Abstract
Background
The prognostic value of epidermal growth factor receptor (EGFR)/phosphorylated EGFR (p-EGFR) expression in nasopharyngeal carcinoma remains controversial. A meta-analysis was performed to investigate prognostic significance of EGFR/p-EGFR expression in patients with nasopharyngeal carcinoma.
Methods
Literatures published before November 2020 were systematically searched in relevant databases, including PubMed, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), and Wan fang databases. STATA 13 statistical software was used to analyze the pooled hazard ratio (HR) and 95% confidence interval (CI). Heterogeneity of the studies was examined by I2. Sensitivity and subgroup analysis were performed to explore sources of heterogeneity. The potential publication bias was assessed using both Egger’s and Begg’s tests.
Results
A total of 20 literatures with 1545 patients were included for the meta-analysis. The meta-analysis results suggested that high expression of EGFR was significantly associated with poor overall survival (OS) (HR = 1.70, 95% CI: 1.24–3.15, P = 0.001) and disease-free survival (DFS) (HR = 2.58, 95% CI: 1.87–3.56, P = 0.000). However, it was not significantly associated with progression-free survival (PFS) (HR = 1.85, 95% CI: 0.90–3.82, P = 0.09) and distant metastasis-free survival (DMFS) (HR = 1.39, 95% CI: 0.73–2.67, P = 0.319). The subgroup analysis indicated that patients with EGFR high expression in studies of higher TNM stage (III–IV) ratio had significantly poor OS (HR = 2.27, 95% CI: 1.09–4.73, P = 0.03), but heterogeneity existed in studies (I2 = 95.1%, P = 0.000). Sensitivity analyses revealed that EGFR expression did not significantly affect OS by an individual study solely, indicating there was inherent heterogeneity in OS cohorts. There was no significant heterogeneity among eight studies in the DFS cohorts (I2 = 0%, P = 0.606). There was significant heterogeneity between EGFR expression and DMFS (I2 = 82.8%, P = 0.000). Sub-group analysis in differentiated carcinoma demonstrated a smaller heterogeneity (I2 = 33.2%). In addition, p-EGFR high expression had no significant correlation with OS (HR = 1.00, 95% CI: 0.88–1.14, P = 0.982) and DMFS (HR = 1.21, 95% CI: 0.96–1.52, P = 0.112). The heterogeneity among p-EGFR and OS studies was small (I2 = 21%, P = 0.26). There was no significant heterogeneity in the DMFS cohorts (I2 = 0%, P = 0.497).
Conclusion
EGFR high-expression was significantly associated with poor OS and DFS, which may serve as a prognostic predictor for nasopharyngeal cancer.
Systematic Review Registration
[https://www.crd.york.ac.uk/PROSPERO], identifier [number CRD42021258457].
Keywords: EGFR, nasopharyngeal carcinoma, meta-analysis, prognosis, p-EGFR
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy that arises from the epithelium of nasopharynx, having obvious regional characteristics and high incidence in China and southeast Asia (1). According to national cancer registry data in China, the incidence and mortality of NPC in Guangxi province rank first (2). Currently, the clinical TNM staging system is the principal prognostic indicator for NPC (3). However, clinical outcomes are different among patients with the same TNM stage (4). It seems that TNM stage alone is insufficient to predict individual clinical outcome. Several studies have shown that varied biological behavior and different prognosis was presented in the NPC patients with the same classification (5–7). Therefore, a reliable prognostic biomarker is necessary to improve individualized patient treatment and predict outcomes.
EGFR, belonging to the receptor tyrosine kinase family, plays an important role in regulation of proliferation and survival of tumor cells (8, 9). After ligand binding, EGFR is activated and forms homodimers or heterodimers, resulting in the phosphorylation and activation of multiple downstream signaling pathways, such as cellular differentiation, proliferation, and carcinogenesis (10, 11). Studies have demonstrated that EGFR is frequently overexpressed in NPC (12, 13). However, the relationship between EGFR expression and prognosis remains controversial. Several researches reported that high expression of EGFR was associated with poor prognosis (14–16), while other studies found no association between EGFR and prognostic value in NPC patients (17–19). Differences in study population’s characteristics and cutoff values may explain the discrepancies among different studies.
Phosphorylated-EGFR (p-EGFR) may be more predictive of patient outcome. Recent studies demonstrated that p-EGFR high-expression was associated with poorer prognosis in patients with sarcoma (20) and non-small cell lung cancer (21). In addition, some studies found that p-EGFR high-expression was closely related to nasopharyngeal cancer development (22, 23). Hence, we performed this updated meta-analysis to evaluate prognostic significance of EGFR/p-EGFR expression in patients with NPC.
Materials and Methods
Search Strategy
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at International Prospective Register of Systematic Reviews (number CRD42021258457). PubMed, Embase, Web of Science, CNKI, and Wan Fang Data were searched to identify relevant studies which were published before November 2020. The following words in English were used for retrieval of relevant studies: ((((((((EGFR) OR EGFR transcription factor) OR (epidermal growth factor receptor)) OR EGFR protein) OR pEGFR) OR phospho-EGFR) OR (phosphorylated signal epidermal growth factor receptor)) OR phosphorylated EGFR transcription factor) OR protein EGFR OR (erbB1)) OR (HER1) AND (((NPC) OR (nasopharyngeal carcinoma)) OR (nasopharyngeal neoplasm)) OR (nasopharyngeal cancer). In addition, the following words in Chinese were searched for relevant studies: nasopharyngeal cancer, EGFR, and phospho-EGFR.
Inclusion Criteria
The following inclusion criteria were used in this study. (1) The tissue samples were from clinically diagnosed nasopharyngeal cancer patients. (2) Immunohistochemical (IHC) assay was performed to examine EGFR/p-EGFR expression. (3) HR and 95% CI was used to evaluate the association between EGFR/p-EGFR overexpression and survival time, or Kaplan-Meier (K-M) curves were used to estimate survival time. (4) When the results were reported in multiple publications, the most complete and recently reported data was extracted.
Exclusion Criteria
The exclusion criteria were as follows: (1) recurrent or metastatic NPC tissue samples, (2) unable to obtain HR and 95% CI date or K-M curves or insufficient data, (3) the results collected from NPC cell lines or animal experiments, and (4) literatures published as letters, reviews, conference abstracts, case reports, or expert consensus.
Data Collection
All articles were independently screened by the two investigators, and those studies not meeting the inclusion criteria were excluded. Any discrepancy was discussed and resolved by seeking opinions from a third party. The content of data extraction includes the following: (1) general information: first author, publication year, country, or region; (2) basic characteristics of studies: types of researches, number of patients, study size, patients’ mean age, follow-up time, detection method, ICH cutoff value, histological differentiation, TNM stage (I–II vs. III–IV), etc.; (3) primary data: HR and 95% CI of survival outcomes, including overall survival (OS) and/or disease-free survival (DFS)/progression-free survival (PFS)/distant metastasis-free survival (DMFS). The HRs and its 95% CI were extracted from the text indirectly or calculated from the K-M survival curve using Engauge Digitizer (version 12.2.1).
Quality Assessment
Quality assessment was performed by two investigators separately according to the method of Hayden et al. (24) and the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) (25), as previously reported by Almangush et al. (26). A score ≥10 was considered to indicate high quality articles.
Statistical Analysis
HRs with 95% CI were used to evaluate the correlation of EGFR/p-EGFR high expression with the survival time of NPC patients. Meta-analysis was performed using Stata software (version 13.0). Heterogeneity among studies was assessed with the Cochran Q test and I2 test. The fixed effects model was used if there was no heterogeneity among studies (P ≥0.1, I2 < 50% in heterogeneity test). Otherwise, it was considered to have significant heterogeneity (P<0.1, I2 ≥ 50% in heterogeneity test), the random effect model was used, and the source of heterogeneity was explored using subgroup analysis or sensitivity analysis. The potential publication bias was evaluated using both Egger’s and Begg’s tests, and P > 0.05 was considered to have no publication bias.
Results
Literature Search Results, Characteristics and Quality Assessment of Included Studies
A total of 1286 studies were identified, among which 680 articles were published in English and 606 in Chinese. After initial screening, 1211 studies were excluded, and 75 trials were retrieved for detailed assessment. After full-text screening, 20 studies with 1545 patients were eligible and included for our systematic review (12, 14–19, 22, 23, 27–35), of which three studies were published in Chinese and the others in English. These eligible studies were published from 2002 to 2019, and 19 of which were on EGFR and 3 on p-EGFR. The literature search flow is shown in Figure 1. The basic characteristics and quality assessment of the included studies are shown in Tables 1 and 2.
Figure 1.
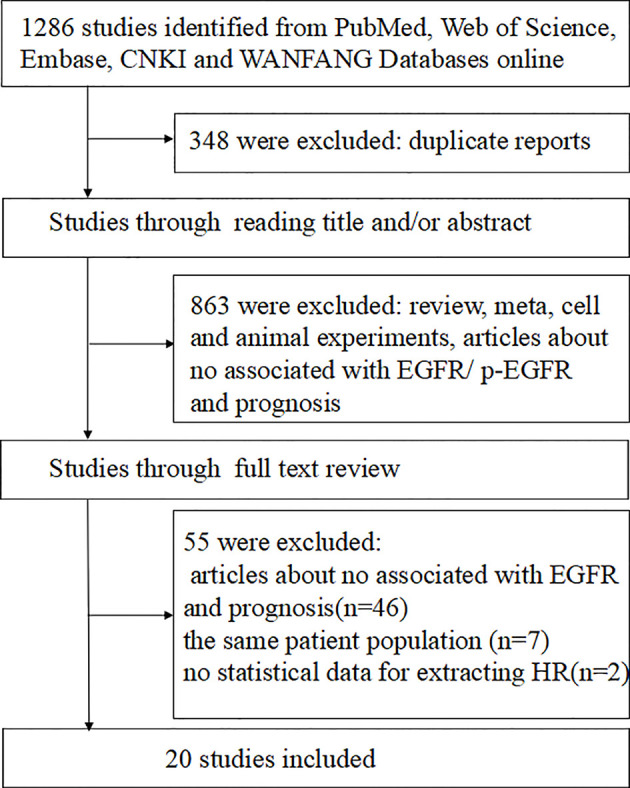
Flow chart of studies selection procedure.
Table 1.
Characteristics of included studies.
| Author | Year | Type | Country | Study type | N | Age | Follo-up | Detection method | Histological differentiation (C vs. UC) | Clinical stage (I–II vs. III– IV) | Clinical outcome | EGFR effect | Treatment | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujii et al. (17) | 2002 | EGFR | Japan | RE | 53 | 49 | 90.9m | IHC | 45,8 | 24,29 | DFS | NS | NACT+RT | 9 |
| Ma et al. (22) | 2003 | EGFR | China (Hong Kong) | PR | 78 | 48 | 46m | IHC | 0,78 | 29,49 | OS | S | CCRT/RT | 10 |
| DFS | S | |||||||||||||
| Chua et al. (27) | 2004 | EGFR | China (Hong Kong) | RE | 54 | NA | 52m | IHC | 0,54 | 23,31 | DFS | S | NACT+ | 10 |
| DMFS | S | RT | ||||||||||||
| Leong et al. (28) | 2004 | EGFR | Singapore | PR | 75 | 46 | 28.6m | IHC | 0,75 | 26,49 | OS | NS | NA | 8 |
| DFS | NS | |||||||||||||
| Wang et al. (29) | 2006 | EGFR | China | RE | 55 | NA | NA | IHC | NA | 7,48 | OS | S | RT | 7 |
| Fang et al. (18) | 2007 | EGFR | China (Taiwan) | RE | 30 | 17 | NA | IHC | 13,17 | 11,19 | OS | NS | CCRT/RT+-AC | 8 |
| DFS | NS | |||||||||||||
| Yuan et al. (23) | 2008 | EGFR | China | RE | 110 | 47 | 65m | IHC | 110,0 | 27,83 | OS | NS | CCRT/RT+-NAC | 7 |
| DMFS | NS | |||||||||||||
| 2008 | p-EGFR | China | RE | 110 | 47 | 65m | IHC | 110,0 | 27,83 | OS | NS | CCRT/RT+-NAC | 7 | |
| DMFS | S | |||||||||||||
| Yuan et al. (36) | 2008 | EGFR | China | RE | 75 | 45 | NA | IHC | 75,0 | 24,51 | OS | S | NA | 8 |
| Taheri-Kadkhoda et al. (14) | 2009 | EGFR | Sweden | RE | 45 | 56 | 96m | IHC | NA | 12,33 | OS DFS | S | NACT+-AC+ERBT | 10 |
| DMFS | S | |||||||||||||
| Huang et al. (12) | 2010 | EGFR | China (Taiwan) | RE | 170 | 46 | 68m | IHC | 76,94 | 71,99 | OS | NS | CCRT/RT | 10 |
| DMFS | NS | |||||||||||||
| 2010 | p-EGFR | China (Taiwan) | RE | 170 | 46 | 68m | IHC | 76,94 | 71,99 | OS | NS | CCRT/RT | 10 | |
| DMFS | NS | |||||||||||||
| Qi (33) | 2010 | EGFR | China | RE | 55 | 45 | 60m | IHC | 55,0 | 13,42 | OS | NS | NACT+-CCRT/CCRT/RT | 8 |
| Kim et al. (30) | 2010 | EGFR | Korea | RE | 38 | 48 | 30m | IHC | 7,31 | 6,32 | OS | NS | NA | 10 |
| PFS | NS | |||||||||||||
| Kim et al. (19) | 2010 | EGFR | Korea | RE | 69 | 50 | 54m | IHC | 9,60 | 17,52 | OS | NS | CCRT/ICRT/RT | 10 |
| Cao et al. (15) | 2011 | EGFR | China | RE | 127 | 45 | 60m | IHC | NA | 0,127 | OS | S | IC+CCRT | 8 |
| DFS | S | |||||||||||||
| Pan et al. (16) | 2013 | EGFR | China | RE | 111 | 46 | NA | IHC | NA | 41,70 | OS | S | CCRT/RT | 9 |
| DFS | S | |||||||||||||
| DMFS | ||||||||||||||
| Zhang et al. (37) | 2014 | EGFR | China | RE | 96 | 49 | NA | IHC | NA | 45,51 | OS | S | CCRT/ICRT+-AC | 9 |
| Wu (34) | 2015 | p-EGFR | China | RE | 107 | 50 | 31m | IHC | 0,107 | 12,95 | OS | NS | ICRT/CCRT | 9 |
| PFS | NS | |||||||||||||
| Kang et al. (31) | 2016 | EGFR | Korea | RE | 46 | 60 | 52m | IHC | NA | 20,26 | OS | NS | CCRT/RT | 10 |
| Mao et al. (32) | 2019 | EGFR | China | RE | 31 | 44 | NA | IHC | NA | 3,28 | OS | S | CCRT/ICRT+-AC, CTX | 9 |
| Wang et al. (35) | 2019 | EGFR | China | RE | 120 | 55 | 43m | IHC | 16,104 | 40,80 | OS | S | CCRT/ICRT+-AC | 8 |
| PFS | S |
RE, retrospective; PR, prospective; N, number of patients; NA, not available; S, significant (identifying EGFR/p-EGFR high-expression as a poor prognostic factor); NS, not significant; IRS, immunoreactive score; IC, induction chemotherapy; NACT, ICRT, induction chemotherapy followed by radiation Therapy; neoadjuvant chemotherapy; CCRT, concurrent chemoradiotherapy; AC, adjuvant chemotherapy; RT, radiotherapy; ERBT, external beam radiotherapy; CTX, cetuximab.
Table 2.
Included studies were evaluated according to the REMARK guidelines.
| Author (year) | Samples | Clinical data | Immunohistochemistry | Prognostication | Statistics | Classical Prognostic Factors |
|---|---|---|---|---|---|---|
| Fujii et al. (17) | A | A | A | I | I | A |
| Ma et al. (22) | A | A | A | A | I | A |
| Chua et al. (27) | A | A | A | I | A | I |
| Leong et al. (28) | A | A | A | A | I | I |
| Wang et al. (29) | I | A | A | I | I | I |
| Fang et al. (18) | A | A | A | A | A | A |
| Yuan et al. (23) | I | A | A | I | A | A |
| Yuan et al. (23) | I | A | A | I | I | I |
| Taheri-Kadkhoda et al. (14) | A | A | A | I | I | A |
| Huang et al. (12) | A | A | A | A | A | A |
| Qi (33) | I | A | A | A | A | I |
| Kim YJ et al. (30) | I | A | A | A | I | I |
| Kim TJ et al. (19) | I | A | A | A | A | A |
| Cao XJ et al. (15) | A | A | A | A | A | A |
| Pan et al. (16) | I | A | A | A | A | A |
| Zhang et al. (37) | A | A | A | I | I | I |
| Wu (34) | I | A | A | I | I | I |
| Kang et al. (31) | I | A | A | I | A | A |
| Mao et al. (32) | A | A | A | A | A | A |
| Wang et al. (35) | I | A | A | A | A | A |
A, Adequate; I, Inadequate.
Meta-Analysis Between EGFR/p-EGFR Expression and Prognosis
EGFR/p-EGFR Expression and OS
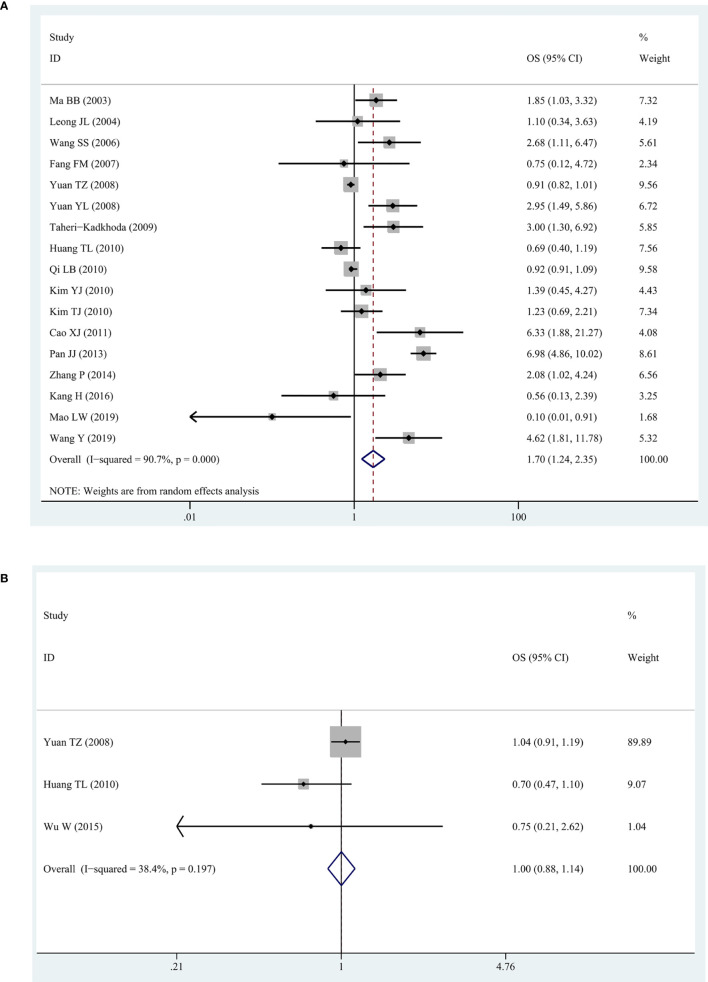
We observed a high degree of heterogeneity among the 17 studies reporting EGFR and OS (I2 = 92%, P = 0. 006). Despite this, the pooled HR indicated a significantly shorter OS in patients with higher expression of EGFR (HR = 1.70, 95% CI: 1.24–2.35, P = 0.001) (Figure 2A). For all three studies about p-EGFR and OS, the pooled HR was 1.00 (95% CI: 0.88–1.14, P = 0.982), indicating that p-EGFR high-expression had no significant correlation with OS in patients with NPC (Figure 2B). In addition, there was no obvious heterogeneity between these studies (I2 = 38.4%, P = 0.197).
Figure 2.
The forest map for relationship between EGFR/p-EGFR and OS in NPC. (A) EGFR and OS. (B) p-EGFR and OS.
EGFR/p-EGFR Expression and DFS/PFS/DMFS
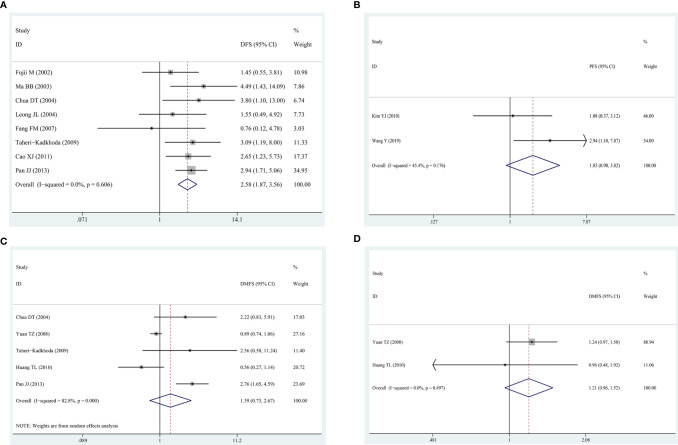
Eight studies exploring the association between EGFR and DFS showed that EGFR high-expression was predictor of poorer DFS (HR = 2.58, 95% CI: 1.87–3.56, P = 0.000; I2 = 0%, P = 0.606) (Figure 3A), which was similar to the results of EGFR and OS. In two studies reporting EGFR and PFS, the pooled HR was 1.85 (95% CI: 0.90–3.82, P = 0.09), suggesting that patients with EGFR high-expression had a poor prognosis and there was an acceptable heterogeneity among studies (I2 = 45.4%, P = 0.176). In the five studies about DMFS, no significant association was found between DMFS and high-expression of EGFR with a pooled HR of 1.39 (95% CI:0.73–2.67, P = 0.319) (Figure 3B), but heterogeneity was significant among the studies (I2 = 82.8%, P = 0.000) (Figure 3C). On the other hand, in two studies reporting p-EGFR and DMFS, the pooled HR was 1.21 (95% CI: 0.96–1.52, P = 0.112) without heterogeneity (I2 = 0%, P = 0.497) (Figure 3D), revealing that high-expression of p-EGFR was not related to DMFS of patients with NPC.
Figure 3.
The forest map for relationship between EGFR/p-EGFR and DFS/PFS/DMFS in NPC. (A) EGFR and DFS. (B) EGFR and PFS. (C) EGFR and DFMS. (D) p-EGFR and DMFS.
Subgroup and Sensitivity Analysis
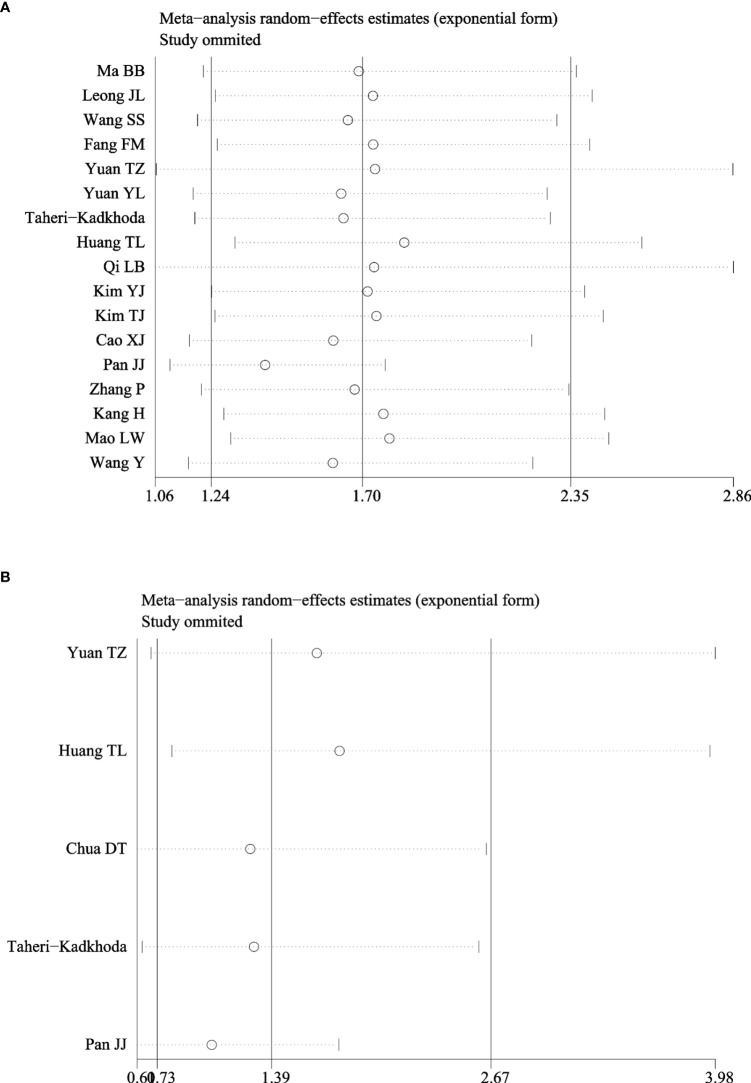
As shown in Table 3, subgroup analyses showed that patients with EGFR high-expression in studies of higher TNM stage (III–IV) ratio divided using a median percentage of TNM stage I–II samples in entire samples had significantly poor OS (HR = 2.27, 95% CI: 1.09–4.73, P = 0.03). However, the heterogeneity still existed in those studies (I2 = 95.1%, P = 0.000). In addition, the prognostic value of EGFR was not significantly associated with the country, sample size, IHC cutoff value, and histological differentiation. Moreover, sensitivity analyses revealed that EGFR expression did not significantly affect OS by an individual study solely, indicating there was inherent heterogeneity in OS cohorts (Figure 4A). A subgroup analysis was performed for studies among EGFR and DMFS, finding that the heterogeneity obviously decreased in differentiated carcinoma subgroup (I2 = 33.2%) (Figure 4B).
Table 3.
Subgroup analysis of relationship between EGFR and OS.
| Marker | Survival outcome | N | Model | HR (95% CI) | P | Heterogeneity (I2, P) |
|---|---|---|---|---|---|---|
| EGFR | OS for Asian | 16 | R | 1.65 (1.19–2.29) | 0.003 | 91.0%, P = 0.000 |
| EGFR | OS for higher rate in differentiated tumor | 3 | R | 1.00 (0.81–1.23) | 0.993 | 82%, P = 0.004 |
| EGFR | OS for higher rate in undifferentiated tumor | 7 | R | 1.38 (0.85–2.23) | 0.189 | 57.4%, P = 0.029 |
| EGFR | OS for cutoff 10% | 7 | R | 1.53 (1.00–2.35) | 0.052 | 95.1%, P = 0.000 |
| EGFR | OS for cutoff 25% | 5 | R | 2.04 (0.92–4.55) | 0.081 | 78.4%, P = 0.001 |
| EGFR | OS for higher TNM stage (I, II vs. III, IV) | 8 | R | 2.27 (1.09–4.73) | 0.03 | 95.1%, P = 0.000 |
| EGFR | OS for lower TNM stage (I, II vs. III, IV) | 9 | R | 1.29 (0.81–2.06) | 0.289 | 65.4%, P = 0.003 |
| EGFR | OS for number of samples (N > 100) | 5 | R | 2.52 (0.84–7.54) | 0.098 | 97%, P = 0.000 |
| EGFR | OS for number of samples (N ≤ 100) | 12 | R | 1.47 (1.00–2.16) | 0.051 | 71.3%, P = 0.000 |
Figure 4.
Sensitivity analysis of hazard ratios of EGFR for OS and DMFS. (A) EGFR and OS. (B) EGFR and DMFS.
Publication Bias
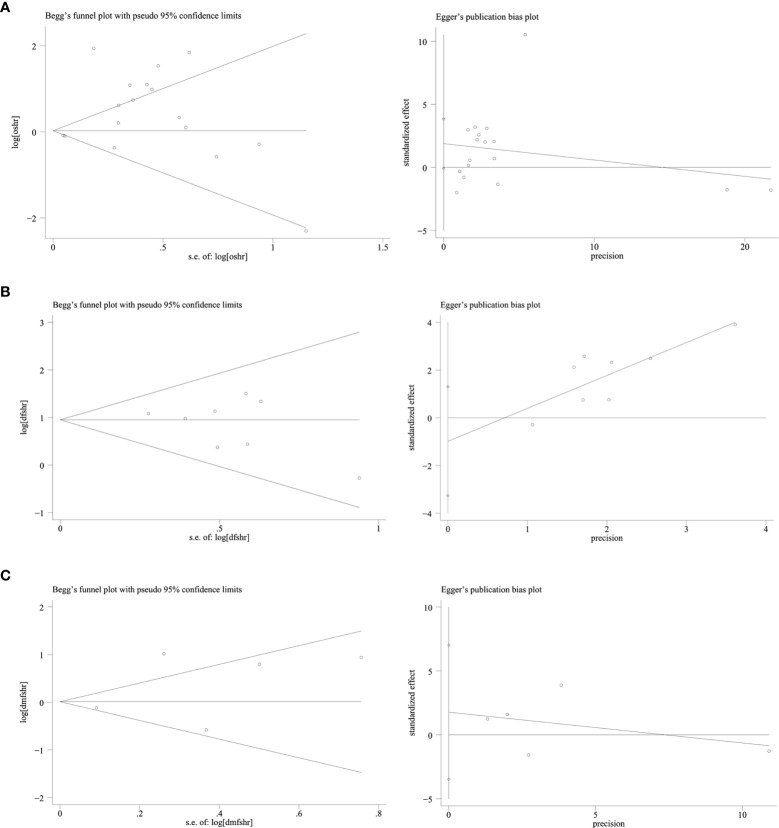
Publication bias was evaluated using Begg’s test and Egger’s test. No significant publication bias was found among studies about EGFR and OS, DFS, and DMFS (all P-values were >0.05) (Figure 5).
Figure 5.
Publication bias funnel plot of EGFR and OS, DFS, DMFS: Begg’s test and Egger’s test. (A) EGFR and OS. (B) EGFR and DFS. (C) EGFR and DMFS.
Discussion
EGFR high-expression and activation of downstream signaling pathways can promote cellular differentiation and contribute to aggressive tumor behaviors, such as increasing metastatic and migratory potential, chemotherapy and radiotherapy resistance, and stemness (38, 39). p-EGFR is an active form of EGFR and is crucial for EGFR signaling (40). It has been reported that p-EGFR was associated with poor prognosis of non-small cell lung cancer patients (21). Besides, patients with high expression of p-EGFR had shorter DMFS compared with those with low p-EGFR expression. However, the prognostic value of EGFR/p-EGFR expression in NPC remains controversial. Thus, the evaluation of relationship between EGFR/p-EGFR expression and prognosis may provide a more suitable strategy for individualized treatment of NPC.
Our meta-analysis showed EGFR could predict the outcome of patients with NPC. The pooled HRs for both OS and DFS indicate an important prognostic role for EGFR in NPC. Furthermore, the results of this meta-analysis are in accordance with the findings of previous meta-analysis (41, 42). However, the association between p-EGFR expression and the prognosis of NPC has not yet been assessed in the previous meta-analysis. In our meta-analysis, high-expression of p-EGFR was not significantly associated with OS (HR = 1.00, 95% CI: 0.88–1.14) and DMFS (HR = 1.21, 95% CI: 0.96–1.52). Additionally, heterogeneity testing displayed significant heterogeneity when analyzing OS and DMFS. Subgroup analyses revealed that patients with EGFR high expression in studies of higher TNM stage (III–IV) ratio had significantly poor OS, but heterogeneity existed in studies (I2 = 95.1%, P = 0.000). EGFR high-expression was not significantly associated with the country, sample size, IHC cutoff value, and histological differentiation. Sensitivity analyses also revealed that EGFR expression did not significantly affect OS by an individual study solely, indicating there was inherent heterogeneity in OS cohorts. In subgroup analysis with EGFR and DMFS, heterogeneity was reduced to I2 = 33.2% when we combined studies of differentiated carcinoma, indicating that the difference in tumor histology may be another source of heterogeneity and undifferentiated carcinoma was more likely to metastasize. In this study, no publication bias was observed according to both Begg’s test and Egger’s test in studies reporting OS, DFS, and DMFS, which proved the stability of our study.
Some of the included studies had deficiencies in some parameters according to the REMARKS guidelines, such as a potential ambiguity in the distinction between OS and disease specific survival in some of the included studies. There is no doubt that our study has serval limitations. Firstly, the studies included mainly focused on the patients in China, with insufficient data to examine the differences in trends by ethnic groups. Secondly, differences in quality of all included studies may affect the reliability of the results. Thirdly, the reliability and stability of the IHC results is related to the detection levels of research institutions and researchers themselves. Finally, we calculated the HR estimates from the K-M survival curves when some of the HRs with 95% CI were not directly extracted from the studies, which may be different from actual value.
In conclusion, EGFR high-expression is associated with shorter OS and DFS, suggesting that it may serve as a potential prognostic factor for patients with NPC. However, p-EGFR expression may not be used as a predictor of survival prognosis in patients with NPC, which needs to be confirmed in additional prospective, multicenter studies in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by National Natural Science Foundation of China (81760544), the Key Research and Development Program Project of Guangxi Zhuang Autonomous Region (Grant No. GuikeAB18221007), the Independent Project of Key Laboratory of Early Prevention & Treatment for Regional High‐Incidence‐Tumor (Grant No. GKE2019-17), Guangxi Science and Nature Foundation Project (2017GXNSFBA198005), the Scientific Research & Technical Development Project of Wuming District, Nanning city (No. 20200214), Liuzhou City Science and technology research projects (2019AF10601), Liuzhou City Science and technology research projects (2018BJ10303), and Department of Health of Guangxi Zhuang Autonomous Region Self-Raised Funds Project (Z20200269 and 20200856). The Basic Ability Enhancement Program for Young and Middle-aged Teachers in Higher Education Institutions of Guangxi (No.2021KY0283, No. 2021KY0091) and Science Foundation for Distinguished Young Scholars of Guangxi University of Chinese Medicine (No. 2020JQ001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal Carcinoma. Lancet (2019) 394(10192):64–80. 10.1016/s0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal Carcinoma Incidence and Mortality in China, 2013. Chin J Cancer (2017) 36(1):90. 10.1186/s40880-017-0257-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun R, Qiu HZ, Mai HQ, Zhang Q, Hong MH, Li YX, et al. Prognostic Value and Differences of the Sixth and Seventh Editions of the UICC/AJCC Staging Systems in Nasopharyngeal Carcinoma. J Cancer Res Clin Oncol (2013) 139(2):307–14. 10.1007/s00432-012-1333-9 [DOI] [PubMed] [Google Scholar]
- 4.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal Carcinoma. Lancet (2016) 387(10022):1012–24. 10.1016/s0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
- 5.Han L, Lin SJ, Pan JJ, Chen CB, Zhang Y, Zhang XC, et al. Prognostic Factors of 305 Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. Chin J Cancer (2010) 29(2):145–50. 10.5732/cjc.009.10332 [DOI] [PubMed] [Google Scholar]
- 6.Chen FM, Zhang YX, Li XF, Gao JF, Ma H, Wang XL, et al. The Prognostic Value of Deficient Mismatch Repair in Stage II-IVa Nasopharyngeal Carcinoma in the Era of IMRT. Sci Rep (2020) 10(1):9690. 10.1038/s41598-020-66678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CL, Guo R, Li JY, Xu C, Mao YP, Tian L, et al. Nasopharyngeal Carcinoma Treated With Intensity-Modulated Radiotherapy: Clinical Outcomes and Patterns of Failure Among Subsets of 8th AJCC Stage IVa. Eur Radiol (2020) 30(2):816–22. 10.1007/s00330-019-06500-5 [DOI] [PubMed] [Google Scholar]
- 8.Paci A, Ciarimboli G, Ferdeghini M. Growth Factors and Oncogenes in Development and Carcinogenesis. Role of the Epidermal Growth Factor System. Minerva Med (1994) 85(9):467–90. [PubMed] [Google Scholar]
- 9.Hardbower DM, Coburn LA, Asim M, Singh K, Sierra JC, Barry DP, et al. EGFR-Mediated Macrophage Activation Promotes Colitis-Associated Tumorigenesis. Oncogene (2017) 36(27):3807–19. 10.1038/onc.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ch’ng S, Low I, Ng D, Brasch H, Sullivan M, Davis P, et al. Epidermal Growth Factor Receptor: A Novel Biomarker for Aggressive Head and Neck Cutaneous Squamous Cell Carcinoma. Hum Pathol (2008) 39(3):344–9. 10.1016/j.humpath.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Chen QY, Liu H, Tang LQ, Mai HQ. Emerging Treatment Options for Nasopharyngeal Carcinoma. Drug Design Dev Ther (2013) 7:37–52. 10.2147/dddt [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang TL, Li CF, Huang HY, Fang FM. Correlations Between Expression of Epidermal Growth Factor Receptor (EGFR), Phosphorylated EGFR, Cyclooxygenase-2 and Clinicopathological Variables and Treatment Outcomes in Nasopharyngeal Carcinomas. Chang Gung Med J (2010) 33(6):619–27. [PubMed] [Google Scholar]
- 13.Yang Y, Xuan J, Yang Z, Han A, Xing L, Yue J, et al. The Expression of Epidermal Growth Factor Receptor and Ki67 in Primary and Relapse Nasopharyngeal Cancer: A Micro-Evidence for Anti-EGFR Targeted Maintenance Therapy. Med Oncol (2012) 29(3):1448–55. 10.1007/s12032-011-0028-4 [DOI] [PubMed] [Google Scholar]
- 14.Taheri-Kadkhoda Z, Magnusson B, Svensson M, Mercke C, Björk-Eriksson T. Expression Modes and Clinical Manifestations of Latent Membrane Protein 1, Ki-67, Cyclin-B1, and Epidermal Growth Factor Receptor in Nonendemic Nasopharyngeal Carcinoma. Head Neck (2009) 31(4):482–92. 10.1002/hed.21002 [DOI] [PubMed] [Google Scholar]
- 15.Cao XJ, Hao JF, Yang XH, Xie P, Liu LP, Yao CP, et al. Prognostic Value of Expression of EGFR and Nm23 for Locoregionally Advanced Nasopharyngeal Carcinoma. Med Oncol (2012) 29(1):263–71. 10.1007/s12032-010-9782-y [DOI] [PubMed] [Google Scholar]
- 16.Pan J, Tang T, Xu L, Lu JJ, Lin S, Qiu S, et al. Prognostic Significance of Expression of Cyclooxygenase-2, Vascular Endothelial Growth Factor, and Epidermal Growth Factor Receptor in Nasopharyngeal Carcinoma. Head Neck (2013) 35(9):1238–47. 10.1002/hed.23116 [DOI] [PubMed] [Google Scholar]
- 17.Fujii M, Yamashita T, Ishiguro R, Tashiro M, Kameyama K. Significance of Epidermal Growth Factor Receptor and Tumor Associated Tissue Eosinophilia in the Prognosis of Patients With Nasopharyngeal Carcinoma. Auris Nasus Larynx (2002) 29(2):175–81. 10.1016/s0385-8146(01)00135-3 [DOI] [PubMed] [Google Scholar]
- 18.Fang FM, Li CF, Chien CY, Rau KM, Huang HY. Immunohistochemical Expression of Epidermal Growth Factor Receptor and Cyclooxygenase-2 in Pediatric Nasopharyngeal Carcinomas: No Significant Correlations With Clinicopathological Variables and Treatment Outcomes. Int J Pediatr Otorhinolaryngol (2007) 71(3):447–55. 10.1016/j.ijporl.2006.11.019 [DOI] [PubMed] [Google Scholar]
- 19.Kim TJ, Lee YS, Kang JH, Kim YS, Kang CS. Prognostic Significance of Expression of VEGF and Cox-2 in Nasopharyngeal Carcinoma and Its Association With Expression of C-Erbb2 and EGFR. J Surg Oncol (2011) 103(1):46–52. 10.1002/jso.21767 [DOI] [PubMed] [Google Scholar]
- 20.Yang JL, Gupta RD, Goldstein D, Crowe PJ. Significance of Phosphorylated Epidermal Growth Factor Receptor and Its Signal Transducers in Human Soft Tissue Sarcoma. Int J Mol Sci (2017) 18(6):1159. 10.3390/ijms18061159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnweber B, Dlaska M, Skvortsov S, Dirnhofer S, Schmid T, Hilbe W. High Predictive Value of Epidermal Growth Factor Receptor Phosphorylation But Not of EGFRvIII Mutation in Resected Stage I Non-Small Cell Lung Cancer (NSCLC). J Clin Pathol (2006) 59(3):255–9. 10.1136/jcp.2005.027615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan CM, et al. Prognostic Significance of Tumor Angiogenesis, Ki 67, P53 Oncoprotein, Epidermal Growth Factor Receptor and HER2 Receptor Protein Expression in Undifferentiated Nasopharyngeal Carcinoma–A Prospective Study. Head Neck (2003) 25(10):864–72. 10.1002/hed.10307 [DOI] [PubMed] [Google Scholar]
- 23.Yuan TZ, Li XX, Cao Y, Qian CN, Zeng MS, Guo X. Correlation of Epidermal Growth Factor Receptor Activation to Metastasis-Free Survival of Nasopharyngeal Carcinoma Patients. Ai zheng (2008) 27(5):449–54. [PubMed] [Google Scholar]
- 24.Hayden JA, Côté P, Bombardier C. Evaluation of the Quality of Prognosis Studies in Systematic Reviews. Ann Internal Med (2006) 144(6):427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PloS Med (2012) 9(5):e1001216. 10.1371/journal.pmed.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almangush A, Heikkinen I, Mäkitie AA, Coletta RD, Läärä E, Leivo I, et al. Prognostic Biomarkers for Oral Tongue Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Br J Cancer (2017) 117(6):856–66. 10.1038/bjc.2017.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic Value of Epidermal Growth Factor Receptor Expression in Patients With Advanced Stage Nasopharyngeal Carcinoma Treated With Induction Chemotherapy and Radiotherapy. Int J Radiat Oncol Biol Phys (2004) 59(1):11–20. 10.1016/j.ijrobp.2003.10.038 [DOI] [PubMed] [Google Scholar]
- 28.Leong JL, Loh KS, Putti TC, Goh BC, Tan LK. Epidermal Growth Factor Receptor in Undifferentiated Carcinoma of the Nasopharynx. Laryngoscope (2004) 114(1):153–7. 10.1097/00005537-200401000-00029 [DOI] [PubMed] [Google Scholar]
- 29.Wang SS, Guan ZZ, Xiang YQ, Wang B, Lin TY, Jiang WQ, et al. Significance of EGFR and P-ERK Expression in Nasopharyngeal Carcinoma. Zhonghua Zhong Liu Za Zhi (2006) 28(1):28–31. [PubMed] [Google Scholar]
- 30.Kim YJ, Go H, Wu HG, Jeon YK, Park SW, Lee SH. Immunohistochemical Study Identifying Prognostic Biomolecular Markers in Nasopharyngeal Carcinoma Treated by Radiotherapy. Head Neck (2011) 33(10):1458–66. 10.1002/hed.21611 [DOI] [PubMed] [Google Scholar]
- 31.Kang H, Kwon M, Park JJ, Kim JP, Woo SH, Ahn SK, et al. Clinical Implications of Human Papilloma Virus and Other Biologic Markers in Nasopharyngeal Cancer. Oral Oncol (2016) 55:e7–10. 10.1016/j.oraloncology.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 32.Mao L, Tan J, Wang F, Luo Y, Liu W, Zeng F, et al. Retrospective Study Comparing Anti-EGFR Monoclonal Antibody Plus Cisplatin-Based Chemoradiotherapy Versus Chemoradiotherapy Alone for Stage II-IVb Nasopharyngeal Carcinoma and Prognostic Value of EGFR and VEGF Expression. Clin Otolaryngol: Off J ENT-UK Off J Netherlands Soc Oto-Rhino-Laryngol Cervico-Facial Surg (2019) 44(4):572–80. 10.1111/coa.13340 [DOI] [PubMed] [Google Scholar]
- 33.Qi LB. Expression and Clinical Significance of EGFR Receptor in Nasopharyngeal Carcinoma. Chin J Primary Med Pharm (2010) 17(20):2755–7. [Google Scholar]
- 34.Wu W. Study on the Relationship Between the Phosphorylated Epidermal Grow the Factor Receptor and the Prognosis of Patients With Nasopharyngeal Carcinoma. Med Innovation China (2015) 12(30):50–3. 10.3969/j.issn.1674-4985.2015.30.017 [DOI] [Google Scholar]
- 35.Wang Y, Wu B, Sun HL, Hu W, Xiong HC, Li CD, et al. Expression of EGFR and HLA-F in Nasopharyngeal Carcinoma and Their Prognostic Value. Zhejiang Med J (2019) 41(17):1826–30. 10.12056/j.issn.1006-2785.2019.41.17.2018-2721 [DOI] [Google Scholar]
- 36.Yuan Y, Zhou X, Song J, Qiu X, Li J, Ye L, et al. Expression and Clinical Significance of Epidermal Growth Factor Receptor and Type 1 Insulin-Like Growth Factor Receptor in Nasopharyngeal Carcinoma. Ann Otol Rhinol Laryngol (2008) 117(3):192–200. 10.1177/000348940811700306 [DOI] [PubMed] [Google Scholar]
- 37.Zhang P, Wu SK, Wang Y, Fan ZX, Li CR, Feng M, et al. p53, MDM2, eIF4E and EGFR Expression in Nasopharyngeal Carcinoma and Their Correlation With Clinicopathological Characteristics and Prognosis: A Retrospective Study. Oncol Lett (2015) 9(1):113–8. 10.3892/ol.2014.2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blume-Jensen P, Hunter T. Oncogenic Kinase Signalling. Nature (2001) 411(6835):355–65. 10.1038/35077225 [DOI] [PubMed] [Google Scholar]
- 39.Hynes NE, Lane HA. ERBB Receptors and Cancer: The Complexity of Targeted Inhibitors. Nat Rev Cancer (2005) 5(5):341–54. 10.1038/nrc1609 [DOI] [PubMed] [Google Scholar]
- 40.Yamano S, Gi M, Tago Y, Doi K, Okada S, Hirayama Y, et al. Role of Deltanp63(Pos)CD44v(pos) Cells in the Development of N-Nitroso-Tris-Chloroethylurea-Induced Peripheral-Type Mouse Lung Squamous Cell Carcinomas. Cancer Sci (2016) 107(2):123–32. 10.1111/cas.12855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun W, Long G, Wang J, Mei Q, Liu D, Hu G. Prognostic Role of Epidermal Growth Factor Receptor in Nasopharyngeal Carcinoma: A Meta-Analysis. Head Neck (2014) 36(10):1508–16. 10.1002/hed.23481 [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Huang J, Wu X, Li X, Zhang J, Xue L, et al. Epidermal Growth Factor Receptor Could Play a Prognostic Role to Predict the Outcome of Nasopharyngeal Carcinoma: A Meta-Analysis. Cancer Biomark: Section A Dis Markers (2014) 14(4):267–77. 10.3233/cbm-140401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.