Abstract
Background
Sporulation is a complex cell differentiation programme shared by many members of the Firmicutes, the end result of which is a highly resistant, metabolically inert spore that can survive harsh environmental insults. Clostridioides difficile spores are essential for transmission of disease and are also required for recurrent infection. However, the molecular basis of sporulation is poorly understood, despite parallels with the well-studied Bacillus subtilis system. The spore envelope consists of multiple protective layers, one of which is a specialised layer of peptidoglycan, called the cortex, that is essential for the resistant properties of the spore. We set out to identify the enzymes required for synthesis of cortex peptidoglycan in C. difficile.
Methods
Bioinformatic analysis of the C. difficile genome to identify putative homologues of Bacillus subtilis spoVD was combined with directed mutagenesis and microscopy to identify and characterise cortex-specific PBP activity.
Results
Deletion of CDR20291_2544 (SpoVDCd) abrogated spore formation and this phenotype was completely restored by complementation in cis. Analysis of SpoVDCd revealed a three domain structure, consisting of dimerization, transpeptidase and PASTA domains, very similar to B. subtilis SpoVD. Complementation with SpoVDCd domain mutants demonstrated that the PASTA domain was dispensable for formation of morphologically normal spores. SpoVDCd was also seen to localise to the developing spore by super-resolution confocal microscopy.
Conclusions
We have identified and characterised a cortex specific PBP in C. difficile. This is the first characterisation of a cortex-specific PBP in C. difficile and begins the process of unravelling cortex biogenesis in this important pathogen.
Keywords: Clostridioides difficile, Sporulation, SpoVD, Peptidoglycan, Penicillin-binding protein
Highlights
-
•
CDR20291_2544 encodes a C. difficile homologue of the B subtilis SpoVD.
-
•
Mutation of spoVDCd completely prevents the formation of heat-resistant spores.
-
•
The SpoVDCd PASTA domain was dispensable for its function.
-
•
SpoVDCd localises to the developing spore.
1. Introduction
C. difficile is the most common cause of nosocomial antibiotic-associated diarrhoea, with an estimated 453,000 infections and 29,300 deaths per year in the USA alone [1]. C. difficile infection (CDI) requires prior disruption to the gut microbiota, most commonly due to an administered antibiotic [2]. As current treatments largely rely on antibiotic therapy, with further consequent damage to the microbiota, recurrent disease is common and is associated with worse patient prognosis [3]. In recent years there have been dramatic changes in C. difficile epidemiology, in particular due to the emergence of the epidemic ribotype 027 lineage, a previously rare ribotype that was responsible for a series of large hospital outbreaks in North America in the early years of this century before spreading worldwide [4].
The spore is an absolute requirement for transmission of disease [5], since it allows the organism to transit the lethal aerobic environment while also providing significant resistance to desiccation, heat and common disinfectants [6]. As a result, the spores shed by an infected individual can survive in the environment for an extended period of time. This environmental contamination is a particular problem in hospital environments where large numbers of susceptible individuals are housed in close proximity. The process of sporulation is still relatively poorly understood, despite significant advances in recent years [7]. We have previously used high-density transposon mutagenesis and TraDIS to identify a subset of C. difficile genes required for formation of mature heat-resistance spores [8]. In total, transposon insertions in 798 genes were found to significantly impact sporulation, many with no clear homology to previously characterised proteins. Very few of these 798 genes have been studied in C. difficile but many have homologues in the well-studied Bacillus subtilis sporulation pathway. However, despite the clear parallels between sporulation in B. subtilis and C. difficile, there are enough critical differences to greatly reduce the value of assumptions based on homology [[9], [10], [11]]. The response regulator Spo0A is the master regulator of sporulation, phosphorylation of which sets in motion a complex asymmetric cell differentiation programme involving the sequential activation of a series of dedicated sigma factors that are in turn responsible for the expression of the individual regulons required for correct spore morphogenesis [12]. The result is the complex multi-layered spore structure that lends robustness to environmental insult. The spore consists of a dehydrated core surrounded by a membrane and peptidoglycan cell wall (primordial wall) derived from the mother cell envelope. Around this is a thick peptidoglycan cortex, synthesised during spore maturation, and a second membrane, formed as a result of engulfment of the prespore by the mother cell. The outer surface consists of multiple layers of highly crosslinked proteins. The order and timing of synthesis of each of these layers is critical and disruption to any of the steps typically results in the formation of defective spores that lack full resistance properties [9].
In B. subtilis the peptidoglycan of the primordial wall and cortex differ in structure allowing differentiation by the cortex lytic hydrolases during germination [13]. The primordial cell wall consists of typical alternating β-1→4-linked N-acetyl glucosamine and N-acetyl muramic acid residues, crosslinked by 4–3 linked peptide stems attached to the muramic acid moieties. In cortex peptidoglycan, every second N-acetyl muramic acid is modified to muramic-δ-lactam, resulting in fewer stem peptides, fewer crosslinks and a more flexible overall structure [14]. The class B penicillin-binding protein (PBP) SpoVD is critical for synthesis of B. subtilis cortex [15]. During sporulation SpoVD is expressed in the mother cell where it interacts with the SEDS protein SpoVE to enable localisation to the asymmetric division septum [16]. An N-terminal transmembrane alpha helix anchors the protein in the membrane, placing the majority of the protein in the inter-membrane space where the cortex is ultimately synthesised [17]. SpoVD consists of a PBP dimerization domain, followed by a transpeptidase domain and a penicillin-binding protein and serine/threonine kinase associated (PASTA) domain, the last of which is dispensable for cortex formation [18].
C. difficile vegetative cell peptidoglycan is superficially similar to that of B. subtilis, albeit with a preponderance of 3-3 cross-linking as a result of l,d-transpeptidase activity [19]. The structure of the C. difficile cortex peptidoglycan also differs, with only approximately half the abundance of muramic-δ-lactam modifications and significant GlcNAc N-deacetylation that is not seen at all in B. subtilis [20,21]. Although the enzymes required for cortex synthesis have yet to be characterised in detail, we have previously shown that CDR20291_2544 is required for sporulation [8] and it has been recently been confirmed that this enzyme is required for sporulation and is the target of cephamycin antibiotics that inhibit spore formation [22]. Here we set out to identify and characterise the major C. difficile cortex PBP.
2. Methods
2.1. Bacterial strains and growth conditions
All bacterial strains, plasmids and oligonucleotides used in this study are described in Table 1. E. coli strains were routinely grown in LB broth and on LB agar, while C. difficile strains were grown in TY broth [23] and on brain heart infusion agar. Cultures were supplemented with chloramphenicol (15 μg/ml), thiamphenicol (15 μg/ml) or cycloserine (250 μg/ml) as appropriate.
Table 1.
Strains, plasmids and oligonucleotides used in this study.
| Strain | Characteristics | Source |
|---|---|---|
| R20291 | C. difficile ribotype 027 strain isolated during an outbreak at Stoke Mandeville hospital, UK in 2006. | [34] |
| R20291ΔpyrE | An R20291 pyrE deletion mutant. | [26] |
| R20291ΔspoVD | R20291 with the entire spoVD gene, except the first and last three codons, deleted. | This study |
| R20291ΔspoVD pyrE::spoVD | R20291ΔspoVD complemented by simultaneous restoration of the wild type pyrE gene and insertion of spoVD under the native promoter between pyrE and the downstream R20291_0189. | This study |
| R20291 SNAP-spoVD | R20291 with the native spoVD locus modified by homologous recombination to add the coding sequence of SNAP to the 5′ end of spoVD. | This study |
| CA434 | E. coli conjugative donor. HB101 carrying R702. | [35] |
| NEB5α |
fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80Δ (lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17. |
New England Biolabs |
|
Plasmid |
Characteristics |
Source |
| pMTL960 | E. coli-C. difficile shuttle vector. | Nigel Minton |
| pRPF150 | Pcwp2-Strep-tag II-secA2 cassette cloned between KpnI and BamHI sites in pMTL960. | [36] |
| pJAK012 | pRPF150 modified to introduce an XhoI site between Strep-tag II encoding sequence and the secA2 gene. | This study |
| pJAK032 | Strep Tag II coding sequence in pJAK012 replaced with a codon-optimized CLIP gene. | This study |
| pFT46 | Plasmid containing a C. difficile codon-optimized copy of the SNAP gene under the control of a tetracycline inducible promoter. | [37] |
| pMTL-YN4 | Allele exchange vector for pyrE-based selection. | [26] |
| pMTL-YN2C | pyrE restoration vector allowing simultaneous insertion of cargo DNA between pyrE and R20291_0189. | [26] |
| pMTL-SC7215 | Allele exchange vector for codA-based selection. | [25] |
| pYAA024 | spoVD deletion: 1200 bp homology arms representing the sequence upstream and downstream of R20291_2544 (spoVD) cloned into pMTL-YN4. Designed to delete all but the first and last 9 bp of spoVD. | This study |
| pYAA027 | SpoVD complementation: spoVD and its native promoter cloned into pMTLYN2C. | This study |
| pYAA031 | Constitutive CLIP-SpoVD: spoVD cloned between XhoI and BamHI sites in pJAK032. | This study |
| pYAA047 | SNAP-SpoVD: 1200 bp upstream of spoVD was fused to the coding sequence of SNAP and the first 1200 bp of spoVD and the subsequent recombination cassette cloned into pMTL-SC7215. | This study |
| pYAA048 | SpoVD(ΔDimerization): pYAA031 modified by deletion of the sequence encoding the SpoVD PBP dimerization domain. | This study |
| pYAA049 | SpoVD(ΔPASTA): pYAA031 modified by deletion of the sequence encoding the SpoVD PASTA domain. | This study |
| pYAA050 | SpoVD(ΔTranspeptidase): pYAA031 modified by deletion of the sequence encoding the SpoVD transpeptidase domain. | This study |
| pYAA051 | SpoVD(ΔDimer & PASTA): pYAA031 modified by deletion of the sequence encoding the SpoVD PBP dimerization and PASTA domains. | This study |
| pYAA052 |
His-SpoVD: spoVD cloned into pET-28a between NcoI and XhoI sites. |
This study |
|
Oligonucleotide |
Sequence |
Use |
| NF1957 | GAGTCAGTTATAGATTCGATACTTGAC | To introduce an XhoI site into pRPF150 by inverse PCR with NF1958 |
| NF1958 | GAGTTTTTCAAATTGTGGATGACTCCAC | To introduce an XhoI site into pRPF150 by inverse PCR with NF1957 |
| RF20 | AAACTCCTTTTTGATAATCTCATGACC | To linearize pMTL-SC7215 with RF311 |
| RF139a | GTCAGAGCTCGTTCTTTATTTAGATTAAATAAAGTCAATG | To clone spoVD into pMTL-YN4 with RF187 |
| RF187a | GTCAGGATCCCTTAGGAATCAGAGAGTAGATAG | To clone spoVD into pMTL-YN4 with RF139 |
| RF226 | GATCGAGCTCGGAGGAACTACTATGGATAAAGATTGTGAAATGAAAAG | To add a 5′ SacI site onto a codon optimized clip gene fragment |
| RF227 | GATCCTCGAGAGCAGCTGCTCCTAATCCTGGTTTTCCTAATC | To add 3xAla codons and a 3′ XhoI site onto a codon optimized clip gene fragment |
| RF311 | TAGGGTAACAAAAAACACCG | To linearize pMTL-SC7215 with RF20 |
| RF323a | GTCAGGATCCGTTTATGGGTATATGTTAATTATCTGTTAC | To clone R20291_2545 and spoVD into pMTL-YN2C with RF324 |
| RF324a | GTCAGAGCTCCTTAGGAATCAGAGAGTAGATAG | To clone R20291_2545 and spoVD into pMTL-YN2C with RF323 |
| RF374a | GATCCTCGAGAGAAAAGTAAAGAGGATAAGTAAGAAAAGG | To clone spoVD into pJAK032 with RF375 |
| RF375a | GTCAGGATCCTTAGTTTTCAAAATATAGGGTTATACTTGAG | To clone spoVD into pJAK032 with RF374 |
| RF461 | CTCAAATCTATTCCCCCTAGTTATCC | To amplify spoVD promoter probe with RF462 for Southern blotting |
| RF462 | GAATCTATGTGGTTATTCAAAAATCTCG | To amplify spoVD promoter probe with RF462 for Southern blotting |
| RF528 | aaatacggtgttttttgttaccctaagtttAAGCTAGAATAGATGGACC | To amplify 1200 bp homology arm upstream of spoVD |
| RF529 | acaatctttatccatATCTATTCCCCCTAGTTATCC | To amplify 1200 bp homology arm upstream of spoVD |
| RF530 | ctagggggaatagatATGGATAAAGATTGTGAAATGAAGAGAACCAC | To amplify SNAP |
| RF531 | cctctttacttttctAGCAGCTGCCCCAAGTCC | To amplify SNAP |
| RF532 | cttggggcagctgctAGAAAAGTAAAGAGGATAAGTAAGAAAAG | To amplify first 1200 bp of spoVD |
| RF533 | tttggtcatgagattatcaaaaaggagtttTAAATCTATACCTGTCTTATCCATAAG | To amplify first 1200 bp of spoVD |
| RF582 | TATATCTCTTGTTTGTTGTTCTAGTGCTTTTG | To delete the coding sequence of the SpoVD PBP Dimerization domain with RF583 |
| RF583 | GCAAAAAAGGTTACTGCAATAGCTATG | To delete the coding sequence of the SpoVD PBP Dimerization domain with RF582 |
| RF584 | GGTTTAACTCCCAAATATTTTAAAGAGTCATTC | To delete the coding sequence of the SpoVD PASTA domain with RF585 |
| RF585 | TAAGGATCCACTAGTAACGGCC | To delete the coding sequence of the SpoVD PASTA domain with RF584 |
| RF586 | AGTATATAAAGAAGAAGAAAAAGCTGAGTATG | To delete the coding sequence of the SpoVD Transpeptidase domain with RF587 |
| RF587 | ATTATTTAACTCATAAGCTTTCTGTACTGC | To delete the coding sequence of the SpoVD Transpeptidase domain with RF586 |
Restriction endonuclease sites are underlined.
2.2. Molecular biology methods
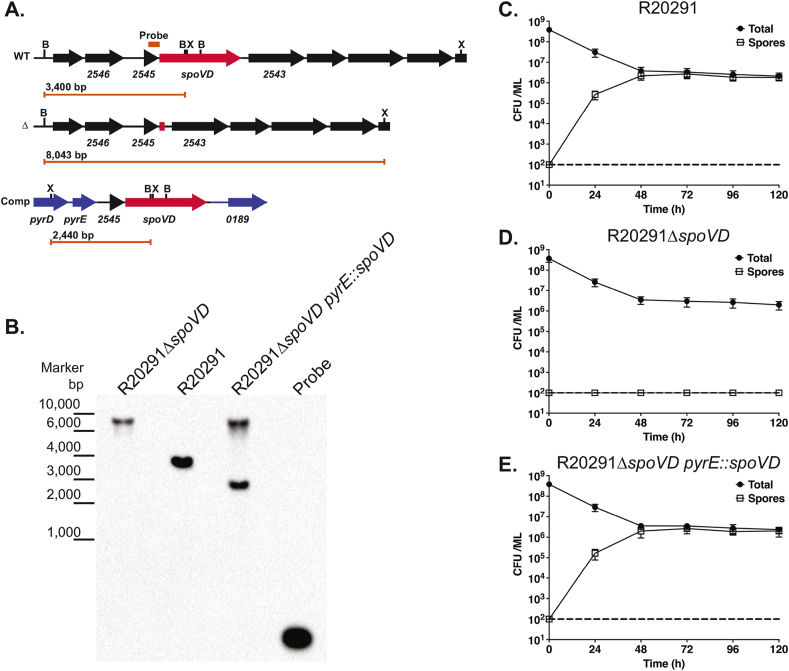
Routine molecular biology techniques were performed according to the manufacturers protocols except where otherwise stated. PCR using Phusion High-Fidelity DNA Polymerase, plasmid isolation and purification of DNA fragments were performed using kits and reagents supplied by Thermo Fisher Scientific according to the manufacturer's instructions. Restriction digestion, ligation and Gibson assembly were performed with enzymes supplied by New England Biolabs. Competent E. coli were transformed using standard methods and plasmid DNA was transferred to C. difficile as described previously [24]. C. difficile mutants were constructed by homologous recombination as described previously [25,26]. Mutants were confirmed by PCR and Southern blotting using the Amersham ECL Direct Labelling and Detection System kit (GE) according to the manufacturer's instructions. A 230 bp probe to the region immediately upstream of spoVDCd was generated by PCD using primer pair RF461/RF462.
2.3. Plasmid construction
pJAK032: pRPF150 was modified by inverse PCR using primer pair NF1957/NF1958 to introduce an XhoI site between the Strep Tag II and SecA2 coding sequences, yielding pJAK012. The Strep Tag II coding sequence was then excised using SacI and XhoI and replaced with a synthetic DNA fragment (IDT gBlock) consisting of a codon-optimized CLIP gene, modified by PCR with primer pair RF226/RF227 to add appropriate SacI and XhoI sites.
pYAA024: Homology arms upstream and downstream of spoVDCd were amplified by PCR using oligonucleotide pairs RF68/RF139 and RF69/RF187. The resulting PCR products were joined together in a SOEing PCR reaction and cloned between the BamHI and SacI sites in pMTL-YN4.
pYAA027: spoVDCd expression appears to be driven from a promoter upstream of CDR20291_2545. In order to ensure complementation at wild type levels a fragment comprising 282 bp upstream of CDR20291_2545, CDR20291_2545 itself and spoVDCd was amplified by PCR using primer pair RF324/RF325 and cloned between BamHI and SacI sites in pMTL-YN2C.
pYAA031: secA2 in pJAK032 was replaced by spoVDCd. spoVDCd was amplified by PCR using primer pair RF374/RF375, digested with BamHI and XhoI and ligated to pJAK032 backbone cut with the same enzymes.
pYAA047: 1200 bp upstream of spoVDCd, the SNAP tag gene from pFT46 and the first 1200 bp of spoVDCd were amplified by PCR using primer pairs, RF528/RF529, RF530/RF531 and RF532/RF533 respectively. pMTL-SC7215 was linearized by PCR using primer pair RF20/RF311. The four DNA fragments were then joined in a Gibson assembly reaction.
pYAA048-050: The coding sequence of the SpoVDCd PBP dimerization domain (pYAA048; primers RF582/RF583), PASTA domain (pYAA049; primers RF584/RF585), or transpeptidase domain (pYAA050; primers RF586/RF587) were deleted by modification of pYAA031 by inverse PCR and subsequent recircularization by ligation.
pYAA051: pYAA048 was further modified to delete the coding sequence of the PASTA domain by inverse PCR with primers RF584/RF585.
2.4. Sporulation efficiency analysis
Overnight cultures of C. difficile R20291 were diluted in BHI broth to an OD600nm of 0.01, incubated for 8 h at 37 °C, diluted to an OD600nm of 0.0001 and finally incubated overnight. This allowed us to obtain cultures in stationary phase with no detectable spores (T = 0). This culture was then incubated for 5 days with vegetative cells and spores enumerated daily. For total viable counts, 10-fold serial dilutions were spotted onto BHIS agar supplemented with 0.1% sodium taurocholate. For total spore counts, the same process was carried out following a 30 min incubation at 65 °C. Colonies were counted after 24 h incubation at 37 °C and the assay was completed in biological triplicates. Formation of phase bright spores was also followed by phase-contrast microscopy at each time point. Samples fixed in 3.7% paraformaldehyde were imaged using a Nikon Eclipse Ti microscope and analysed using Fiji [27].
2.5. Microscopy
Bacterial samples were harvested by centrifugation, washed once with PBS and fixed in 4% paraformaldehyde. For phase-contrast microscopy, samples were mounted in 80% glycerol and imaged using a Nikon Ti Eclipse inverted microscope. Samples for transmission electron microscopy were fixed as above before additional fixation in 3% glutaraldehyde, 0.1 M cacodylate buffer. Fixed samples were then treated with 1% OsO4, dehydrated in ethanol and embedded in araldite resin. Embedded samples were sectioned at 85 nm on a Leica UC6 ultramicrotome, transferred onto coated copper grids, further stained with uranyl acetate and lead citrate and visualised using a FEI Tecnai BioTWIN TEM at 80 kV fitted with a Gatan MS600CW camera.
For fluorescence confocal microscopy, bacteria were grown in TY broth containing 500 nM HADA [28], labelled with 250 nM SNAP-Cell TMR-Star (New England Biolabs) and grown under anaerobic conditions for a further 60 min. Following labelling, cells were harvested at 8000×g for 2 min at 4 °C and washed twice in 1 ml ice cold PBS. Cells were resuspended in PBS and fixed in a 4% paraformaldehyde at room temperature for 30 min with agitation. Cells were washed three times in 1 ml ice cold PBS, immobilized by drying to a coverslip and mounted in SlowFade Diamond (Thermo Fisher Scientific). Images were captured using a Zeiss AiryScan confocal microscope.
3. Results
3.1. C. difficile produces a SpoVD homologue that is required for sporulation
The C. difficile R20291 genome encodes 10 putative penicillin-binding proteins (PBPs) (Table 2) and one predicted monofunctional transglycosylase (CDR20291_2283). In our previous transposon mutagenesis study only two of these, CDR20291_0712 and 0985, were identified as essential for growth in vitro [8]. However, five of the PBPs were required for formation of heat-resistant spores, including two with homology to the B. subtilis cortex specific PBP SpoVD, CDR20291_1067 and 2544. Of these only CDR20291_2544 has the C terminal PASTA domain that is characteristic of the B. subtilis sporulation-specific PBPs [18]. CDR20291_2544 (SpoVDCd) shares 40.1% amino acid identity with B. subtilis SpoVD and has the same predicted overall organisation, with an N terminal predicted transmembrane helix, followed by a PBP dimerization domain (PF03717), a transpeptidase domain (PF00905) and the C terminal PASTA domain (PF03793). spoVD is located immediately downstream of CDR20291_2545 (Fig. 2A), encoding a protein with weak homology to B. subtilis FtsL (18.8% amino acid identity). Despite the weak similarity, the C. difficile and B. subtilis proteins are very similar in size (115 and 117 amino acids respectively), have a similar PI (9.57 and 9.63 respectively) and both have a high proportion of lysine residues (22.6% and 14.5% respectively). CDR20291_2545 and spoVDCd appear to be in an operon, with the promoter upstream of CDR20291_2545. In our earlier TraDIS screen, CDR20291_2545 was also found to be required for sporulation, although this may have been due to polar effects on spoVDCd.
Table 2.
Putative C. difficile PBPs.
| C. difficile R20291 gene designation | Best B. subtilis strain 168 hit | Amino acid identity | Essential in vitro? |
|---|---|---|---|
| 0712 | PonA | 27.3% | Yes |
| 2544 | SpoVD | 40.1% | No but required for sporulation |
| 1067 | SpoVD | 27.9% (PbpB 26.6%) | No but required for sporulation |
| 1131 | DacF | 43.8% | No but required for sporulation |
| 1318 | PbpX | 21.3% (PbpE 20.4%) | No |
| 2048 | DacF | 31.5% | No but required for sporulation |
| 0441 | DacF | 30.3% | No |
| 0985 | PbpA | 21.2% | Yes |
| 3056 | PbpX | 20.1% | No but required for sporulation |
| 2390 | DacB | 27.5% | No |
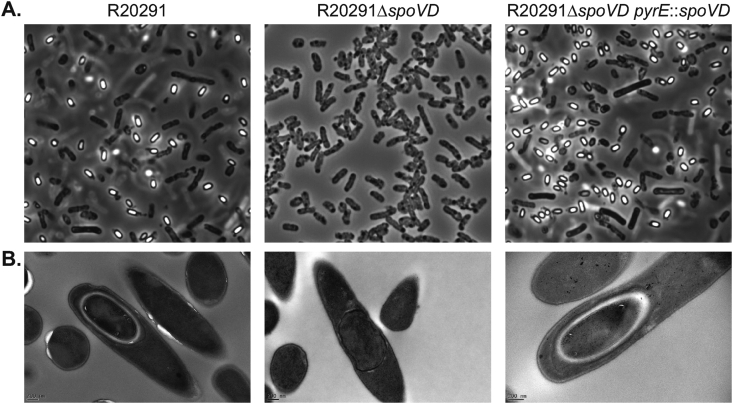
Fig. 2.
Microscopic analysis of sporulation. Phase-contrast light microscopy (A.) and negative stained TEM (B.) of the wild type parental strain (R20291), spoVDCd mutant (R20291ΔspoVD) and complemented strain (R20291ΔspoVD pyrE:spoVD). A. Cultures were imaged at day 5 of the sporulation assays shown in Fig. 1. Spores are visible as ovoid phase bright objects i, while vegetative cells are phase dark bacilli. B. TEM imaging of developing spores clearly shows normal spore development in R20291 and R20291ΔspoVD pyrE:spoVD; the densely stained core surrounded by a thick, largely unstained cortex layer. Cultures of R20291ΔspoVD contained no morphologically normal developing spores, although fully engulfed prespores without a cortex (example shown) were common.
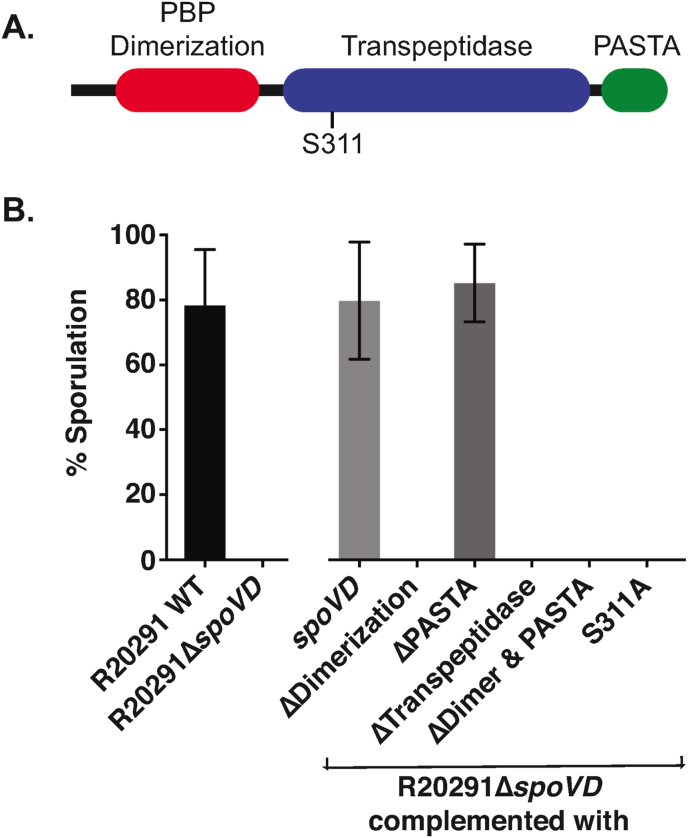
To confirm a role in sporulation, we constructed a clean spoVDCd deletion by homologous recombination and then complemented this mutant by integrating the CDR20291_2545-spoVDCd cassette under the control of the native promoter into the chromosome between the pyrE and R20291_0189 genes (referred to here as R20291ΔspoVD pyrE:spoVD; Fig. 1A and B). We then analysed the ability of each strain to form heat-resistant spores. In our assay, a stationary phase culture of wild type R20291 gradually accumulated spores, accounting for 81% of the viable counts after 3 days (Fig. 1C). In the same assay R20291ΔspoVD formed no detectable spores, even after 5 days of incubation (Fig. 1D). Complementation completely restored sporulation to wild type levels (Fig. 1E). Examination by phase-contrast microscopy confirmed the presence of abundant mature phase bright spores in 5 day old cultures of wild type R20291 and the complemented strain R20291ΔspoVD pyrE:spoVD (Fig. 2A). In contrast no phase bright objects were observed in cultures of R20291ΔspoVD. When visualised at higher magnification using TEM of thin sections, no morphologically normal spores were observed in cultures of R20291ΔspoVD (Fig. 2B). Membrane-bound prespores were present, but these structures were irregular in shape and crucially lacked the cortex and protein coat layers seen in R20291 and the complemented strain developing spores. SpoVDCd is predicted to consist of 3 domains: a PBP dimerization domain, a transpeptidase domain and a PASTA domain (Fig. 3A). To identify which of these were required for viable spore formation, CLIP-spoVDCd was placed under the control of a constitutive promoter (Pcwp2) in a C. difficile expression vector and a panel of mutants, lacking one or more of these domains, were constructed (Table 1). These plasmids were all transferred into R20291ΔspoVD and the ability of the expressed CLIP-SpoVDCd variant to restore sporulation was evaluated. Only proteins including both the dimerization and transpeptidase domains (SpoVD(ΔPASTA) or full-length SpoVD) restored normal sporulation (Fig. 3B), the PASTA domain was dispensable as observed previously in B. subtilis [18]. This observation was supported by TEM examination, with morphologically normal spores only observed when the full-length or SpoVD(ΔPASTA) proteins were expressed (not shown).
Fig. 1.
Sporulation requires SpoVDCd. A. Genomic organisation of the native spoVDCd locus (WT), following deletion of the spoVDCd gene (Δ) and following complementation by insertion of R20291_2545 and spoVDCd between the pyrE and R20291_0189 genes (Comp). The locations of XmnI (X) and BsrGI (B) sites are indicated, as is the annealing site of the Southern blot probe. The length of the diagnostic restriction product containing the probe sequence is also shown below each locus diagram. B. Southern blot analysis of a spoVDCd mutant (R20291ΔspoVD), the wild type parental strain (R20291) and complemented strain (R20291ΔspoVD pyrE:spoVD). A DNA ladder is shown on the left hand side. The predicted fragment sizes and annealing site of the probe are shown in panel A. C.-E. Sporulation efficiencies of the wild type (C.), spoVDCd mutant (D.) and complemented strains (E.). Stationary phase cultures were incubated anaerobically for 5 days with samples taken daily to enumerate total colony forming units (CFUs) and spores, following heat treatment to kill vegetative cells. Experiments were performed in duplicate on biological triplicates with mean and standard deviation shown. The dotted horizontal line indicates the limit of detection of the experiment.
Fig. 3.
The contribution of SpoVDCd domains to sporulation. A. The domain organisation of SpoVDCd showing Pfam predictions [33]. B. Sporulation efficiency of R20291, R20291ΔspoVD and R20291ΔspoVD complemented in trans using plasmids expressing a series of mutant CLIP-SpoVDs under the control of a constitutive promoter: full-length CLIP-SpoVDCd (spoVD); CLIP-SpoVDCd lacking the PBP dimerization domain (ΔDimerization), PASTA domain (ΔPASTA), transpeptidase domain (ΔTranspeptidase) or both PBP dimerization and PASTA domains (ΔDimer & PASTA); CLIP-SpoVDCd lacking the active site nucleophile serine (S311A). Shown is the sporulation efficiency after 5 days in broth culture, expressed as number of spores as a percentage of total viable CFUs. Experiments were conducted in duplicate on biological triplicates and mean and standard deviations are shown.
B. subtilis SpoVD, and the wider family of class B PBPs, share a conserved active site consisting of 3 non-contiguous motifs that are brought into close proximity in the folded enzyme, SxxK, SxN and KTG [29]. The first of these motifs contains the essential serine nucleophile. SpoVDCd has all three motifs, with Ser311 as the predicted nucleophile. SpoVDCd S311A supplied in trans was also incapable of complementing the sporulation defect observed in a spoVDCd deletion mutant (Fig. 3B), confirming a role for this residue in cortex synthesis.
3.2. Subcellular localisation of SpoVDCd
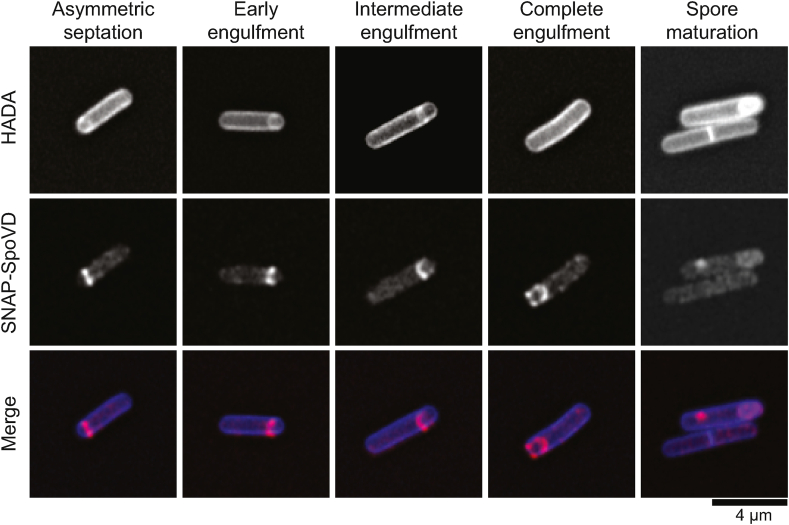
To visualise the cellular localisation of SpoVDCd, we fused the coding sequence for SNAP to the 5′ end of the spoVDCd gene and transferred this to the C. difficile genome in the native locus and under the control of the native promoter. SNAP was then labelled with the fluorescent reagent TMR-Start, while newly synthesised peptidoglycan was labelled with the fluorescent d-amino acid HADA [28]. Using Airyscan confocal microscopy we observed weak punctate fluorescence around the periphery of the cell, localizing to the asymmetric division septum once the cell had committed to sporulation (Fig. 4A). Fluorescence then tracked the asymmetric membrane through engulfment (Fig. 4B and C), eventually surrounding the prespore (Fig. 4D). Localisation of SNAP-SpoVDCd clearly preceded significant cortex synthesis as we visualised localisation around the spore without concomitant HADA accumulation (Fig. 4D). Following further spore maturation (Fig. 4E), accumulation of new HADA-labelled peptidoglycan co-localized with SNAP-SpoVDCd.
Fig. 4.
Subcellular localisation of SpoVDCd. R20291 SNAP-spoVDCd was grown for 24 h in TY broth containing the fluorescent d-amino acid HADA (500 nM) to label de novo synthesised peptidoglycan. The bacteria were then further stained with SNAP-Cell TMR-Star (250 nM) to label SNAP-SpoVDCd, fixed, mounted in SlowFade Diamond mountant and imaged using a Zeiss AiryScan confocal microscope. Shown are representative cells demonstrating the sequential stages of sporulation: asymmetric septum placement, early, intermediate and complete prespore engulfment respectively, and spore maturation.
4. Discussion
C. difficile is the most common cause of hospital acquired infection in the USA and Europe [30,31]. The formation of a robust spore form is crucial for transmission of infection between patients and for persistence and relapse following treatment [5]. However, despite their importance in C. difficile pathogenesis, we still know surprisingly little about the underlying molecular mechanisms of sporulation and germination, in part due to a lack of effective genetic tools until recently [32]. Much can be learned from the parallels with the well-studied but distantly related species B. subtilis, however there are significant differences in the sporulation pathways between the Bacillales and Clostridiales and even homologous proteins can play subtly different roles [[9], [10], [11]]. Here we have identified and characterised a C. difficile homologue of the B. subtilis spore cortex PBP SpoVD. We have confirmed that this protein is required for sporulation in C. difficile.
Bioinformatic analysis of the C. difficile genome identified 10 genes encoding proteins with significant homology to characterised B. subtilis PBPs. In a previous transposon mutagenesis screen we determined that only two of these are essential for growth in vitro, but five were required for formation of heat-resistant spores [8]. One of these (R20291_2544) encodes a protein sharing 40.1% amino acid identity with B. subtilis SpoVD. Despite this relatively weak homology, the two proteins share a similar overall domain organisation and are encoded in a similar genomic context. To determine if this protein played a role in C. difficile sporulation we constructed a clean deletion mutant that we found to be incapable of producing viable spores. Microscopic examination of this mutant allowed us to visualise fully engulfed prespores but these structures lacked any obvious cortex. This sporulation defect was fully complemented by integration of spoVDCd (and the upstream R20291_2545 and native promoter) in a distal chromosomal locus. These observations clearly demonstrated that SpoVDCd plays a crucial role in C. difficile sporulation and is required for the synthesis of cortex peptidoglycan. We then demonstrated that the sporulation defect in a spoVDCd mutant could be complemented by expression in trans of a mutant CLIP-SpoVDCd lacking the C terminal PASTA domain but that mutation of the PBP dimerization or transpeptidase domains resulted in a non-functional SpoVDCd. This is in full agreement with previous B. subtilis studies that showed that the PASTA domain was dispensable for cortex synthesis [18]. By comparison with the B. subtilis sequence we were also able to putatively identify the active site nucleophile serine as S311 and confirmed this role by mutation to alanine, resulting in a non-functional SpoVDCd.
It has been shown previously that B subtilis SpoVD localises to the asymmetric septum upon initiation of sporulation and ultimately to the developing spore following engulfment [17]. To visualise this process in C. difficile we generated a strain expressing SNAP-SpoVDCd under the control of the native promoter. Super-resolution fluorescence microscopy imaging of this strain showed clear localisation of SNAP-SpoVDCd to the asymmetric septum and to the developing spore. Intriguingly we also observed weak punctate fluorescence staining around the periphery of the mother cell. This could be indicative of mislocalisation as a result of the N terminal SNAP fusion or could suggest a broader role for SpoVDCd in vegetative cell peptidoglycan synthesis. To test this latter possibility, we examined the peptidoglycan composition of wild type and spoVDCd mutant cells but observed no obvious differences. However, given the enormous potential for redundancy with 10 encoded PBPs it is possible that small differences could be missed in this analysis.
Sporulation of C. difficile represents one of the most pressing clinical challenges in tackling recurrent disease in individual patients as well as preventing outbreaks in nosocomial settings. However, this cell differentiation pathway also represents a promising target for the development of C. difficile-specific therapeutics. Indeed it has been shown that inhibition of sporulation with the SpoVD-targeting cephamycin cefotetan prevents relapse in a mouse model of CDI [22]. In order to exploit this potential fully however, we must first develop a deeper understanding of both the complex regulatory processes that underpin sporulation as well as the function of the effector proteins that direct differentiation. Here we have identified and characterised a PBP that is absolutely required for production of viable spores and that we believe is a promising target for future therapeutics aimed at preventing recurrent disease and transmission.
Funding
This work was supported by a PhD studentship from the Higher Committee for Education and Development in Iraq for Y.A.A. and by the Medical Research Council (P.O., grant number MR/N000900/1) and the Wellcome Trust (J.A.K., grant number 204877/Z/16/Z). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author contributions
Y.A.A. and R.P.F. designed and coordinated the study. Y.A.A., P.O. and J.A.K. performed the experiments. R.P.F. wrote the paper with input from all co-authors.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We would like to thank Darren Robinson and Christa Walther (The Wolfson Light Microscopy Facility, University of Sheffield) for their light microscopy support and training, Stephane Mesnage (University of Sheffield) for assistance with peptidoglycan analysis, Chris Hill (Electron Microscopy Unit, University of Sheffield) for thin sectioning and transmission electron microscopy, Nigel Minton (University of Nottingham) for supplying plasmids for homologous recombination, Adriano Henriques for supplying pFT46, and Simon Jones and Shuwen Ma (University of Sheffield) for synthesis of HADA.
Handling editor: Maja Rupnik
References
- 1.Lessa F.C., Mu Y., Bamberg W.M., Beldavs Z.G., Dumyati G.K., Dunn J.R. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smits W.K., Lyras D., Lacy D.B., Wilcox M.H., Kuijper E.J. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupnik M., Wilcox M.H., Gerding D.N. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 4.He M., Miyajima F., Roberts P., Ellison L., Pickard D.J., Martin M.J. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013;45:109–113. doi: 10.1038/ng.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deakin L.J., Clare S., Fagan R.P., Dawson L.F., Pickard D.J., West M.R. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012;80:2704–2711. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer C., Hutt L.P., Burky R., Joshi L.T. Biocide resistance and transmission of Clostridium difficile spores spiked onto clinical surfaces from an American healthcare facility. Appl. Environ. Microbiol. 2019;85(17) doi: 10.1128/AEM.01090-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu D., Sorg J.A., Sun X. Clostridioides difficile biology: sporulation, germination, and corresponding therapies for C. difficile infection. Front Cell Infect Microbiol. 2018;8:29. doi: 10.3389/fcimb.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dembek M., Barquist L., Boinett C.J., Cain A.K., Mayho M., Lawley T.D. High-throughput analysis of gene essentiality and sporulation in Clostridium difficile. mBio. 2015;6 doi: 10.1128/mBio.02383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fimlaid K.A., Bond J.P., Schutz K.C., Putnam E.E., Leung J.M., Lawley T.D. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paredes C.J., Alsaker K.V., Papoutsakis E.T. A comparative genomic view of clostridial sporulation and physiology. Nat. Rev. Microbiol. 2005;3:969–978. doi: 10.1038/nrmicro1288. [DOI] [PubMed] [Google Scholar]
- 11.Underwood S., Guan S., Vijayasubhash V., Baines S.D., Graham L., Lewis R.J. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J. Bacteriol. 2009;191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paredes-Sabja D., Shen A., Sorg J.A. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 2014;22:406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore M.E., Bandyopadhyay D., Dean A.M., Linnstaedt S.D., Popham D.L. Production of muramic delta-lactam in Bacillus subtilis spore peptidoglycan. J. Bacteriol. 2004;186:80–89. doi: 10.1128/JB.186.1.80-89.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meador-Parton J., Popham D.L. Structural analysis of Bacillus subtilis spore peptidoglycan during sporulation. J. Bacteriol. 2000;182:4491–4499. doi: 10.1128/jb.182.16.4491-4499.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel R.A., Drake S., Buchanan C.E., Scholle R., Errington J. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J. Mol. Biol. 1994;235:209–220. doi: 10.1016/s0022-2836(05)80027-0. [DOI] [PubMed] [Google Scholar]
- 16.Fay A., Meyer P., Dworkin J. Interactions between late-acting proteins required for peptidoglycan synthesis during sporulation. J. Mol. Biol. 2010;399:547–561. doi: 10.1016/j.jmb.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidarta M., Li D., Hederstedt L., Bukowska-Faniband E. Forespore targeting of SpoVD in Bacillus subtilis is mediated by the N-terminal part of the protein. J. Bacteriol. 2018;200 doi: 10.1128/JB.00163-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukowska-Faniband E., Hederstedt L. The PASTA domain of penicillin-binding protein SpoVD is dispensable for endospore cortex peptidoglycan assembly in Bacillus subtilis. Microbiology. 2015;161:330–340. doi: 10.1099/mic.0.000011. [DOI] [PubMed] [Google Scholar]
- 19.Peltier J., Courtin P., El Meouche I., Lemee L., Chapot-Chartier M.P., Pons J.L. Clostridium difficile has an original peptidoglycan structure with a high level of N-acetylglucosamine deacetylation and mainly 3-3 cross-links. J. Biol. Chem. 2011;286:29053–29062. doi: 10.1074/jbc.M111.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coullon H., Rifflet A., Wheeler R., Janoir C., Boneca I.G., Candela T. N-Deacetylases required for muramic-delta-lactam production are involved in Clostridium difficile sporulation, germination, and heat resistance. J. Biol. Chem. 2018;293:18040–18054. doi: 10.1074/jbc.RA118.004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz O.R., Sayer C.V., Popham D.L., Shen A. Clostridium difficile lipoprotein GerS Is required for cortex modification and thus spore germination. mSphere. 2018;3 doi: 10.1128/mSphere.00205-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srikhanta Y.N., Hutton M.L., Awad M.M., Drinkwater N., Singleton J., Day S.L. Cephamycins inhibit pathogen sporulation and effectively treat recurrent Clostridioides difficile infection. Nat. Microbiol. 2019;4:2237–2245. doi: 10.1038/s41564-019-0519-1. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy B., Sonenshein A.L. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 1998;27:107–120. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 24.Kirk J.A., Fagan R.P. Heat shock increases conjugation efficiency in Clostridium difficile. Anaerobe. 2016;42:1–5. doi: 10.1016/j.anaerobe.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartman S.T., Kelly M.L., Heeg D., Heap J.T., Minton N.P. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Appl. Environ. Microbiol. 2012;78:4683–4690. doi: 10.1128/AEM.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng Y.K., Ehsaan M., Philip S., Collery M.M., Janoir C., Collignon A. Expanding the repertoire of gene tools for precise manipulation of the Clostridium difficile genome: allelic exchange using pyrE alleles. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuru E., Tekkam S., Hall E., Brun Y.V., Van Nieuwenhze M.S. Synthesis of fluorescent D-amino acids and their use for probing peptidoglycan synthesis and bacterial growth in situ. Nat. Protoc. 2015;10:33–52. doi: 10.1038/nprot.2014.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauvage E., Kerff F., Terrak M., Ayala J.A., Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 30.Aguado J.M., Anttila V.J., Galperine T., Goldenberg S.D., Gwynn S., Jenkins D. Highlighting clinical needs in Clostridium difficile infection: the views of European healthcare professionals at the front line. J. Hosp. Infect. 2015;90:117–125. doi: 10.1016/j.jhin.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen A. Expanding the Clostridioides difficile genetics toolbox. J. Bacteriol. 2019;201 doi: 10.1128/JB.00089-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Gebali S., Mistry J., Bateman A., Eddy S.R., Luciani A., Potter S.C. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stabler R.A., He M., Dawson L., Martin M., Valiente E., Corton C. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 2009;10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purdy D., O'Keeffe T.A., Elmore M., Herbert M., McLeod A., Bokori-Brown M. Conjugative transfer of clostridial shuttle vectors from Escherichia coli to Clostridium difficile through circumvention of the restriction barrier. Mol. Microbiol. 2002;46:439–452. doi: 10.1046/j.1365-2958.2002.03134.x. [DOI] [PubMed] [Google Scholar]
- 36.Fagan R.P., Fairweather N.F. Clostridium difficile has two parallel and essential Sec secretion systems. J. Biol. Chem. 2011;286:27483–27493. doi: 10.1074/jbc.M111.263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira F.C., Saujet L., Tome A.R., Serrano M., Monot M., Couture-Tosi E. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003782. [DOI] [PMC free article] [PubMed] [Google Scholar]