Main text
Recently, Micklethwaite et al.1 and Bishop et al.2 reported results of a phase I first-in-human clinical trial of CD19-directed allogeneic chimeric antigen receptor (CAR)-T cells in 10 patients with relapsed or persistent B cell malignancies after matched-related allogeneic hematopoietic stem cell transplantation (HSCT). CAR-T cells were produced via electroporation with a Piggybac (PB) transposon vector, encoding a CD19 CAR (second generation, with a 4-1BB co-stimulatory domain) driven by a human elongation factor-1 alpha (EF1a) promoter, terminated by an SV40 polyadenylation signal, and flanked by chicken hypersensitivity site 4 (cHS4) β-globin insulator sequences. Clinical responses (five patients with continuous complete remission at median follow-up of 18 months) were promising and comparable to those seen with retroviral CD19-CAR vectors. Surprisingly, two patients developed donor-derived T cell lymphoma. While the exact causes are still under investigation, clinical trials with PB-based CAR-T cells were voluntarily suspended at the investigators’ research centers. The authors discuss a number of mechanisms that may have contributed to T cell transformation and have performed work to address potential underlying causes, which we put into perspective here regarding lessons learned in gene therapy trials with integrating retroviral vectors.
Most current ex vivo gene therapies, including already approved products, are based on integrating retroviral vectors. However, retroviral vectors present some challenges, such as limited vector cargo capacity and demanding and costly production with limited capacity worldwide. Transposon-based vector systems, such as PB and Sleeping Beauty (SB) represent suitable alternatives. These two-component transposon vector systems are less complex and can be handled under lower biosafety level regulations.
In the new study, patient 2 was treated with CAR-T cells starting in January 2018 and showed a transient response to the treatment. Four months after the first CAR-T cell infusion, a paraaortic lymph node tumor appeared that was shown to be CAR-positive and of donor origin 12 months later. The patient died of sepsis and multiorgan failure 2 months thereafter. A second T cell lymphoma case was found during follow-up screening of the remaining patients. Patient 8 displayed an asymptomatic mediastinal tumor and cervical lymphadenopathy 12 months after CAR-T cell therapy and is in remission after CHOP (cyclophosphamide, doxorubicin or etoposide, vincristine, prednisolone) treatment and secondary allogeneic HSCT.
T cells are generally considered to have a low risk for transformation, as evidenced by thousands of patients treated with CAR- or T cell receptor (TCR)-mediated T cell therapies that were based on genetic modification with retroviral vectors. However, examples of (benign) monoclonal CAR-T cell proliferation have been documented in patients receiving T cells equipped with CD19 and CD22 CARs.3,4 In addition, mature T cells have been reported to be resistant to transformation even after retroviral oncogene expression.5,6 However, several risk factors for developing T cell lymphoma, including insertional dysregulation, expression of transgenes that confer a survival advantage, and the suppression of a polyclonal TCR repertoire have been described. In Bishop et al.,2 CAR overexpression could have contributed to a survival advantage of individual T cell clones. However, the CAR-T cells failed to expand in response to CD19 in vitro and the authors found no permanent signaling. On the other hand, for most leukemias, it is essentially impossible to derive cell lines growing in vitro. Therefore, growth and signaling characteristics in cell culture do not necessarily recapitulate the in vivo situation, where essential signals might be provided by lymphoid stroma tissue. Thus, it remains possible that expression of a CAR has some dominant negative effect on T cell (receptor) diversity that was shown to suppress T cell transformation.5,6
The risk of insertional oncogenesis correlates with the use of increasing vector copy numbers/integrations, possibly due to the increased risk of cooperative oncogenesis in a multiple hit model. This is particularly relevant in settings of preferred integration in vulnerable gene structures, such as promoter/enhancer sequences and gene bodies, and when stronger promoter/enhancer elements are employed. In hematopoietic stem and progenitor cell (HSPC)-based gene therapy trials, the number of vector copy number (VCN) integrants should not be higher than five (https://www.who.int/biologicals/expert_committee/BS.2019.2373_Lentiviral_vector_IS_Study_Report_final.pdf). In patient 2 of the current study, a VCN of 24 was observed in malignant cells (and comparable numbers in non-malignant cells), which may have contributed to the risk of insertional dysregulation.
Of potential clinical importance, the authors reported widespread copy number gains and losses in malignant CAR-T cells. This could indicate major chromosomal instability, potentially exacerbated by the electroporation procedure and transposase activity. The authors detected an embryonic transcriptomic signature, including well-known transcription factors involved in hematopoietic transformation and malignancies. PB-based vectors exhibit an integration preference for transcriptional start sites as well as promoter/enhancer sequences.7 This integration preference is associated with a higher risk of insertional dysregulation of neighboring genes. In both patients who developed T cell lymphomas in the current study, integrations (apparently not resulting in transcriptional dysregulation) into the transcription factor gene BACH2 were observed, which was previously associated with proliferation and survival of T cells in HIV1-infected individuals.8 One patient also had insertions nearby FYN, which is a SYK-related kinase involved in TCR signaling.
While the EF1a short promoter has a good safety record in hematopoietic cells when used in retroviral gene therapy, the authors used a longer version of EF1a that harbors strong splice donor and acceptor sites. This may have contributed to the aberrant read-through transcripts and the “transcriptional shadow” of up to 1,000 kb that the authors described. Noteworthy, insulators would largely decrease enhancer effects on the neighboring host genome, but not read-through transcripts mediated by aberrant splicing and leaky polyadenylation.
The authors suggest that their production methodology was likely the predominant factor in malignant transformation. The methods included an electroporation step with a single high-voltage pulse, high concentrations of the transposable element in DNA form as well as the PB transposase in RNA form, and the subsequent expansion of the CAR-T cells on irradiated peripheral blood mononuclear cell feeders with interleukin-15 supplementation. Future studies should evaluate the effects of the electroporation procedure on non-specific DNA damage and chromosomal instability. Moreover, potential cytotoxicity of the overexpressed transposase should be taken into consideration.9
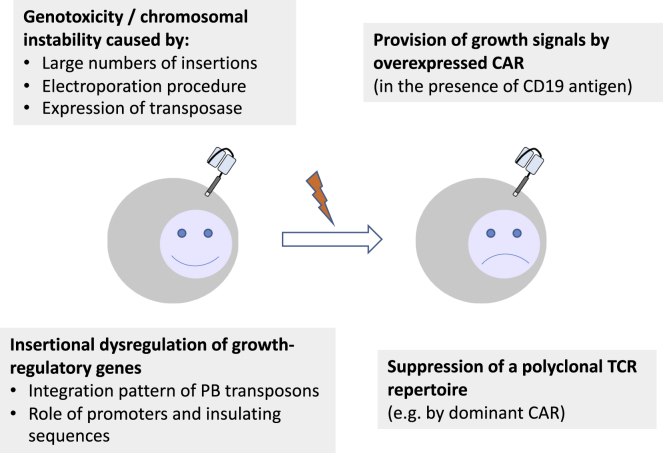
Other factors might still contribute to the different risks seen in studies using very similar vectors. Those might include (but are not restricted to) the transgene, the ex vivo culture conditions, and/or the underlying disease. The rapid kinetics of lymphomagenesis in the two patients suggest a very efficient mechanism of transformation. Since allogeneic cells were used, disease-specific factors might be excluded, and the focus should be on the risks associated with the genetic modification discussed above (Figure 1).
Figure 1.
Mechanisms that potentially contributed to malignant transformation of CD19-CAR T cells generated using PB transposons
In summary, the authors have invested considerable effort laying the basis to understand the underlying causes of the two adverse events in this first-in-human clinical trial using this PB-mediated CAR-T cell production. We agree with their conclusion that future clinical studies should aim for low copy numbers (3–5) to help avoid unwanted toxicities. In addition, further work is urgently needed to more completely elucidate the role of the electroporation procedure and whether the addition of a genome-modifying transposase further contributes to DNA damage and genomic instability. Moreover, transposases with a more random integration pattern (e.g., SB) or with a more targeted integration pattern should be further explored. More generally, the observed high incidence (2/10) of leukemia in the above trial should remind us that gene therapy is still an experimental approach and, even in such a well-studied context, new techniques need to be adopted with care. Since contribution of CAR signaling to the observed transformation could not be completely excluded, strategies aimed at further increasing the fitness of CAR-T cells (such as deletion of checkpoint signals) require particular caution.
Acknowledgments
This work was supported by the REBIRTH Center for Translational Regenerative Medicine funded through the State of Lower Saxony (MWK: ZN3440).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Axel Schambach, Email: schambach.axel@mh-hannover.de.
Boris Fehse, Email: fehse@uke.de.
References
- 1.Micklethwaite K.P., Gowrishankar K., Gloss B.S., Li Z., Street J.A., Moezzi L., Mach M.A., Sutrave G., Clancy L.E., Bishop D.C. Investigation of product derived lymphoma following infusion of piggyBac modified CD19 chimeric antigen receptor T-cells. Blood. 2021 doi: 10.1182/blood.2021010858. Published online May 11, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop D.C., Clancy L.E., Simms R., Burgess J., Mathew G., Moezzi L., Street, J.A., Sutrave, G., Atkins, E., McGuire, H.M. Development of CAR T-cell lymphoma in two of ten patients effectively treated with piggyBac modified CD19 CAR T-cells. Blood. 2021 doi: 10.1182/blood.2021010813. Published online May 19, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah N.N., Qin H., Yates B., Su L., Shalabi H., Raffeld M., Ahlman M.A., Stetler-Stevenson M., Yuan C., Guo S. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 2019;3:2317–2322. doi: 10.1182/bloodadvances.2019000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newrzela S., Al-Ghaili N., Heinrich T., Petkova M., Hartmann S., Rengstl B., Kumar A., Jäck H.M., Gerdes S., Roeder I. T-cell receptor diversity prevents T-cell lymphoma development. Leukemia. 2012;26:2499–2507. doi: 10.1038/leu.2012.142. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich T., Rengstl B., Muik A., Petkova M., Schmid F., Wistinghausen R., Warner K., Crispatzu G., Hansmann M.L., Herling M. Mature T-cell lymphomagenesis induced by retroviral insertional activation of Janus kinase 1. Mol. Ther. 2013;21:1160–1168. doi: 10.1038/mt.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogol-Döring A., Ammar I., Gupta S., Bunse M., Miskey C., Chen W., Uckert W., Schulz T.F., Izsvák Z., Ivics Z. Genome-wide Profiling Reveals Remarkable Parallels Between Insertion Site Selection Properties of the MLV Retrovirus and the piggyBac Transposon in Primary Human CD4(+) T Cells. Mol. Ther. 2016;24:592–606. doi: 10.1038/mt.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesana D., Santoni de Sio F.R., Rudilosso L., Gallina P., Calabria A., Beretta S., Merelli I., Bruzzesi E., Passerini L., Nozza S. HIV-1-mediated insertional activation of STAT5B and BACH2 trigger viral reservoir in T regulatory cells. Nat. Commun. 2017;8:498. doi: 10.1038/s41467-017-00609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galla M., Schambach A., Falk C.S., Maetzig T., Kuehle J., Lange K., Zychlinski D., Heinz N., Brugman M.H., Göhring G. Avoiding cytotoxicity of transposases by dose-controlled mRNA delivery. Nucleic Acids Res. 2011;39:7147–7160. doi: 10.1093/nar/gkr384. [DOI] [PMC free article] [PubMed] [Google Scholar]