −1.00 (−1.24 to −0.76) |
| Uterine cancer | 1808 | 0.06 | 1808 | 0.12 | — | — | −1.66 (−1.89 to −1.42) |
| Multiple myeloma | 1680 | 0.05 | 627 | 0.04 | 1053 | 0.07 | 0.47 (0.18 to 0.77) |
| Nonmelanoma skin cancer | 1466 | 0.04 | 559 | 0.03 | 906 | 0.06 | −0.34 (−0.63 to −0.05) |
| Mesothelioma | 990 | 0.03 | 466 | 0.03 | 523 | 0.03 | −0.79 (−1.14 to −0.43) |
| Prostate | 875 | 0.02 | — | — | 875 | 0.05 | −0.01 (−0.4 to 0.38) |
| Other malignant neoplasms | 51 453 | 1.73 | 21 445 | 1.46 | 30 007 | 1.99 | −0.04 (−0.09 to 0.01) |
CI, confidence interval; EAPC, estimated annual percentage change.
Heterogeneity of cancer types
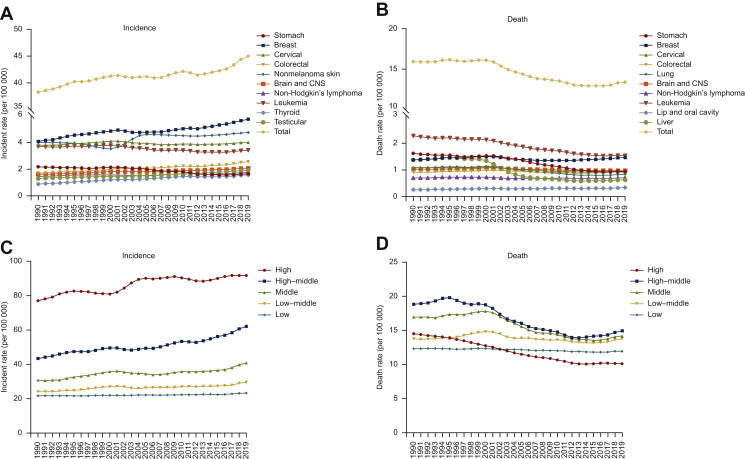
The burden varied greatly between the 29 types of cancer. The top five ranking cancer types overall in terms of new cases were breast cancer (5.72/100 000), nonmelanoma skin cancer (4.74/100 000), cervical cancer (4.01/100 000), leukemia (3.41/100 000), and colorectal cancer (2.56/100 000), with leukemia (1.51/100 000), breast cancer (1.45/100 000), brain and CNS cancer (0.98/100 000), colorectal cancer (0.95/100 000), and stomach cancer (0.93/100 000) being the main contributors to cancer-associated deaths among AYAs (Tables 1 and 2, Supplementary Data File S1, available at https://doi.org/10.1016/j.esmoop.2021.100255). The analysis of the long-term trends by cancer types showed that rising incidence rates in AYAs were driven mainly by the increased detection of breast cancer, nonmelanoma skin cancer, colorectal cancer, brain and CNS cancer, testicular cancer, and thyroid cancer (Figure 1A, Table 1, Supplementary Table S1A, available at https://doi.org/10.1016/j.esmoop.2021.100255). Cancer death rates in AYAs declined steadily since 2000, which was mainly due to the reduced mortality with leukemia, stomach cancer, lung cancer and liver cancer, with EAPCs of −1.58 (95% CI −1.63 to −1.54), −2.12 (95% CI −2.18 to −2.06), −1.38 (95% CI −1.45 to −1.31), and −3.98 (95% CI −4.05 to −3.91), respectively (Figure 1B, Table 2, Supplementary Table S1B, available at https://doi.org/10.1016/j.esmoop.2021.100255). Notably, these trends indicated the improvements in cancer surveillance, detection, and treatment in recent years.
Figure 1.
Worldwide trends of incidence and death rate by cancer type and SDI quintile among 15-39-year olds from 1990 to 2019.
(A) Top 10 cancer types in terms of incidence; (B) top 10 cancer types in terms of death rates; (C) incidence rates in different SDI quintiles; (D) death rates in different SDI quintiles.
CNS, central nervous system; SDI, sociodemographic index.
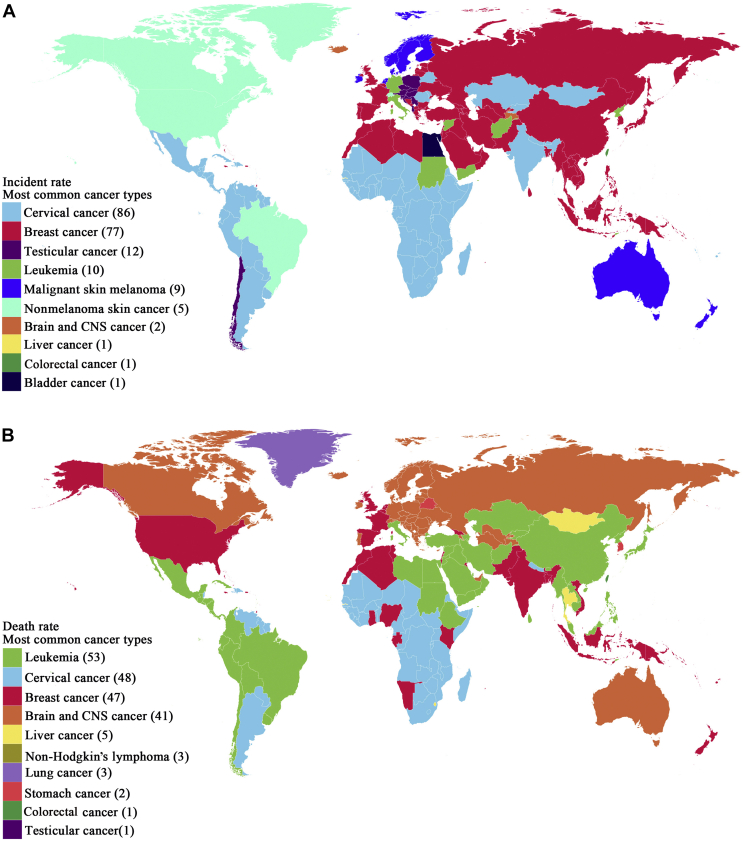
Breast cancer and cervical cancer were the most frequent cancer types in AYAs for most countries in terms of incidence in 2019, accounting for 169, 859 (12.7%), and 119 258 (8.9%) of the total new cases, respectively (Figure 2A, Table 1, Supplementary Table S2A, available at https://doi.org/10.1016/j.esmoop.2021.100255). Other cancers with a high incidence included testicular cancer [57 400 (4.3%); rate 1.93/100 000 people per year], leukemia [101 206 (7.6%); rate 3.41/100 000 people per year], and malignant skin melanoma [37 265 (2.8%); rate 1.25 per 100 000 people per year]. The top five ranking cancer types for most countries in terms of mortality were leukemia [45 105 (11.3%); rate 1.51 per 100 000 people per year], breast cancer [43 087 (10.8%); rate 1.45 per 100 000 people per year], brain and CNS cancer [29 105 (7.3%); rate 0.98 per 100 000 people per year], cervical cancer [27 168 (6.8%); rate 0.91 per 100 000 people per year], and liver cancer [18 572 (4.6%); rate 0.62 per 100 000 people per year] (Figure 2B and Table 2, Supplementary Table S2B, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Figure 2.
Global map of the most common cancer type by country in terms of (A) incidence cases and (B) cancer-related death cases among 15-39-year olds in 2019. Numbers in brackets are the number of countries where this type of cancer is the most common.
CNS, central nervous system.
Age-specific burden
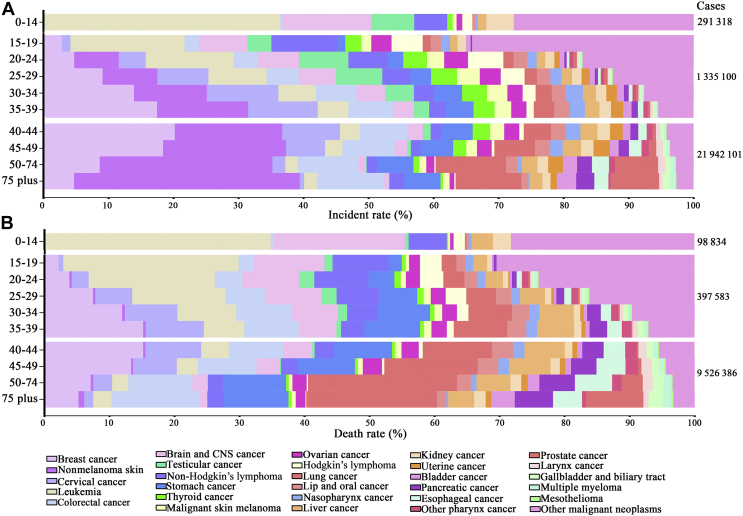
The cancer burden of AYAs was significantly higher than that of children (0-14 years old), with a 4.6 times greater burden for incidence and 4.0 times greater burden for deaths; however, it was significantly lower than that in patients aged 40 years or older (Figure 3, Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100255). The spectrum of cancers occurring among AYAs was also distinct from those diagnosed in younger or older age groups. The most frequent cancer types in children were leukemia, brain and CNS cancer, testicular cancer, and non-Hodgkin’s lymphoma, the incidence of which decreased gradually with increasing age in AYAs and accounted for a small proportion in the older age group (Figure 3, Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100255). Breast cancer (ranked first for new cases and second for deaths overall), cervical cancer (ranked third for new cases and sixth for deaths overall), colorectal cancer (ranked fifth for new cases and fourth for deaths overall), and stomach cancer (ranked ninth for new cases and fifth for deaths overall) were observed more frequently among AYAs than among children. Nonmelanoma skin cancer (ranked second for new cases and twenty-sixth for deaths overall) was observed more frequently among young adults than among children or adolescents, though to a lesser extent than that observed in older adults (Tables 1 and 2, Figure 3A, Supplementary Table S3A, available at https://doi.org/10.1016/j.esmoop.2021.100255). In addition, even among AYAs, the cancer characteristics of incident cases varied according to the age interval of 5 years (Supplementary Table S3A, available at https://doi.org/10.1016/j.esmoop.2021.100255). The proportion of leukemia, brain and CNS cancer, testicular cancer, non-Hodgkin’s lymphoma, and Hodgkin’s lymphoma cases decreased with increasing age, whereas the proportions of breast cancer, cervical cancer, colorectal cancer, stomach cancer, and lung cancer cases increased (Figure 3A, Supplementary Table S3A, available at https://doi.org/10.1016/j.esmoop.2021.100255). A similar transition was observed in deaths, except that the mortality rate of nonmelanoma skin cancer was low because of its good prognosis (Figure 3B, Supplementary Table S3B, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Figure 3.
Worldwide distribution of cancer type by (A) age group and incidence, and (B) death cases in 2019.
CNS, central nervous system.
Variations according to SDI levels
The SDI-based regional analysis showed that the burden of cancer in AYAs was higher in low SDI regions than in high SDI regions. Although the incidence of cancer in the high SDI regions was approximately 4.0 times greater than that in the low SDI regions (rates, 91.81 versus 23.21 per 100 000 people per year), the mortality burden in the high SDI region was lower than that in the low SDI region (rates, 10.10 versus 11.97 per 100 000 people per year; Figure 1C and D, Tables 3 and 4, Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100255). In addition, the EAPC analysis showed a significant decreasing trend for death rates in the high to the middle SDI regions. The analysis revealed that in the high SDI regions, the EAPC was −1.47 (95% CI −1.49 to −1.45); the high–middle SDI regions, −1.37 (95% CI −1.38 to −1.35); and in the middle SDI regions, −1.02 (95% CI −1.04 to −1.01). No obvious changes were observed in the low SDI regions (EAPC, −0.16; 95% CI −0.18 to −0.14) and in the low–middle SDI regions (EAPC, −0.20; 95% CI −0.22 to −0.18) (Figure 1D, Table 4, Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100255). These findings indicated that the global burden of cancer in AYAs varied across SDI regions and was disproportionally greater in the low- and low–middle SDI regions than in other regions.
Table 3.
Worldwide incidence and rates by SDI and regions for the 29 cancer types in 1990 and 2019 among 15-39-year olds and the change in the trends from 1990 to 2019
| 1990 |
2019 |
1990-2019 EAPC, No. (95% CI) | |||
|---|---|---|---|---|---|
| Incident cases | Rate per 100 000 | Incident cases | Rate per 100 000 | ||
| Sex | |||||
| Female | 479 401 | 44.22 | 766 692 | 52.32 | 0.37 (0.36 to 0.38) |
| Male | 359 935 | 32.43 | 568 407 | 37.82 | 0.38 (0.37 to 0.39) |
| Sociodemographic index | |||||
| High SDI | 248 945 | 77.05 | 304 186 | 91.81 | 0.57 (0.56 to 0.58) |
| High–middle SDI | 210 024 | 43.43 | 321 162 | 62.15 | 0.99 (0.98 to 1.00) |
| Middle SDI | 230 698 | 30.90 | 381 888 | 40.83 | 0.70 (0.69 to 0.71) |
| Low–middle SDI | 107 196 | 24.07 | 217 792 | 29.61 | 0.51 (0.50 to 0.53) |
| Low SDI | 42 055 | 21.69 | 103 945 | 23.21 | 0.18 (0.16 to 0.19) |
| Region | |||||
| Africa | |||||
| Central Sub-Saharan Africa | 4335 | 20.88 | 10 463 | 20.19 | −0.20 (−0.22 to −0.19) |
| Eastern Sub-Saharan Africa | 16 904 | 24.03 | 39 376 | 23.61 | −0.21 (−0.22 to −0.20) |
| Western Sub-Saharan Africa | 11 622 | 16.34 | 32 628 | 18.21 | 0.39 (0.38 to 0.41) |
| Southern Sub-Saharan Africa | 7478 | 34.10 | 11 965 | 35.52 | −0.24 (−0.25 to −0.22) |
| North Africa and Middle East | 32 025 | 23.58 | 92 962 | 35.94 | 1.47 (1.46 to 1.49) |
| America | |||||
| Andean Latin America | 4833 | 31.27 | 10 928 | 42.55 | 0.97 (0.96 to 0.98) |
| Caribbean | 5392 | 36.35 | 7864 | 43.38 | 0.32 (0.31 to 0.33) |
| Central Latin America | 22 198 | 32.54 | 44 804 | 44.36 | 1.04 (1.02 to 1.05) |
| High-income North America | 131 451 | 116.25 | 161 026 | 132.52 | 0.55 (0.54 to 0.56) |
| Southern Latin America | 9272 | 48.58 | 15 991 | 62.87 | 0.79 (0.78 to 0.80) |
| Tropical Latin America | 23 695 | 36.84 | 43 422 | 48.73 | 1.00 (0.99 to 1.01) |
| Asia | |||||
| Central Asia | 11 886 | 41.74 | 16 297 | 43.01 | −0.48 (−0.49 to −0.47) |
| East Asia | 204 664 | 36.09 | 318 991 | 61.85 | 1.47 (1.46 to 1.48) |
| High-income Asia Pacific | 30 944 | 45.81 | 29 060 | 55.30 | 0.91 (0.90 to 0.92) |
| South Asia | 91 146 | 21.10 | 208 127 | 27.06 | 0.69 (0.68 to 0.71) |
| Southeast Asia | 56 624 | 28.77 | 98 028 | 36.09 | 0.51 (0.50 to 0.52) |
| Europe | |||||
| Central Europe | 25 360 | 55.10 | 23 641 | 66.38 | 0.72 (0.71 to 0.73) |
| Eastern Europe | 44 349 | 51.69 | 50 680 | 73.84 | 0.98 (0.97 to 0.99) |
| Western Europe | 98 068 | 68.07 | 108 039 | 82.447 | 0.43 (0.42 to 0.44) |
| Oceania | |||||
| Australasia | 6293 | 77.17 | 8821 | 90.78 | 0.32 (0.31 to 0.33) |
| Other Oceania countries | 788 | 29.95 | 1976 | 36.31 | 0.59 (0.58 to 0.60) |
CI, confidence interval; EAPC, estimated annual percentage change; SDI, sociodemographic index.
Table 4.
Worldwide deaths and rates by SDI and regions for the 29 cancer types among 15-39-year olds in 1990 and 2019 and the change in the trends from 1990 to 2019
| 1990 |
2019 |
1990-2019 EAPC, No. (95% CI) | |||
|---|---|---|---|---|---|
| Death cases | Rate | Death cases | Rate per 100 000 | ||
| Sex | |||||
| Female | 176 497 | 16.28 | 20 3011 | 13.85 | −0.92 (−0.94 to −0.90) |
| Male | 172 732 | 15.56 | 194 571 | 12.94 | −0.95 (−0.97 to −0.93) |
| Sociodemographic index | |||||
| High SDI | 46 779 | 14.47 | 33 476 | 10.10 | −1.47 (−1.49 to −1.45) |
| High–middle SDI | 90 769 | 18.77 | 76 939 | 14.89 | −1.37 (−1.38 to −1.35) |
| Middle SDI | 126 469 | 16.93 | 132 396 | 14.15 | −1.02 (−1.04 to −1.01) |
| Low–middle SDI | 61 075 | 13.71 | 100 905 | 13.72 | −0.20 (−0.22 to −0.18) |
| Low SDI | 23 960 | 12.36 | 53 608 | 11.97 | −0.16 (−0.18 to −0.14) |
| Region | |||||
| Africa | |||||
| Central Sub-Saharan Africa | 2395 | 11.53 | 5382 | 10.39 | −0.45 (−0.47 to −0.43) |
| Eastern Sub-Saharan Africa | 9307 | 13.20 | 20 041 | 12.01 | −0.44 (−0.46 to −0.42) |
| Western Sub-Saharan Africa | 6379 | 8.97 | 16 443 | 9.17 | 0.07 (0.05 to 0.09) |
| Southern Sub-Saharan Africa | 3613 | 16.47 | 5059 | 15.02 | −0.65 (−0.66 to −0.63) |
| North Africa and Middle East | 16 692 | 12.29 | 30 084 | 11.63 | −0.28 (−0.30 to −0.26) |
| America | |||||
| Andean Latin America | 2449 | 15.85 | 3811 | 14.84 | −0.32 (−0.31 to −0.34) |
| Caribbean | 2254 | 15.19 | 2865 | 15.80 | −0.03 (−0.05 to −0.01) |
| Central Latin America | 9453 | 13.85 | 13 971 | 13.83 | −0.03 (−0.05 to −0.01) |
| High-income North America | 16 326 | 14.43 | 11 830 | 9.73 | −1.56 (−1.57 to −1.54) |
| Southern Latin America | 3534 | 18.51 | 3876 | 15.24 | −0.79 (−0.81 to −0.77) |
| Tropical Latin America | 9157 | 14.23 | 12 468 | 13.99 | −0.04 (−0.06 to −0.02) |
| Asia | |||||
| Central Asia | 5665 | 19.89 | 6563 | 17.32 | −1.16 (−1.18 to −1.15) |
| East Asia | 118 957 | 20.98 | 85 630 | 16.60 | −1.50 (−1.52 to −1.49) |
| High-income Asia Pacific | 9900 | 14.65 | 4441 | 8.45 | −1.96 (−1.98 to −1.94) |
| South Asia | 52 871 | 12.23 | 100 793 | 13.10 | 0.07 (0.05 to 0.09) |
| Southeast Asia | 30 603 | 15.54 | 40 558 | 14.93 | −0.40 (−0.42 to −0.39) |
| Europe | |||||
| Central Europe | 9542 | 20.73 | 5183 | 14.55 | −1.36 (−1.37 to −1.34) |
| Eastern Europe | 17 513 | 20.41 | 13 303 | 19.38 | −0.92 (−0.93 to −0.90) |
| Western Europe | 21 064 | 14.62 | 13 262 | 10.12 | −1.56 (−1.58 to −1.54) |
| Oceania | |||||
| Australasia | 1116 | 13.69 | 1005 | 10.34 | −1.2 (−1.22 to −1.18) |
| Other Oceania countries | 429 | 16.30 | 1002 | 18.41 | 0.41 (0.39 to 0.43) |
CI, confidence interval; EAPC, estimated annual percentage change; SDI, sociodemographic index.
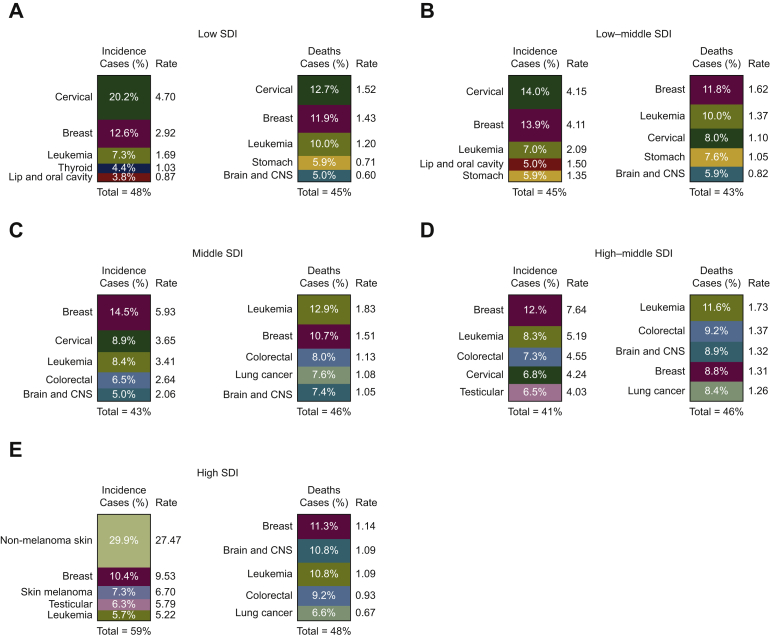
With regard to cancer profile across the SDI regions, the top five cancer incident or mortality cases accounted for >40% of the total of new incident or mortality cases across all the SDI regions (Figure 4, Supplementary Data File S2, available at https://doi.org/10.1016/j.esmoop.2021.100255). Cervical cancer, breast cancer, and leukemia were all in the top five cancers in terms of incidence of cases across all the SDI regions, except for the high SDI region. In the high SDI regions, the most frequent cancer type was skin cancer, including nonmelanoma skin cancer (29.9%, rate 27.47 per 100 000 people per year) and skin melanoma (7.3%, rate 6.70 per 100 000 people per year; Figure 4, Supplementary Data File S2, available at https://doi.org/10.1016/j.esmoop.2021.100255). Notably, cervical cancer was the most frequently diagnosed cancer in the low SDI and low–middle SDI regions, while it ranked third, fifth, and eighth in the middle, high–middle, and high SDI regions, respectively. With respect to mortality, leukemia, brain and CNS cancer, and breast cancer were among the five leading causes of cancer-related deaths across all the SDI levels. Cervical and stomach cancers were the remaining leading causes of cancer death in the low and low–middle SDI regions, while colorectal and lung cancers were the remaining largest contributors to the mortality burden in the middle, high–middle, and high SDI regions (Figure 4, Supplementary Data File S2, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Figure 4.
Worldwide distribution of the top five cancer types by SDI quintile in terms of incidence and death cases among 15-39-year olds in 2019.
(A) Low SDI; (B) low–middle SDI; (C) middle SDI; (D) high–middle SDI; (E) high SDI.
CNS, central nervous system; SDI, sociodemographic index.
Geographical differences
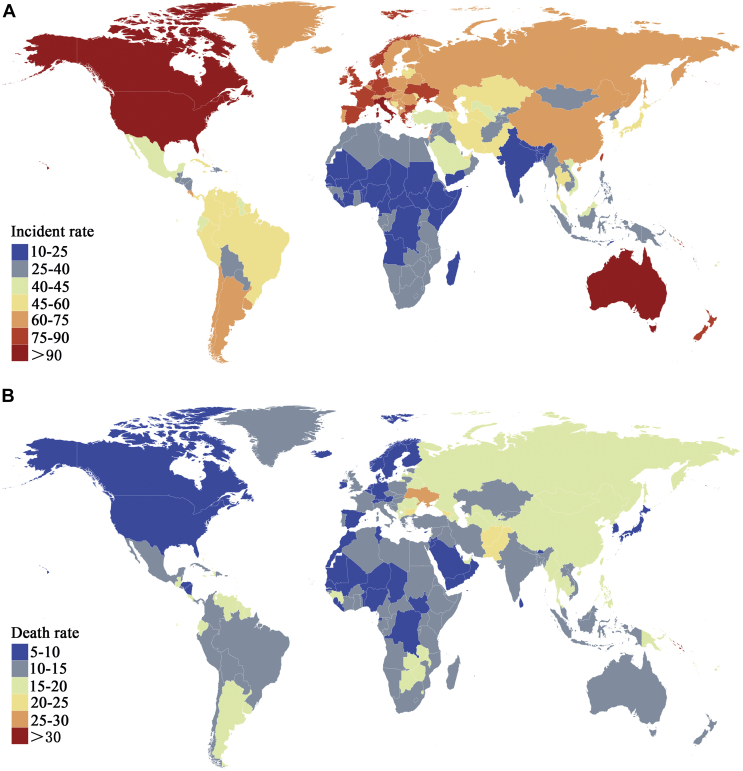
Based on the data in the 21 GBD regions over the last three decades, the incidence rates among AYAs was the greatest in the developed regions (especially in the high-income regions of North America, Australasia, and Western Europe), whereas Africa and parts of Asia (south Asia and southeast Asia) had the lowest incidence rates (Figure 5A). The EAPC analysis showed a downward trend of incidence in four GBD regions (most parts of Africa and Central Asia) and an upward trend of incidence in the other 17 GBD regions (Table 3, Supplementary Table S5A, available at https://doi.org/10.1016/j.esmoop.2021.100255). A significant upward trend of incidence was observed in four regions, including North Africa and the Middle East (1.47 per 100 000 people per year), East Asia (1.47 per 100 000 people per year), Central Latin America (1.04 per 100 000 people per year), and tropical Latin America (1.00 per 100 000 people per year; Table 3). Although the incidence rate was the highest in the developed countries, these regions conversely had the lowest death rate (Figure 5, Tables 3 and 4, Supplementary Data File S3, available at https://doi.org/10.1016/j.esmoop.2021.100255). The top three lowest mortality burdens were in high-income North America, high-income Asia Pacific, and Western Europe, coinciding with the regions with the most significant decrease in EAPC, −1.56 (95% CI −1.57 to −1.54), −1.96 (95% CI −1.98 to −1.94), and −1.56 (95% CI −1.58 to −1.54; Table 4, Supplementary Table S5B, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Figure 5.
Global map of (A) incidence and (B) death rate for total cancers by country among 15-39-year olds in 2019.
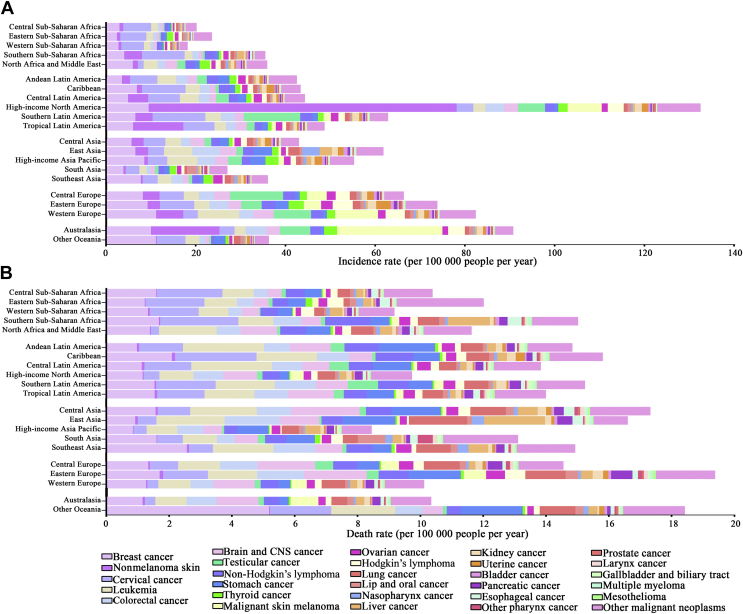
Additionally, cancer profiles varied substantially across the geographical regions. For instance, the incidence rate for breast cancer varied from 2.4 per 100 000 people per year in Eastern Sub-Saharan Africa to 11.1 per 100 000 people per year in Western Europe and Australia (Figure 6, Supplementary Data File S4, available at https://doi.org/10.1016/j.esmoop.2021.100255). The incidence of cervical cancer varied 10-fold with the lowest in North Africa and the Middle East (1.2 per 100 000 people per year) and highest in Southern Latin America (11.7 per 100 000 people per year). The incidence of leukemia varied fourfold to sixfold with the lowest in most parts of Africa and South Asia and highest in Western Europe, high-income Asia Pacific, and East Asia (Figures 2A and 6A, Supplementary Data File S4, available at https://doi.org/10.1016/j.esmoop.2021.100255). The greatest variation was seen in the incidence of cervical cancer, which was higher in high-income North America than in all the other regions. With respect to cancer-associated mortality, the greatest mortality burden for breast cancer was observed in Oceania (5.1 per 100 000 people per year), Southeast Asia (2.5 per 100 000 people per year), and the Caribbean (2.0 per 100 000 people per year). The mortality burden of cervical cancer varied 10-fold, ranging from 0.25 per 100 000 people per year in North Africa and the Middle East to 2.5 per 100 000 people per year in the Caribbean and Southern Sub-Saharan Africa. The five regions with the highest mortality rates for leukemia were Andean Latin America, Central Latin America, East Asia, Central Asia, and other Oceania countries, all with rates of >2.0 per 100 000 people per year (Figures 2B and 6B, Supplementary Data File S4, available at https://doi.org/10.1016/j.esmoop.2021.100255).
Figure 6.
Incidence and death rates for the 29 cancer types by geographical region among 15-39-year olds in 2019.
(A) Incidence rates; (B) death rates.
CNS, central nervous system.
Discussion
To the best of our knowledge, this is the first comprehensive analysis of worldwide cancer burden in AYAs in recent years, adding detailed profiles to the limited available epidemiological data of cancers in AYAs. Overall, the global incidence of cancer in AYAs in 2019 was 44.99 per 100 000 people per year, and the corresponding death rate was 13.39 per 100 000 people per year. We reported population-based trends in cancer incidence and mortality, with a focus on the considerable variations in cancer types within this population when stratified by age, sex, SDI, and geographical regions. Our study highlighted the need for tailored cancer control measures in this neglected subpopulation.
The death rate of AYAs with cancers showed a significant decreasing trend from 1990 to 2019, which became more pronounced from 2000. Consistent with established epidemiological data, the reduction of cancer burden in AYAs was mainly from the contributions of the high and high–middle SDI regions,2,6 suggesting that there were variations in the cancer burden in the different SDI regions within this age groups. Previous studies have shown that cancer survival rates between different income countries varied for reasons such as variations in cancer screening, detection modalities, insurance status, specialized health care availability, treatment used, and the knowledge of cancer prevention.17, 18, 19 These findings indicated that rational allocation of existing or given resources is required to reduce the burden of cancer in AYAs in developing countries.
Female predominance is a peculiarity of the cancer burden in AYAs. Cervical and breast cancers were the most common cancer types in AYAs in most countries. These sex-based characteristics of cancer burden should be considered as important aspects when designing cancer control measures for AYAs. For example, the leading reason for adverse outcomes in AYAs with breast cancers is that they are more likely to have familial cancer predisposition genes than older women are, and in addition, a substantial proportion of them are found to have distant metastases at the time of diagnosis.3,20,21 Enhanced screening for predisposing genes may be an important measure in the early diagnosis of breast cancer and may, thus, reduce the cancer burden in AYAs. The incidence rate of cervical cancer in the low SDI regions was five times higher than that in the high SDI regions. The difficulty in reducing cervical cancer risk is associated with an elevated transmission of human papillomavirus (HPV) in the increasing young urban population in developing countries.3,22 Therefore a national HPV vaccination program of HPV-naïve people may have a significant effect in curbing the incidence trends.
AYA cancers in the low SDI regions were more likely to be associated with chronic infections, including infections with HPV, hepatitis B/C virus, Helicobacter pylori, Epstein–Barr virus, and human herpesvirus 8, than were the cases in the high SDI region. The analysis of the GLOBOCAN 2012 data showed that 30% of all cancers were linked to chronic infections in the low SDI countries compared with 10% in the very high SDI countries.6 Our data showed that the burden of cervical cancer was ranked first in both low and middle–low SDI regions; however, the burden of other infection-associated cancers such as lymphomas and liver cancer decreased significantly. These results emphasize the effectiveness of the cancer control measures of the past decade while providing a direction for future strategies to reduce the cancer burden in AYAs.
It should be noted that the implications of the cancer burden in AYAs are beyond the number of incident cases and deaths. Survivors of AYA cancers often live for a long time after their diagnosis, resulting in an increased risk of the development of cancer- and treatment-related ‘late effects,’ including secondary malignancies,8,23,24 cardiovascular diseases,25,26 endocrine dysfunctions,27 neurocognitive impairments,9,28 exercise dysfunctions, and psychological distress.29 Consequently, the cancer burden in AYAs is characterized mainly by the proportion of quality of life lost at an individual level. It also results in considerable losses to society in terms of social structure. In addition, despite a decline in productivity, AYA cancer survivors generally have higher annual medical expenditures than adults without a history of cancer.30,31 In particular, this situation is worse in the low SDI regions, where most patients paid out-of-pocket for both the diagnosis and treatment owing to the paucity of health insurance. Therefore, in addition to specific medical measures such as effective prevention, timely diagnoses, and high-quality care, some early screening programs with a limited cost for AYAs might have a significant effect, particularly those targeting cervical cancer and breast cancer. Besides, collaborative work taking into account economic, social, and psychological aspects should be tailored to reduce the cancer burden among this specific age group.
The limitations of the GBD data in accessing cancer incidence and mortality have been described extensively elsewhere.15,32 Briefly, the accuracy of the results depended on the quality and availability of the GBD data at a given time. Furthermore, the classification of cancer in the GBD was not identical to the recommended classification for cancers in AYAs.33 Moreover, the GBD Study was based on countries and regions, resulting in a lack of analysis on the influence of race. Finally, the cancer incidence rate may have been influenced by the evolution of cancer diagnostic technology and criteria.
Conclusion
In summary, this study provided comprehensive estimates of the worldwide cancer burden among AYAs. Our results show that the global cancer burden among AYAs is distinct from that in younger or older age groups and varies significantly by sex, age, SDI, and GBD regions. Although there has been rapid progress in the treatment and improvement in the prognosis of cancer over the last decade, the cancer burden in the AYA population is considerable in the low SDI regions. These findings provide a direction for the rational allocation of limited resources and the formulation of policies.
Acknowledgements
Not applicable.
Funding
The research was supported by National Natural Science Foundation of China (Grant Nos 81873451 and 81900154).
Disclosure
The authors declare that they have no competing interests.
Data sharing
The datasets and material used and/or analyzed during this study are available from the Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool).
Contributor Information
J. Jin, Email: jiej0503@zju.edu.cn.
Q. Han, Email: hanqingmei9212@zju.edu.cn.
Supplementary data
References
- 1.US Department of Health and Human Services. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Report of the Adolescent and Young Adult Oncology Progress Review Group. 2006. NIH publication; 2-3.
- 2.Miller K.D., Fidler-Benaoudia M., Keegan T.H. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 3.Trama A., Botta L., Steliarova-Foucher E. Cancer burden in adolescents and young adults: a review of epidemiological evidence. Cancer J. 2018;24(6):256–266. doi: 10.1097/PPO.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 4.Chao C., Bhatia S., Xu L. Incidence, risk factors, and mortality associated with second malignant neoplasms among survivors of adolescent and young adult cancer. JAMA Netw Open. 2019;2:e195536. doi: 10.1001/jamanetworkopen.2019.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleyer A., Barr R., Hayes-Lattin B. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 6.Fidler M.M., Gupta S., Soerjomataram I. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–1589. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 7.Suh E., Stratton K.L., Leisenring W.M. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21(3):421–435. doi: 10.1016/S1470-2045(19)30800-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bright C.J., Reulen R.C., Winter D.L. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20(4):531–545. doi: 10.1016/S1470-2045(18)30903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jim H.S.L., Jennewein S.L., Quinn G.P. Cognition in adolescent and young adults diagnosed with cancer: an understudied problem. J Clin Oncol. 2018;36(27):2752–2754. doi: 10.1200/JCO.2018.78.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams S.C., Herman J., Lega I.C. Young adult cancer survivorship: recommendations for patient follow-up, exercise therapy, and research. JNCI Cancer Spectr. 2020;5(1):pkaa099. doi: 10.1093/jncics/pkaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott A.R., Stoltzfus K.C., Tchelebi L.T. Trends in cancer incidence in US adolescents and young adults, 1973-2015. JAMA Netw Open. 2020;3(12):e2027738. doi: 10.1001/jamanetworkopen.2020.27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Meer D.J., Karim-Kos H.E., van der Mark M. Incidence, survival, and mortality trends of cancers diagnosed in adolescents and young adults (15-39 years): a population-based study in the Netherlands 1990-2016. Cancers (Basel) 2020;12(11):3421. doi: 10.3390/cancers12113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIRTUM Working Group. CCM. AIEOP Working Group Italian cancer figures, report 2012: cancer in children and adolescents. Epidemiol Prev. 2013;37(1 Suppl 1):1–225. [PubMed] [Google Scholar]
- 14.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q., Mao L., Shao L. Global, regional, and national burden of chronic myeloid leukemia, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Front Oncol. 2020;10:580759. doi: 10.3389/fonc.2020.580759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z., Jiang Y., Yuan H. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Coleman M.P. Cancer survival: global surveillance will stimulate health policy and improve equity. Lancet. 2014;383(9916):564–573. doi: 10.1016/S0140-6736(13)62225-4. [DOI] [PubMed] [Google Scholar]
- 18.Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringhini S., Berkman L., Dugravot A. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II Cohort Study, 1985-2009. Am J Epidemiol. 2012;175(12):1275–1283. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson R.H., Anders C.K., Litton J.K. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65(12):e27397. doi: 10.1002/pbc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janavičius R. Founder BRCA1/2 mutations in Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010;1:397–412. doi: 10.1007/s13167-010-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuzick J., Castanon A., Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20-29 in the UK. Br J Cancer. 2010;102:933–939. doi: 10.1038/sj.bjc.6605528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai E., Buchanan N., Townsend J. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–4891. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S. Adolescents and young adults with cancer and the risk of subsequent primary neoplasms: not just big children. Lancet Oncol. 2019;20(4):466–467. doi: 10.1016/S1470-2045(18)30941-0. [DOI] [PubMed] [Google Scholar]
- 25.Chao C., Xu L., Bhatia S. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2016;34(14):1626–1633. doi: 10.1200/JCO.2015.65.5845. [DOI] [PubMed] [Google Scholar]
- 26.Rugbjerg K., Mellemkjær L., Boice J.D. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943-2009. J Natl Cancer Inst. 2014;106(6):dju110. doi: 10.1093/jnci/dju110. [DOI] [PubMed] [Google Scholar]
- 27.Shanis D., Merideth M., Pulanic T.K. Female long-term survivors after allogeneic hematopoietic stem cell transplantation: evaluation and management. Semin Hematol. 2012;49(1):83–93. doi: 10.1053/j.seminhematol.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelstein K., Spiegler B.J., Fung S. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro-Oncol. 2011;13(5):536–545. doi: 10.1093/neuonc/nor015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak M., Zebrack B.J., Meeske K.A. Trajectories of psychological distress in adolescent and young adult patients with cancer: a 1-year longitudinal study. J Clin Oncol. 2013;31(17):2160–2166. doi: 10.1200/JCO.2012.45.9222. [DOI] [PubMed] [Google Scholar]
- 30.Guy G.P., Jr., Yabroff K.R., Ekwueme D.U. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff (Millwood) 2014;33(6):1024–1031. doi: 10.1377/hlthaff.2013.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magrath I., Epelman S. Cancer in adolescents and young adults in countries with limited resources. Curr Oncol Rep. 2013;15(4):332–346. doi: 10.1007/s11912-013-0327-3. [DOI] [PubMed] [Google Scholar]
- 32.Kyu H.H., Stein C.E., Boschi Pinto C. Causes of death among children aged 5-14 years in the WHO European Region: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Child Adolesc Health. 2018;2(5):321–337. doi: 10.1016/S2352-4642(18)30095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birch J.M., Alston R.D., Kelsey A.M. Classification and incidence of cancers in adolescents and young adults in England 1979-1997. Br J Cancer. 2002;87:1267–1274. doi: 10.1038/sj.bjc.6600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.