Abstract
Context.
The effects of COVID-19 on the adrenocortical system and its hormones are not well known.
Objectives.
We studied serum cortisol, serum adrenocorticotropic hormone (ACTH), and their ratio in hospitalized non-critically ill COVID-19 patients.
Design.
A prospective case-control study.
Methods.
The study participants were divided into 2 groups. Group 1 consisted of 74 COVID-19 patients. The second group consisted of 33 healthy persons. Early admission above hormones levels was determined and compared between the study groups. Besides that, COVID-19 patients were grouped according to their Glasgow Coma Score (GCS), CURB-65 score, and intensive care unit (ICU) requirement, and further sub-analyses were performed.
Results.
There were no significant differences in the mean age or gender distribution in both groups. In the patients’ group, the serum ACTH concentration was lower than in the healthy group (p<0.05). On the other hand, the serum cortisol levels and cortisol/ACTH ratio of the patients’ group were significantly higher than of the healthy controls (p<0.05, all). Further analyses showed that, although serum cortisol and ACTH levels were not high, the cortisol/ACTH ratio was higher in COVID-19 patients with low GCS (<15) than patients with normal GCS (=15) (p<0.05). In COVID-19 in patients with different CURB-65 scores, the cortisol/ACTH ratio was significantly different (p<0.05), while serum cortisol and ACTH were not different in groups (p>0.05). Serum cortisol levels and cortisol/ACTH ratio were higher but ACTH level was lower in the ICU needed COVID-19 patients than in patients who do not need ICU (p<0.05).
Conclusion.
Our pilot study results showed that the cortisol/ACTH ratio would be more useful than serum cortisol and/or ACTH levels alone in evaluating the adrenocortical system of COVID-19 patients. Still, further detailed studies are needed to confirm these.
Keywords: COVID-19, cortisol, ACTH, CURB-65, coronavirus, pandemic
Introduction
COVID-19 is caused by the new coronavirus (SARS-CoV-2). It first started in December 2019 in China (1). Then it spread to the most parts of the world. In Turkey, the first COVID-19 positive case was reported on March 10, 2020 (2). Mortality is increased in patients older than 50 years as well as in men (3). The World Health Organization (WHO) and most of the countries’ health authorities are updating the guidelines of the approach and treatment of this new viral disease continuously. Although steroid therapy was not recommended at the beginning of the COVID-19 pandemic, these days WHO recommend its use in critically ill COVID-19 patients (4, 5). A new study by Tan et al. reported that a high serum cortisol level was associated with increased mortality in COVID-19 patients (6). So, deciding to start steroid therapy in non-critically ill COVID-19 patients with a high basal serum cortisol level would be challenging to health professionals. Although rare, there are reports of adrenal gland injuries (infarction and/or hemorrhage) during COVID-19 infection (7–9). As we know, the main measurable serum hormones of the adrenocortical system are adrenocorticotrophic hormone - ACTH (made by the pituitary gland) and cortisol that is secreted by the adrenal glands. In a study by Alici O (10), community-acquired pneumonia’s patients’ serum cortisol levels (but not serum ACTH levels) were higher than in healthy controls. Additionally, both ACTH and cortisol serum levels of these patients increased significantly during the febrile period (p<0.05), as expected. The adrenocortical system has an important immunomodulatory role during viral infection. It can modulate cytokine release and/or cell proliferation (11). Although serum cortisol levels have been studied in COVID-19 patients, serum ACTH levels and their ratio (i.e. cortisol/ACTH) have not been studied yet. So, in this pilot study, we will try to study these.
Methods
This prospective observational study has been approved by Bakirkoy Dr. Sadi Konuk Training & Research Hospital’s ethical committee. Ethics was conducted according to The Declaration of Helsinki. Written informed consent has been taken from participants or their family members before enrollment. The centers of this study were the Internal Medicine Department of Istanbul Kanuni Sultan Suleyman Training & Research Hospital, Istanbul, Turkey, and the Internal Medicine Department of Basaksehir Cam & Sakura State Hospital, Istanbul, Turkey.
Participants were divided into 2 groups:
Group 1: Mild-severe COVID-19 patients. This group consisted of COVID-19 patients defined as a mild disease (patients with no or mild pneumonia) or severe disease (patients with dyspnea, hypoxia, or >50% lung involvement on imaging within 24 to 48 hours) according to the classification of disease severity (12). The COVID-19 patients with the critical disease (patients with respiratory failure, shock, or multiorgan dysfunction) were excluded from the study.
Group 2: Age and sex-matched healthy volunteers that had no possible clinical and/or laboratory features of COVID-19 and had a negative PCR test of COVID-19 at the time of enrolment (that was done for employment or travel purpose).
Inclusion criteria:
- Age > 18 years old (all groups),
- Ability to give written consent (participant or his/her 1st-degree relative).
Exclusion criteria;
- Critically ill COVID-19 patients (both groups),
- Severe COVID-19 patients that needed corticosteroid therapy at admission (both groups),
- Using medication(s) that could affect the study parameters levels,
- History of pituitary and/or adrenal gland operation (both groups).
- Presence of chronic inflammation or disease(s) (group 2),
- Presence of previous COVID-19 infection history (group 2),
- Presence of adrenocortical disease (Cushing’s disease, adrenal insufficiency) (both groups),
- High serum C-reactive protein levels (group 2 only).
Within 24 hours of admission or application to the hospital, fasting blood samples were taken for the below laboratory parameters. Venous blood sampling was carried out in the early morning after overnight fasting in all participants. All venous blood samples were collected by venipuncture into K3-EDTA plasma, collected using siliconized glass tubes or plastic tubes. The only pre-cooled sampling vials were used. After drawing the blood, the vials were put immediately on ice. A cooled centrifuge was used to separate the plasma. Samples were measured immediately or frozen at -20 ºC (± 5º C). Then, the patient was managed according to the Turkish Ministry of Health’s COVID-19 management guidelines (13). The investigators did not act the selection of treatment protocols.
The following laboratory blood tests were performed on the blood samples obtained from all participants: serum cortisol (mcg/dL) and serum ACTH (pg/mL).
Biochemical Analysis
These laboratory measurements are in routine use at university hospitals. Still, to standardize the measurements, all the above-mentioned study blood test measurements were performed at Kanuni Sultan Suleyman Training & Research Hospital’s central laboratory. Internal quality control and external quality assurance are applied to monitor the accuracy and precision of tests. Adrenocorticotropic hormone and cortisol levels were determined using Roche Elecys immunoassay analyzer (Roche Diagnostic GmbH Mannheim, Germany). The cortisol measuring range is 1.5-1750 nmol/L or 0.054-63.4 μg/dL (defined by the Limit of Detection and the maximum of the master curve, or up to 17500 nmol/L or 634 μg/dL for 10-fold diluted samples). Adrenocorticotropic hormone measuring range is 1.00-2000 pg/mL or 0.220-440 pmol/L (defined by the Lower Detection Limit and the maximum of the master curve). The limit of detection was determined following the Clinical and Laboratory Standards Institute EP17-A requirements. Before calculating the ratio of cortisol to ACTH, the 2 parameters were converted to the same measurement units (i.e. mcg/dL).
To diagnose the possible adrenal insufficiency in patients with serum cortisol <5 nmol/L, a standard dose (250 μg) corticotropin stimulation test was planned. Adrenal insufficiency was considered when the peak cortisol level at 30 or 60 minutes was below 18 nmol/L.
Glasgow Coma Score (GCS) was calculated in all COVID-19 patients and patients were divided into 2 groups as GCS <15 and GCS = 15.
CURB-65 score was calculated in all COVID-19 patients who were grouped according to the CURB-65 score (i.e. CURB-65 score 0, CURB-65 score-1, CURB-65 score-2).
The primary aim of this study was to determine and compare the above-mentioned parameters between group 1 and 2 participants.
A secondary aim was to compare the above parameters in group 1 COVID-19 patients according to GCS, CURB-65 scores, and intensive care unit (ICU) needs (14, 15).
Data availability
Data are available to researchers (for research purpose only) on request by directly contacting the corresponding author.
Statistical analysis
All categorical variables were summarized with frequencies and percentages whereas the numerical variables were summarized with mean and standard deviations (SD) or medians and interquartile ranges (Q1, Q3) according to their distributions. The numerical variables were compared across two groups with Mann Whitney U test or t-test and three or more groups by the Kruskal-Wallis test or ANOVA depending on their distribution. The assumption of normality was assessed with Shapiro Wilks’ test in addition to visual inspections of QQ and PP plots. The homogeneity of variance was assessed using Levene’s test. The significant group comparisons of numerical variables for three or more groups were followed by post-hoc tests. Pairwise comparisons were conducted using the Mann-Whitney U test where Benjamini-Hochberg adjusted p-values were evaluated. The p-value smaller than 0.05 represents statistical significance. The R version 4.0.2 (2020-06-22) was employed for all analyses.
Results
A total of 107 [61 females (F) / 46 males (M)] participants were enrolled in this study. The mean (SD) age of the participants was 57.206 (13.529) years. Group 1 consisted of 74 (43F / M 31) COVID-19 patients. The second group consisted of 33 (18F / 15M) healthy persons. The mean (SD) age of both groups was 58.486 (14.430) and 54.333 (10.908) years, respectively. There was no significant difference in mean age and gender (F / M ratio) between the two study groups (P>0.05, in both).
In our participants, there was no one with a high suspicion of adrenal insufficiency (i.e. serum cortisol less than 5 nmol/L) that needed urgent ACTH stimulation test and/or urgent steroid therapy.
Comparing serum cortisol, ACTH and their ratio between the two study groups showed that serum cortisol and cortisol/ACTH ratio levels were significantly higher in group 1, but serum ACTH levels were significantly higher in group 2 participants (Table 1 and Fig. 1).
Figure 1.
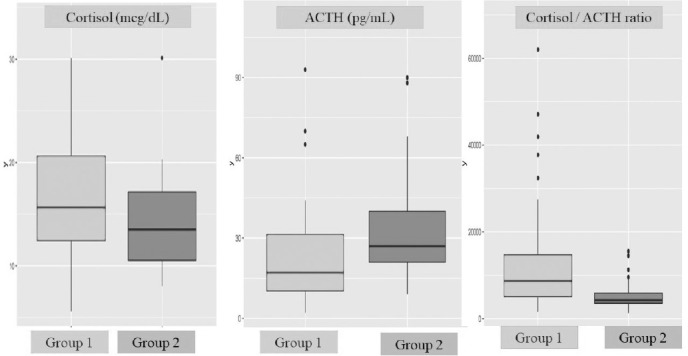
Comparison of serum cortisol, ACTH, and their ratio between COVID-19 patients and healthy controls.
Table 1.
Comparison of COVID-19 patients study parameters with healthy controls
| COVID-19 patients (n=74) | Healthy controls (n=33) | Total (n=107) |
p value | |
|---|---|---|---|---|
| Age | NS | |||
| Mean (SD) | 58.486 (14.430) | 54.333 (10.908) | 57.206 (13.529) | |
| Median (Q1,Q3) | 58.500 (47.500, 70.750) | 54.000 (48.000, 63.000) | 58.000 (48.000, 67.500) | |
| Min – Max | 19.000 - 84.000 | 23.000 - 72.000 | 19.000 - 84.000 | |
| Gender | NS | |||
| Female | 43 (58.1%) | 18 (54.5%) | 61 (57.0%) | |
| Male | 31 (41.9%) | 15 (45.5%) | 46 (43.0%) | |
| Serum cortisol | 0.034 | |||
| Mean (SD) | 16.443 (5.940) | 14.104 (4.772) | 15.721 (5.688) | |
| Median (Q1,Q3) | 15.655 (12.443, 20.617) | 13.520 (10.520, 17.150) | 15.000 (11.470, 18.910) | |
| Min – Max | 5.600 - 34.050 | 8.040 - 30.120 | 5.600 - 34.050 | |
| Serum ACTH | 0.002 | |||
| Mean (SD) | 23.408 (20.173) | 33.000 (19.551) | 26.366 (20.383) | |
| Median (Q1,Q3) | 17.000 (10.250, 31.400) | 27.000 (21.000, 40.000) | 21.000 (13.000, 33.000) | |
| Min – Max | 2.000 - 114.000 | 9.000 - 90.000 | 2.000 - 114.000 | |
| Cortisol/ACTH ratio | <0.001 | |||
| Mean (SD) | 12879.091 (12957.387) | 5507.039 (3396.952) | 10605.467 (11437.214) | |
| Median (Q1,Q3) | 8651.103 (5062.446, 14696.930) | 4203.226 (3558.824, 5876.000) | 6297.059 (4184.524, 12132.579) | |
| Min - Max | 1578.947 - 70333.333 | 1243.333 - 15522.222 |
NS: not significant.
The median (min-max) days of medical word stay of the patients’ group was 8 (3-18) days. Further comparison of group 1 COVID-19 patients’ parameters according to GCS (=15 or <15), CURB-65 pneumonia severity index and outcome (requirement of ICU care or discharge from hospital) were done. Age and gender were not significantly different between COVID-19 patients with normal GCS (15) (n=66) and those with low GCS (<15) (n=8) (p>0.05, both).
Although serum cortisol and ACTH levels were not significantly different between the above-mentioned two patients’ sub-groups, the cortisol/ACTH ratio of normal GCS patients was significantly lower than the low GCS patients’ sub-group (Table 2).
Table 2.
Comparison of COVID-19 patients group according to Glasgow Coma Score (GCS)
| GCS=15 (n=66) | GCS <15(n=8) | Total (n=74) | p value | |
|---|---|---|---|---|
| Age | NS | |||
| Mean (SD) | 58.182 (14.503) | 61.000 (14.501) | 58.486 (14.430) | |
| Median (Q1, Q3) | 58.500 (47.500, 70.750) | 58.000 (49.250, 68.500) | 58.500 (47.500, 70.750) | |
| Min - Max | 19.000 - 83.000 | 47.000 - 84.000 | 19.000 - 84.000 | |
| Gender | NS | |||
| Female | 39 (59.1%) | 4 (50.0%) | 43 (58.1%) | |
| Male | 27 (40.9%) | 4 (50.0%) | 31 (41.9%) | |
| Cortisol (mcg/dL) | NS | |||
| Mean (SD) | 16.152 (5.833) | 18.840 (6.679) | 16.443 (5.940) | |
| Median (Q1, Q3) | 15.655 (12.340, 20.533) | 16.120 (14.127, 23.530) | 15.655 (12.443, 20.617) | |
| Min - Max | 5.600 - 34.050 | 11.340 - 30.130 | 5.600 - 34.050 | |
| ACTH (pg/mL) | NS | |||
| Mean (SD) | 24.655 (20.800) | 13.125 (9.538) | 23.408 (20.173) | |
| Median (Q1, Q3) | 17.500 (11.250, 32.900) | 11.500 (5.500, 18.750) | 17.000 (10.250, 31.400) | |
| Min - Max | 2.000 - 114.000 | 3.000 - 28.000 | 2.000 - 114.000 | |
| Cortisol/ACTH ratio | 0.012 | |||
| Mean (SD) | 11716.224 (12334.477) | 22472.744 (14840.306) | 12879.091 (12957.387) | |
| Median (Q1, Q3) | 7782.860 (4889.423, 12388.824) | 17120.588 (12095.312, 33731.250) | 8651.103 (5062.446, 14696.930) | |
| Min - Max | 1578.947 - 70333.333 | 5042.857 - 47100.000 | 1578.947 - 70333.333 |
NS: not significant.
Comparison of patients group in relation to CURB-65 score of 0 (n=36), 1 (n=24), and 2 (n=14) is shown in Table 3. As expected, the age was different between the above three CURB-65 score sub-group COVID-19 patients. Also, the cortisol/ACTH ratio was significantly different between the above-mentioned three CURB-65 score subgroups. A pairwise Wilcoxon post-hoc test revealed that age in the CURB-65 score 0 sub-group was significantly lower than both the CURB-65 score 1 and 2 sub-groups. Cortisol/ACTH ratio of CURB-65 score 2 patients was significantly higher only from CURB-65 score 0 patients(see Table 3 for mean [SD], median, and p values). Although the p-value for serum cortisol was 0.044, post-hoc analysis showed no significant differences between the groups (p > 0.05, in all).
Table 3.
Comparison of COVID-patients study parameters according to CURB-65 scores
| Score 0 (n=36) | Score 1 (n=24) | Score 2 (n=14) | Total (n=74) | p value | |
|---|---|---|---|---|---|
| Age (years) | < 0.001 | ||||
| Mean (SD) | 49.194 (11.326) | 66.792 (11.572) | 68.143 (10.960) | 58.486 (14.430) | |
| Median (Q1, Q3) | 51.000 (44.750, 58.000) | 71.000 (62.000, 74.000) | 69.500 (64.250, 75.250) | 58.500 (47.500, 70.750) | |
| Min - Max | 19.000 - 64.000 | 31.000 - 83.000 | 49.000 - 84.000 | 19.000 - 84.000 | |
| *Posthoc | a | b | b | ||
| Gender | NS | ||||
| Female | 19 (52.8%) | 15 (62.5%) | 9 (64.3%) | 43 (58.1%) | |
| Male | 17 (47.2%) | 9 (37.5%) | 5 (35.7%) | 31 (41.9%) | |
| *Posthoc | - | - | - | ||
| Cortisol (mcg/dL) | 0.044 | ||||
| Mean (SD) | 14.990 (6.190) | 16.775 (5.322) | 19.611 (5.290) | 16.443 (5.940) | |
| Median (Q1, Q3) | 14.380 (10.105, 18.620) | 16.460 (14.422, 18.670) | 20.870 (14.348, 22.452) | 15.655 (12.443, 20.617) | |
| Min - Max | 5.600 - 28.410 | 6.590 - 34.050 | 12.320 - 30.130 | 5.600 - 34.050 | |
| *Posthoc | a | a | a | ||
| ACTH (pg/mL) | NS | ||||
| Mean (SD) | 25.861 (23.119) | 22.050 (16.042) | 19.429 (18.810) | 23.408 (20.173) | |
| Median (Q1, Q3) | 17.500 (13.000, 30.200) | 17.000 (9.750, 33.500) | 10.500 (7.500, 27.000) | 17.000 (10.250, 31.400) | |
| Min - Max | 2.000 - 114.000 | 3.000 - 65.000 | 3.000 - 70.000 | 2.000 - 114.000 | |
| *Posthoc | a | a | a | ||
| Cortisol/ACTH ratio | 0.017 | ||||
| Mean (SD) | 9984.849 (10930.832) | 12468.869 (9897.552) | 21024.663 (18783.642) | 12879.091 (12957.387) | |
| Median (Q1, Q3) | 6370.752 (4509.746, 11481.731) | 9776.042 (5648.359, 13366.176) | 14238.304 (8710.172, 26858.333) | 8651.103 (5062.446, 14696.930) | |
| Min - Max | 1578.947 - 62000.000 | 3623.188 - 41900.000 | 2520.000 - 70333.333 | 1578.947 - 70333.333 | |
| *Posthoc | a | ab | b |
*Pairwise Wilcoxon test (P value of the same letters is >0.05, and of the different letters [i.e, a versus b] is <0.05).
Comparison of COVID-19 patients that needed ICU admission (n=6) with those that were discharged without any ICU care admission (n=68) is shown in Table 4. Again, the age and gender were not different between these two sub-groups (p>0.05). Serum cortisol levels were higher in the ICU needed group, but serum ACTH levels were higher in the opposite group. Lastly, the cortisol/ACTH ratio was higher in ICU needed group (see Table 4 for mean [SD], median, and p values).
Table 4.
Comparison of COVID-19 patients group according to outcomes [needing intensive care unit (ICU) admission or discharge]
| Discharge (n=68) | ICU (n=6) | Total (n=74) | p value | |
|---|---|---|---|---|
| Age | NS | |||
| Mean (SD) | 58.426 (14.842) | 59.167 (9.368) | 58.486 (14.430) | |
| Median (Q1, Q3) | 58.500 (47.000, 71.000) | 59.500 (52.750, 64.750) | 58.500 (47.500, 70.750) | |
| Min - Max | 19.000 - 84.000 | 47.000 - 72.000 | 19.000 - 84.000 | |
| Gender | NS | |||
| Female | 40 (58.8%) | 3 (50.0%) | 43 (58.1%) | |
| Male | 28 (41.2%) | 3 (50.0%) | 31 (41.9%) | |
| Cortisol (mcg/dL) | 0.005 | |||
| Mean (SD) | 15.838 (5.651) | 23.302 (5.080) | 16.443 (5.940) | |
| Median (Q1, Q3) | 15.210 (12.283, 19.017) | 23.705 (21.495, 25.742) | 15.655 (12.443, 20.617) | |
| Min - Max | 5.600 - 34.050 | 15.090 - 30.130 | 5.600 - 34.050 | |
| ACTH (pg/mL) | 0.039 | |||
| Mean (SD) | 24.532 (20.556) | 10.667 (8.214) | 23.408 (20.173) | |
| Median (Q1, Q3) | 17.500 (11.750, 32.700) | 8.000 (4.750, 15.000) | 17.000 (10.250, 31.400) | |
| Min - Max | 2.000 - 114.000 | 3.000 - 24.000 | 2.000 - 114.000 | |
| Cortisol/ACTH ratio | 0.001 | |||
| Mean (SD) | 11135.607 (10601.948) | 32638.576 (20861.643) | 12879.091 (12957.387) | |
| Median (Q1, Q3) | 7782.860 (4968.269, 12342.941) | 29938.889 (18375.327, 36393.750) | 8651.103 (5062.446, 14696.930) | |
| Min - Max | 1578.947 - 62000.000 | 12554.167 - 70333.333 | 1578.947 - 70333.333 |
NS. Not significant.
Discussion
As far as we know, only the study of Tan and colleagues compared serum cortisol levels of COVID-19 with non-COVID-19 participants (6). The median serum cortisol level of COVID-19 patients was 619 nmol/L (22.43 mcg/dL), while of the non-COVID-19 patients was 519 nmol/L (18.81mcg/dL) (p<0.0001). Additionally, they found that a baseline admission serum cortisol ≤744nmol/L (26.96mcg/dL) was associated with less mortality, and baseline serum cortisol levels above this 744nmol/dL associated with higher mortality (i.e. a median survival of 35 versus 15 days, respectively). They did not compare the serum cortisol levels with disease severity indices. Also, they did not determine and compared serum ACTH levels (and serum cortisol/ACTH ratio) between the 2 study groups (6, 16). Although our present study does not include critically ill or severe COVID-19 patients that needed steroid therapy at admission, the mean and median serum cortisol concentrations of the patients’ group were significantly higher than in the healthy controls (p=0.034). On the other hand, serum ACTH concentrations of our patients’ group were significantly lower than in the healthy controls (p=0.002). As a result, the serum cortisol/ACTH ratio of the patients’ group was significantly higher than in the healthy group (p<0.001) (see Table 1 for means, medians, etc.). As shown in Figure 1, the difference in median cortisol /ACTH ratio is more prominent than in the others. Using the ratio of cortisol/ACTH in the diagnosis of primary hypoadrenalism and/or Cushing’s disease is suggested by some investigators (17, 18). So, using this ratio in future similar studies of COVID-19 infected patients would add more information on the effect of this disease on the hypothalamic-adrenocortical axis.
Sub-analysis of our COVID-19 patients’ group serum cortisol levels according to GCS or CURB-65 score showed no significant difference. Comparison of patients’ serum ACTH concentrations yielded a similar result (p>0.05, in all). Surprisingly, the cortisol /ACTH ratio was significantly higher in COVID-19 patients with GSC<15 (in comparison to patients with GCS=15), and in patients with CURB-65 score 2 (in comparison to patients with a CURB-65 score 0) (p was 0.012, and 0.017, respectively) (Tables 2, 3). None of our study COVID-19 patients has died. But 6 of the total 74 patients (i.e. 8.11%) needed ICU admission during the hospitalization period. The median serum ACTH concentration of the ICU needed patients was lower than the non-ICU needed subgroups (p=0.039). On the other hand, the median serum cortisol level and cortisol/ACTH ratio of these 6 ICU needed patients were significantly higher than the other subgroup patients (p were 0.005, and 0.001, respectively) (Table 4).
Unfortunately, the national COVID-19 management guideline at the time of data collection of this study did not recommend the routine use of steroids in mild-severe non-ICU care required patients. The results of the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial regarding the efficacy of dexamethasone were announced afterwards (19). Also, critically ill COVID-19 patients were not included in this study. So, except at ICU admission, none of the study patients received any steroid therapy during the stay in our medical department. As a result, we could not see the role of baseline serum cortisol, ACTH (and especially their ratio) on predicting the response to steroid therapy in this challenging viral disease. Future studies might point out these.
Our study was a prospective observational one. We, as investigators, did not influence the management of the study patients’ group. This may be considered a strength. It has certain weaknesses. One of them is not including critically ill COVID-19 patients. Another limitation is not containing repeated measurements of the study laboratory parameters. As known, some participants related confounding factors might affect study parameters (ACTH, cortisol levels, etc.). The effect of these confounding factors on cortisol/ACTH ratio levels would be less than their effect on serum cortisol and/or ACTH levels alone. So, using this ratio in such studies would be more appropriate. Still, as a pilot study, it may lead to further detailed studies in this field.
In conclusion, our pilot study results showed that serum cortisol, ACTH, and cortisol/ACTH ratio was significantly different in COVID-19 patients (in comparison to healthy controls). Further comparison of these study parameters in COVID-19 subgroup patients (concerning GCS, CURB-65 score, and ICU care needs) showed the unique useful parameter is cortisol/ACTH ratio. Further studies are needed to confirm these.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kodaz H. Successful Treatment Strategy of Turkey against COVID-19 Outbreak. Eurasian J Med Oncol. 2020 doi: 10.14744/ejmo.2020.12345. [DOI] [Google Scholar]
- 3.Older Adults and COVID-19 | CDC. n.d.. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html accessed 8 September 2020).
- 4.WHO updates guidance on corticosteroids in COVID-19 patients. 2020. https://www.pharmaceutical-technology.com/news/who-corticosteroids-covid-advice/ accessed 12 September 2020.
- 5.WHO updates guidance on corticosteroids in COVID-19 patients. n.d.. https://www.pharmaceutical-technology.com/news/who-corticosteroids-covid-advice/ accessed 7 September 2020.
- 6.Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT, Comninos AN, Abbara A, Dhillo WS. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(8):659–660. doi: 10.1016/S2213-8587(20)30216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R, Guruparan T, Siddiqi S, Sheth R, Jacyna M, Naghibi M, Vrentzou E. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep. 2020;6(3):20200075. doi: 10.1259/bjrcr.20200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel M, Feldman I, Levine M, Frank Y, Bogot NR, Benjaminov O, Kurd R, Breuer GS, Munter G. Bilateral Adrenal Hemorrhage in Coronavirus Disease 2019 Patient: A Case Report. J Clin Endocrinol Metab. 2020;105(12):dgaa487. doi: 10.1210/clinem/dgaa487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somasundaram NP, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Dissanayake HA, Yogendranathan N, Gamage KKK, de Silva NL, Sumanatilleke M, Katulanda P, Grossman AB. The Impact of SARS-Cov-2 Virus Infection on the Endocrine System. J Endocr Soc. 2020;4(8):bvaa082. doi: 10.1210/jendso/bvaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alici O, Koca C, Kaya A, Karakurt F, Carlioglu A, Kosar A, Kanbay M. Responses of anterior pituitary hormones to fever during community-acquired infections. Eur J Gen Med. 2008;5(4):216–221. [Google Scholar]
- 11.Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 2003;16(2):141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- 12.Coronavirus disease 2019 (COVID-19): Clinical features - UpToDate. 2020. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-clinical-features?search=severityofcovid§ionRank=1&usagetype=default&anchor=H4100426989&source=machineLearning&selectedTitle=2~150&display_rank=2#H4100426989 accessed 8 September 2020.
- 13.COVID-19 (Sars-Cov-2 Infection) Guide Republic Of Turkey Ministry Of Health Directorate General Of Public Health. 2020 [Google Scholar]
- 14.Jain S, Iverson LM. Glasgow Coma Scale. [Updated 2020 Jun 23]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020 Available from: https://www.ncbi.nlm.nih.gov/books/NBK513298/ [PubMed] [Google Scholar]
- 15.Nazik S, Kokturk N, Baha A, Ekim N. CURB 65 or CURB(S) 65 for community acquired pneumonia? European Respıratory Journal. 2012:40. [Google Scholar]
- 16.Pal R, Banerjee M, Bhadada SK. Cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020;8(10):809. doi: 10.1016/S2213-8587(20)30304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MK, Vasikaran S, Doery JC, Wijeratne N, Prentice D. Cortisol: ACTH ratio to test for primary hypoadrenalism: a pilot study. Postgrad Med J. 2013;89(1057):617–620. doi: 10.1136/postgradmedj-2012-131723. [DOI] [PubMed] [Google Scholar]
- 18.Selek A, Cetinarslan B, Canturk Z, Tarkun I, Akyay OZ, Cabuk B, Ceylan S. The Utility of Preoperative ACTH/Cortisol Ratio for the Diagnosis and Prognosis of Cushing’s Disease. J Neurosci Rural Pract. 2018;9(1):106–111. doi: 10.4103/jnrp.jnrp_308_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with COVID-19 - Preliminary Report. N Engl J Med. 2020:NEJMoa2021436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to researchers (for research purpose only) on request by directly contacting the corresponding author.


