Abstract
This study evaluated the therapeutic efficacy of Schisandrin A on systemic colibacillosis of chickens. One hundred and eighty, 1-day-old Hailan Brown chickens were divided into 6 groups of 30 chickens each and assigned to the following treatments: 1) uninfected/untreated control; 2) infected Escherichia coli; 3) infected-plus low dose of Schisandrin A therapy (50 mg/kg); 4) infected-plus medium dose of Schisandrin A therapy (100 mg/kg); 5) infected-plus high dose of Schisandrin A therapy (200 mg/kg) and 6) infected-plus antimicrobial therapy (florfenicol). Each group of chickens was placed in cages with a photoperiod of 12 h of light and 12 h of dark. Feed and water for all groups were provided ad libitum for the duration of the study. On d 14, all the chickens except the uninfected control group were intraperitoneally inoculated with a fresh culture of E. coli containing 1 × 108 CFU/mL. The parameters measured included: average daily weight gain (ADG), percent survivability, liver index, serum activity of enzymes (ALT and AST), hepatic and intestinal concentrations of TNF-α, IL-1β, IL-6, IL-8, and LPS, expression of tight junction proteins (occludin, ZO-1, and claudin-1), relative abundance of bacterial species and histopathological changes in hepatic and intestinal tissue. The results showed that the medium and high doses of Schisandrin A ameliorated the detrimental effects of colibacillosis on weight gain. Regarding organ indexes, E. coli infection induced a significant increase in liver index, all the doses of Schisandrin A produced a significant reduction of liver index in comparison to the E. coli infected control. Serum activity of ALT and AST enzymes significantly increased due to E. coli infection, with the exception of the low dose of Schisandrin A for AST enzyme activity, all the Schisandrin A treatments significantly lowered enzyme activity in comparison to the E. coli infected control. Regarding concentrations of inflammatory markers in hepatic and intestinal, E. coli infection caused a significant increase in TNF-α, IL-1β, IL-6, and IL-8, except the lowest dose of Schisandrin A for IL-1β, the rest of the doses tested were able to significantly reduced the concentrations of inflammatory markers. Concentrations of LPS in hepatic and intestinal tissues were significantly increased by E. coli infection, all doses of Schisandrin A significantly reduced the concentration of LPS in hepatic and intestinal tissue. E. coli infection significantly reduced the expression of 2 tight junction proteins (ZO-1 and Claudin-1), the higher doses of Schisandrin A were effective in significantly increasing the expression of these tight junction proteins when compared with the E. coli infected control. Taken together, these results show that Schisandrin A has potential as an alternative therapy for the treatment of colibacillosis in chickens.
Key words: avian colibacillosis, Schisandrin A, gut-liver axis
INTRODUCTION
Avian colibacillosis is an infectious disease caused by pathogenic Escherichia coli characterized by hepatitis and diarrhea. E. coli infection can pollute chickens and eggs and cause serious food safety problems. It is one of the important reasons for the economic losses of global poultry producers (Ma et al., 2018). Antibiotics, such as florfenicol have a therapeutic effect on avian colibacillosis. However, the overuse of these drugs has led to the emergence of drug-resistant strains, and there are antibiotic residues in human food, such as chickens and eggs. These drugs can enter the environment with animal and human feces, causing pollution and affecting human health (Casella et al., 2018). Therefore, the search for a safe and effective antibiotic substitute is one of the key issues in the poultry industry and public health research.
The liver is directly connected to the gut through portal vein circulation. It is constantly exposed to bacterial products (e.g., E. coli endotoxin and lipopolysaccharide), which is known as the gut-liver axis. Microbiome can be considered as one of the driving forces affecting the gut-liver axis. A healthy microbial community achieves anti-inflammatory and proinflammatory effects through its metabolites. In recent years, many studies have confirmed that intestinal microorganisms can affect liver health through the gut-liver axis. Green tea extract can limit the inflammatory response of LPS-TLR4-NFκB and reduce the occurrence of nonalcoholic steatohepatitis through the intestinal hepatic axis pathway (Dey et al., 2020). Mesenteric congestion caused by portal blood flow interruption can induce endotoxin mediated toll-like receptor 4 expression, leading to an increased burden of liver cancer (Orci et al., 2018). However, E. coli infection often leads to an imbalance of intestinal flora and liver inflammation (Sun et al., 2020)
Schisandra chinensis is a traditional Chinese medicinal herb, which is often used as a substitute for tea. Studies have confirmed that S. chinensis can treat liver diseases (Li et al., 2020). Schisandrin A is one of the main components of Schisandrae lignin, which has good hepatoprotective and antibacterial effect. (Fan et al., 2019) showed that many components in Schisandrae lignin could significantly prevent intrahepatic cholestasis and liver necrosis induced by cholic acid. Hakala et al. (2015) studied the 6 main components of Schisandrae lignin and found that these could inhibit the formation of Chlamydia pneumoniae inclusion body and the generation of infectious offspring.
Schisandrin A is beneficial to liver health and has a certain antibacterial effect. The effect of Schisandrin A on the liver and intestinal injury caused by E. coli remains unclear. Therefore, in this study, chickens infected with E. coli were used to explore the mechanism of Schisandrin A by regulating intestinal microflora and gut-liver axis. The results provide a basis for the treatment of chicken colibacillosis with Schisandrin A.
MATERIALS AND METHODS
Reagents
Schisandrin A (purity ≥98%) was purchased from Chengdu Zhibiaohua Pure Biotechnology Co., Ltd., Chengdu, China. Alanine aminotransferase (ALT) activity test kit and aspartate aminotransferase (AST) activity test kit were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Tumor necrosis factor (TNF)-α, interleukin 1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and lipopolysaccharide (LPS) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Shanghai Enzyme-Linked Biotechnology Company, Shanghai, China. Lysogeny broth (LB) medium was purchased from Beijing Soleibao Biotechnology Co., Ltd., Beijing, China.
Bacterial Strain
The E. coli strain was presented by the Preventive Veterinary Laboratory of Animal Medical College, Hebei Agricultural University, Hebei, China. The strains were inoculated in 3 mL LB medium and cultured at 220 rpm for 12 h at 37°C. The inoculating ring was used to dip the bacterial solution into the LB agar medium and cultured at 37°C for 12 h. A single colony was inoculated in 3 mL LB medium and cultured at 220 rpm for 12 h at 37°C. Finally, aseptic phosphate buffered saline (PBS) was used to adjust the concentration of strain to 1 × 108 colony forming unit (CFU)/mL for further analyses.
Animals and Treatment
A total of 180 one-day-old male Hailan brown chickens (weigh of 40 ± 5 g) were purchased from Hebei Dawu Group Breeding Chicken Co., Ltd., Hebei, China. These were randomly divided into 6 groups: uninfected/untreated control; infected E. coli; infected-low dose of Schisandrin A therapy (50 mg/kg); infected-medium dose of Schisandrin A therapy (100 mg/kg); infected-high dose of Schisandrin A therapy (200 mg/kg) and infected-antimicrobial therapy (florfenicol), the dose reference (Yuan et al., 2020). Each group of chickens was placed in cages with a photoperiod of 12 h of light and 12 h of dark. All groups were given ad libitum access to feed and water. On d 14, all the chickens except for the uninfected control group were intraperitoneally inoculated with a fresh culture of E. coli containing 1 × 108 CFU/mL. After intraperitoneal injection of E. coli, Schisandrin A was given immediately for 5 d, and the chickens were killed by inhaling CO2. The experiments met the requirements of animal ethics of Hebei Agricultural University.
Analysis of Liver and Duodenum ALT, AST, TNF-α, IL-1β, IL-6, IL-8, and LPS Content in Chickens
The tissues were added into PBS homogenate, and centrifugation was used to extract supernatant. The tissue content of ALT, AST, TNF-α, IL-1β, IL-6, IL-8 and LPS in chickens were detected according to the kit's instructions. ELISA kit was purchased from Shanghai Meilian Biotechnology Co., Ltd., Shanghai, China. The supernatant and marker horseradish peroxidase (HRP) were added to the antibody-coated 96-well plate and incubated at 37°C for 1 h. The color-developing solution was added and incubated at 37°C for 15 min in the dark. The absorbance of each well was measured at 450 nm wavelength.
Analysis of Serum ALT and AST Content in Chicken
The blood of the chicken was collected with a clotting tube. The coagulant tube was placed in a refrigerator at 4°C for 1 h and centrifuged at low speed to separate the serum. The serum content of ALT and AST in chickens was detected using ALT and AST kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the kits. The serum and marker HRP were added to the antibody-coated 96-well plate and incubated at 37°C for 1 h. Color-developing solution was added and incubated at 37°C for 15 min in the dark, and the absorbance of each well was measured at 450 nm wavelength.
Histopathological Analysis
Liver and duodenum tissues were fixed with 4% paraformaldehyde for 24 h, dehydrated with different concentrations of ethanol, embedded in paraffin, and sectioned. The slices were baked in a constant temperature oven at 60°C for 4 h, dewaxed with xylene and rehydrated with alcohol. Finally, the sections were stained with hematoxylin and eosin (H&E). Histopathological changes were observed under a light microscope.
Analysis of mRNA Relative Transcriptional Levels in the Duodenum of Chicken by Quantitative Polymerase Chain Reaction
Total RNA extraction kit (Promega Biotechnology Co., Ltd., Beijing, China) was used to extract RNA from duodenum tissues of chickens. The total RNA was transcribed to obtain complementary DNA (cDNA) samples. The primers used were synthesized by Takara Biotechnology Co., Ltd., Dalian, China. Primer design and synthesis were entrusted to Dalian Bao Biological Company, Dalian, China. The sequence is shown in Table 1.
Table 1.
Real-time PCR primer sequences.
| Gene ID | Sequence | GC% | Tm | length(bp) |
|---|---|---|---|---|
| Occludin | CCTTGTTGGCCATGTGCAG | 57.9 | 63.7 | 78 |
| GGTCCACGGTGCAGTAGTGGTA | 59.1 | 64.4 | ||
| ZO-1 | TGGCAATCAACTTTGGGTAGCA | 45.5 | 64.8 | 156 |
| ATCCACAGAGGCAACTGAACCATA | 45.8 | 64.1 | ||
| Claudin-1 | CTCCCAAGCAGCTGCATATCTC | 54.5 | 63.5 | 148 |
| GCTCAGTCAGGCTAAGAACACCAA | 50 | 64.1 | ||
| β-actin | ATTGTCCACCGCAAATGCTTC | 47.6 | 64.4 | 113 |
| AAATAAAGCCATGCCAATCTCGTC | 41.7 | 64.5 |
Note: β-actin is an internal reference gene.
Gene Sequencing of Intestinal Flora
Fecal samples (100 mg) were added into 2 mL centrifuge tubes and extracted according to the operation steps of the EZNA Stool DNA Kit. After obtaining the purified genomic DNA (gDNA), the absorbance of total DNA at 260 nm and 280 nm was detected by UV spectrophotometer, and the concentration of total DNA and the ratio of OD 260/OD 280 were observed. The purified gDNA was stored at −20°C. The library was constructed by TruSeq DNA PCR-Free Sample Preparation Kit. The constructed library was quantified by qubit and quantitative polymerase chain reaction. After qualifying the library, it was sequenced by HiSeq2500 PE250.
Statistical Analysis
The test results were analyzed with Statistical Package for the Social Sciences (SPSS) 19.0 software, and the results were expressed as mean (x) ± standard deviation. Compared with the control, P < 0.05 indicates a significant difference.
RESULTS
Average Daily Gain and Organ Index
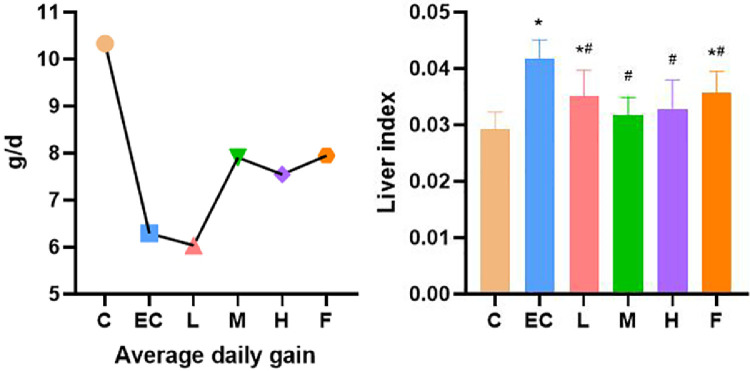
E. coli could significantly reduce the average daily gain (ADG) of chickens. After treatment, the declining trends of Schisandrin A medium (100 mg/kg) and high (200 mg/kg) dose groups were alleviated. Compared with the control group, the liver index of E. coli infection group was increased significantly, while the liver index of Schisandrin A groups (50,100, and 200 mg/kg) was decreased significantly (Figure 1).
Figure 1.
Average daily gain (ADG) and organ index. Note: C: Control group; EC: E. coli infection group; L: Schisandrin A low dose group; M: Schisandrin A medium dose group; H: Schisandrin A high dose group; F: positive drug group. * Significant difference with control group (P < 0.05). # Significant difference with E. coli infection group (P < 0.05).
Expression of LPS in Liver and Duodenum
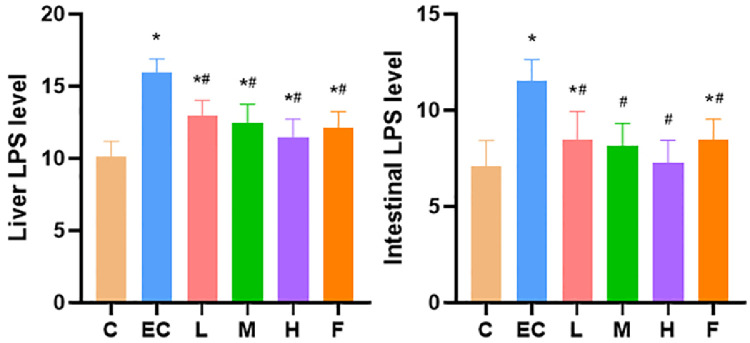
Compared with the control group, the level of LPS in the liver and duodenum of the E. coli infection group was significantly higher. The levels of LPS in Schisandrin A (50, 100, and 200 mg/kg) and infected-plus antimicrobial therapy were significantly lower than those in the E. coli infection group (Figure 2).
Figure 2.
Expression of LPS in liver and duodenum. Note: C: Control group; EC: E. coli infection group; L: Schisandrin A low dose group; M: Schisandrin A medium dose group; H: Schisandrin A high dose group; F: positive drug group. * Significant difference with control group (P < 0.05). # Significant difference with E. coli infection group (P < 0.05).
Expression of Inflammatory Factors in Liver and Duodenum
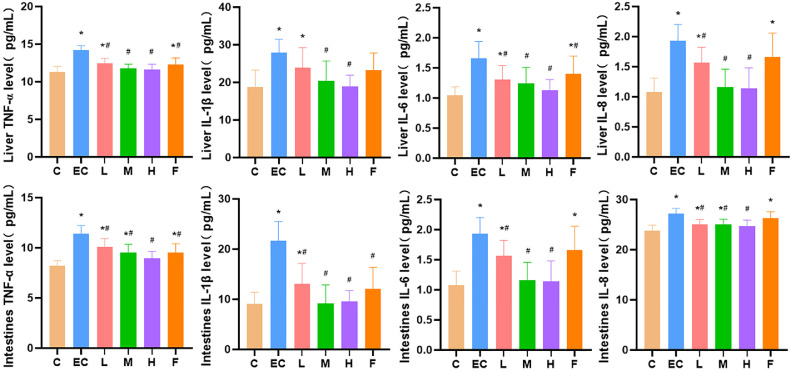
The liver and duodenum levels of TNF-α, IL-1β, IL-6, and IL-8 in the E. coli infection group were significantly higher than those in the control group. In contrast, the liver and duodenum levels of TNF-α, IL-6, and IL-8 in Schisandrin A groups (50, 100 and 200 mg/kg) and infected-plus antimicrobial therapy were significantly decreased in a dose-dependent manner. The liver and duodenum levels of IL-1β in medium (100 mg/kg) and high (200 mg/kg) dose groups of Schisandrin A were significantly lower than those in the E. coli infection group. The liver levels of IL-1β in the low dose group of Schisandrin A (50 mg/kg) and infected-plus antimicrobial therapy were decreased, but there was no statistical significance (Figure 3).
Figure 3.
Expression of liver and duodenum inflammatory factors. Note: C: Control group, EC: E. coli infection group, L: Schisandrin A low dose group, M: Schisandrin A medium dose group, H: Schisandrin A high dose group, F: positive drug group. * Significant difference with control group (P < 0.05). # Significant difference with E. coli infection group (P < 0.05).
Serum Indexes of Liver Function
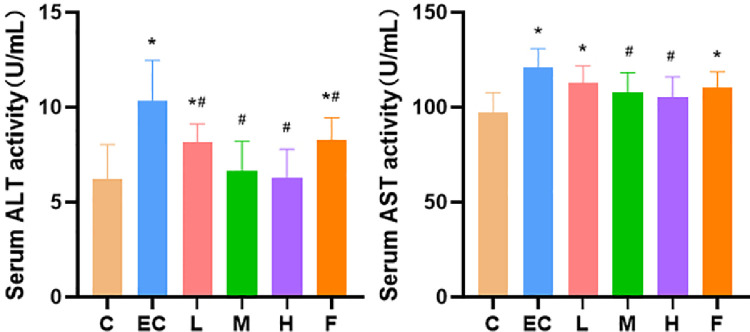
Compared with the control group, the serum ALT and AST levels of chickens infected with E. coli were significantly increased. Schisandrin A dose (50, 100, and 200 mg/kg) and infected-plus antimicrobial therapy significantly alleviated the phenomenon of increased serum ALT level caused by E. coli infection. Schisandrin A medium (100 mg/kg) and high (200 mg/kg) dose groups significantly reduced the serum AST level. The expression of Schisandrin A in low dose (50 mg/kg) and infected-plus antimicrobial therapy was decreased. However, there was no statistical significance (Figure 4).
Figure 4.
Serum indexes of liver function. Note: C: Control group, EC: E. coli infection group, L: Schisandrin A low dose group, M: Schisandrin A medium dose group, H: Schisandrin A high dose group, F: positive drug group. * Significant difference with control group (P < 0.05). # Significant difference with E. coli infection group (P < 0.05).
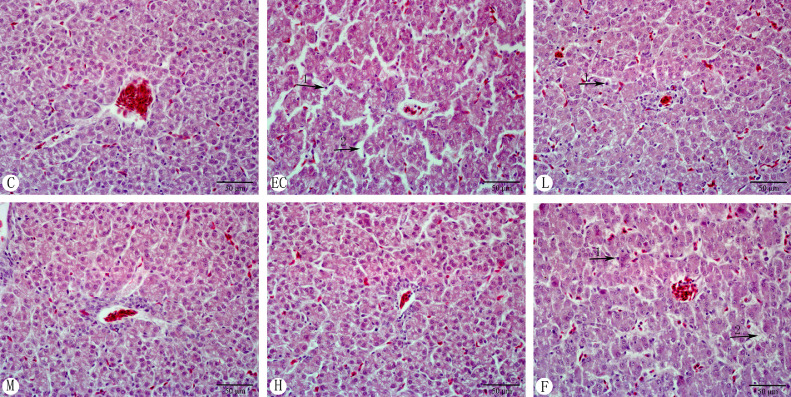
Histopathological Changes of Chicken Liver
As shown in Figure 5, the liver structure of the control group was clear. The liver cord was arranged in order, and the nucleus was clear. In the E. coli infection group, the structure of the hepatic lobule was disordered. The hepatic cord was broken (arrow 1), and the nucleus was condensed and ruptured (arrow 2). Compared with the E. coli infection group, the nuclear damage of the infected-plus antimicrobial therapy was reduced (arrow 2), but the arrangement of hepatic cords was irregular (arrow 1). In the low dose Schisandrin A group (50 mg/kg), the hepatic cord was clear, and the degree of hepatocyte necrosis was reduced (arrow 1). In the medium (100 mg/kg) and high (200 mg/kg) dose Schisandrin A groups, the structure of hepatic lobules was intact. The hepatic cords were arranged in order, and the necrosis of hepatocytes was significantly reduced, which was similar to that in the control group.
Figure 5.
Histopathological changes of chicken liver (H&E staining, 400 ×). Note: C: Control group; EC: E. coli infection group; L: Schisandrin A low dose group; M: Schisandrin A medium dose group; H: Schisandrin A high dose group; F: positive drug group.
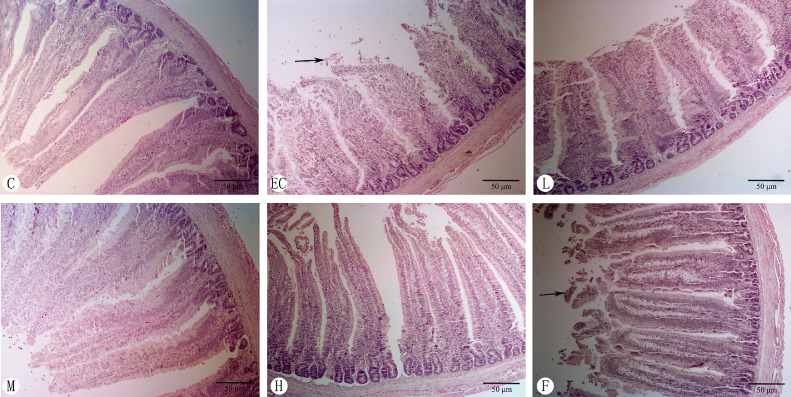
Histopathological Changes of Chicken Duodenum
As shown in Figure 6, in the control group, the morphology and structure of intestinal mucosa were complete, and the villi were arranged. In the E. coli infection group, the villi were arranged, with different heights. The villi were shortened, swollen, and shed (arrow). In the infected-plus antimicrobial therapy, the length of intestinal villi increased, but a large number of villi fell off (arrow). Compared with the E. coli infection group, the length of villi in medium (100 mg/kg) and high (200 mg/kg) dose of Schisandrin A groups increased significantly.
Figure 6.
Histopathological changes of chicken duodenum (H&E staining, 200 ×). Note: A: Control group, B: E. coli infection group, C: Schisandrin A low dose group, D: Schisandrin A medium dose group, e: Schisandrin A high dose group, F: positive drug group.
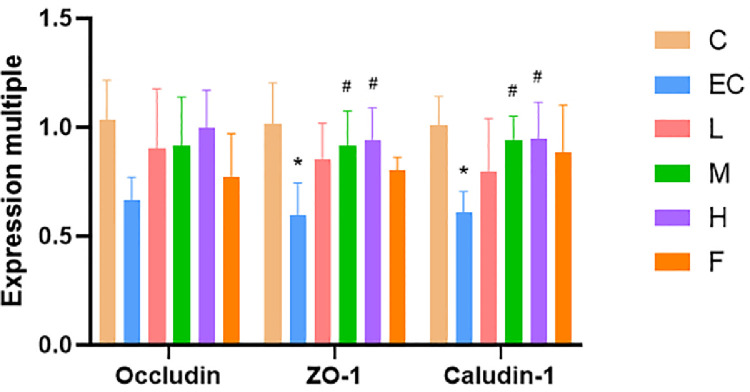
Expression of Intestinal Tight Junction Protein
The expression levels of occludin, ZO-1, and caludin-1 were decreased significantly after E. coli infection, while the expression levels of ZO-1 and caludin-1 were increased significantly in the medium (100 mg/kg) and high (200 mg/kg) dose of Schisandrin A groups. The expression of occludin in the Schisandrol A groups was also increased but was not statistically significant (Figure 7).
Figure 7.
Expression of intestinal tight junction protein. Note: C: Control group, EC: E. coli infection group, L: Schisandrin A low dose group, M: Schisandrin A medium dose group, H: Schisandrin A high dose group, F: positive drug group. * Significant difference with control group (P < 0.05). # Significant difference with E. coli infection group (P < 0.05).
Analysis of Microbial Diversity of Intestinal Contents
Data Preprocessing Statistics and Quality Control
The data (Raw PE) obtained by sequencing of illustra novaseq was spliced and quality controlled, and the chimeric filter was carried out to obtain the effective data (Effective Tags), which can be used for subsequent analysis. The statistical results of each step in the process of data processing are shown in Table 2.
Table 2.
Data preprocessing statistics and quality control.
| Samples | Raw PE(#) | Effective Tags(#) | AvgLen (nt) | GC (%) | Effective (%) |
|---|---|---|---|---|---|
| C1 | 85,018 | 61,984 | 253 | 53.41 | 72.91 |
| C2 | 102,349 | 65,723 | 253 | 52.72 | 64.21 |
| C3 | 111,288 | 69,731 | 253 | 53.28 | 62.66 |
| EC1 | 107,430 | 65,886 | 253 | 52.77 | 61.33 |
| EC2 | 87,497 | 65,608 | 253 | 56.69 | 74.98 |
| EC3 | 100,112 | 69,698 | 253 | 52.87 | 69.62 |
| H1 | 91,106 | 65,097 | 253 | 53.47 | 71.45 |
| H2 | 100,561 | 66,046 | 253 | 52.6 | 65.68 |
| H3 | 98,677 | 62,482 | 253 | 52.22 | 63.32 |
Note: Raw PE refers to the original PE reads; effective tags refer to the tag sequences that are finally used for subsequent analysis after filtering the chimera; avglen refers to the average length of effective tags; GC (%) refers to the content of GC bases in effective tags; effective (%) refers to the percentage of the number of effective tags and raw PE.
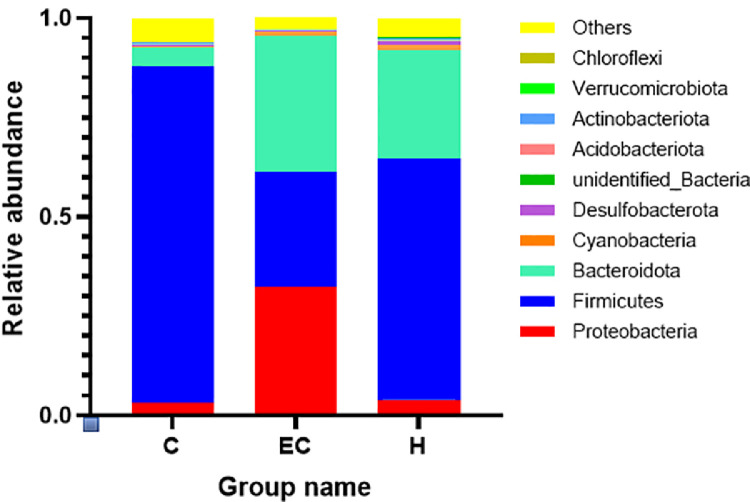
Species Relative Abundance at the Phylum Level.
At the gate level, the dominant bacteria in the control group were Firmicutes, followed by Bacteroidetes and Proteobacteria accounted for less. Compared with the control group, the abundance of Proteobacteria and Bacteroidetes in the E. coli infection group was increased significantly, and the proportion of Firmicutes was decreased. Compared with the E. coli infection group, the abundance of Proteobacteria in the high dose Schisandrin A group (200 mg/kg) was decreased, while the abundance of Firmicutes was increased (Figure 8).
Figure 8.
Relative abundance of species at phylum level.
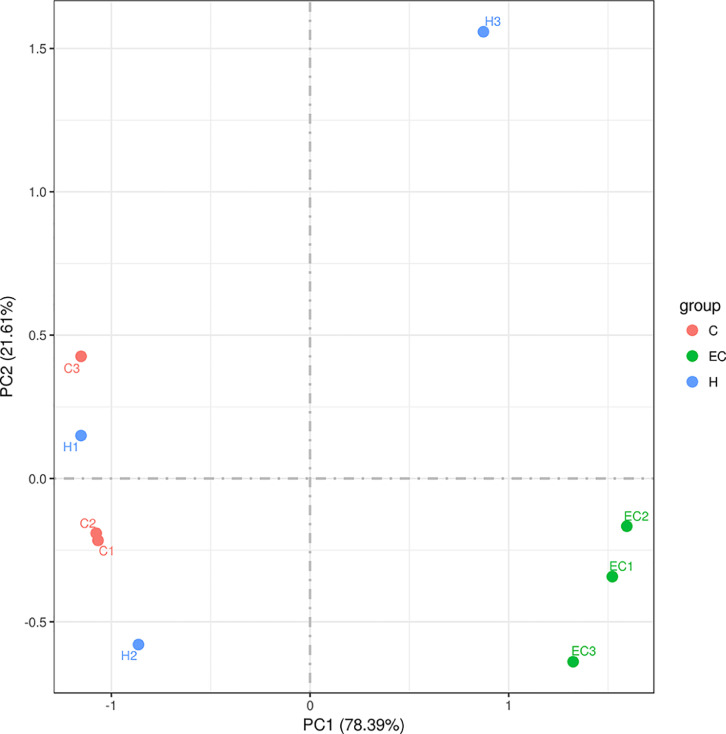
Principal Component Analysis
Principal component analysis (PCA) is a technique to simplify data analysis, which can identify the most important elements and structures in the data. PC1, PC2, and PC3 represent the first, second, and third principal components, respectively. The percentage of principal components represents the contribution rate of principal components to sample differences, which measures the information of principal components extracted from the original information. The distance between the sample points indicates the similarity of the classification distribution in the sample. In the coordinate system, the closer the distance between two points, the higher the similarity. In this study, the community similarity of control and Schisandrin A high dose (200 mg/kg) groups was higher, while the community difference of the E. coli infection group was larger than control and Schisandrin A high dose (200 mg/kg) groups (Figure 9).
Figure 9.
Principal component analysis.
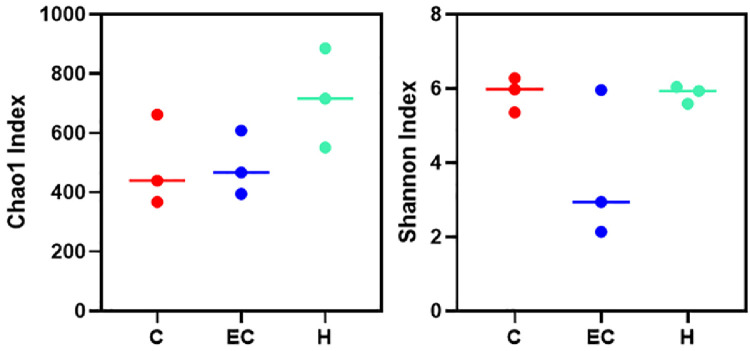
Changes of Chao1 and Shannon Indexes
Chao1 and Shannon indexes are important components of the α diversity index. The Chao1 index is used to estimate the total number of operational taxonomic units (OTUs) in the sample. Shannon index was used to evaluate the diversity of microbial community. The Chao1 index of Schisandrin A high dose (200 mg/kg) group was higher than that of control and E. coli infection groups. The Shannon index of control and Schisandrin A high dose (200 mg/kg) groups was higher than that of the E. coli infection group (Figure 10).
Figure 10.
Chao1 and shannon index.
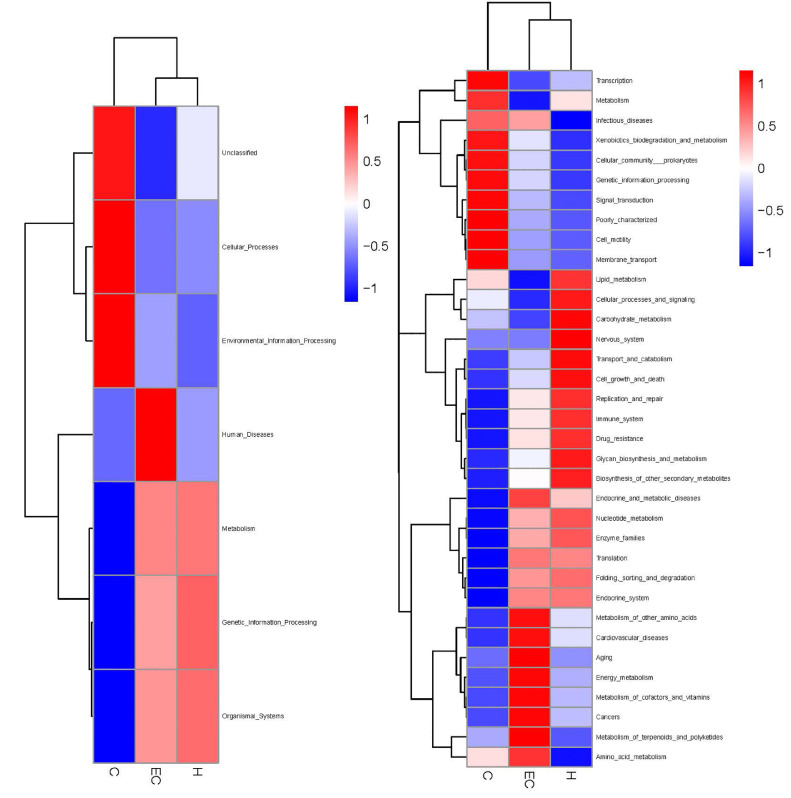
Cluster Analysis of Functional Relative Abundance
According to the functional annotation and abundance information of the samples in Kyoto Encyclopedia of Genes and Genomes (KEGG) database, the top 35 functions and their abundance information in each sample were selected by using the function prediction method of Tax4fun, and the thermal map was drawn. The clustering was carried out from the functional difference level. In the E. coli infection group, the gene function related to human diseases was increased significantly, involving cardiovascular disease, cancer, aging and amino acid and energy metabolism. The gene function of the Schisandrin A high dose (200 mg/kg) group was mainly related to the immune system, replication and repair, and lipid and carbohydrate metabolism (Figure 11).
Figure 11.
Clustering heat map of functional relative abundance.
DISCUSSION
Avian colibacillosis is a common bacterial infectious disease in chickens with high mortality, which seriously endangers the development of the chicken industry. Schisandrin A could treat chicken colibacillosis and significantly improve the weight loss caused by E. coli infection.
The liver and intestine are connected with the portal vein, which contains nutrients, food antigens, bacterial products and environmental toxins from the intestine. When the liver is damaged, the function of Kupffer cells (KCs) in the liver can be inhibited. Bacteria and endotoxin in the intestine invade the blood circulation system, leading to enterogenous infection. Endotoxin activates KCs and releases a variety of inflammatory factors. The interaction of these inflammatory factors aggravates the damage of the intestinal mucosal barrier. LPS is the key component of E. coli cell wall. E. coli can induce intestinal function damage in the body. The damaged intestine directly exposes the liver to intestinal endotoxin LPS, which induces the secretion of inflammatory cytokines and mediates liver damage (Giuffrè et al., 2020). Schisandrin A has a good anti-inflammatory effect (Cui et al., 2020). Study has shown that Schisandrin A can reduce the secretion of pro-inflammatory cytokines (including TNF-α and IL-1 β) induced by LPS (Kwon et al., 2018), which is consistent with the results of our study. In this study, that Schisandrin A could significantly reduce the expression of LPS, TNF-α, IL-1 β, IL-6, and IL-8 in hepatic and intestinal tissues of chickens.
ALT and AST are the most sensitive indicators of liver injury (Goorden et al., 2013). ALT is distributed in the cytoplasm of hepatocytes, and AST is mainly distributed in the cytoplasm and mitochondria of hepatocytes. In the case of liver injury, the permeability of hepatocyte membrane increases, and transaminase is released into the blood. The content of transaminase in serum is directly proportional to the degree of liver injury. Study reported that after intraperitoneal injection of E. coli, the levels of ALT and AST in serum of mice were increased (Zhang et al., 2018), which is consistent with the results of our study. Cellulosic hepatitis occurs in chickens after infecting with E. coli. E. coli can change the structure of liver lobule, damage liver cord, and break nucleus. Schisandrin A can alleviate liver injury. After treatment with Schisandrin A, the liver index was significantly increased, and the serum AST and ALT levels were significantly decreased. The structure of hepatic lobules was clear. The hepatic cords were radially arranged, and the cytoplasm of hepatocytes was uniform. Schisandrin A could protect the liver by improving the structure of hepatic lobules and reducing the permeability of hepatocyte membrane.
Intestinal mucosa is the most important mucosal system in poultry. The integrity of its morphology, structure and function is an effective barrier, which maintains intestinal health (Ghosh et al., 2020). Intestinal villus is an important index of nutrient absorption (Lang et al., 2019). The higher the villus height, the better the development of intestinal epithelial cells. The decrease of villus height can lead to the impairment of intestinal absorption of food and increase the possibility of diarrhea (Zhang et al., 2019). Endotoxin stimulation of E. coli can cause intestinal injury, decreasing villus height of small intestine (Tunisi et al., 2019). The results showed that Schisandrin A could significantly improve the reduction of villus height and the shedding of villus epithelium induced by E. coli.
Tight junction (TJ) is a multiprotein complex, which forms a selective membrane between adjacent epithelial cells. It is a barrier for the intestinal tract to regulate ions and prevent inflammatory molecules (Lee, 2015). TJs can provide a physical barrier to luminal inflammatory molecules. Impaired integrity and structure of the TJ barrier result in chronic inflammation in different tissues (Suzuki, 2020). TJs are the results of the interaction among claudin, occludin, junctional adhesion molecules (JAMs), and ZO-1. Occludin plays an important role in the structure and permeability of intestinal epithelium. Occludin gene deletion significantly increases intestinal permeability in mice (Al-Sadi et al., 2011). ZO protein binds to the N-terminal half region of many TJ proteins, while the C-terminal region interacts with the actin cytoskeleton and cytoskeleton-related proteins, connecting TJ proteins with structural systems, such as epithelial cells and desmosomes. ZO plays a key role in the regulation of TJ assembly. When ZO-1 was inactive, the connection between claudins and occludin and the establishment of the barrier were significantly blocked (Umeda et al., 2004). Claudin is a tetrapeptide TJ protein, which plays an important role in the acellular ion permeability between epithelial cells and is the key component of TJs (Amasheh et al., 2015). Occludin and ZO-1 are widely expressed in the epithelial layer. Under inflammatory conditions, occludin, claudin-1 and ZO-1 are reduced or even dissolved, which increases the permeability of intestinal epithelium and reduces the mechanical barrier function of epithelial cells. In this study, the expression levels of occludin and ZO-1 protein in the duodenum of chickens infected with E. coli were significantly lower than those of the control group, indicating that E. coli could make the TJ separation of chicken duodenum epithelial cells, increase the intercellular space and the permeability and reduce the barrier function of the epithelium. The expression of occludin, claudin-1 and ZO-1 in medium and high dose groups of Schisandrin A were higher than those in the E. coli infection group, and the changes of claudin-1 and ZO-1 were significant. These results indicate that Schisandrin A can promote the secretion of occludin, claudin-1 and ZO-1 proteins and reduce the gap and permeability of duodenal epithelial cells, thus slowing down the damage of epithelial barrier function caused by E. coli infection.
The abundance of beneficial bacteria in the intestine is important to maintain the balance of intestinal microecosystems and the integrity of the intestinal barrier. The regulation of intestinal flora can affect liver function and endotoxin (Hofer, 2017). Zhang et al. (2012) used penicillin to induce intestinal flora imbalance and dextran sulfate sodium (DSS) to cause intestinal mucosal damage. These results showed that long-term exposure to penicillin or DSS could lead to a continuous increase of circulating LPS and IL-6, indicating that flora imbalance and mucosal damage contribute to endotoxemia and systemic inflammation. This study showed that E. coli induced changes in the structure and abundance of intestinal microflora in chickens. At the phylum level, E. coli downregulated the abundance of Firmicutes and upregulated the abundance of Proteobacteria and Bacteroides. Firmicum is the dominant bacteria in the control group, which can maintain the balance of flora. Proteobacteria contain pathogenic bacteria, such as E. coli and Salmonella, which can damage intestinal mucosa. PCA showed that the community similarity of control and Schisandrin A high dose groups was higher. The community difference of the E. coli infection group was larger than the other 2 groups. The comparison of alpha diversity showed that the Chao1 and Shannon indexes of the high-dose Schisandrin A group were higher than those of the E. coli infection group, indicating that the diversity of intestinal microflora was increased after Schisandrin A treatment. The results of function prediction of Tax4fun showed that the gene function of intestinal flora associated with human diseases was increased significantly in the E. coli infection group, involving cardiovascular disease, cancer, aging, and amino acid and energy metabolism. The expression levels of these pathogenic genes were downregulated in the high-dose Schisandrin A group. According to the results, Schisandrin A can effectively regulate the intestinal flora disorder caused by E. coli infection, reduce the number of harmful bacteria and increase the number of beneficial bacteria.
E. coli invades the intestine, resulting in intestinal flora disorder, damaged and deformed intestinal epithelial villi, decreased TJ protein expression and increased intestinal permeability. Therefore, endotoxin LPS of E. coli can enter the blood and reach the liver from the portal vein, causing rupture of liver cord, cell necrosis and other damage, thus increasing the levels of inflammatory factors. Schisandrin A can regulate the intestinal flora disorder caused by E. coli, improve the damage caused by E. coli, enhance the integrity of the intestinal mucosal barrier, reduce the entry of intestinal endotoxin into the blood and protect the liver through the gut-liver axis.
CONCLUSIONS
Schisandrin A has a therapeutic effect on avian colibacillosis. It can alleviate inflammation, regulate intestinal flora and improve the intestinal wall barrier based on the gut-liver axis. It has a good protective effect on the liver and intestinal injury caused by E. coli.
ACKNOWLEDGEMENTS
This study was financially supported by Key Research grants of Hebei (21326602D) and Precision Animal Husbandry Discipline Group Con-struction Project of Hebei Agricultural University (1090064).
Disclosures
The authors declare no conflict of interest.
REFERENCES
- Amasheh S., Fromm M., Günzel D. Claudins of intestine and nephron - a correlation of molecular tight junction structure and barrier function. Acta Physiol. 2015;201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R., Khatib K., Guo S., Ye D., Youssef M., Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella T., Haenni M., Madela N.K., De Alk, Kalir P.L., De Aln, Costa D., Jean-Yves M., Lelles Nmc. Extended-spectrum cephalosporin- resistant Escherichia coli isolated from chickens and chicken meat in Brazil is associated with rare and complex resistance plasmids and pandemic ST lineages. J. Antimicrob. Chemother. 2018;73:3293–3297. doi: 10.1093/jac/dky335. [DOI] [PubMed] [Google Scholar]
- Cui L., Zhu W., Yang Z., Song X., Xu C., Cui Z., Xiang L. Evidence of anti-inflammatory activity of Schizandrin A in animal models of acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393:2221–2229. doi: 10.1007/s00210-020-01837-x. [DOI] [PubMed] [Google Scholar]
- Dey P., Olmstead B.D., Sasaki G.Y., Vodovotz Y., Bruno R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020;84:10845. doi: 10.1016/j.jnutbio.2020.108455. [DOI] [PubMed] [Google Scholar]
- Fan S., Liu C., Jiang Y., Gao Y., Bi H. Lignans from Schisandra sphenanthera protect against lithocholic acid-induced cholestasis by pregnane X receptor activation in mice. J. Ethnopharmacol. 2019;245 doi: 10.1016/j.jep.2019.112103. [DOI] [PubMed] [Google Scholar]
- Ghosh S.S., Wang J., Yannie P.J., Shobha G. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocrine Soc. 2020;4 doi: 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrè M., Campigotto M., Campisciano G., Comar M., Crocè L.S. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;318:G889–G906. doi: 10.1152/ajpgi.00161.2019. [DOI] [PubMed] [Google Scholar]
- Goorden S., Buffart T.E., Bakker A., Buijs M.M. Liver disorders in adults: ALT and AST. Ned. Tijdschr. Geneeskd. 2013;157:A6443. [PubMed] [Google Scholar]
- Hakala E., Hanski L., Uvell H., Yrjnen T., Vuorela P.M. Dibenzocyclooctadiene lignans from Schisandra spp. selectively inhibit the growth of the intracellular bacteria Chlamydia pneumoniae and Chlamydia trachomatis. J. Antibiot. 2015;68:609–614. doi: 10.1038/ja.2015.48. [DOI] [PubMed] [Google Scholar]
- Hofer U. Microbiome: the microbiota’s cryptic message. Nat. Rev. Microbiol. 2017;15:708–709. doi: 10.1038/nrmicro.2017.138. [DOI] [PubMed] [Google Scholar]
- Kwon D.H., Cha H.J., Choi E.O., Leem S.H., Kim G.Y., Moon S.K., Chang Y.C., Yun S.J., Hwang H., Kim B. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018;41:264–274. doi: 10.3892/ijmm.2017.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W., Hong P., Li R., Zhang H., Huang Y., Zheng X. Growth performance and intestinal morphology of Hyline chickens fed diets with different diet particle sizes. J. Anim. Physiol. A Anim. Nutr. 2019;103:518–524. doi: 10.1111/jpn.13046. [DOI] [PubMed] [Google Scholar]
- Lee S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.S., Huang Q.F., Guan L.H., Zhang H.Z., Hui-Chang B.I. Targeted bile acids and gut microbiome profiles reveal the hepato-protective effect of WZ tablet (Schisandra sphenanthera extract) against LCA-induced cholestasis. Chin. J. Nat. Med. 2020;18:211–218. doi: 10.1016/S1875-5364(20)30023-6. [DOI] [PubMed] [Google Scholar]
- Ma S.T., Ding G.J., Huang X.W., Wang Z.W., Wang L., Yu M.L., Shi W., Jiang Y.P., Tang L.J., Xu Y.G., Li Y.J. Immunogenicity in chickens with orally administered recombinant chicken-borne Lactobacillus saerimneri expressing FimA and OmpC antigen of O78 avian pathogenic Escherichia coli. J. Med. Microbiol. 2018;67:441–451. doi: 10.1099/jmm.0.000679. [DOI] [PubMed] [Google Scholar]
- Orci L.A., Lacotte S., Delaune V., Slits F., Oldani G., Lazarevic V., Rossetti C., Rubbia-Brandt L., Morel P., Toso C. Effects of the gut-liver axis on ischemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J. Hepatol. 2018;68:978. doi: 10.1016/j.jhep.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Sun Q., Zhang S., Liu X., Huo Y., Li X. Effects of a probiotic intervention on Escherichia coli and high-fat diet-induced intestinal microbiota imbalance. Appl. Microbiol. Biotechnol. 2020;104:1243–1257. doi: 10.1007/s00253-019-10304-4. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of the intestinal barrier by nutrients: the role of tight junctions. Anim. Sci. J. 2020;91:e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunisi L., Forte N., Fernández-Rilo A.C., Mavaro I., Capasso R., D'Angelo L., Milić N., Cristino L., Marzo V.D., Palomba L. Orexin-A prevents lipopolysaccharide-induced neuroinflammation at the level of the intestinal barrier. Front. Endocrinol. (Lausanne) 2019;10:219. doi: 10.3389/fendo.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K., Matsui T., Nakayama M., Furuse K., Sasaki H., Furuse M., Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- Yuan M., Peng L.Y., Wei Q., Li J.H., Song K., Chen S., Huang J.N., Yu J.L., An Q., Yi P.F., Shen H.Q., Fu B.D. Schizandrin attenuates lung lesions induced by Avian pathogenic Escherichia coli in chickens. Microb. Pathog. 2020;142 doi: 10.1016/j.micpath.2020.104059. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Kong X.B., Huo J.R., Wang L., Liu Y., Yang X.H., Tian Y., Hou Z.J., Chen F., Chen X.Y. Comparative study on sepsis models induced by Escherichia coli subtypes. Tianjin Med. J. 2018;46 doi: 10.11958/20180370. [DOI] [Google Scholar]
- Zhang L.X., Ding Y.L., Yan-Nan B.I., Miao Z.Q., Chen M.J., Wang F.L. Effects of Pathogenic E. coli K99 from bovine on growth of intestinal villi and expression of ITF mRNA in mice. Progr. Vet. Med. 2019;40:91–99. [Google Scholar]
- Zhang H.L., Yu L.X., Yang W., Tang L., Yan L., Wu H., Zhai B., Tan Y.X., Lei S., Liu Q. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 2012;57:803–812. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]