Abstract
Context.
Large variations in thyroid volume (TV) have been reported in Hashimoto’s thyroiditis (HT). The need for long-term levo-thyroxine (L-T4) administration in order to control TV, as well as to normalise thyroid function, has not been well defined.
Subjects and Methods.
Retrospective data on TV in 94 adult women with HT were analysed in an ambulatory setting in Liguria, an area of moderate iodine sufficiency. TV was evaluated by means of ultrasonography (US). Thyroid function, anthropometric data, smoking habits and pharmaceutical drugs were registered at each examination.
Results.
At the baseline, an atrophic gland was noted in 16% of the women, and goitre in 13%. The women were evaluated 56 and 102 months after the baseline examination. At the time of each examination, 50%, 78% and 83% of women, respectively, were on L-T4 treatment. Baseline TV was not significantly different in women on/off L-T4 treatment. However, in those on L-T4, TV decreased significantly over the period of follow-up, while in those without L-T4 treatment, it did not change. By the end of the study, the percentage of L-T4-treated women with an atrophic gland had increased to 27%, and that of women with goitre had dropped to 6%; in untreated women, only minor changes were noted. There was a significant negative correlation between TV% change and baseline TSH levels in HT women on L-T4 treatment.
Conclusion.
The majority of HT women living in an area of moderate iodine sufficiency have normal TV. Moreover, long-term L-T4 treatment can be used to control TV, as well as to maintain normal thyroid parameters.
Keywords: Hashimoto’s thyroiditis, women, thyroid volume, levo-thyroxine treatment
Introduction
Hashimoto’s thyroiditis (HT) is an autoimmune disease; its incidence is estimated to be 0.8 per 1000 per year in men and 3.5 per 1000 per year in women (1). It is now clear that HT is part of a spectrum of autoimmune diseases with variable histological, morphological and functional involvement of the gland (1). The prevalence of HT depends on age, gender and ethnicity (1). The pathology of the disease involves the formation of anti-thyroid antibodies that attack the thyroid tissue, causing progressive fibrosis (1). Apoptosis of thyroid follicular cells occurs in HT, probably as a result of its iodine-induced suppression of autophagy (2). HT is currently sub-classified as IgG4 thyroiditis and non-IgG4 thyroiditis (3). Younger patients and men have the highest prevalence of IgG4 thyroiditis, which is manifested on diagnosis as enlargement of the thyroid size and diffuse low sonographic echogenicity, and rapidly progresses to subclinical hypothyroidism (4). Painful HT is a rare variant of the disease and mostly affects women (5). In most HT patients, levo-thyroxine (L-T4) substitution is needed, owing to the impairment of thyroid function (1). In HT patients treated with L-T4, hypothyroidism rarely converts to hyperthyroidism (6). The diagnosis of HT is based on the presence of antibodies against thyroid peroxidase (TPOAb) and/or thyroglobulin (TgAb) and grey-scale ultrasonography (US) features (1); other, more sophisticated, imaging techniques do not seem to add anything to the diagnosis (7, 8).
Although thyroid volume (TV) is reported to be normal in about 50% of HT patients (7, 9), a greater dispersion of TV values is observed in HT patients than in controls (10-12). Indeed, TV is reduced (atrophic thyroiditis) in about 19-28% of subjects (7, 13) and increased (goitre) in 19-46% (7, 9, 12). Patients with smaller TV have more pronounced hypothyroidism (10, 12), whereas those with sub-clinical hypothyroidism (SCH) predominantly have goitre (11). During the course of HT disease, TV changes, and this may reflect changes in biologic function, stromal changes, lymphocytic infiltration, oedema, and vascularity (1, 10).
For 30 years, it has been postulated that L-T4 can be used to reduce TV in HT patients. Indeed, in paediatric and adolescent HT patients, it has been observed that L-T4 treatment causes a reduction in TV (14-16). Moreover, in euthyroid (17, 18), hypothyroid (19) and SCH (19) adults with HT, short-term prophylactic L-T4 treatment has been shown to reduce both TV and markers of autoimmune thyroiditis. However, the available data are discordant. Padberg et al. (17) reported only a reduction in serological markers, but not in TV, in euthyroid HT patients after 1 year on L-T4. Conversely, in hypothyroid adults on L-T4 treatment for 2 years, Hegedus et al. (20) reported a reduction in large goitres in hypothyroid patients, but no changes in TPOAb levels. Furthermore, in a small group of euthyroid women with HT, Aksoy et al. (18) reported a reduction in TV and a concomitant decrease in TgAb after 2 years of L-T4 administration. More recently, Kawasaki et al. (9) observed that, in euthyroid HT subjects with large goitres, one year of L-T4 treatment did not change TV.
The recent literature contains few data on TV changes in HT patients, and, to our knowledge, no observational data covering a period as long as 9 years have been reported. The aim of our study was to observe changes in TV in a series of women with HT on or off L-T4 treatment. In an era in which the widespread use of suppressive L-T4 is not recommended in post-menopausal women (21), we believe that the effectiveness of chronic L-T4 administration on TV in HT women should be reassessed.
Materials and methods
Subjects
A total of 3552 outpatient medical files collected in the period 2008-2019 were retrospectively searched in order to anonymously pick out women with HT who had undergone long-term ultrasonography (US) follow-up. We based the diagnosis of HT on the documentation of TPOAb positivity and a heterogeneous hypoechoic pattern on US ultrasound examination. Exclusion criteria were: age <18 years; unavailable data on current pharmacological treatments; other chronic or serious diseases which tend to influence thyroid function tests; and pregnancy or lactation in the 12 months before examination. The study group comprised 94 women aged 49.8 ± 14.5 years (±SD; median 48.5 years, range 18-82 years) living in Savona (50%), Genoa (48%) and nearby districts (2%). All women were examined on inclusion and underwent 1 or 2 further examinations in the subsequent years (up to 10 years).
Methods
Body mass index (BMI) was calculated on the basis of the weight (kg) and height (m) reported in the medical files, according to the following formula: kg/m2. Body surface area (BSA: m2) was calculated by means of the Du Bois and Du Bois formula. TV was calculated by means of US, as previously reported (22), by using the depth, width and length of each lobe, as reported in medical files. TV was obtained by combining the volumes of both lobes.
All US examinations were performed by the same experienced endocrinologist (MG); over the period considered, three different machines were used (MyLab 70-XV Esaote, Genoa, Italy; UF-850 XTD Fukuda Denshi, Tokyo, Japan; Logiq V2 General Electric, Milwaukee, WI, USA); these were equipped with linear probes working at 7.5 MHz. Intra-observer variability was 11.5%.
In our districts, normal TV in women is 8.0 mL (IQR 6.7 – 9.8 mL; range 3.2-19.8 mL) (23). Arbitrarily, thyroid atrophy and goitre were defined as TV <3rd (4.6 mL) and >97th percentile (14.2 mL), respectively (23).
Laboratory data
Free-T4 (f-T4) and TSH levels were sought in the medical files; the values recorded on the dates closest to that of TV evaluation were considered. In the districts where our population was living, the laboratories of the Public Health Service used the Cobas automated analytic platform (Roche Diagnostics, Mannheim, Germany). Normal ranges are 12.0 to 22.0 pmol/L for f-T4 and 0.3 to 4.2 mIU/L for TSH. The functional sensitivity of the TSH assay is 0.03 mIU/L, with intra- and inter-assay coefficients of variation of 3% and 7%, respectively. Several commercial methods were used for TPOAb evaluation during the study period, and judgements of negative TPOAb values were assigned according to the normal range reported by the manufacturers.
Statistical methods
Categorical variables were described as percentages, and continuous variables as mean, median and interquartile range (IQR) values. Continuous variables were compared by means of the two-tailed Kruskal-Wallis analysis of variance, Mann-Whitney test, and Wilcoxon test when applicable. For categorical variables, percentages were compared by means of the Fisher exact test. Relationships among variables were sought by applying the Spearman correlation coefficient (Sr). P values less than 0.05 were considered statistically significant. All statistical analyses were performed by means of GraphPad 8.4.0 software (San Diego, CA, USA). Data collection and subsequent analysis were performed in compliance with the Helsinki Declaration. As this evaluation was retrospective, no formal request was made to the Liguria Ethics Committee. All patients gave written consent for data collection.
Results
Eighty-eight women were evaluated 56 ± 1 months (median 56 months; IQR 47-64 months) and 41 women 102 ± 3 months (100 months; 88-118 months) after the baseline examination. Six women missed the intermediate evaluation, but attended the final examination, and one woman was lost to the end-of-study examination. At the moment of data analysis, 52 women were still waiting for their 3rd scheduled examination. At the time of each examination (baseline, 56 months and 102 months), 50% (n=47), 78% (n=69) and 83% (n=34) of women, respectively, were on L-T4 treatment. Table 1 reports clinical data on all women at the baseline. For the purpose of comparison, the women were also subdivided into two groups: those on L-T4 (n=48) and those without L-T4 (n=19) treatment. Women who started L-T4 after the baseline examination were excluded from these two groups. None of the women who were on L-T4 at the baseline, or who started L-T4 after this time-point, discontinued the drug during the follow-up period. The only significant difference between the two groups was in fT4 levels (P=0.01) (Table 1).
Table 1.
Some clinical data of patients at the baseline evaluation. Significance is reported between subjects with (on) L-T4 and without (off) L-T4 therapy for the entire period of TV evaluation. Significance values are: (a) P=0.10, (b) P=0.18, (c) P=0.19, (d) P=0.29, (e) P=0.01 on Mann-Whitney test; (f) P=0.36 on Fisher’s test.
| All (n=94) | On L-T4 (n=48) | Off L-T4 (n=19) | |
|---|---|---|---|
| Age (year); mean ± SD | 49.8 ± 14.5 | 52.5 ± 15.0 | 46.1 ± 11.6 (a) |
| BMI (kg/m2); median, IQR | 23.4; 21.2-26.7 | 24.0; 22.3-27.9 | 23.5; 20.7-26.1 (b) |
| BSA (m2); median, IQR | 1.65; 1.57-1.72 | 1.67; 1.59-1.73 | 1.61; 1.53-1.72 (b) |
| Thyroid volume (mL), median, IQR | 8.3; 5.6-11.1 | 7.1; 5.1-10.1 | 9.1; 6.2-12.3 (c) |
| Former/current smokers (%) | 37 52% | 15/40 (38%) | 8/15 (53%) (f) |
| Non-smokers (%) | 34 48% | 25/40 (62%) | 7/15 (47%) |
| TSH (mIU/L); median, IQR | 3.23; 1.69-6.17 | 2.44; 1.42-4.02 | 1.61; 1.34-3.44 (d) |
| f-T4 (pmol/L); median, IQR | 14.2; 11.7-16.2 | 15.2; 12.4-17.4 | 12.9; 10.4-15.2 (e) |
| L-T4 (µg/kg bw/day); median, IQR | - | 0.98; 0.88-1.43 | n.e |
| Other ongoing drugs; % none |
45% |
42% |
42% |
| hypotensive drugs | 18% | 23% | 16% |
| psychotropic drugs | 18% | 17% | 21% |
| anti-reabsorptive drugs/vitamin D/Ca | 16% | 21% | 21% |
| hormonal contraceptive/HRT | 12% | 13% | 16% |
| anti-secretory drugs | 10% | 8% | 16% |
| statins | 10% | 4% | 10% |
| steroids/NSAIDs | 8% | 10% | 10% |
| H1-inhibitors | 3% | 6% | 0% |
NSAIDs =nonsteroidal anti-inflammatory drugs.
At the baseline, atrophic glands and goitres were noted in 16% and 13% of women, respectively. In the whole population, TV declined from 8.3 mL (5.6-11.1 mL) at the baseline to 7.3 mL (4.8-10.2 mL) at the intermediate examination and to 6.9 mL (5.2-10.2 mL) at the last examination (P=0.20). Baseline TV was not significantly different (P=0.19) in women on/off L-T4 treatment (Table 1). At the baseline, atrophic glands and goitres were recorded in 19% and 10%, respectively, of L-T4-treated women, and in 11% and 5% of those without L-T4 treatment.
In women on L-T4 for the entire study period, TV decreased from 7.1 mL (5.1-10.2 mL) at the baseline to 6.0 mL (4.4-9.6 mL) at the last examination (P=0.03), while in untreated women, it increased from 9.1 mL (6.2-12.3 mL) to 10.0 mL (6.6-12.2 mL) (P=0.99). TSH and f-T4 levels did not change from the baseline (Table 1) to the last examination in either L-T4-treated [TSH 1.97 mIU/L (0.92-3.50 mIU/L), P=0.37; f.T4 15.2 pmol/L (12.4-17.4 pmol/L), P=0.30] or L-T4-untreated women [TSH 2.39 mIU/L (1.93-4.68 mIU/L), P=0.11; f-T4 12.9 pg/mL (10.4-15.2 pmol/L), P=0.11].
By the end of the study, the percentage of L-T4-treated women with atrophic glands had increased to 27%, while that of those with goitre had decreased to 6%; in the off-L-T4 group, minor changes were noted (atrophic gland 5%, goitre 5%). Figure 1 reports the percentage change from the baseline to the last examination in the two groups of women. There was a significant difference (P=0.03) in changes between the groups. Figure 2 reports the negative correlation between TV% change and baseline TSH levels in women on L-T4 treatment (n=48; Sr -0.41, P=0.004); no correlation between TSH and TV % change was noted in the 19 women without L-T4 treatment. No other correlation between TV % change and initial TV, age, BMI, BSA, smoking or f-T4 levels was noted in either group.
Figure 1.
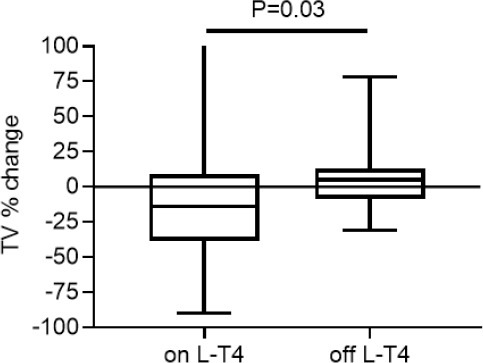
Median, IQR (box) and range (whiskers) of the percentage changes from baseline to last examination in women on or off LT4 treatment for the entire study period.
Figure 2.
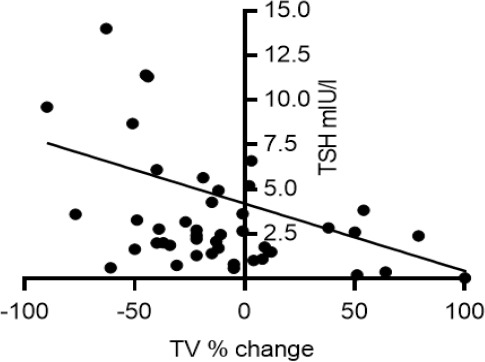
Correlation between baseline TSH levels and TV change observed from baseline to last examination in HT women on L-T4 treatment for the entire study period (n=48; Sr -0.41, P=0.004).
Discussion
US is the imaging tool most commonly used to determine the volume and structure of the thyroid gland. It is non-invasive, relatively inexpensive and readily accessible. US evaluation is one of the examinations suggested in the diagnosis of HT (1, 7, 8).
Normative values of TV in Italy are lacking. The introduction of iodine supplementation in salt was regulated by Italian law in 2005 (24). Recently, we retrospectively searched for data on urinary iodine concentration (UIC) and TV in subjects living in Liguria in medical files recorded since 2010. Conte et al. (25) reported a median UIC of 113 µg/L in 415 subjects living in Savona, Genoa and nearby districts. Several years since the introduction of iodine supplementation, about 50-60% of the Ligurian population now have an adequate iodine intake (25). In 382 selected women of median age of 34 years living in the above-mentioned areas since 2008, and free from laboratory-and US-detected thyroid abnormalities, Giusti & Sidoti (23) reported a median TV of 8.0 mL. In this group of women, the 3rd and 97th percentiles of TV were set at 4.6 mL and 14.2 mL, respectively (23). While information is lacking as to whether a gland of below normal volume should be regarded as displaying hypoplasia, data from this study indicated goitre in <5% of subjects (23), as reported in several other similar evaluations (26, 27).
In the present study, median TV was 8.3 mL at the first examination, but a large variability in TV was noted, as already reported in HT (12). In our HT women, the cumulative frequency of goitre and thyroid atrophy was less than 30%. We were not able to diagnose women with IgG4 HT, a condition in which SCH and goitre are more frequent. In our opinion, however, given that our patients were middle-aged women and IgG4 thyroiditis has an overall low incidence in HT (3), it was not indispensable to know their IgG4 levels.
In a small group of women, younger than ours, with HT and SCH, Gonzalez-Aguilera et al. (6) reported a median TV of 7.4 mL, without significant changes after spontaneous variation of thyroid function. Kawasaki et al. (9) reported that TV was normal in 54% of subjects with HT, but found a slight, moderate or severe goitre in 39%, 5% and 2%, respectively, of the remaining subjects. On the other hand, in HT women living in an iodine-sufficient area, Höfling et al. (7) reported normal (10.7 mL) TV in 62% of subjects and similar numbers of cases of goitre and thyroid atrophy in the remaining subjects. In a group of 144 adult patients with newly diagnosed HT and with normal thyroid function or subclinical/overt hypothyroidism, Carle et al. (12) judged that goitre and thyroid atrophy constituted extremes within a normal range of TV.
In the long interval between one examination and the next, we observed an increasing trend in the need to begin L-T4 administration and a slight reduction in TV only in women on long-term L-T4 therapy. A reduced percentage of goitre and an increased percentage of thyroid atrophy seem to be favoured by L-T4 administration, as well as by the natural history of HT (10, 13). The median and IQR TSH levels that we recorded were always in the normal ranges and no deleterious events occurred, as has been observed during L-T4 therapy in a range of clinical hyperthyroxinaemia (21).
The physiological effects of TSH on thyroid cells are both early (increased iodine uptake, increased hormone synthesis and release) and late (cell proliferation). It is therefore not surprising that we found a reduction in TSH-related growth of the thyroid gland, which is in line with the negative correlation that we observed between TSH levels and TV changes. Indeed, a negative correlation between TSH and TV has already been reported by some authors in young (14) and adult (28) HT subjects. No other significant correlation was noted in our study of HT women. Smoking is reported not to be associated to an increased incidence of HT in an area of mild iodine deficiency (29). In accordance with that observation, we did not find any correlation between TV changes and smoking. In addition, like us, Kawasaki et al. (9) did not report correlation with BMI and age in HT. Finally, in our small cohort of women, BSA was unrelated to long-term TV changes.
The effectiveness of L-T4 treatment on TV in HT has been a matter of debate, and one problem is that some patients receive L-T4 replacement therapy before the evaluation of TV. This phenomenon was observed in our study, and this previous treatment could influence the US pattern, antibody levels, and subsequent TV changes.
Several years ago, in 90 HT subjects with a median age of 12 years at the beginning of L-T4 administration, Svensson et al. (15) reported a significantly larger reduction in TV after 2.8 years among patients with SCH and overt hypothyroidism than among euthyroid patients, irrespective of whether goitre was present or not at the baseline. In a randomized study involving subjects aged <18 years, Dörr et al. (16) reported a decline in mean TV after 30 months in the L-T4-treated group but not in the control group. However, only few subjects were evaluated and, at the end of the study (36 months), mean TV was almost identical in both groups (16). In a small group of 21 adult HT subjects randomized to L-T4 or not, TV was found not to have changed after 12 months of therapy (17). In another group of 33 HT subjects, almost all adult women with normal thyroid function, who were randomised to L-T4 or observation for 15 months, a reduction in TV was noted only in the L-T4-treated group (18). These and our data suggest that L-T4 treatment is beneficial, a conviction that is also supported by the fact that the age-related progression of thyroid hypofunction is the most common consequence of HT.
Recently, Brčić et al. (30) conducted a genome-wide association study in HT in order to verify the hypothesis that TV in this condition is genetically determined. In 370 HT subjects, unselected for gender, some of whom were on L-T4 treatment, these authors reported that TV was lower in L-T4-treated subjects (mean 8.7 mL) than in L-T4-untreated subjects (10.6 mL). However, it was not clear from this study whether the lower TV was linked only to a greater expression of the genetic loci involved in apoptosis in the treated group than in the untreated group. Moreover, the study was not randomized, L-T4 was started because of progression of hypothyroidism, and the time from the beginning of L-T4 treatment to data analysis was not reported (30).
Our study has several limitations: a) it must be considered that the ellipsoid formula per se cannot provide an adequate mathematical description of TV; b) our cohort of subjects was small, which might have limited the statistical power of the analysis; c) as the study was performed at a single out-patient clinic with a specific population, the results may not be representative of the entire extra-regional area; d) neither UIC nor selenium levels were available; e) the COVID-19 pandemic prevented us from extending our follow-up data. The strengths of our study were the availability of a detailed clinical history comprising pharmacological anamnesis, and the absence of inter-observer variability.
In conclusion, our data indicated that the majority of women with HT living in an area of moderate iodine sufficiency had normal TV. Moreover, in these subjects, long-term L-T4 treatment can be undertaken in order to control TV, as well as to maintain normal thyroid function parameters.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
We thank Bernard Patrick for revising the English language of the paper.
Statement of Ethics
All procedures performed were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Owing to the retrospective nature of the study, no formal request was made to our regional ethics committee. All patients provided written informed consent for medical file data collection.
Author contributions
MG conceived the study, carried out US evaluations, conducted data collection and statistical analysis, and drafted the manuscript. MS participated in the conception of the study and data collection. The authors have read and approved the final manuscript.
References
- 1.Mincer DL, Jialal I. Hashimoto thyroiditis. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 2.Xu C, Wu F, Mao C, Wang X, Zheng, Bu L, Mou X, Zhou Y, Yuan G, Wang S, Xiao Y. Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J Autoimmun. 2016;75:50–57. doi: 10.1016/j.jaut.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Rotondi M, Carbone A, Coperchini F, Fonte R, Chiovato L. IgG4-related thyroid autoimmune disease. Eur J Endocrinol. 2019;180(5):R175–R183. doi: 10.1530/EJE-18-1024. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of Hashimoto’s thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010;95(3):1309–1317. doi: 10.1210/jc.2009-1794. [DOI] [PubMed] [Google Scholar]
- 5.Peng CC, Huai-En Chang R, Pennant M, Huang HK, Munir KM. A literature review of painful Hashimoto thyroiditis: 70 published cases in the past 70 years. J Endocr Soc. 2020;4(2):1–15. doi: 10.1210/jendso/bvz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Aguilera B, Betea D, Lutteri L, Cavalier E, Geenen V, Beckers A, Valdes-Socin H. Conversion to Graves disease from Hashimoto thyroiditis: a study of 24 patients. Arch Endocrinol Metab. 2018;62(6):609–614. doi: 10.20945/2359-3997000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Höfling DB, Marui S, Buchpiguel CA, Cerri GG, Chammas MC. The end-diastolic velocity of thyroid arteries is strongly correlated with the peak systolic velocity and gland volume in patients with autoimmune thyroiditis. J Thyroid Res. 2017 doi: 10.1155/2017/192497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kara T, Ateş F, Durmaz MS, Akyürek N, Durmaz FD, Öztürk B, Öztürk M. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto’s thyroiditis patients. J Ultrasound. 2020;23(4):543–551. doi: 10.1007/s40477-020-00437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki M, Ito M, Danno H, Kousaka K, Nakamura T, Yoshioka W, Kasahara T, Kudo T, Nishihara E, Fukata S, Nishikawa M, Nakamura H, Toyoda N, Miyauchi A. The association between thyroid hormone balance and thyroid volume in patients with Hashimoto thyroiditis. Endocrinol J. 2019;66(9):763–768. doi: 10.1507/endocrj.EJ19-0063. [DOI] [PubMed] [Google Scholar]
- 10.Nordmeyer JP, Shafeh TA, Heckmann C. Thyroid sonography in autoimmune thyroiditis. A prospective study on 123 patients. Acta Endocrinol (Copenh) 1990;122(3):391–395. doi: 10.1530/acta.0.1220391. [DOI] [PubMed] [Google Scholar]
- 11.Raber W, Gessel RW, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12(8):725–731. doi: 10.1089/105072502760258712. [DOI] [PubMed] [Google Scholar]
- 12.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Jørgensen T, Laurberg P. Thyroid volume in hypothyroidism due to autoimmune disease follows a unimodal distribution: evidence against primary thyroid atrophy and autoimmune thyroiditis being distinct diseases. J Clin Endocrinol Metab. 2009;94(3):833–839. doi: 10.1210/jc.2008-1370. [DOI] [PubMed] [Google Scholar]
- 13.Duarte GC, Araujo LGQ, Filho FM, Filho CMA, Cendoroglo MS. Ultrasonographic assessment of thyroid volume in oldest-old individuals. Arch Endocrinol Metab. 2017;61(3):269–275. doi: 10.1590/2359-3997000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaruratanasirikul S, Leethanaporn K, Khuntigij P, Sriplung H. The clinical course of Hashimoto’s thyroiditis in children and adolescents: 6 years longitudinal follow-up. J Pediatr Endocrinol Metab. 2001;14(2):177–184. doi: 10.1515/jpem.2001.14.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Svensson J, Ericsson UB, Nilsson P, Olsson C, Jonsson B, Lindberg B, Ivarsson S-A. Levothyroxine treatment reduces thyroid size in children and adolescents with chronic autoimmune thyroiditis. J Clin Endocrinol Metab. 2006;91(5):1729–1734. doi: 10.1210/jc.2005-2400. [DOI] [PubMed] [Google Scholar]
- 16. Dörr HG, Bettendorf M, Binder G, Karges B, Kneppo C, Schmidt H, Voss E, Wabitsch M, Dötsch J. Levothyroxine treatment of euthyroid children with autoimmune Hashimoto thyroiditis: results of a multicenter, randomized, controlled trial. Horm Res Paediatr. 2015;84(4):266–274. doi: 10.1159/000437140. [DOI] [PubMed] [Google Scholar]
- 17.Padberg S, Heller K, Usadel KH, Schumm-Draeger PM. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11(3):249–255. doi: 10.1089/105072501750159651. [DOI] [PubMed] [Google Scholar]
- 18. Aksoy DY, Kerimoglu U, Okur H, Canpinar H, Karaagaoglu E, Yetgin S, Kansu E, Gedik O. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52(3):337–343. doi: 10.1507/endocrj.52.337. [DOI] [PubMed] [Google Scholar]
- 19.Romaldini JH, Biancalana MM, Figueiredo DI, Farah CS, Mathias PC. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6(3):183–188. doi: 10.1089/thy.1996.6.183. [DOI] [PubMed] [Google Scholar]
- 20.Hegedus L, Hansen JM, Feldt-Rasmussen U, Hansen BM, Hoier-Madsen M. Influence of thyroxine treatment on thyroid size and anti-thyroid peroxidase antibodies in Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 1991;35(3):235–238. doi: 10.1111/j.1365-2265.1991.tb03528.x. [DOI] [PubMed] [Google Scholar]
- 21.Biondi B, Cooper DS. Thyroid hormone suppression therapy. Endocrinol Metab Clin North Am. 2019;48(1):227–237. doi: 10.1016/j.ecl.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Massaro F, Vera L, Schiavo M, Lagasio C, Caputo M, Bagnasco M, Minuto F, Giusti M. Ultrasonography thyroid volume estimation in hyperthyroid patients treated with individual radioiodine dose. J Endocrinol Invest. 2007;30(4):318–322. doi: 10.1007/BF03346299. [DOI] [PubMed] [Google Scholar]
- 23.Giusti M, Sidoti M. Normal thyroid volume in subjects evaluated in a primary ambulatory setting in Liguria. Minerva Endocrinol. 2021 doi: 10.23736/S2724-6507.20.03312-X. 10.23736/S0391-1977.20.03312-X Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Italian Parliament Low 55/2005. Disposizioni finalizzate alla prevenzione del gozzo endemico e di altre patologie da carenza iodica. Gazzetta Ufficiale della Repubblica Italiana 91. 2005 [Google Scholar]
- 25.Conte L, Comina M, Monti E, Sidoti M, Vannozzi O, Di Ciolo L, Lillo L, Giusti M. Urinary iodine concentration in a cohort of adult outpatients with thyroid disease in Liguria 14 years after the law on salt iodization. Nutrients. 2019 doi: 10.3390/nu12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghervan C. Thyroid and parathyroid ultrasound. Med Ultrason. 2011;13(1):80–84. [PubMed] [Google Scholar]
- 27.Doupis J, Stavrianos C, Saltiki K, Mantzou E, Mastokostopoulos A, Philippou G, Alevizaki M. Thyroid volume, selenium levels and nutritional habits in a rural region in Albania. Hormones (Athens) 2009;8(4):296–302. doi: 10.14310/horm.2002.1246. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Huang H, Zeng J, Sun C. Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: a cross-sectional study. BMC Public Health. 2013 doi: 10.1186/1471-2458-13-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rendina D, De Palma D, De Filippo G, De Pascale F, Muscariello R, Ippolito R, Fazio V, Fiengo A, Benvenuto D, Strazzullo P, Galletti F. Prevalence of simple nodular goiter and Hashimoto’s thyroiditis in current, previous, and never smokers in a geographic area with mild iodine deficiency. Horm Metab Res. 2015;47(3):214–219. doi: 10.1055/s-0034-1387702. [DOI] [PubMed] [Google Scholar]
- 30.Brčić L, Barić A, Benzon B, Brekalo M, Gračan S, Kaličanin D, Škrabić V, Zemunik T, Barbalić M, Novak I, Pešutić Pisac V, Punda A, Boraska Perica V. AATF and SMARCA2 are associated with thyroid volume in Hashimoto’s thyroiditis patients. Sci Rep. 2020 doi: 10.1038/s41598-020-58457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


