Abstract
Toxoplasma gondii infection, when acquired as an acute infection during pregnancy, can have substantial adverse effects on fetuses. We present the case of a 19-year-old pregnant woman with no previous relevant medical history. The ultrasound in the third trimester showed brain and abdominal abnormalities such as congenital hydrocephalus, volume loss of the brain parenchyma, and hepatosplenomegaly. The laboratory test showed positive IgM for Toxoplasma gondii. MRI was performed for better assessment and it shows the lesions in the central nervous system and other organs with more details.
Keywords: congenital toxoplasmosis, fetal mri, abdominal manifestations, toxoplasmosis, cns manifestations, hydrocephalus
Introduction
Human toxoplasmosis is a cosmopolitan zoonosis caused by Toxoplasma gondii. The overall prevalence of acute Toxoplasma infection in pregnant women is estimated at 0.6% (95% CI 0.4e0.7%) and is indicated that annually ~201,600 children are born with congenital toxoplasmosis [1,2]. Humans and other animals develop a systemic infection by the ingestion of contaminated food. Tachyzoites infect nucleated host cells and utilize monocytes, macrophages, and dendritic cells to escape the host immune defense, bypass the blood-brain barrier and the placenta barricade, and spread and form systemic disease [3].
Women who have acute toxoplasmosis during pregnancy can transmit organisms to their fetuses through the placenta. Tachyzoites bypass the placental blood barrier and invade the fetal organs to propagate and compromise the embryonic developmental process [3,4].
Several factors affect the risk of mother-to-child transmission, including the gestational age at the time of acute Toxoplasma infection, the virulence of the parasite strain or genotype, the parasite load during acute Toxoplasma infection, and the delay in the initiation of treatment following acute maternal infection [2,5]. The most common congenital manifestation of toxoplasmosis are hydrocephalus, chorioretinitis, intracerebral calcification, mental retardation, and loss of hearing, and very rarely, death may also occur [6,7]. The diagnosis of congenital toxoplasmosis can be made during pregnancy by serological methods with no detectable serum IgG anti-Toxoplasma antibodies but detection of specific IgM and/or IgA and/or IgE antibodies, with low avidity serum anti-Toxoplasma IgG antibodies or seroconversion from IgG negative to IgG positive in cases of sequential testing during gestation [2,8].
We present a case with severe manifestations of toxoplasmosis studied by fetal MRI to assess its compromise in the brain and organs in the abdomen.
Case presentation
The patient is a 19-year-old pregnant woman without any significant clinical history. No alterations were found in the ultrasounds done in the first and second trimesters (Figure 1), and no symptoms were reported.
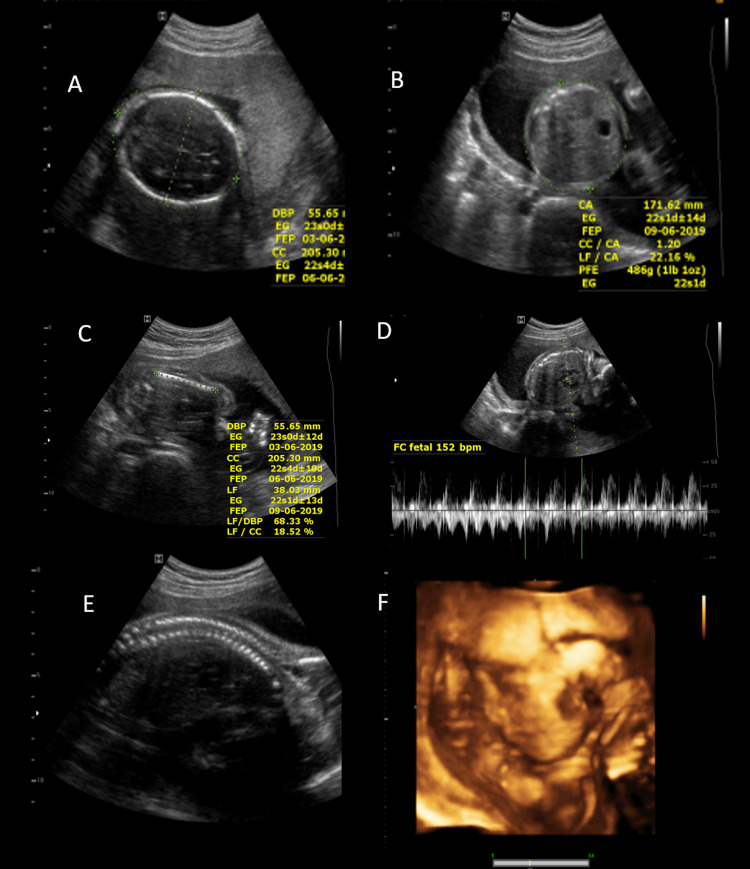
Figure 1. Second-trimester ultrasound.
Ultrasound in the second trimester showed active gestation of 22ss 3d by biometry with normal fetal morphology. Brain (A), abdomen (B), limbs (C), heart (D), and spine (E) were normal without malformations. 3D ultrasound (F) shows the normal morphology of the face.
In the 31st week of gestation, ultrasound showed dilation of lateral ventricles where both lateral ventricles measure 26 mm at the atrium level with no cystic brain changes. No abnormalities were seen in the liver and spleen. The remaining structures demonstrated no morphological alterations.
In the presence of congenital hydrocephalus of an unspecified etiology and with the epidemiological history of contact with cats, she was tested for a TORCH (Toxoplasmosis, Rubella, Cytomegalovirus, and Herpes simplex virus) profile, obtaining positive serology for Toxoplasma gondii IgM (> 5000 IU/ml) with low levels of IgG (170.3 IU/ml) and negative serology for Rubeola, Herpes 1-2, and Cytomegalovirus (Table 1). Polymerase chain reaction (PCR) in the amniotic fluid was not performed.
Table 1. Laboratory testing.
| Pathogen | Units | Results | Normal values |
| Toxoplasma Gondii IgG (Elisa method) | IU/ml | 170.3 | Negative<10 Positive>20 |
| Toxoplasma Gondii IgM (Elisa method) | IU/ml | >5000 | Negative<300 Positive>350 |
| Rubeola IgG (Elisa method) | IU/ml | 94 | Negative<10 Positive>20 |
| Rubeola IgM (Elisa method) | IU/ml | 2.9 | Negative<2.5 Positive>3.5 |
| Herpes 1 IgG 1 (Elisa method) | IU/ml | 6.1 | Negative<20 Positive>30 |
| Herpes 2 IgG 2 (Elisa method) | IU/ml | 6 | Negative<20 Positive>30 |
| Cytomegalovirus IgG (Elisa method) | IU/ml | 8 | Negative<25 Positive>40 |
| Cytomegalovirus IgM (Elisa method) | IU/ml | 1.6 | Negative<10 Positive>15 |
The MRI performed at 32 weeks of gestation showed the hydrocephalus more evident. Fetal MRI showed a multisystem compromise with severe loss of volume and alteration of the brain and cerebellum that included corticosubcortical cystic degeneration and more dilatation of the lateral ventricles (compared to the previous ultrasound). The right atrium of the lateral ventricle measured 33 mm of transverse diameter and the left 28 mm. The fetal MRI also showed hepatosplenomegaly and signs suggestive of chorioretinitis (Figures 2, 3).
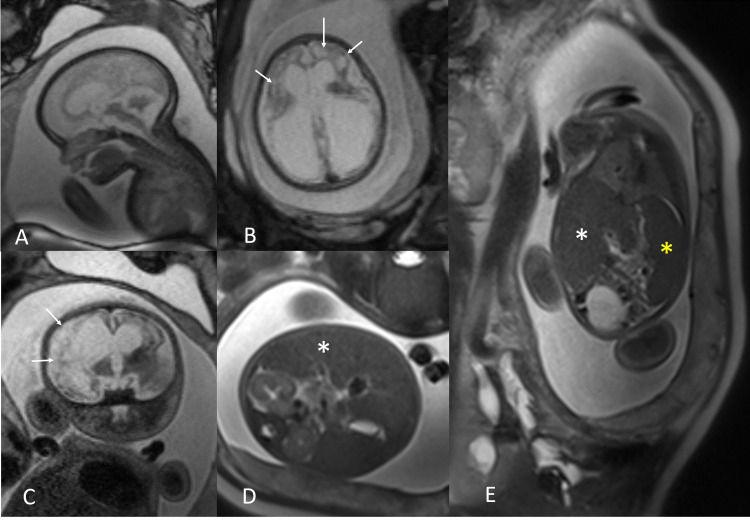
Figure 2. Fetal MRI.
Sagittal (A), axial (B), and coronal (C) T2-weighted MRI images of the brain show severe loss of parenchymal volume in both cerebral hemispheres with corticosubcortical involvement, both thalamus, basal ganglia, and cerebellum. The cerebral parenchyma is thin with cystic degeneration (arrows in B and C), and there is dilation of the lateral ventricles. Axial (D) and coronal (E) T2-weighted MRI images of the abdomen show the right hepatic lobe reaching the lower pole of the right kidney related to hepatomegaly (white asterisk in D and E) and enlargement of the spleen (yellow asterisk in E).
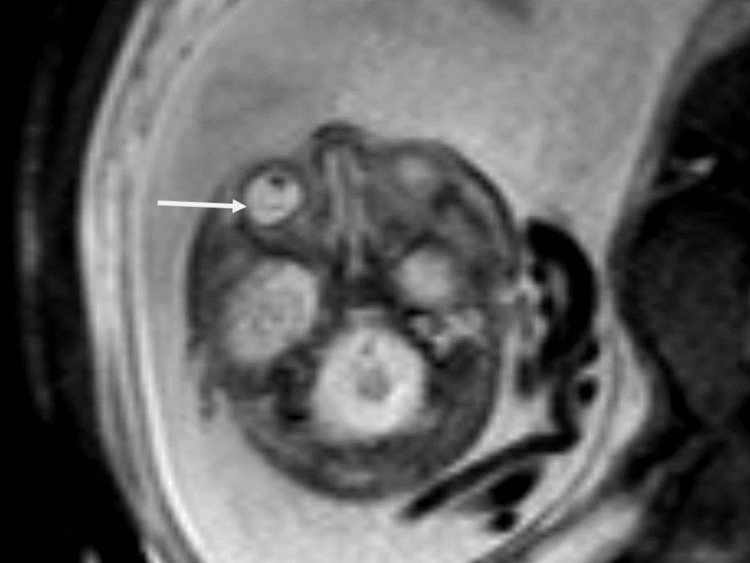
Figure 3. Eye globe abnormalities.
Axial T2-weighted image at the level of the eye globe shows the detachment of the choroid and retina suggestive of chorioretinitis (arrow).
Discussion
Toxoplasmosis is an endemic disease caused by Toxoplasma gondii, a protozoan transmitted to humans by the ingestion of undercooked meat containing the parasite's cysts, contaminated vegetables, or by contact with cat feces [9]. Congenital infection for toxoplasmosis has been found in South America in 3.3 cases per 10,000 [10,11]. The parasite enters the fetus through the placental barrier after primary maternal infection. The risk of fetal transmission increases as gestational age increases but the severity of the disease decreases [12,13].
In our case, the fetal toxoplasmosis infection was diagnosed by serology as part of the prenatal investigation of hydrocephalus, evidenced initially in the ultrasound of the third trimester and the severe damage of the brain and hepatosplenomegaly evidenced in the MRI one week later. The patient had positive IgM with low levels of IgG; other tests for infection were negative (Table 1) [14,15].
The ultrasound findings associated with fetal toxoplasmosis are ventricular dilation, brain calcifications, hepatomegaly, splenomegaly, ascites, pleural effusion, and pericardial effusion [16-18]. In the present case, severe hydrocephalus and polyhydramnios were evident at 31 weeks of gestation, which were not present in previous ultrasound examinations according to the report. A fetal MRI was performed for better assessment of the abnormal findings in the third-trimester ultrasound. The MRI showed loss of the parenchymal volume of both cerebral hemispheres and cerebellum with corticosubcortical cystic degeneration, dilation of the lateral ventricles, and hepatosplenomegaly. Because ultrasound findings can only detect very obvious malformations, other subtle findings can be overlooked; therefore, MRI of the fetal brain is used to better delineate subtle malformations [19]. Differential diagnosis by imaging includes Cytomegalovirus [20] and Aicardi-Goutières syndrome; hydrocephaly is also present in aqueductal stenosis, Chiari malformation type 2, and Dandy-Walker malformation, but they are not associated with hepatosplenomegaly. In the present clinical case, MRI is helpful for an accurate evaluation of malformations, providing more significant details of the damage and guiding the diagnosis of fetal pathology with more precision.
In our case, the pregnant woman had performed routine examinations in the first and second trimesters. No pathological findings were found; however, in the third-trimester ultrasound, there was evidence of a significant pathology related to toxoplasmosis, and it was confirmed in greater detail in the fetal MRI. Additionally, the close time difference between the ultrasound and the fetal MRI (one week) showed the rapid evolution of the findings in congenital toxoplasmosis with the development of hepatosplenomegaly and the cystic changes of the brain not seen in the ultrasound.
The limitation of this study is that we could not present the images of the ultrasound performed initially in the third trimester because the patient came from the country's interior and we could access only the report. We could not repeat another serological test for toxoplasmosis three weeks later, as is recommended, due to the demise of the fetus. A PCR of the amniotic fluid was also not performed. However, the laboratory tests done on the mother, epidemiological history, and imaging findings helped make the diagnosis.
Conclusions
Congenital toxoplasmosis is widespread in developing countries and causes severe damage in fetuses' brains and affects the abdomen, demonstrating its multisystemic compromise. Ultrasound is the initial exam to assess fetal anomalies.
Findings on fetal MRI in affected fetuses establishes a good correlation with the ultrasound findings, helping to determine the impact of the disease on the central nervous system and to assess the involvement of other organs, making fetal MRI an excellent technique to complete the study and derive more information.
Gynecologists, pediatricians, and radiologists need to be aware of imaging manifestations of congenital toxoplasmosis and how MRI imaging can help do a more detailed evaluation of these manifestations.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Rostami A, Riahi SM, Gamble HR, et al. Clin Microbiol Infect. 2020;26:673–683. doi: 10.1016/j.cmi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Acute toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. Rostami A, Riahi SM, Contopoulos-Ioannidis DG, et al. PLoS Negl Trop Dis. 2019;13:0. doi: 10.1371/journal.pntd.0007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maternal and congenital toxoplasmosis, currently available and novel therapies in horizon. Oz HS. Front Microbiol. 2014;5:385. doi: 10.3389/fmicb.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seroprevalence of toxoplasmosis in pregnant women and its associated factors among hospital and community populations in Lambayeque, Peru. Silva-Díaz H, Arriaga-Deza EV, Failoc-Rojas VE, et al. http://www.scielo.br/scielo.php. Rev Soc Bras Med Trop. 2020;53:0. doi: 10.1590/0037-8682-0164-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congenital toxoplasmosis. McAuley JB. J Pediatric Infect Dis Soc. 2014;3 Suppl 1:0–5. doi: 10.1093/jpids/piu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remington JS, Klein JO, Wilson CB, Baker CJ. New York: W.B. Saunders; 2006. Infectious Diseases of the Fetus and Newborn Infant (Sixth Edition) [Google Scholar]
- 7.Mother-to-child transmission and diagnosis of Toxoplasma gondii infection during pregnancy. Singh S. https://pubmed.ncbi.nlm.nih.gov/17642985/ Indian J Med Microbiol. 2003;21:69–76. [PubMed] [Google Scholar]
- 8.Congenital toxoplasmosis: clinical features, outcomes, treatment, and prevention. Singh S. Trop Parasitol. 2016;6:113–122. doi: 10.4103/2229-5070.190813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Best cases from the AFIP: cerebral toxoplasmosis. Lee GT, Antelo F, Mlikotic AA. Radiographics. 2009;29:1200–1205. doi: 10.1148/rg.294085205. [DOI] [PubMed] [Google Scholar]
- 10.Newborn screening for congenital infectious diseases. Neto EC, Rubin R, Schulte J, Giugliani R. Emerg Infect Dis. 2004;10:1068–1073. doi: 10.3201/eid1006.030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Incidence of congenital toxoplasmosis estimated by neonatal screening: relevance of diagnostic confirmation in asymptomatic newborn infants. Carvalheiro CG, Mussi-Pinhata MM, Yamamoto AY, De Souza CB, Maciel LM. Epidemiol Infect. 2005;133:485–491. doi: 10.1017/s095026880400353x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imaging of congenital central nervous system infections. Neuberger I, Garcia J, Meyers ML, Feygin T, Bulas DI, Mirsky DM. Pediatr Radiol. 2018;48:513–523. doi: 10.1007/s00247-018-4092-1. [DOI] [PubMed] [Google Scholar]
- 13.Pediatric intracranial infections. Parmar H, Ibrahim M. Neuroimaging Clin N Am. 2012;22:707–725. doi: 10.1016/j.nic.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Hidropesía fetal como signo ecográfico de toxoplasmosis congénita. Lacunza-Paredes RO, Boza-Marroquín M. http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2304-51322012000100010 Rev Peru Ginecol Obstet. 2012;58:55–58. [Google Scholar]
- 15.Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Sensini A. Clin Microbiol Infect. 2006;12:504–512. doi: 10.1111/j.1469-0691.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 16.Fetal toxoplasmosis: ultrasonographic signs. Hohlfeld P, MacAleese J, Capella-Pavlovski M, Giovangrandi Y, Thulliez P, Forestier F, Daffos F. Ultrasound Obstet Gynecol. 1991;1:241–244. doi: 10.1046/j.1469-0705.1991.01040241.x. [DOI] [PubMed] [Google Scholar]
- 17.Ultrasound features of fetal toxoplasmosis: a contemporary multicenter survey in 88 fetuses. Codaccioni C, Picone O, Lambert V, et al. Prenat Diagn. 2020;40:1741–1752. doi: 10.1002/pd.5756. [DOI] [PubMed] [Google Scholar]
- 18.Too late prenatal diagnosis of fetal toxoplasmosis: a case report. Nowakowska D, Respondek-Liberska M, Golab E, Stray-Pedersen B, Szaflik K, Dzbenski TH, Wilczynski J. Fetal Diagn Ther. 2005;20:190–193. doi: 10.1159/000083903. [DOI] [PubMed] [Google Scholar]
- 19.Neuroimaging findings of congenital toxoplasmosis, cytomegalovirus, and zika virus infections: a comparison of three cases. Werner H, Daltro P, Fazecas T, Zare Mehrjardi M, Araujo Júnior E. J Obstet Gynaecol Can. 2017;39:1150–1155. doi: 10.1016/j.jogc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 20.The MRI spectrum of congenital cytomegalovirus infection. Diogo MC, Glatter S, Binder J, Kiss H, Prayer D. Prenat Diagn. 2020;40:110–124. doi: 10.1002/pd.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]