Abstract
Objectives
To evaluate the antimicrobial susceptibility patterns of Pseudomonas aeruginosa isolates collected from the lower respiratory tract of cystic fibrosis (CF) patients.
Methods
We susceptibility tested 273 contemporary P. aeruginosa isolates from 39 hospitals worldwide (17 countries) by the reference broth microdilution method.
Results
Ceftazidime/avibactam [MIC50/90, 2/8 mg/L; 96.0% susceptible (S)] was the most active agent, followed by ceftolozane/tazobactam (MIC50/90, 1/4 mg/L; 90.5% S), ceftazidime (MIC50/90, 2/>32 mg/L; 80.6% S), piperacillin/tazobactam (MIC50/90, 4/128 mg/L; 80.2% S) and tobramycin (MIC50/90, 2/>16 mg/L; 76.6% S). Ceftazidime/avibactam retained activity against P. aeruginosa isolates non-susceptible to meropenem (86.5% S to ceftazidime/avibactam), piperacillin/tazobactam (85.2% S to ceftazidime/avibactam) or ceftazidime (79.2% S to ceftazidime/avibactam). MDR phenotype was observed among 36.3% of isolates, and 88.9% and 73.7% of MDR isolates were susceptible to ceftazidime/avibactam and ceftolozane/tazobactam, respectively. Against isolates non-susceptible to meropenem, piperacillin/tazobactam and ceftazidime, susceptibility rates were 78.9% for ceftazidime/avibactam and 47.4% for ceftolozane/tazobactam. Ceftazidime/avibactam was active against 65.4% of ceftolozane/tazobactam-non-susceptible isolates and ceftolozane/tazobactam was active against 18.2% of ceftazidime/avibactam-non-susceptible isolates.
Conclusions
Ceftazidime/avibactam and ceftolozane/tazobactam exhibited potent and broad-spectrum activity against P. aeruginosa isolated from CF patients worldwide, but higher susceptibility rates for ceftazidime/avibactam compared with ceftolozane/tazobactam were observed among the resistant subsets. Ceftazidime/avibactam and ceftolozane/tazobactam represent valuable options to treat CF pulmonary exacerbations caused by P. aeruginosa.
Introduction
Pseudomonas aeruginosa is the most common cultured respiratory pathogen in individuals with cystic fibrosis (CF) and is associated with a more rapid decline in lung function. More than 50% of CF individuals aged 18 years and older in the USA are infected with P. aeruginosa, of whom approximately one-third are infected with an MDR strain. Over time, most individuals with CF become chronically infected with P. aeruginosa, and a mucoid phenotype of P. aeruginosa frequently becomes the predominant form found in culture. Moreover, chronic P. aeruginosa infection is associated with greater rates of morbidity and mortality irrespective of pulmonary function.1–3
Ceftazidime/avibactam and ceftolozane/tazobactam are β-lactamase inhibitor combinations approved by the US FDA and by the EMA to treat hospital-acquired bacterial pneumonia, including ventilator-associated bacterial pneumonia (HABP/VABP), complicated intra-abdominal infections (cIAIs) in combination with metronidazole, and complicated urinary tract infections (cUTIs), including pyelonephritis.4–6 Both compounds have demonstrated potent activity against clinical P. aeruginosa isolates, including MDR isolates, but studies evaluating these two compounds against P. aeruginosa from CF patients are very limited.7 The objective of this study was to evaluate the in vitro activity and spectrum of these two combinations and comparator agents currently used to treat Gram-negative infections against contemporary P. aeruginosa isolated from CF patients.
Materials and methods
Isolates were collected as part of SENTRY Antimicrobial Surveillance Program.8 Medical centres were asked to collect consecutive bacterial isolates from lower respiratory tract sites of CF patients from January 2018 to December 2019. Each participant centre could contribute up to 40 P. aeruginosa isolates. Only isolates from invasive sampling, including transtracheal aspiration, bronchoalveolar lavage, protected brush samples or qualified sputum samples were accepted.
The isolate collection included 273 P. aeruginosa isolates from 39 medical centres in 17 countries. The majority of isolates were from the USA (n = 130; 47.6%) and 11 European countries (n = 117; 42.9%). The European country that contributed the largest number of isolates was Spain (n = 43; two medical centres), followed by Poland (n = 20; one medical centre), Sweden (n = 16; one medical centre), and France (n = 15; two medical centres; Table S1, available as Supplementary data at JAC-AMR Online). Species identification was performed at the participating medical centres and confirmed at JMI Laboratories by standard biochemical tests and using the MALDI Biotyper (Bruker Daltonics, Billerica, MA, USA) according to the manufacturer instructions, where necessary. Isolates were categorized as MDR or XDR according to criteria defined in 2012 by the joint European and US Centres for Disease Control. These criteria define MDR as non-susceptible to ≥1 agent in ≥3 antimicrobial classes and XDR as susceptible to ≤2 classes.9 The antimicrobial classes and drug representatives in this analysis included cephalosporins (ceftazidime and cefepime), carbapenems (imipenem and meropenem), broad-spectrum penicillins combined with a β-lactamase-inhibitor (piperacillin/tazobactam), fluoroquinolones (ciprofloxacin and levofloxacin), aminoglycosides (gentamicin, tobramycin and amikacin) and the polymyxins (colistin).
Broth microdilution test methods were conducted according to CLSI criteria to determine the antimicrobial susceptibility of ceftazidime/avibactam, ceftolozane/tazobactam (inhibitor at fixed concentration of 4 mg/L for both combinations) and comparator agents.10 Concurrent quality control (QC) testing was performed to ensure proper test conditions and procedures. All QC results were within published ranges. CLSI and EUCAST susceptibility interpretive criteria were used to determine susceptibility/resistance rates for ceftazidime/avibactam, ceftolozane/tazobactam and comparator agents.11,12
Isolates resistant to either ceftazidime/avibactam or ceftolozane/tazobactam were submitted to WGS. Total genomic DNA was extracted and used as input material for library construction. DNA libraries were prepared using the Nextera XTTM library construction protocol and index kit (Illumina, San Diego, CA, USA) and sequenced on a MiSeq sequencer (Illumina) using a minimum of 20% coverage, as previously described.13
Results
Ceftazidime/avibactam (MIC50/90, 2/8 mg/L; 96.0% susceptible per CLSI and EUCAST) was the most active agent, followed by ceftolozane/tazobactam (MIC50/90, 1/4 mg/L; 90.5% susceptible per CLSI and EUCAST), ceftazidime (MIC50/90, 2/>32 mg/L; 80.6% susceptible per CLSI), piperacillin/tazobactam (MIC50/90, 4/128 mg/L; 80.2% susceptible per CLSI) and tobramycin (MIC50/90, 2/>16 mg/L; 76.6%/63.7% susceptible per CLSI/EUCAST). Colistin (MIC50/90, 0.5/1 mg/L) was active against 98.2% of isolates when EUCAST breakpoint criteria were applied (Table 1 and Figure 1).
Table 1.
Activity of ceftazidime/avibactam, ceftolozane/tazobactam and comparator antimicrobial agents against 273 P. aeruginosa isolates collected from CF patients (2018–19)
| Antimicrobial agent | mg/L |
CLSIa |
EUCASTa |
|||||
|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | |
| Ceftazidime/avibactam | 2 | 8 | 96.0 | 4.0 | 96.0 | 4.0 | ||
| Ceftolozane/tazobactam | 1 | 4 | 90.5 | 2.9 | 6.6 | 90.5 | 9.5 | |
| Ceftazidime | 2 | >32 | 80.6 | 3.7 | 15.8 | c | 80.6c | 19.4 |
| Cefepime | 8 | 32 | 71.4 | 13.9 | 14.7 | c | 71.4c | 28.6 |
| Piperacillin/tazobactam | 4 | 128 | 80.2 | 8.4 | 11.4 | c | 80.2c | 19.8 |
| Meropenem | 0.5 | 16 | 72.9 | 5.1 | 22.0 | 72.9 | 13.6 | |
| Imipenem | 1 | >8 | 66.3 | 6.2 | 27.5 | c | 72.5c | 27.5 |
| Levofloxacin | 2 | 16 | 40.3 | 18.7 | 41.0 | c | 40.3c | 59.7 |
| Tobramycin | 2 | >16 | 76.6 | 8.1 | 15.4 | 63.7b | 36.3 | |
| Amikacin | 16 | >32 | 65.9 | 14.7 | 19.4 | 65.9b | 34.1 | |
| Gentamicin | 8 | >16 | 46.9 | 18.7 | 34.4 | |||
| Colistin | 0.5 | 1 | 98.2 | 1.8 | 98.2 | 1.8 | ||
Criteria as published by CLSI11 and EUCAST.12
Infections originating from the urinary tract. For systemic infections, aminoglycosides must be used in combination with another active therapy.
An arbitrary susceptible breakpoint of ≤0.001 mg/L and/or >50 mm has been published by EUCAST indicating that susceptible should not be reported for this organism–agent combination and intermediate should be interpreted as susceptible increased exposure.12
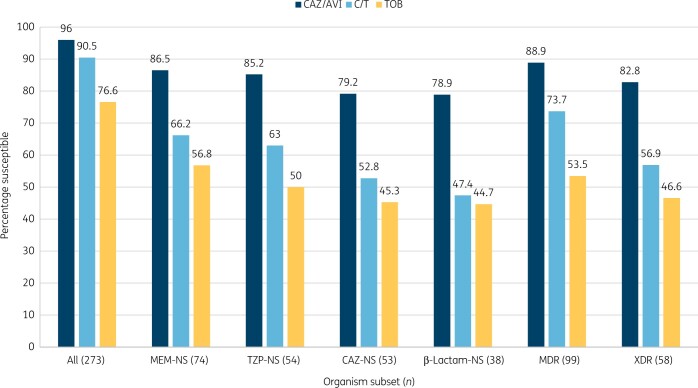
Figure 1.
Antimicrobial activity of ceftazidime/avibactam (CAZ/AVI), ceftolozane/tazobactam (C/T) and tobramycin (TOB) tested against P. aeruginosa resistant subsets collected from CF patients (2018–19). MEM, meropenem; NS, non-susceptible; TZP, piperacillin/tazobactam; CAZ, ceftazidime; β-lactam-NS, isolates not susceptible to meropenem, piperacillin/tazobactam and ceftazidime.
The ceftazidime/avibactam susceptibility rate was slightly higher in the USA compared with Europe (98.5% versus 94.0%); whereas susceptibility rates for cefepime (66.9% in the USA and 75.2% in Europe), meropenem (70.0% versus 77.8%), imipenem (61.5% versus 72.6%), levofloxacin (36.2% versus 45.3%), tobramycin (71.5% versus 86.3%), amikacin (59.2% versus 71.8%) and gentamicin (40.0% versus 55.6%) were lower in the USA compared with Europe. Moreover, susceptibility rates for ceftolozane/tazobactam, ceftazidime, piperacillin/tazobactam and colistin were similar in both geographic regions (0.4% to 2.1% difference; Table S2).
Interestingly, higher susceptibility rates for ceftazidime/avibactam compared with ceftolozane/tazobactam were more noticeable within the resistant subsets. When tested against meropenem-non-susceptible isolates (n = 74), susceptibility rates were 86.5% for ceftazidime/avibactam (MIC50/90, 4/16 mg/L) and 66.2% for ceftolozane/tazobactam (MIC50/90, 2/>16 mg/L; Table 2 and Figure 1). Similarly, against piperacillin/tazobactam-non-susceptible isolates (n = 54), susceptibility rates were 85.2% for ceftazidime/avibactam (MIC50/90, 4/32 mg/L) and 63.0% for ceftolozane/tazobactam (MIC50/90, 4/>16 mg/L). Against ceftazidime-non-susceptible isolates (n = 53), susceptibility rates were 79.2% for ceftazidime/avibactam (MIC50/90, 4/32 mg/L) and 52.8% for ceftolozane/tazobactam (MIC50/90, 4/>16 mg/L; Table 2 and Figure 1).
Table 2.
Cross-resistance among β-lactams and β-lactamase inhibitor combinations when tested against P. aeruginosa isolates collected from CF patients (2018–19)
| Antimicrobial | Percentage susceptible by resistant subset (no. of isolates)a |
||||
|---|---|---|---|---|---|
| MEM-NS (74) | TZP-NS (54) | CAZ-NS (53) | C/T-NS (26) | CAZ/AVI-NS (11) | |
| MEM | 0.0 | 20.4 | 17.0 | 3.8 | 9.1 |
| TZP | 41.9 | 0.0 | 15.1 | 3.8 | 27.2 |
| CAZ | 40.5 | 16.7 | 0.0 | 3.8 | 0.0 |
| C/T | 66.2 | 63.0 | 52.8 | 0.0 | 18.2 |
| CAZ/AVI | 86.5 | 85.2 | 79.2 | 65.4 | 0.0 |
CAZ, ceftazidime; MEM, meropenem; TZP, piperacillin/tazobactam; C/T, ceftolozane/tazobactam; AVI, avibactam; NS, non-susceptible.
Isolates were categorized as non-susceptible based on CLSI criteria.
When tested against isolates non-susceptible to meropenem, piperacillin/tazobactam, and ceftazidime (n = 38), susceptibility rates were 78.9% for ceftazidime/avibactam (MIC50/90, 8/32 mg/L) and only 47.4% for ceftolozane/tazobactam (MIC50/90, 8/>16 mg/L; Figure 1). Moreover, 65.4% of ceftolozane/tazobactam-non-susceptible isolates remained susceptible to ceftazidime/avibactam and only 18.2% of ceftazidime/avibactam-non-susceptible isolates remained susceptible to ceftolozane/tazobactam (Table 2).
MDR and XDR phenotypes were observed in 36.3% (n = 99) and 21.2% (n = 58) of isolates, respectively. Susceptibility rates against the MDR and XDR subsets were 88.9% and 82.8% for ceftazidime/avibactam and 73.7% and 56.9% for ceftolozane/tazobactam, respectively (Figure 1).
Possible mechanisms of resistance to ceftazidime/avibactam or ceftolozane/tazobactam and MLST were evaluated by analysing WGS data and these results are summarized in Table 3. Among the 29 isolates resistant to either one of these agents, 9 isolates were resistant to both agents, 2 isolates were resistant to ceftazidime/avibactam and susceptible to ceftolozane/tazobactam, and 17 isolates were resistant to ceftolozane/tazobactam and susceptible to ceftazidime/avibactam; 28 of these 29 isolates were available to be submitted to WGS. An ESBL or carbapenemase was observed in only 3 isolates: 1 isolate that was resistant to ceftolozane/tazobactam and susceptible to ceftazidime/avibactam had a GES-1 and 2 isolates that were resistant to both compounds had a VIM-type β-lactamase (VIM-2 and VIM-20). Fifteen isolates, including 3 isolates resistant to both compounds and 12 isolates resistant to ceftolozane/tazobactam and susceptible to ceftazidime/avibactam, had early terminations/deletions in the OprD incompatible to a functional protein. Moreover, a great genetic diversity was observed among these isolates, with 26 MLSTs observed among the 28 isolates submitted to WGS.
Table 3.
Summary of WGS results of isolates resistant to either ceftazidime/avibactam or ceftolozane/tazobactam
| Collection number | Susceptibility |
Carbapenemase/ESBL | PDC | Major OprD changes | MLST | |
|---|---|---|---|---|---|---|
| CAZ/AVI | C/T | |||||
| 1077179 | R | R | none | PDC-16 | deletions starting in aa 185 | 298 |
| 1084588 | R | R | none | PDC-35 | early termination (Q166X) | 235 |
| 1111576 | R | R | none | PDC-179 | none | 633-like |
| 1111644 | R | R | none | PDC-97 | frameshift starting in aa 415 | 3694 |
| 1118232 | R | R | VIM-2 | PDC-3 | frameshift starting in aa 29 | 111 |
| 1127896 | R | R | none | PDC-97 | early termination (W138X) | 1497-like |
| 1131386 | R | R | none | PDC-20 | none | 508-like |
| 1131387 | R | R | VIM-20 | PDC-1 | frameshift starting in aa 28 | 175 |
| 1131419 | R | R | NT | NT | NT | NT |
| 1131383 | R | S | none | PDC-1 | none | 500 |
| 1067332 | R | S | none | PDC-3 | none | 903-like |
| 1044749 | S | R | none | PDC-3 | none | 260 |
| 1044750 | S | R | none | PDC-129 | none | 260 |
| 1047619 | S | R | none | PDC-168 | early termination (W277X) | 132 |
| 1055361 | S | R | none | PDC-147 | deletion (M1→K34) | 809 |
| 1067334 | S | R | none | PDC-8 | deletion (S267→X442) | 17 |
| 1067975 | S | R | none | PDC-31 | deletions starting in aa 208 | 1233 |
| 1072979 | S | R | none | PDC-97 | deletions starting in aa 208 | 3473-likea |
| 1084581 | S | R | GES-1 | PDC-35 | early termination (T103X) | 235 |
| 1102272 | S | R | none | PDC-116 | deletion (S267→X442) | 274-like |
| 1118222 | S | R | none | PDC-8 | early termination (W415X) | 258 |
| 1120958 | S | R | none | PDC-1 | frameshift starting at aa 391 | 2587 |
| 1126957 | S | R | none | PDC-5 | deletion (I411→X442) | 262-like |
| 1130447 | S | R | none | PDC-116 | early termination (Q158X) | 274-like |
| 1130449 | S | R | none | PDC-31 | frameshift starting at aa 23 | 2708 |
| 1130451 | S | R | none | PDC-71 | none | 390-like |
| 1131423 | S | R | none | PDC-24 | deletion (M1→A10) | 198 |
| 1132066 | S | R | none | PDC-169 | deletions starting in aa 208 | 282 |
aa, amino acid; CAZ/AVI, ceftazidime/avibactam; C/T, ceftolozane/tazobactam; PDC, Pseudomonas-derived cephalosporinases; R, resistant; S, susceptible; NT, not tested/sequenced.
Double loci variant. All other ‘likes’ were single loci variants.
Discussion
Many studies have shown that ceftazidime/avibactam and ceftolozane/tazobactam are both very active and exhibit similar coverage against P. aeruginosa isolates causing infections in US hospitals.14–17 It has also been shown that these compounds remain active against most isolates resistant to other β-lactams, but susceptibility rates of ceftazidime/avibactam and ceftolozane/tazobactam may vary more widely when testing resistant subsets of P. aeruginosa. Grupper et al.18 evaluated 290 carbapenem-resistant P. aeruginosa isolates from 34 US medical centres against these two compounds and found susceptibility rates of 81% for ceftazidime/avibactam and 91% for ceftolozane/tazobactam. Humphries et al.19 studied 309 β-lactam-resistant P. aeruginosa isolates from three institutions located in the Los Angeles area and found that 61.8% and 72.5% of these isolates were susceptible to ceftazidime/avibactam and ceftolozane/tazobactam, respectively. Moreover, 36.4% of the ceftazidime/avibactam-resistant isolates were susceptible to ceftolozane/tazobactam and only 9.1% of ceftolozane/tazobactam-resistant isolates were susceptible to ceftazidime/avibactam.19 These two studies showed slightly greater susceptibility for ceftolozane/tazobactam compared with ceftazidime/avibactam.18,19 In contrast, this investigation found a greater spectrum of activity for ceftazidime/avibactam compared with ceftolozane/tazobactam.
We evaluated 273 P. aeruginosa isolates from CF patients in 17 countries. Ceftazidime/avibactam (96.0% susceptible) was slightly more active than ceftolozane/tazobactam (90.5% susceptible) against the entire collection of organisms. Interestingly, a greater coverage of ceftazidime/avibactam over ceftolozane/tazobactam was more evident when testing resistant subsets of organisms. For example, among isolates non-susceptible to meropenem, piperacillin/tazobactam and ceftazidime, which were collected from 21 medical centres in 9 countries, susceptibility rates were 78.9% for ceftazidime/avibactam and only 47.4% for ceftolozane/tazobactam. WGS results of isolates resistant to either ceftazidime/avibactam or ceftolozane/tazobactam showed that β-lactamase production was responsible for resistance to these agents in only 10.7% (3/28) of isolates. The most common WGS finding was the presence of OprD alterations, and we could not identify any specific mechanism that could be responsible for resistance to one or both compounds.20
Differences in the activities of these two β-lactamase inhibitor combinations against drug-resistant P. aeruginosa reflect the variety of resistance mechanisms expressed by these organisms and illustrate how these mechanisms may have different impacts on each of these compounds. Mechanisms of resistance to ceftazidime/avibactam and ceftolozane/tazobactam are complex and caused by the presence and interaction of multiple mutation-driven resistance mechanisms.21 Therefore, the activity of these two compounds, and especially the rates of cross-resistance between them, may vary widely depending on selective pressure due to previous antibiotic usage and other factors.
In summary, ceftazidime/avibactam exhibited potent activity and broad spectrum against P. aeruginosa from CF patients and retained activity against isolates resistant to other antipseudomonal β-lactams as well as against MDR and XDR isolates. Our results indicated that ceftazidime/avibactam and ceftolozane/tazobactam represents a valuable option to treat CF patients with respiratory tract infections caused by P. aeruginosa.
Supplementary Material
Acknowledgements
We would like to thank all participants of the SENTRY Antimicrobial Surveillance Program for providing bacterial isolates. We would also like to thank Amy Chen, Judy Oberholser and Joshua Maher for editorial assistance.
Funding
This study at JMI Laboratories was supported by Allergan (prior to its acquisition by AbbVie). Allergan (now AbbVie) was involved in the design and decision to present these results and JMI Laboratories received compensation fees for services in relation to preparing the manuscript. Allergan (now AbbVie) did not have involvement in the collection, analysis and interpretation of data.
Transparency declarations
JMI Laboratories contracted to perform services in 2019–20 for Albany College of Pharmacy and Health Sciences, Allecra Therapeutics, Allergan, AmpliPhi Biosciences Corp., Amicrobe Advanced Biomaterials, Amplyx, Antabio, American Proficiency Institute, Arietis Corp., Arixa Pharmaceuticals Inc., Astellas Pharma Inc., Athelas, Basilea Pharmaceutica Ltd, Bayer AG, Becton, Boston Pharmaceuticals, Bugworks Research Inc., CEM-102 Pharmaceuticals, Cidara Therapeutics Inc., Cipla Pharmaceuticals; CorMedix Inc., DePuy Synthes, Destiny Pharma, Discuva Ltd, Dr. Falk Pharma GmbH, Emery Pharma, Entasis Therapeutics, US Food and Drug Administration, Fox Chase Chemical Diversity Center Inc., Gateway Pharmaceutical LLC, GenePOC Inc., Geom Therapeutics Inc., GlaxoSmithKline plc, Harvard University, Helperby, HiMedia Laboratories, F. Hoffmann-La Roche Ltd, ICON plc, Idorsia Pharmaceuticals Ltd, Iterum Therapeutics plc, Laboratory Specialists Inc., Melinta Therapeutics Inc., Merck & Co. Inc., Microchem Laboratory, Micromyx, MicuRx Pharmaceuticals Inc., Mutabilis Co., Nabriva Therapeutics plc, NAEJA-RGM, Novartis AG, Paratek Pharmaceuticals Inc., Pfizer Inc., Polyphor Ltd, Pharmaceutical Product Development, LLC, Prokaryotics Inc., Qpex Biopharma Inc., Roivant Sciences Ltd, Safeguard Biosystems, Scynexis Inc., SeLux Diagnostics Inc., Shionogi and Co. Ltd, SinSa Labs, Spero Therapeutics, Summit Pharmaceuticals International Corp., Synlogic, T2 Biosystems Inc., Taisho Pharmaceutical Co. Ltd, TenNor Therapeutics Ltd, Tetraphase Pharmaceuticals, Theravance Biopharma, University of Colorado, University of Southern California-San Diego, University of North Texas Health Science Center, VenatoRx Pharmaceuticals Inc., Viosera Therapeutics, Vyome Therapeutics Inc., Wockhardt, Yukon Pharmaceuticals Inc., Zai Lab and Zavante Therapeutics Inc. There are no speakers’ bureaus or stock options to declare.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC-AMR Online.
References
- 1.Crull MR, Somayaji R, Ramos KJ. et al. Changing rates of chronic Pseudomonas aeruginosa infections in cystic fibrosis: a population-based cohort study. Clin Infect Dis 2018; 67: 1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerson J, Rosenfeld M, McNamara S. et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34: 91–100. [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Morgan WJ, Butler SM. et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 2007; 151: 134–9.e1. [DOI] [PubMed] [Google Scholar]
- 4.van Duin D, Bonomo RA.. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63: 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AVYCAZ® (Ceftazidime-Avibactam). https://www.allergan.com/assets/pdf/avycaz_pi.
- 6.Merck & Co. Inc. ZERBAXA®. https://www.merck.com/product/usa/pi_circulars/z/zerbaxa/zerbaxa_pi.pdf.
- 7.Nolan PJ, Jain R, Cohen L. et al. In vitro activity of ceftolozane-tazobactam and ceftazidime-avibactam against Pseudomonas aeruginosa isolated from patients with cystic fibrosis. Diagn Microbiol Infect Dis 2021; 99: 115204. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrmeister AS, Jones RN, Sader HS. et al. Global surveillance of antimicrobial resistance: 20 years of experience with the SENTRY Program. Open Forum Infect Dis 2019; 6: S1–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—Eleventh Edition: M07. 2018.
- 11.CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Informational Supplement: M100. 2020.
- 12.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0, January 2020. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf.
- 13.Castanheira M, Doyle TB, Smith CJ. et al. Combination of MexAB-OprM overexpression and mutations in efflux regulators, PBPs and chaperone proteins is responsible for ceftazidime/avibactam resistance in Pseudomonas aeruginosa clinical isolates from US hospitals. J Antimicrob Chemother 2019; 74: 2588–95. [DOI] [PubMed] [Google Scholar]
- 14.Shirley M.Ceftazidime-avibactam: a review in the treatment of serious Gram-negative bacterial infections. Drugs 2018; 78: 675–92. [DOI] [PubMed] [Google Scholar]
- 15.Zhanel GG, Chung P, Adam H. et al. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014; 74: 31–51. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Flamm RK, Carvalhaes CG. et al. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against Gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017-2018). Diagn Microbiol Infect Dis 2020; 96: 114833. [DOI] [PubMed] [Google Scholar]
- 17.Sader HS, Carvalhaes CG, Streit JM. et al. Antimicrobial activity of ceftazidime-avibactam, ceftolozane-tazobactam and comparators tested against Pseudomonas aeruginosa and Klebsiella pneumoniae isolates from United States medical centers in 2016–2018. Microb Drug Resist 2021; 27: 342–9. [DOI] [PubMed] [Google Scholar]
- 18.Grupper M, Sutherland C, Nicolau DP.. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother 2017; 61: e00875-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphries RM, Hindler JA, Wong-Beringer A. et al. Activity of ceftolozane-tazobactam and ceftazidime-avibactam against β-lactam-resistant Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother 2017; 61: e01858-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correa A, Del Campo R, Perenguez M. et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob Agents Chemother 2015; 59: 2421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraile-Ribot PA, Cabot G, Mulet X. et al. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother 2018; 73: 658–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.