Abstract
We explore the utility of bioengineered human tissues, individually or connected into physiological units, for biological research. While much smaller and simpler than their native counterparts, these tissues are complex enough to approximate distinct tissue phenotypes: molecular, structural and functional. Unlike organoids which form spontaneously and recapitulate development, “organs on a chip” are engineered to display some specific functions of whole organs. Looking back, we discuss the key developments of this emerging technology. Thinking forward, we focus on the challenges faced to fully establish, validate ad utilize the fidelity of these models for biological research.
Introduction
The field tissue engineering, created in the late 1980s, was defined as “the application of the principles and methods of engineering and life sciences toward the fundamental understanding of structure-function relationships in normal and pathologic mammalian tissue and the development of biological substitutes to restore, maintain, or improve function” (Langer and Vacanti, 1993). Interestingly, this definition of the field and its fundamental approach to enable cells to form tissues by using biomaterial scaffolds, molecular and physical regulatory signals have not changed. It is now possible to buy living skin grafts for restoration of large non-healing burns from one of the pioneers in the field, Organogenesis. Tissue engineered blood vessels will hopefully follow soon, as the clinical trials in atrioventricular shunt patients are being completed (Kirkton et al., 2019). Thus far, most success has been achieved with tissues that are either thin (skin, blood vessels, bladder) or avascular (cartilage), or having high ability for regeneration (bone). Across the board, tissue engineering is based on providing the cells with the 3-dimensional tissue-specific environment, in strike contrast to cell monolayers. In parallel, the field of microfluidics developed rapidly with the invention of soft lithography by the Whitesides lab, and the ability to prototype devices using polydimethylsiloxane (PDMS), a soft silicone-based material. Consequently, the number of reports using microfluidics increased from only a few in early 2000s to over 16,000 per year over the last five years (Zhang and Radisic, 2017).
“Organs-on-a-chip” (OOC) evolved in the late 1990s, with the idea of “human body on a chip” for studies of human physiology being introduced shortly thereafter by the Shuler lab (Sin et al., 2004). OOC gained traction with the landmark “lung-on-a-chip” study by the Ingber group (Huh et al., 2010) that recreated an epithelial/endothelial barrier on a stretchable PDMS membrane mimicking the breathing motion. This simple design recapitulated the barrier function of the lung. Another example are the engineered strips of contractile cardiac muscle that were matured enough to become similar to native trabeculae (Ronaldson-Bouchard et al., 2018; Zhao et al., 2019).
While the term OOC suggests that mini-organs are grown on a chip, it is important to note that this elusive goal has not been achieved. Instead, these systems contain small tissue constructs designed to reproduce just one or a few specific functional properties of the entire organ, as for example: barrier function of the skin, lung vasculature, muscle contractility or liver metabolism (Figure 1). The simplicity of these models is a major advantage, as it allows direct assessments of the effects of genetic and environmental factors on cellular and tissue function. Clear distinction between OOC and organoids is also important (Takebe et al., 2017) (Figure 1).
Figure 1. Cell microenvironment in organ-on-a-chip (OOC) engineering.
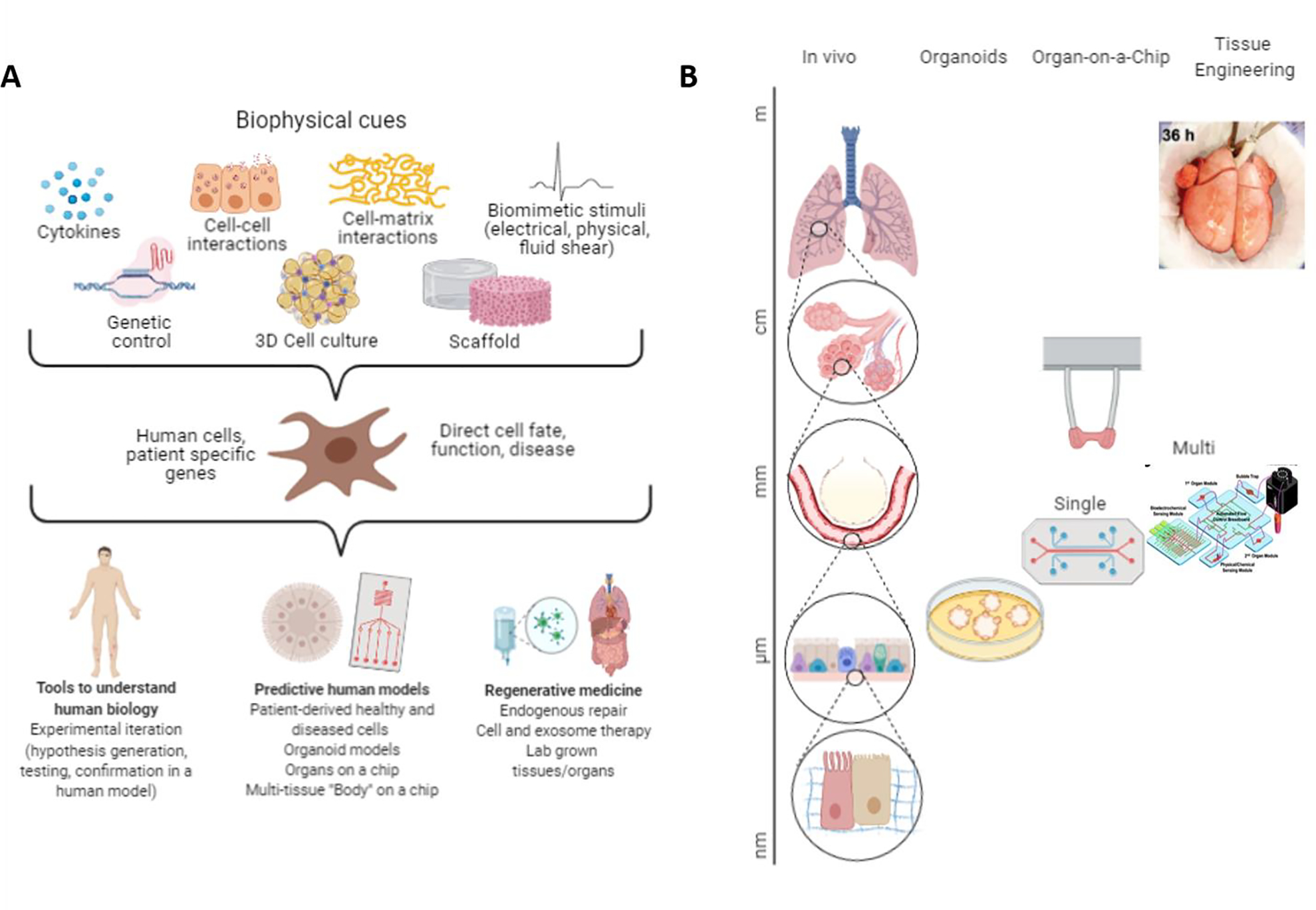
A OOCs rely on the use of cells, biomaterials and culture systems (bioreactors) to recreate environments to reproduce key functional properties of the tissue or organ of interest. B Living cell-made structures in OOC devices are on the order of μm-mm in size, similar to organoids, whereas regenerative engineering recreates structures on the order of mm-cm.
Organoids naturally form into multi-cellular structures that provide faithful models for studying early development and some diseases (Clevers, 2016), in contrast, to OOC that utilize bioengineering tools to assemble matured tissue constructs that display distinct organ functions.
Organs-on-a-chip (OOC).
Approaches from tissue engineering are harnessed in OOC which utilize cell culture on scaffolds, physical signals (fluid-dynamic, mechanical, electrical) and microfabrication of culture spaces and channels (Figure 1). Through these features, OOCs provide improved consistency of tissue structure and phenotypes for studies of organ-level functions, while often incorporating only a few cell types found in the native organ (Huh et al., 2010). Examples of OOCs include heart muscle (Ronaldson-Bouchard et al., 2018; Zhao et al., 2019), liver (Schepers et al., 2016, et al., 2019), alveolar unit of the lung (Huh et al., 2010), blood-brain-barrier (Vatine et al., 2019; Vernetti et al., 2017), kidney glomerulus and proximal tubule, (Zhou et al., 2016, Xie et al., 2020, Homan et al., 2016) neuromuscular junction (Afshar Bakooshli et al., 2019), vascular network (Zhang et al., 2016), skin (Abaci et al., 2018), retina (Achberger et al., 2019), pancreas (Bauer et al., 2017), gut (Kim et al., 2016), bone marrow (Chou et al., 2020b). placenta (Blundell et al., 2016) and tumors (Lai et al., 2020b) being used to study tissue maturation, regeneration and disease.
By coupling multiple OOCs together through vascular perfusion of a shared blood substitute, or supernatant exchange one can study organ-organ interactions and systemic diseases such as cancer (Jeon et al., 2015; Oyirifi et al., 2019; Xu et al., 2016), inflammation (Lin et al., 2019; Trapecar et al., 2020) or infection (Grassart et al., 2019).
We discuss the tissue engineering paradigm and its application to human OOCs, from a minimally functional single tissue units to multiple tissues connected into physiological units. Our focus is on the utility of OOC in biological research, where living cells can be investigated in the native-like contexts of development, physiology or disease. We also discuss some of the challenges and prospects in this rapidly evolving field providing human tissue constructs that approximate the molecular makeup, form and function of their native counterparts for quantitative biological studies.
Tissue engineering paradigm
Here we summarize some of the key developments, lessons learned and the tools we now have available for OOC studies. More detail can be found in several excellent reviews (de Graaf et al., 2019; Low et al., 2020; Marx et al., 2016; Takebe et al., 2017; Zhang and Radisic, 2017).
Initially, tissue engineering focused on creating tissues from primary cells sourced from young animals with high regenerative ability (TE 1.0). The original paradigm involved cell seeding into biomaterial scaffolds and either direct implantation or bioreactor cultivation to reach a certain level of functional competence. In the second phase (TE 2.0), the concept of isomorphous tissue regeneration was introduced, meaning that the scaffolds were designed to degrade at the rate of new tissue formation. With the advent of human induced pluripotent stem cells, tissue engineering entered its third phase (TE 3.0). The patient specific cells and scaffolds are being harnessed to create individualized approaches to organ regeneration, and to restore the anatomy and function of the original tissue. In parallel, the individualized approaches were extended into the in vitro modeling of biological processes using OOC. Adoption of methodologies developed for regenerative engineering, and advances in microfabrication and soft-lithography facilitated the rapid development of OOCs.
Cells
As the actual “tissue engineers”, cells are indispensable for tissue formation, either being supplied exogenously or mobilized in vivo. The right cell phenotypes are needed for regulating cell function and remodeling cellular environment (Guilak et al., 2009). One of the key findings during early years of tissue engineering was that the co-culture of different cell types that comprise native tissues truly improve the tissue outcomes (Aleman et al., 2019; Alimperti et al., 2017). The supporting cells (fibroblasts, pericytes, vasculature) in the stromal environment largely determine tissue functionality, through molecular and physical signaling and deposition of extracellular matrix. Also, the stroma can dramatically change in the context of disease, particularly in systemic diseases such as fibrosis and cancer.
Another key factor determining the biological fidelity of engineered tissues in all areas of application is the maturity of their molecular, structural and functional phenotypes. One can enhance tissue maturation by: (i) developmental engineering, using developmental cues and extended culture times (Keung et al., 2014; Musah et al., 2017); (ii) biomimetic engineering, by replicating the in vivo environment (e.g. mechanical stimuli, 3D culture, multiple cell types) (Guilak et al., 2009; Ronaldson-Bouchard et al., 2018; Zhao et al., 2019); and (iii) bioactivation, by activating certain pathways (via endogenous signaling, environmental stimuli, transcriptomic factors). While the first two approaches are well established, bioactivation is just emerging. Matured engineered tissues can also help advance our understanding of epigenetic regulation of diseases.
Scaffolds
Most cells require a scaffold to serve as a structural and logistic template for attachment, tissue formation and remodeling. In general, the scaffold is designed to mimic the composition, structure and biomechanics of the native tissue matrix. The role of the scaffold is only temporary, and it should biodegrade at the rate allowing deposition and remodeling of new extracellular matrix. Tissue engineering has largely driven the evolution of permanent scaffolding materials (such as nylon) into those biodegrading at a given rate, and inert scaffolds (polyesthers) into those with bioactive properties (chemically modified materials with ligands, conductive polymers). Preparations of native tissue matrix from decellularized tissues are becoming increasingly, as for example mineralized bone matrix as a scaffold for bone tissue (Chen et al., 2020), or fibrin for the formation of muscle, (Ronaldson-Bouchard et al., 2019).
The field is now starting to explore adaptive-responsive biomaterials that can sense and actuate cells or respond to the local environment to drive functional restoration of complex tissue structures. This new class of scaffold materials will allow a shift from predetermined scaffold properties to scaffolds that can change in response to cellular and environmental signals, or on demand.
Bioreactors
Certain conditions and duration of culture are needed for the collections of cells to organize into functional tissue units. Approaches that rely on mimicking the native organ environment have become increasingly successful, including the use of medium flow to mimic blood perfusion, and electrical and mechanical stimuli to drive the maturation of muscle, neural tissues or bone (Alimperti et al., 2017; Blundell et al., 2016; de Graaf et al., 2019; Ronaldson-Bouchard et al., 2018; Vatine et al., 2019; Zhao et al., 2019). Culture systems (bioreactors) are designed to control environmental conditions, exchange oxygen, nutrients, and metabolites, and provide the molecular and physical regulatory factors (Chen et al., 2020).
The requirements for bioreactor design and operation can be different for OOC than for engineering large grafts for regenerative medicine, and vary from one tissue type to another. In general, clinically sized tissue grafts cannot be kept alive without vascular perfusion, as a hypoxic core rapidly forms once the oxygen diffusion length exceeds ~100–200 μm. The only exception are thin tissues, such as skin or bladder. However, vascularization is important for achieving biological fidelity of cultured tissues, as it supports paracrine interactions between the cells. Small sizes of tissues in OOCs allow control of microenvironmental cues across short diffusional distances, thereby maintaining tissue viability and function. Dynamic mechanical stresses can be imposed through organ specific pressures, mechanical stress, and pulsatile blood flow, along with the provision of spatiotemporal regulation of biochemical signals and concentration gradients. It is interesting that unmet challenges in one area (regenerative medicine) propelled another area (OOC) into a fast-track application of tissue engineering.
Increased complexity may be needed to mature tissues and create high-fidelity biological models of health and disease. More complex questions require more complex models, while straightforward models (i.e. analyzing the effects of a single point gene mutation) can be well served by rather simple approaches that avoid confounding effects and facilitate interpreting the results. As complexity increases so does the physiological relevance of the developed model (Oyirifi et al., 2019). However, increasing complexity reduces the level of user control and increases biological variability, which can complicate interpretation of the results. To determine the necessary level of complexity, it can be useful to first determine the context of use for the model and then work backwards to define which variables need to be recapitulated to answer a specific question in a most straightforward way.
Cells respond to the entire context of their environment, in vivo and in vitro: cytokines, surrounding cells, extracellular matrix and physical forces (Figure 1). Cell fate is a result of the combined effects of environmental factors, which can lead both to the favorable (differentiation, self-renewal) and unfavorable outcomes (apoptosis, de-differentiation). The purpose of tissue engineering is to enable the cell to conduct its biological function by providing an appropriate environment for the formation, maintenance or maturation of a specific tissue. In turn, we may also create a specific pathological environment if the goal is to establish a model of injury or disease to evaluate therapeutic modalities. These considerations are the scientific premise for biomimetic principles of tissue engineering. Some of the universal requirements are to include multiple cell types, scaffolds with appropriate ECM signaling and physical signals, and to achieve some level of maturation. More sophisticated models incorporate provisions for vascular perfusion, tissue connectiveness, systemic factors (immune cells, cytokines) and for some tissues (such as the heart and bone) the structural and mechanical anisotropy. Incorporation of inducible cell reporters further allows longitudinal dynamic studies of the dynamics and progression of disease, injury and healing.
Single OOC models
The scientific premise for engineering single OOC devices is that appropriate function will follow the appropriate form, because creating of a cell-matrix structure emulating that in a native organ will guide the cells to assume an appropriate function. Based on the function they are modelling, OOC approaches can be broadly classified into: (1) Barrier function devices and (2) Parenchymal function devices. In terms of hardware design, two general trends emerge: 1) Closed devices, with sealed channels and pump driven flow, and (2) Open devices that resemble well plates and are perfused by gravity driven flow or using rocking platforms. Each design has advantages and disadvantages (Figure 2). Whereas pump driven flow is highly controllable and allows complex flow patterns, device operation, fluid and cell sampling can be more difficult. In contrast, open devices allow facile tissue and media retrieval, at the expense of less precise environmental control.
Figure 2. Organ-on-a-chip (OOC) devices drive biological research.
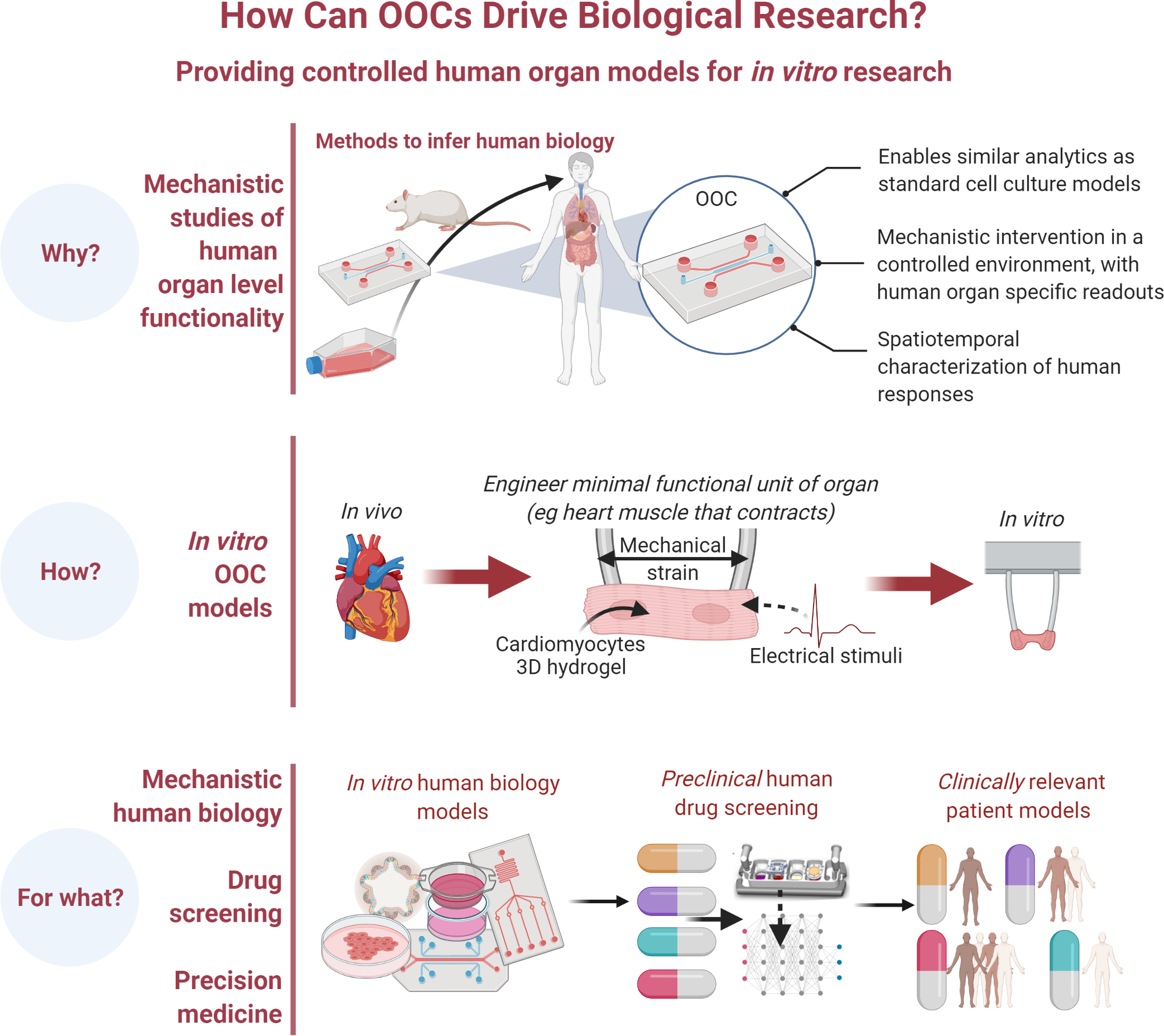
OOC devices uniquely enable studies of human organ-level functionality using standard methods validated in 2D and animal studies through systems aimed at reproducing a single organ or multiple organs. They are already used in human disease modelling, drug screening and precision medicine.
The recent years have seen an explosive growth of companies commercializing OOC devices (~30 companies in 7 years) (Zhang et al., 2018), fueled by their promise to transform drug discovery (Esch et al., 2015). The one-size-fits all paradigm in biological and medical research can now be overcome by using OOCs made using donor-specific genetically defined cells. This approach opens a whole range of possibilities for individualized studies and parsing out the effects of genetic and environmental factors (Figure 3). An overview of single OOC models is shown in Figure 4, with comprehensive details provided in recent reviews.
Figure 3. Biological question drives design considerations.
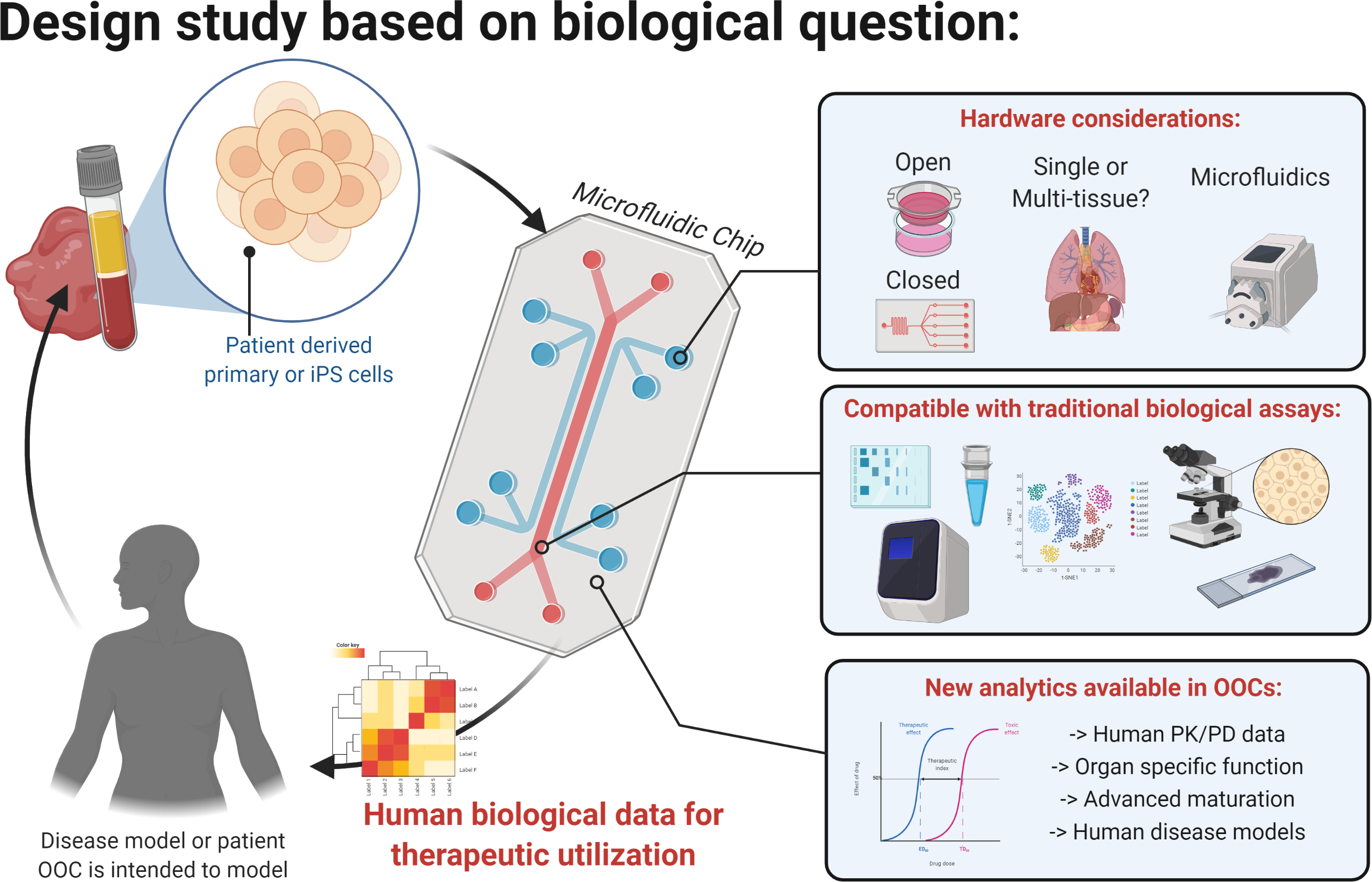
The configuration of OOC can be selected depending on the biological question studied, among the open or closed, single or multi-organ OOCs, with gravity or pump driven flow. OOCs are compatible with traditional assays such as immunofluorescence, - omics, or the use of ion or membrane and calcium dyes. They enable cell maturation, not routinely possible in 2D culture, and the tissue specific readouts such as contraction force, barrier function, and impulse propagation.
Figure 4. Representative examples of single OOCs for studies of organ functions.
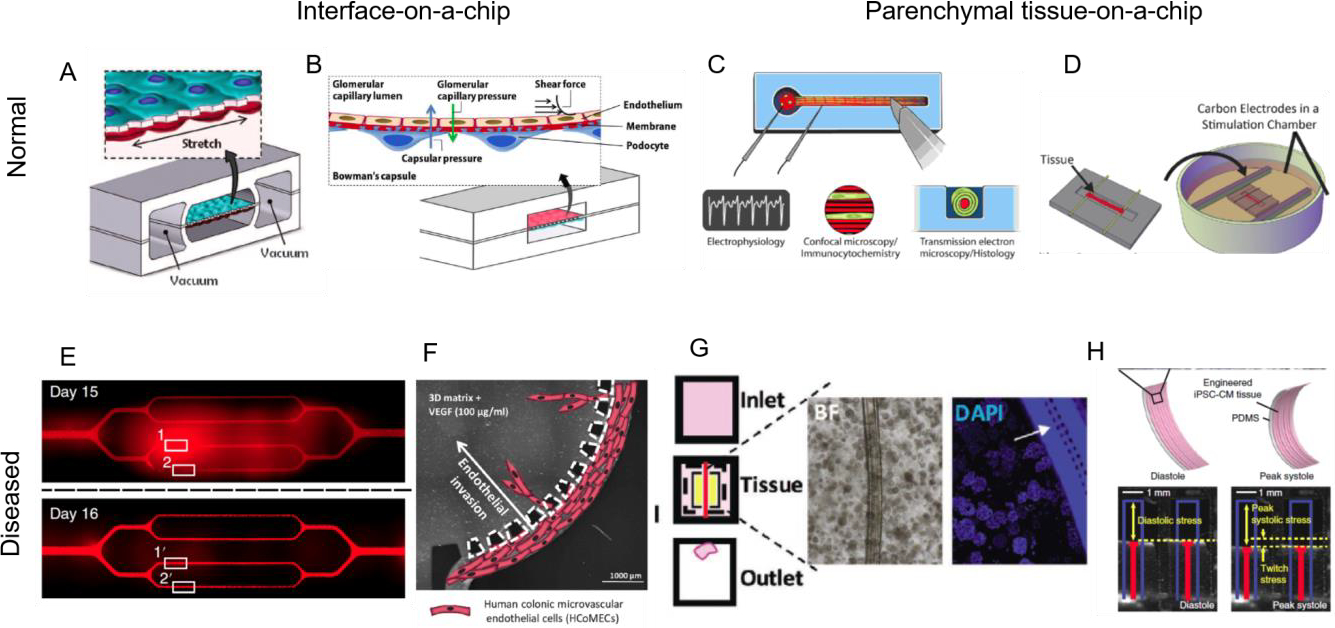
In the category of interface on-a-chip devices, A lung-on-a-chip device recreates epithelial/endothelial barrier function, reproduced with permission from (Huh et al., 2010). B Glomerulus-on-a-chip recreates podocyte/endothelial barrier function, reproduced with permission from (Zhou et al., 2016). C In the category of parenchymal tissue devices peripheral nerve-on-a-chip can be used to study electrophysiological properties due to drug toxicity, reproduced with permission from (Sharma et al., 2019a). D Biowire II platform established functional hallmarks of human ventricular and atrial myocardium, reproduced with permission from (Zhao et al., 2019). Interface on-a-chip devices can be used to study increases in permeability due to disease: E sickle cell occlusion of vasculature-on-a-chip, reproduced with permission from (Qiu et al., 2017) or F endothelial invasion in colorectal tumor, reproduced with permission from (Carvalho et al., 2019b). Parenchymal tissue-on-a-chip devices can be used to recreate G pancreatic cancer microenvironment, reproduced with permission from (Lai et al., 2020a). H cardiomyopathy of a genetic disease, Barth syndrome, reproduced with permission from (Wang et al., 2014).
Studies of tissue development and function
(a). Barrier function
Barrier function was the first one to be established using OOC approach in a lung-on-a-chip device (Huh et al., 2010) (Figure 4A) that can recapitulate many epithelial barriers. For example, by cultivating podocytes on one side of the membrane and endothelial cells on the other side, it is possible to establish a barrier that selectively filters species according to molecular weight, thus mimicking the function of kidney glomerulus (Figure 4B). While large molecules (albumin, antibodies such as IgG) remain in the endothelial compartment, small molecules (inulin) can cross the barrier (Zhou et al., 2016). The barrier function weakens at the higher flow rate/pressure, thus exhibiting hallmarks of hypertensive nephropathy. Also, the curvature affects gene expression in endothelial cells and stability of vasculature in collagen hydrogel (Mandrycky et al., 2020), an important consideration for epithelial/endothelial barriers. A great degree of complexity in shape control was achieved by 3D printing of a proximal tubule-on-a-chip (Homan et al., 2016), microfluidic spinning of glomerulus-on-a-chip (Xie et al., 2020) and stereolithographic printing of microvasculature and lung alveoli in photopolymerizable hydrogels using food colors as photoabsorbers (Grigoryan et al., 2019).
(b). Parenchymal function
A nerve-on-a-chip system consisting of a spheroid embedded into a PDMS based well with a single extending channel enabled co-culture of human Schwan cells and neurons with robust myelin formation enabling delineation of electrophysiological responses upon application of various signals and agents (Sharma et al, 2019a) (Figure 4C).
Heart muscle has been extensively studied in OOC devices since cardiovascular disease still kills more people than all cancers combined (American Heart Association, 2015). The need for human cardiac tissue models is highlighted by recent drug recalls due to cardiotoxicity (e.g. Micturin, Fen-phen, Seldane, Vioxx, Avandia) that were due at least in part to low ability of animal models and cell cultures to predict the patients’ responses (Piccini et al., 2009). However, immature phenotypes of human heart muscle derived from iPS cells have limited their utility.
Human heart muscle has been grown from iPSC in hydrogel anchored at the two ends (Figure 4D) that also allowed to measure the contraction force through the deflection of PDMS posts (Hinson et al., 2015) or cantilevers (Wang et al., 2014) that provide resistance to contractions (Figure 4H). In later studies, PDMS has been replaced by inert plastics (Zhao et al., 2019) (Figure 4D). Electrical stimulation of heart muscle formed from early-stage iPSC-derived cardiomyocytes at a gradually increasing frequency markedly advanced tissue maturation (Ronaldson-Bouchard et al., 2018, Nunes et al., 2013). Such “intensity training” of heart muscle resulted in adult-like gene expression profiles, oxidative metabolism, remarkably organized ultrastructure with physiologic sarcomere length and density of mitochondria, and networks of transverse tubules, positive force–frequency relationship and functional calcium handling (Ronaldson-Bouchard et al., 2018). The atrial vs ventricular specification was achieved by ramping the stimulation frequency at two different rates (Zhao et al., 2019) and used to model left ventricular hypertrophy, using cells from patients.
Anchoring the forming tissue also enabled engineering of skeletal muscle and neuromuscular junction (NMJ). In one study, patient-derived muscle progenitors were mixed with iPS cell-derived motor neurons (Afshar Bakooshli et al., 2019). In another study, the first patient-specific model of NMJ was established using muscle cells and optogenetically edited motoneurons, to model Myasthenia gravis, an autoimmune disease resulting in NMJ dysfunction (Vila et al., 2019). Similar to cardiac models, the skeletal muscle models can also be matured using electrical stimulation (Khodabukus et al., 2019) and derived from human iPSCs (Rao et al., 2018).
Liver-on-a-chip is one of the most commonly used OOCs, as a model of metabolic function and drug toxicity. The importance of heterotypic interactions between hepatocytes and fibroblasts has been recognized early on (Hui and Bhatia, 2007) resulting in a commercialized Hepatopac® system. Sensing of drug injury was demonstrated through incorporation of Kuppfer cells in Organovos’s 3D printed liver spheroids (Norona et al., 2019). Emulate designed a rat, dog, and human Liver-Chip containing primary hepatocytes and liver sinusoidal endothelial cells, with or without Kupffer and stellate cells. This study found liver toxicity and fibrosis from compounds that were discontinued upon rodent studies, proving that OOC can detect similar effects in rodent based devices, and predict liver injury in humans (Jang et al., 2019). Recent PDMS-free OOC with separate hepatic and vascular channels, incorporating primary human hepatocytes and sinusoidal endothelial cells, stellate and Kupffer cell lines) recapitulated the liver acinus and oxygen zonation (Li et al., 2018).
Lymph node-on-a-chip was modeled using a PDMS channel with two inlets and two outlets, supporting a monolayer of dendritic cells to which T-cells can be bound during flow in an antigen specific manner (Moura Rosa et al., 2016).
Human eye-on-a-chip presents the next frontier in OOC, capturing imagination at the same time. The challenge is particularly difficult due to the concavely hemispherical retina structure, a wide field of view, high resolution, and adaptivity to the optical environment. Human corneal and conjunctival cells were combined in a hemispherical device in the shape of the eye. The patterned cells are periodically lubricated by a hydrogel lid to create a blinking eye on a chip (Seo et al., 2019). A mimic of human retina with all seven cell types was created in an OOC with organoids and microvascular channels (Achberger et al., 2019).
(c). Microvasculature
AngioChip was shown to support the assembly of different types of parenchymal cells in a mechanically tunable matrix surrounding a perfusable microchannel network lined with endothelial cells. The design of AngioChip decouples the engineered vessel network and parenchymal tissue, enabling extensive remodeling while maintaining open-vessel lumens. The incorporation of nanopores and micro-holes in the vessel walls enhances intercellular crosstalk in vascularized heart and liver OOC (Zhang et al., 2016). This platform can be scaled down to a footprint of a 96-well plate (Figure 4G), using the inVADE system, requiring only 100,000 cells per tissue and enabling facile connections of tumor and liver tissues (Lai et al., 2017). Up to 128 independent vascularized colons-on-a-chip were formed in a 384-well plate platform with gravity flow that supported modeling of colon inflammation with innate immune function (Rajasekar et al., 2020). OOC model of vascularized bone was also created by inducing microvascular flow into the ECM populated by bone forming cells (Jusoh et al., 2015). Recent studies are also focusing on capillary, venous, arterial, and lymphatic vascular networks (Grigoryan et al, 2019; Fleischer et al, 2020).
Studies of injury and disease
Whereas endothelial barrier can capture hallmark functions of some organs, studies of cell trafficking and crosstalk require the formation of perfusable vasculature (Figure 4E–G). Endothelial networks can be maintained for a month, using a branching multichannel device with interpenetrating hydrogel recapitulating the blood vessel intima, to study the inflammatory mediators of barrier function and sickle cell occlusion (Qiu et al., 2017) (Figure 4E).
As fibrosis affects essentially all organs as the end stage of various diseases and injuries, recent OOC studies have focused on recapitulating fibrosis (Hayward et al., 2020). This approach is especially important in modelling cancer microenvironment, due to the recognized role of cancer associated fibroblasts in driving and promoting cancer metastasis. A multi-layered lung-on-a-chip with patterned vasculature, lung channels was used to form cystic fibrosis-like epithelium and study neutrophil migration (Mejias et al., 2020). To generate fibrotic interstitium, fibroblasts from donors with idiopathic pulmonary fibrosis and healthy fibroblasts treated with TGF-β1 were incorporated into the fibrin/collagen hydrogel at the air/liquid interface. (Mejias et al., 2020).
The effects of ionizing radiation and myeloerythroid toxicity after chemotherapy were studied in a bone marrow OOC, created using an approach similar to the lung OOC, with two parallel channels separated by the porous membrane. The vascular compartment was lined with endothelial cells, whereas the bone marrow compartment was created by filling the parallel channels with fibrin gel encapsulated CD34+ cells that supported differentiation and maturation of multiple blood cell lineages over 4 weeks (Chou et al., 2020a). The alcoholic fatty liver disease was modeled by exposing Emulate liver OOC to the ethanol levels consistent with those in the human blood, resulting in structural changes in bile caniculi and accumulation of lipid in hepatocytes (Nawroth et al., 2020).
A tumor OOC was used to generate organotypic patient-specific blood vessel models using normal and renal carcinoma associated primary CD31+ cells. RNA sequencing of blood vessels allowed selection of candidate drugs, ultimately leading to the testing of sirolimus and nintedanib (Figure 4F). These models are becoming invaluable for assessing the efficacy of drug-loaded nanoparticles in emerging therapies (Carvalho et al., 2019a).
Synovium OOC containing embedded organic-photodetector arrays has been developed to study rheumatoid arthritis. Patient-derived primary synovial organoids were cultivated in the absence and presence of tumor necrosis factor, by noninvasive detection of changes in tissue architecture via light scattering (Rothbauer et al., 2020).
OOCs have been invaluable for the studies of SARS-CoV-2 and other viral infections associated with organ dysfunction (Tang et al., 2020). Emulate’s lung OOC was used for screening FDA approved protease inhibitors to curb the injury resulting from influenza, and inhibit pseudotyped SARS-CoV-2 viral entry (Si et al., 2020). The heart OOC demonstrated profound contractile dysfunction (Marchiano et al., 2020), consistent with the recent reports of cardiac side effects in asymptomatic and recovering COVID-19 infected individuals (Topol, 2020). Importantly, OOCs uniquely allow delineating the effects of SARS-CoV-2 infection on the main functional cell types in the organ from indirect effects of inflammatory cytokines.
Multiple “organs on a chip” models of disease
Studies of human physiology necessitate the use of holistic models that capture how cells, tissues, and organs work in conjunction with one another during normal homeostasis or disease. Multi-OOCs rely on connection via recirculating media that enables communication via secreted factors, extracellular vesicles and circulating cells. Here we describe the multi-OOCs used to study the systemic development and function of multiple connected tissues, progression of disease, and their utility for therapeutic screening. An overview of multi-OOC models is shown in Figure 5.
Figure 5. Representative examples of multi organ-on-a-chip devices and their utility.
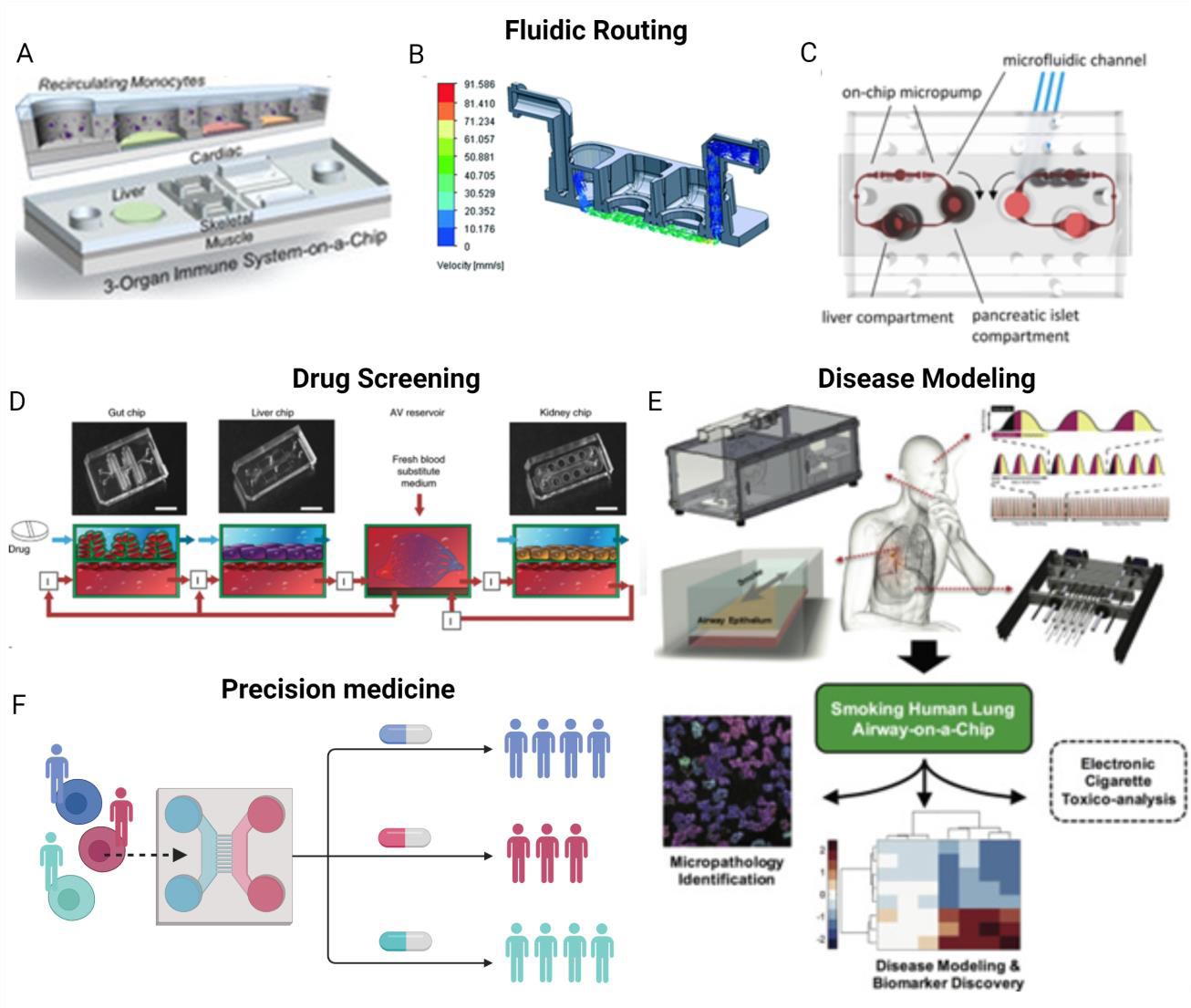
Single OOCs can be connected by fluidic routing to facilitate inter-organ communication via A recirculating shared media perfused above cells, reproduced with permission from (Sasserath et al., 2020), B pump-driven recirculation below engineered tissues, reproduced with permission from (Chramiec et al., 2020), and C on-chip micropumps, reproduced with permission from (Bauer et al., 2017). These multi OOC devices can be used for D human drug screening, reproduced with permission from (Herland et al., 2020), E disease modeling, reproduced with permission from (Benam et al., 2016), and F precision medicine approaches.
Combining single organ models to create multi-organ models
The single organ models can be fluidically connected to create multi-organ models, by co-culture in shared media (Wikswo et al., 2013), external media transfer (Vernetti et al., 2017), recirculation using pneumatic pressure-driven actuation (Satoh et al., 2018), and peristaltic pumps (Chramiec et al., 2020) (Figure 5A–C). Advanced circuit designs account for the blood flow each organ receives in vivo (Sasserath et al., 2020).
Most multi-organ settings include liver, as the primary site of drug metabolism, required for prodrug transformation into its active form. More advanced systems are now being developed to provide a tissue specific niche for each tissue module, mimic the systemic vascular network, and include routing of culture media and biosensors for on-line readouts (Achberger et al., 2019; Trapecar et al., 2020; Xiao et al., 2017). Such systems allow scaling of organ volumes and blood flow to match the in vivo situation, while enabling high throughput screening and extended culture times (Marx et al., 2016). Linking multiple tissues by vascular perfusion has impact on each tissue (by increasing its biological fidelity through cellular cross-talk within in vivo like tissue environment) as well as on the entire multi-organ model (by providing systemic components – such as metabolism, immunity, clearance that are needed for physiological studies). The advantages of microfluidic multi-organ models support their continued development despite additional complexities (Sung et al., 2010).
Modeling development, physiology, and systemic disease
Multi-organ models are designed for a range of applications in drug screening, disease modeling and precision medicine studies (Figure 5).
To disentangle the complexity of the human bone marrow, a multi-OOC was developed that connected the periarterial, perisinusoidal, mesenchymal, and osteoblastic components of the human bone marrow niche (Aleman et al., 2019).
The female reproductive system has been modeled in a multi-OOC to study hormonal signaling during the menstrual cycle and pregnancy-like endocrine loops by connecting ovary, fallopian tube, uterus, cervix, and liver modules via microfluidic routing (Xiao et al., 2017).
Using microfluidics to connect a module containing human pancreatic islet microtissues with a module containing liver spheroids, the hormonal feedback between the two organs could be modeled in vitro (Bauer et al., 2017). These advances show promise for decoupling the behaviors of individual organs during homeostasis and identifying factors driving metabolic and hormonal diseases.
Systemic diseases provide a real opportunity for the use of multi-organ platforms, as their mechanisms are not adequately recapitulated in animal models. An excellent example is the physiomimetic multi-organ platform containing gut and liver modules connected by fluidic circulation containing circulating Treg and Th17 immune cells to create a human multi-organ model of ulcerative colitis (Trapecar et al., 2020). The system was paired with multi-omics to reveal that short-chain fatty acids either improved or worsened the disease, and that these opposing responses were dictated by CD4+ T cell-effector function. This study uniquely demonstrated how human multi-organ systems can be leveraged to better understand the immune and metabolic regulation of human pathophysiology.
The development of degenerative brain diseases like Alzheimer’s and Parkinson’s disease has been connected with the gut health (Alkasir et al., 2017). Recapitulating the inter-organ brain-gut-immune axis using multi-OOCs studies of how these organ systems evolve in response to one another. While the progression from healthy to diseased state is difficult to model in vivo, multi-organ platforms provide human tissues and bioengineering tools to do just this. The inclusion of patient microbiome into multi-organ models allows predictive insights into how a patient will respond to a drug therapy (McCracken et al., 2014).
Cancer metastasis is another systemic disease that would greatly benefit from multi-OOCs. Cancer cells have a complex interplay with their surrounding microenvironment, immune system, and metastatic sites. The advanced control offered by multi-OOCs enables decoupling of these networks and identification of key drivers of cancer progression, immune evasion, and drug resistance. Microfluidic models allow studying various components of the metastatic process: cancer cell escape into the bloodstream (intravasation), changes that occur while in circulation, and cancer cell invasion into target tissues (extravasation). Cancer cell intravasation has been studied using cancer, immune, and vascular multi-organ models, revealing the role of TNFα in increasing endothelial permeability and cancer cell escape (Zervantonakis et al., 2012). Multi-organ models provide tissue specific insight into cancer cell extravasation (Xu et al., 2016), as lung cancer cells acquired an invasive phenotype when co-cultured with healthy lung cells, to populate distant bone, brain, and liver organs within the multi-OOC. Breast cancers have similarly been modeled to show propensity for metastasis to bone (Jeon et al., 2015), a result commonly seen clinically but difficult to model in animal systems.
Human OOCs for therapeutic screening
Multi-OOCs provide methodology to evaluate therapeutic safety, efficacy, immunogenicity and pharmacokinetics/pharmacodynamics (PK/PD) in physiologically relevant human settings, prior to clinical trials, allowing refinement of clinical strategies to better direct the right drug, at the right dose and right time, to the right patient. Multi-organ models usually include the target organ of interest (i.e., tumor) and the organs related to drug metabolism (i.e. liver and kidney) and off-target toxicity (i.e. liver, heart). An example is the model with microfluidically linked liver, tumor and marrow established by the OOC pioneer Schuler, using flow rates and residence times matching those in the human body (Sung and Shuler, 2009). The liver module was able to metabolize the prodrug Tegafur into 5-fluoroucil, and this active drug induced cell death, as expected. Notably, liver cells cultured in a standard 96 well plate were unable to show this expected response.
Models of human drug PK/PD (Phase I)
The initial driver for developing these OOC models was the need to identify human drug toxicities and human therapeutic indexes – drug concentrations that are high enough to show an effect but low enough to avoid toxicity. OOCs accurately describe drug adsorption, distribution, metabolism, elimination and toxicity (ADMET) as seen clinically. For example, naphthalene was converted in the liver module into its reactive metabolites and transferred to the lung module, where it depleted cellular glutathione levels and caused the accumulation of hydrophobic compounds in the fat module (Viravaidya et al., 2004).
The inclusion of endothelium can support the functionality of many OOC systems (Schepers et al., 2016) by separating tissue compartments while enabling their communication, and is critical in modeling drug transport. A multi-organ human neurovascular unit developed by coupling blood brain barrier module (containing human astrocytes and pericytes above a channel lined with brain microvascular endothelial cells) with the brain module (containing primary human brain neurons) recapitulated the blood brain barriers response to methamphetamine, revealing its selective penetration and the previously unknown metabolic coupling between neurons and the microvascular brain endothelium (Maoz et al., 2018).
Lung OOCs enable studies of inhaled drugs, pollution, and smoking (Benam et al., 2016). Similarly, skin OOCs enable drug entry through the skin, allowing screening of drug delivery and the safety of chemicals and cosmetics in contact with the skin (Abaci et al., 2018; Pires de Mello et al., 2020). Combining computational models with experimental multi-organ systems, PK parameters were measured for cisplatin and nicotine delivered intravenously or orally, to match the PK values demonstrated clinically (Herland et al., 2020).
Models of human safety and efficacy (Phase II/III)
Multi-OOCs have been able to accurately model both the on target effects of cancer therapies and off target heart and liver toxicity, for drugs involving liver metabolism (McAleer et al., 2019). The system was designed so that the drug first passed through the liver module, where it was converted into its active form and subsequently delivered to downstream tissue modules via fluidic recirculation.
Multi-organ model screening of the anti-cancer drug Linsitinib recapitulated clinical findings: limited efficacy of the drug in treating bone tumors and limited cardiac toxicity in the human setting (Chramiec et al., 2020). These results are in direct contrast to animal models which showed both high efficacy and high potential for cardiac toxicity. Notably, clinical trials more closely matched with the OOC, supporting the utility of this model for predictive insights into the potential of drugs before clinical trials. Insights into which patients are more likely to respond to a drug, could help refine the scope of clinical trials.
Personalized medicine
The top ten clinical drugs in the United States only work in 4–25% of patients (Schork, 2015). For systemic diseases (i.e. cancer, autoimmune diseases, fibrosis, infection, inflammation), patient variability in disease presentation and therapeutic outcomes complicates the development of successful interventions. The use of patient derived cells in multi-OOCs will equip biologists with tools to mechanistically interrogate and understand disease onset, progression, and treatment in a personalized manner. The utility of multi-organ models for such work has been demonstrated already, including the role of gut health in immune regulation of patients with ulcerative colitis (Trapecar et al., 2020) and responses to smoking in patients with COPD (Benam et al., 2016).
Perspective and challenges
Looking forward, the key question is what is necessary for OOCs to become broadly used. We summarize here the challenges and prospects for realizing the full potential of OOC in biological research.
1. OOC availability -
Current OOCs are developed in academic labs as artisanal devices, hand-made at a production throughput of just a set of devices per day. Standardization and quality control that are essential for reproducible functionality of these devices vary from one design to another, and are limited. To support the broad use in biological research, standardized OOCs need to become as available as are cell culture plates, and reliably manufactured on industrial scale. The first steps in this direction have been made, as several companies (Emulate, inSphero, Mimetas, Tissuse, Nortis, CN Bio) now offer OOCs for culturing one or more tissue types, enabling entry into the field for new groups. Biological experimentation is expected to continue driving the development of OOCs and advancing their performance, that require mass-production of standardized, inexpensive and configurable devices.
2. Inert OOC materials -
PDMS is currently commonly used for making the entire OOCs or their components (such as elastic anchors), including the commercial OOCs listed above, because of its ease of processing. As PDMS absorbs hydrophobic compounds, and most critically oxygen and many drugs, it hinders control of their concentrations in the cellular environment (Toepke and Beebe, 2006). While the need to replace PDMS with inert plastics has been long recognized, some progress has been made only recently (Zhao et al., 2019), by accounting for drug absorption (Herland et al., 2020) or applying inert coatings (Herland et al., 2020).
3. On-line functional readouts
Noninvasive data acquisition in longitudinal studies where the same biological sample is repeatedly evaluated over time, are invaluable for increasing consistency of experimentation and capturing the biological dynamics. This way, each sample becomes an experiment by itself ( “n=1 study”, Lillie et al., 2011), allowing monitoring of the evolution of biological events, or responses to perturbation. Because these studies generate large volumes of data, OOCs are now starting to attract the artificial intelligence and machine learning approaches to data interpretation and experimental design. Also, the concept of a “digital twin” (a physical or computational replica of the actual system) is being extended to OOCs to advance studies of emergent cellular behaviors. Cells themselves can be tracked in space and time using inducible reporters of cell state or function (Mathur et al., 2015)) or on-demand activation (e.g., using optogenetic methods, Vila et al., 2019). Many studies have taken advantage of measuring in real time the contraction frequency and force generation of heart and skeletal muscle, from deflection of the anchors at the two ends of the tissue (Mannhardt et al., 2016). Likewise, calcium flux measurements are already well established for a number of tissue systems (Goldfracht et al., 2019).
4. Establish and maintain mature tissue phenotypes
An essential requirement for utility of OOCs is the authenticity of molecular, structural and functional tissue phenotypes, which in turn involves cell types, maturity, and long-term culture with tissue-tissue interactions.
Primary cells have a well-established identity and heterogeneity, but the access to these cells can be limited (e.g., neural and heart cells) thus not allowing patient-specific studies. In contrast, iPS cells are routinely derived from small samples of blood and differentiated into a number of lineages that can be used to derive tissues of interest. iPS cells provide consistency, comparisons across the labs, and patient-specific OOCs that allow parsing out genetic and environmental factors, studies of genetic diseases, and biologic diversity. A long-standing limitation of iPSCs - the immaturity of the resulting tissues, is being addressed by inclusion of supporting cells, metabolic, and physical regulatory factors (Abaci et al., 2018; Aleman et al., 2019; Alimperti et al., 2017; Bauer et al., 2017; Benam et al., 2016; Blundell et al., 2016; Keung et al., 2014; Kostrzewski et al., 2020; Lin et al., 2019; Musah et al., 2017; Ronaldson-Bouchard et al., 2018; Trapecar et al., 2020; Zhao et al., 2019).
The required level of maturity and the proper benchmarks are not clearly defined and depend on the biological question. The molecular, structural and functional features can develop at different rates and to a different extent. An example is the engineered heart muscle matured by electromechanical conditioning with the force generation and conduction developing more slowly and to a lesser extent than structural features (Ronaldson-Bouchard et al., 2018; Ronaldson-Bouchard et al., 2019).
Maintaining the individual tissue phenotypes for weeks to months while allowing their communication remains a challenge. The new OOC designs that utilize physiological principles of tissue and organ communication are needed to overcome the limitations of sharing medium by all tissue types.
5. Biological complexity
A great value would come from establishing vascular connections, innervation, immune system and tissue interfaces in OOCs, and the ability to build complexity on-demand. studies will also depend on adding innervation, immune system and microbiome. The immune and endocrine systems are particularly important, as they interact with all organ systems in the body and are poorly modeled in animals (Habert et al., 2014). A major challenge is the derivation of innate, adaptive and tissue resident immune cells from iPS cells. Bone marrow OOC with functional multipotent hemopoietic pool of stem cells (HSCs) (Chou et al., 2020b) could provide a renewable source of immune cells in OOCs. Current OOCs rely on adding immune cells into the tissues or perfusate. For myeloid cells, one does not need to match the tissues with the donor immune cells, but with lymphoid cells matching may be needed. Another approach is to use a common HLA-null iPS cell line to generate “agnostic” tissues that can be combined with the patient’s immune cells. Recent studies also started establishing patient-specific microbiota in OOCs (Cho and Blaser, 2012).
5. Address patient diversity
Humans are living longer than ever before, and healthcare is entering the era of healthy aging and precision medicine. The forward-thinking healthcare would greatly benefit from OOC, which can provide patient-specific models of human pathophysiology. Notably, OOC enable systematic studies of the diversity of population with respect to racial/ethnic background, sex and age, to help address the current health disparity. By looking for commonalities between the clinical and in vitro data, we could identify shared mechanisms related to disease risk, discover early-stage biomarkers, monitor disease progression, and determine optimal therapeutic treatment regimens in a personalized manner.
Summary
Our goal was to explore the use of OOC in biological research, and explain why they have so rapidly evolved in recent years. We distinguish OOC from organoids and describe the design and applications of OOC representing a single tissue unit (e.g., bone marrow, neuromuscular junction, lung alveolum) and multiple units linked to recapitulate more complex physiological functions (e.g., cancer metastasis, infection). Importantly, OOC do not recapitulate the entire organ, but instead approximate just one or few organ-level functions: barrier function of the lung, contractile function of the heart or filtration in kidney.
We propose that OOC are en-route to become broadly accepted in biological research, as they can offer biologic fidelity along with experimental control not provided otherwise, while still being sufficiently easy to use. We believe that OOCs will realize this promise through the development of: (i) Standardized user-friendly designs that are readily available at low cost, (ii) Real-time measurements, (iii) Maintenance of tissue phenotypes over weeks to months, while allowing their communication by vascular perfusion, (iv) Incorporation of innervation, immune system, metabolism and microbiome, if needed. Biological experimentation requires that these features are complemented by versatility of design configurations and operating conditions allowing the basic designs to be customized for addressing a specific question.
Organ-on-a-chip technologies promise to bridge a longstanding gap between animal models and in vivo human studies, but several challenges remain for making this technology broadly utilizable.
Acknowledgements
This work was funded by the National Institutes of Health (UH3 EB025765, P41 EB027062, 3R01 HL076485, CA249799), National Science Foundation (NSF16478), Canadian Institutes of Health Research (CIHR) (FDN-167274), Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN 326982-10), NSERC-CIHR (CHRP 493737-16), and NASA (NNX16AO69A). MR was supported by Killam Fellowship and Canada Research Chair.
Footnotes
Competing interest declaration
G.V.N, K.R-B. and M. R. are co-founders and equity holders of TARA Biosystems that uses Biowire II platform for commercial drug testing. M.R. and G.V.N. receive consulting fees and royalty from TARA Biosystems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abaci HE, Coffman A, Doucet Y, Chen J, Jackow J, Wang E, Guo Z, Shin JU, Jahoda CA, and Christiano AM (2018). Tissue engineering of human hair follicles using a biomimetic developmental approach. Nature communications 9, 5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, Nikolova M, Cora V, Antkowiak L, Haq W, et al. (2019). Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar Bakooshli M, Lippmann ES, Mulcahy B, Iyer N, Nguyen CT, Tung K, Stewart BA, van den Dorpel H, Fuehrmann T, Shoichet M, et al. (2019). A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar ME, Abraha HY, Bakooshli MA, Davoudi S, Thavandiran N, Tung K, Ahn H, Ginsberg HJ, Zandstra PW, and Gilbert PM (2020). A 96-well culture platform enables longitudinal analyses of engineered human skeletal muscle microtissue strength. Scientific reports 10, 6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleman J, George SK, Herberg S, Devarasetty M, Porada CD, Skardal A, and Almeida-Porada G (2019). Deconstructed Microfluidic Bone Marrow On-A-Chip to Study Normal and Malignant Hemopoietic Cell–Niche Interactions. 15, 1902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alimperti S, Mirabella T, Bajaj V, Polacheck W, Pirone DM, Duffield J, Eyckmans J, Assoian RK, and Chen CS (2017). Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell-endothelial cell-regulated barrier function. Proceedings of the National Academy of Sciences of the United States of America 114, 8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alkasir R, Li J, Li X, Jin M, and Zhu B (2017). Human gut microbiota: the links with dementia development. Protein & cell 8, 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Heart Association, C., NIH (2015). American Heart Association – 2015 Heart Disease and Stroke Update
- 9.Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, Andersson S, Ewart L, Haynes WG, Maschmeyer I, Winter A, et al. (2017). Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Scientific reports 7, 14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benam KH, Novak R, Nawroth J, Hirano-Kobayashi M, Ferrante TC, Choe Y, Prantil-Baun R, Weaver JC, Bahinski A, Parker KK, et al. (2016). Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell systems 3, 456–466.e454. [DOI] [PubMed] [Google Scholar]
- 11.Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, and Huh D (2016). A microphysiological model of the human placental barrier. Lab on a chip 16, 3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho MR, Barata D, Teixeira LM, Giselbrecht S, Reis RL, Oliveira JM, Truckenmuller R, and Habibovic P (2019a). Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Science advances 5, eaaw1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Wu JY, Kennedy KM, Yeager K, Bernhard JC, Ng JJ, Zimmerman BK, Robinson S, Durney KM, Shaeffer C, et al. (2020). Tissue engineered autologous cartilage-bone grafts for temporomandibular joint regeneration. Sci Transl Med 12. [DOI] [PubMed] [Google Scholar]
- 14.Cho I, and Blaser MJ (2012). The human microbiome: at the interface of health and disease. Nature Reviews Genetics 13, 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bolukbasi O, Joyce CE, Moreira Teixeira LS, et al. (2020a). On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nature biomedical engineering 4, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chramiec A, Teles D, Yeager K, Marturano-Kruik A, Pak J, Chen T, Hao L, Wang M, Lock R, Tavakol DN, et al. (2020). Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab on a chip 20, 4357–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clevers H (2016). Modeling Development and Disease with Organoids. Cell 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- 18.de Graaf MNS, Cochrane A, van den Hil FE, Buijsman W, van der Meer AD, van den Berg A, Mummery CL, and Orlova VV (2019). Scalable microphysiological system to model three-dimensional blood vessels. APL bioengineering 3, 026105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esch EW, Bahinski A, and Huh D (2015). Organs-on-chips at the frontiers of drug discovery. Nature reviews Drug discovery 14, 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfracht I, Efraim Y, Shinnawi R, Kovalev E, Huber I, Gepstein A, Arbel G, Shaheen N, Tiburcy M, Zimmermann WH, et al. (2019). Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta biomaterialia 92, 145–159. [DOI] [PubMed] [Google Scholar]
- 21.Grassart A, Malarde V, Gobaa S, Sartori-Rupp A, Kerns J, Karalis K, Marteyn B, Sansonetti P, and Sauvonnet N (2019). Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host & Microbe 26, 435–444.e434. [DOI] [PubMed] [Google Scholar]
- 22.Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science (New York, NY) 364, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, and Chen CS (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell stem cell 5, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habert R, Muczynski V, Grisin T, Moison D, Messiaen S, Frydman R, Benachi A, Delbes G, Lambrot R, Lehraiki A, et al. (2014). Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction (Cambridge, England) 147, R119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harberts J, Fendler C, Teuber J, Siegmund M, Silva A, Rieck N, Wolpert M, Zierold R, and Blick RH (2020). Toward Brain-on-a-Chip: Human Induced Pluripotent Stem Cell-Derived Guided Neuronal Networks in Tailor-Made 3D Nanoprinted Microscaffolds. ACS nano 14, 13091–13102. [DOI] [PubMed] [Google Scholar]
- 26.Hayward KL, Kouthouridis S, and Zhang B (2020). Organ-on-a-Chip Systems for Modeling Pathological Tissue Morphogenesis Associated with Fibrosis and Cancer. ACS Biomaterials Science & Engineering. [DOI] [PubMed] [Google Scholar]
- 27.Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, et al. (2020). Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nature biomedical engineering 4, 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, et al. (2015). HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science (New York, NY) 349, 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, and Lewis JA (2016). Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Scientific reports 6, 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood L, Balling R, and Auffray C (2012). Revolutionizing medicine in the 21st century through systems approaches. 7, 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, and Ingber DE (2010). Reconstituting organ-level lung functions on a chip. Science (New York, NY) 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui EE, and Bhatia SN (2007). Micromechanical control of cell-cell interactions. Proceedings of the National Academy of Sciences of the United States of America 104, 5722–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang KJ, Otieno MA, Ronxhi J, Lim HK, Ewart L, Kodella KR, Petropolis DB, Kulkarni G, Rubins JE, Conegliano D, et al. (2019). Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med 11. [DOI] [PubMed] [Google Scholar]
- 34.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, and Kamm RD (2015). Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. 112, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jusoh N, Oh S, Kim S, Kim J, and Jeon NL (2015). Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab on a chip 15, 3984–3988. [DOI] [PubMed] [Google Scholar]
- 36.Keung W, Boheler KR, and Li RA (2014). Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem cell research & therapy 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khodabukus A, Madden L, Prabhu NK, Koves TR, Jackman CP, Muoio DM, and Bursac N (2019). Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle. Biomaterials 198, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Li H, Collins JJ, and Ingber DE (2016). Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proceedings of the National Academy of Sciences of the United States of America 113, E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SLM, Niklason LE, and Prichard HL (2019). Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med 11, eaau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostrzewski T, Maraver P, Ouro-Gnao L, Levi A, Snow S, Miedzik A, Rombouts K, and Hughes D (2020). A Microphysiological System for Studying Nonalcoholic Steatohepatitis. Hepatology communications 4, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai BFL, Huyer LD, Lu RXZ, Drecun S, Radisic M, and Zhang B (2017). InVADE: Integrated Vasculature for Assessing Dynamic Events. 27, 1703524. [Google Scholar]
- 42.Lai BFL, Lu RXZ, Hu Y, Davenport Huyer L, Dou W, Wang EY, Radulovich N, Tsao MS, Sun Y, and Radisic M (2020b). Recapitulating Pancreatic Tumor Microenvironment through Synergistic Use of Patient Organoids and Organ-on-a-Chip Vasculature. Advanced Functional Materials 30, 2000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langer R, and Vacanti JP (1993). Tissue engineering. Science (New York, NY) 260, 920–926. [DOI] [PubMed] [Google Scholar]
- 44.Li X, George SM, Vernetti L, Gough AH, and Taylor DL (2018). A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab on a chip 18, 2614–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, and Schork NJ (2011). The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personalized medicine 8, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Z, Li Z, Li EN, Li X, Del Duke CJ, Shen H, Hao T, O’Donnell B, Bunnell BA, Goodman SB, et al. (2019). Osteochondral Tissue Chip Derived From iPSCs: Modeling OA Pathologies and Testing Drugs. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low LA, Sutherland M, Lumelsky N, Selimovic S, Lundberg MS, and Tagle DA (2020). Organs-on-a-Chip. Advances in experimental medicine and biology 1230, 27–42. [DOI] [PubMed] [Google Scholar]
- 48.Mandrycky C, Hadland B, and Zheng Y (2020). 3D curvature-instructed endothelial flow response and tissue vascularization. Science advances 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mannhardt I, Breckwoldt K, Letuffe-Breniere D, Schaaf S, Schulz H, Neuber C, Benzin A, Werner T, Eder A, Schulze T, et al. (2016). Human Engineered Heart Tissue: Analysis of Contractile Force. Stem cell reports 7, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maoz BM, Herland A, FitzGerald EA, Grevesse T, Vidoudez C, Pacheco AR, Sheehy SP, Park TE, Dauth S, Mannix R, et al. (2018). A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nature biotechnology 36, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchiano S, Hsiang T-Y, Higashi T, Khanna A, Reinecke H, Yang X, Pabon L, Sniadecki NJ, Bertero A, Gale M, et al. (2020). SARS-CoV-2 infects human pluripotent stem cell-derived cardiomyocytes, impairing electrical and mechanical function. bioRxiv, 2020.2008.2030.274464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marx U, Andersson TB, Bahinski A, Beilmann M, Beken S, Cassee FR, Cirit M, Daneshian M, Fitzpatrick S, Frey O, et al. (2016). Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. Altex 33, 272–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, et al. (2015). Human iPSC-based cardiac microphysiological system for drug screening applications. Scientific reports 5, 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, Rumsey JW, Martin C, Schnepper M, Wang Y, Schuler F, et al. (2019). Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. 11, eaav1386. [DOI] [PubMed] [Google Scholar]
- 55.McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai Y-H, Mayhew CN, Spence JR, Zavros Y, et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mejias JC, Nelson MR, Liseth O, and Roy K (2020). A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab on a chip 20, 3601–3611. [DOI] [PubMed] [Google Scholar]
- 57.Moura Rosa P, Gopalakrishnan N, Ibrahim H, Haug M, and Halaas O (2016). The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab on a chip 16, 3728–3740. [DOI] [PubMed] [Google Scholar]
- 58.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, et al. (2017). Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature biomedical engineering 1, 0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawroth JC, Petropolis DB, Manatakis DV, Maulana TI, Burchett G, Schlünder K, Witt A, Shukla A, Hamilton G, Seki E, et al. (2020). Modeling alcoholic liver disease in a human Liver-Chip. bioRxiv, 2020.2007.2014.203166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norona LM, Nguyen DG, Gerber DA, Presnell SC, Mosedale M, and Watkins PB (2019). Bioprinted liver provides early insight into the role of Kupffer cells in TGF-beta1 and methotrexate-induced fibrogenesis. PloS one 14, e0208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, et al. (2013). Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature methods 10, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oyirifi A, Joyce KM, Helferich WG, and Imoukhuede PIJTFJ (2019). 2D vs 3D–Triple negative breast cancer spheroid formation induces quantitative heterogeneity of VEGF and PDGF receptor profiles and modulates cytosolic phosphorylation. 33, 647.630–647.630. [Google Scholar]
- 63.Piccini JP, Whellan DJ, Berridge BR, Finkle JK, Pettit SD, Stockbridge N, Valentin JP, Vargas HM, and Krucoff MW (2009). Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the Critical Path Initiative. Am Heart J 158, 317–326. [DOI] [PubMed] [Google Scholar]
- 64.Pires de Mello CP, Carmona-Moran C, McAleer CW, Perez J, Coln EA, Long CJ, Oleaga C, Riu A, Note R, Teissier S, et al. (2020). Microphysiological heart-liver body-on-a-chip system with a skin mimic for evaluating topical drug delivery. Lab on a chip 20, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajasekar S, Lin DSY, Abdul L, Liu A, Sotra A, Zhang F, and Zhang B (2020). IFlowPlate-A Customized 384-Well Plate for the Culture of Perfusable Vascularized Colon Organoids. Advanced materials 32, e2002974. [DOI] [PubMed] [Google Scholar]
- 66.Rao L, Qian Y, Khodabukus A, Ribar T, and Bursac N (2018). Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nature communications 9, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, and Vunjak-Novakovic G (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ronaldson-Bouchard K, Yeager K, Teles D, Chen T, Ma S, Song L, Morikawa K, Wobma HM, Vasciaveo A, Ruiz EC, et al. (2019). Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nature Protocols 14, 2781–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothbauer M, Holl G, Eilenberger C, Kratz SRA, Farooq B, Schuller P, Olmos Calvo I, Byrne RA, Meyer B, Niederreiter B, et al. (2020). Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. Lab on a chip 20, 1461–1471. [DOI] [PubMed] [Google Scholar]
- 70.Sasserath T, Rumsey JW, McAleer CW, Bridges LR, Long CJ, Elbrecht D, Schuler F, Roth A, Bertinetti-LaPatki C, Shuler ML, et al. (2020). Differential Monocyte Actuation in a Three-Organ Functional Innate Immune System-on-a-Chip. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 7, 2000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satoh T, Sugiura S, Shin K, Onuki-Nagasaki R, Ishida S, Kikuchi K, Kakiki M, and Kanamori T (2018). A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab on a chip 18, 115–125. [DOI] [PubMed] [Google Scholar]
- 72.Schepers A, Li C, Chhabra A, Seney BT, and Bhatia S (2016). Engineering a perfusable 3D human liver platform from iPS cells. Lab on a chip 16, 2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schork NJ (2015). Personalized medicine: Time for one-person trials. Nature 520, 609–611. [DOI] [PubMed] [Google Scholar]
- 74.Seo J, Byun WY, Alisafaei F, Georgescu A, Yi YS, Massaro-Giordano M, Shenoy VB, Lee V, Bunya VY, and Huh D (2019). Multiscale reverse engineering of the human ocular surface. Nature medicine 25, 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma AD, McCoy L, Jacobs E, Willey H, Behn JQ, Nguyen H, Bolon B, Curley JL, and Moore MJ (2019a). Engineering a 3D functional human peripheral nerve in vitro using the Nerve-on-a-Chip platform. Scientific Reports 9, 8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Nurani A, Zhu DY, Goyal G, Gilpin SE, et al. (2020). Human organs-on-chips as tools for repurposing approved drugs as potential influenza and COVID19 therapeutics in viral pandemics. bioRxiv, 2020.2004.2013.039917. [Google Scholar]
- 77.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, and Shuler ML (2004). The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnology progress 20, 338–345. [DOI] [PubMed] [Google Scholar]
- 78.Sung JH, Kam C, and Shuler ML (2010). A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab on a chip 10, 446–455. [DOI] [PubMed] [Google Scholar]
- 79.Sung JH, and Shuler ML (2009). A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab on a chip 9, 1385–1394. [DOI] [PubMed] [Google Scholar]
- 80.Takebe T, Zhang B, and Radisic M (2017). Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell stem cell 21, 297–300. [DOI] [PubMed] [Google Scholar]
- 81.Tang H, Abouleila Y, Si L, Ortega-Prieto AM, Mummery CL, Ingber DE, and Mashaghi A (2020). Human Organs-on-Chips for Virology. Trends in microbiology 28, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toepfer CN, Sharma A, Cicconet M, Garfinkel AC, Mücke M, Neyazi M, Willcox JAL, Agarwal R, Schmid M, Rao J, et al. (2019). SarcTrack. 124, 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toepke MW, and Beebe DJ (2006). PDMS absorption of small molecules and consequences in microfluidic applications. Lab on a chip 6, 1484–1486. [DOI] [PubMed] [Google Scholar]
- 84.Topol EJ (2020). COVID-19 can affect the heart. Science (New York, NY) 370, 408–409. [DOI] [PubMed] [Google Scholar]
- 85.Trapecar M, Communal C, Velazquez J, Maass CA, Huang YJ, Schneider K, Wright CW, Butty V, Eng G, Yilmaz O, et al. (2020). Gut-Liver Physiomimetics Reveal Paradoxical Modulation of IBD-Related Inflammation by Short-Chain Fatty Acids. Cell systems 10, 223–239.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, et al. (2019). Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell stem cell 24, 995–1005.e1006. [DOI] [PubMed] [Google Scholar]
- 87.Vernetti L, Gough A, Baetz N, Blutt S, Broughman JR, Brown JA, Foulke-Abel J, Hasan N, In J, Kelly E, et al. (2017). Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Scientific reports 7, 42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vila OF, Uzel SGM, Ma SP, Williams D, Pak J, Kamm RD, and Vunjak-Novakovic G (2019). Quantification of human neuromuscular function through optogenetics. Theranostics 9, 1232–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viravaidya K, Sin A, and Shuler ML (2004). Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnology progress 20, 316–323. [DOI] [PubMed] [Google Scholar]
- 90.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, et al. (2014). Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature medicine 20, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, and Matloff WJ (2013). Scaling and systems biology for integrating multiple organs-on-a-chip. Lab on a chip 13, 3496–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, et al. (2017). A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nature communications 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie R, Korolj A, Liu C, Song X, Lu RXZ, Zhang B, Ramachandran A, Liang Q, and Radisic M (2020). h-FIBER: Microfluidic Topographical Hollow Fiber for Studies of Glomerular Filtration Barrier. ACS central science 6, 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Z, Li E, Guo Z, Yu R, Hao H, Xu Y, Sun Z, Li X, Lyu J, and Wang Q (2016). Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Applied Materials & Interfaces 8, 25840–25847. [DOI] [PubMed] [Google Scholar]
- 95.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, and Kamm RD (2012). Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proceedings of the National Academy of Sciences of the United States of America 109, 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang B, Korolj A, Lai BFL, and Radisic M (2018). Advances in organ-on-a-chip engineering. Nature Reviews Materials 3, 257–278. [Google Scholar]
- 97.Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Masse S, Kim J, Reis L, et al. (2016). Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nature materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang B, and Radisic M (2017). Organ-on-a-chip devices advance to market. Lab on a chip 17, 2395–2420. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, et al. (2019). A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927.e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou M, Zhang X, Wen X, Wu T, Wang W, Yang M, Wang J, Fang M, Lin B, and Lin H (2016). Development of a Functional Glomerulus at the Organ Level on a Chip to Mimic Hypertensive Nephropathy. Scientific reports 6, 31771. [DOI] [PMC free article] [PubMed] [Google Scholar]


