Abstract
Background
It remains uncertain if prior use of oral anticoagulants (OACs) in COVID-19 outpatients with multimorbidity impacts prognosis, especially if cardiometabolic diseases are present. Clinical outcomes 30-days after COVID-19 diagnosis were compared between outpatients with cardiometabolic disease receiving vitamin K antagonist (VKA) or direct-acting OAC (DOAC) therapy at time of COVID-19 diagnosis.
Methods
A study was conducted using TriNetX, a global federated health research network. Adult outpatients with cardiometabolic disease (i.e. diabetes mellitus and any disease of the circulatory system) treated with VKAs or DOACs at time of COVID-19 diagnosis between 20-Jan-2020 and 15-Feb-2021 were included. Propensity score matching (PSM) was used to balance cohorts receiving VKAs and DOACs. The primary outcomes were all-cause mortality, intensive care unit (ICU) admission/mechanical ventilation (MV) necessity, intracranial haemorrhage (ICH)/gastrointestinal bleeding, and the composite of any arterial or venous thrombotic event(s) at 30-days after COVID-19 diagnosis.
Results
2275 patients were included. After PSM, 1270 patients remained in the study (635 on VKAs; 635 on DOACs). VKA-treated patients had similar risks and 30-day event-free survival than patients on DOACs regarding all-cause mortality, ICU admission/MV necessity, and ICH/gastrointestinal bleeding. The risk of any arterial or venous thrombotic event was 43% higher in the VKA cohort (hazard ratio 1.43, 95% confidence interval 1.03–1.98; Log-Rank test p = 0.029).
Conclusion
In COVID-19 outpatients with cardiometabolic diseases, prior use of DOAC therapy compared to VKA therapy at the time of COVID-19 diagnosis demonstrated lower risk of arterial or venous thrombotic outcomes, without increasing the risk of bleeding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-021-01368-6.
Keywords: Coronavirus disease 2019, SARS-CoV-2, Thrombosis, Anticoagulant, Vitamin K antagonist, Direct-acting oral anticoagulants, Bleeding
Introduction
Oral anticoagulants (OACs), including vitamin K antagonists (VKAs) and direct-acting OACs (DOACs), have been used for thromboprophylaxis in different clinical scenarios. In the pivotal clinical trials of stroke prevention in atrial fibrillation (AF), DOACs were non-inferior to warfarin for preventing stroke/systemic embolism (SE), with lower rates of intracranial haemorrhage (ICH) in comparison with warfarin [1–4]. Similarly, in venous thromboembolism (VTE), dabigatran, rivaroxaban, apixaban and edoxaban were non-inferior to conventional therapy in terms of efficacy and caused less bleeding in a broad spectrum of patients [5–9]. These trials evidences are supported by data from real world and observational studies, where DOACs have demonstrated significantly lower rates for major bleeding and a positive net clinical benefit compared to VKAs [10–13]. In VTE patients, the use of DOACs has also been associated with a lower risk of VTE recurrence even after anticoagulant discontinuation [14, 15].
Coronavirus Disease 2019 (COVID-19) has shown to trigger endothelial dysfunction, inflammatory and hypercoagulable states [16–18]. The risk of thrombosis is increased, and thromboembolic complications are relatively frequent in these patients, particularly in those patients with intensive care unit (ICU) admission [19–22]. Thus, anticoagulation is now well-established for the management of COVID-19 patients [23–25]. However, a common limitation is that most of the evidence to date refers to the hospitalization context. Thus, it remains uncertain if prior OAC therapy in outpatients, especially amongst patients with multimorbidity, would potentially influence the severity and clinical outcomes after COVID-19 diagnosis.
The aim of this study was to compare clinical outcomes 30-days after COVID-19 diagnosis between outpatients with cardiometabolic disease on chronic VKA or DOAC therapy at time of COVID-19 diagnosis, using a propensity score matching (PSM) approach.
Methods
Data from TriNetX, a global federated health research network with real-time updates of anonymised electronic medical records (EMRs) mainly from the United States (US), were used. The network includes healthcare organisations (HCOs, academic medical centres, specialty physician practices and community hospitals), with accumulated data for more than 71 million patients. Approximately, 18 million adult patients had a visit in a TriNetX HCO during 2020.
The inclusion criteria were ≥ 18 years and outpatient with COVID-19 and cardiometabolic disease recorded in EMRs between 20 January 2020 and 15 February 2021. COVID-19 was identified using criteria provided by TriNetX based on Centers for Disease Control and Prevention (CDC) coding guidelines [26]. COVID-19 status was determined using codes in EMRs or a positive test result identified with COVID-19-specific laboratory codes. Specifically, COVID-19 was identified by one or more of the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes in the EMRs of the patients (Additional file 1: Table S1). The inclusion date start was set as 20 January 2020 because COVID-19 was first confirmed in the US on this date, and the TriNetX network is predominately US-based [27]. Cardiometabolic disease was defined as the combination of diabetes mellitus (ICD-10-CM code: E08-E13) and any disease of the circulatory system (ICD-10-CM code: I00-I99). In addition, all patients should have OAC therapy in the one-year period prior to COVID-19 recorded in their EMRs, and remained on this therapy at COVID-19 diagnosis. During 1-year period prior to COVID-19 diagnosis, patients must not be hospitalized to ensure they are stable outpatients.
All patients were then stratified by OAC prescription. The DOAC group included outpatients who received either dabigatran, apixaban, rivaroxaban or edoxaban for at least 1 year before COVID-19 diagnosis, whereas the VKA group included outpatients who received warfarin under the same conditions. Patients were excluded if they received concomitant anticoagulant therapy (oral or parenteral). Baseline demographics, comorbidities and medication use were also captured from the patient EMRs.
The searches were run in TriNetX on 30 April 2021, which allowed for at least 30-days of follow-up for all participants from the time all conditions were fulfilled. When the searchers were run, there were 61 participating HCOs within the TriNetX research network.
Follow-up and clinical outcomes
All patients were followed-up for up to 30-days after COVID-19 diagnosis. Primary outcomes included all-cause mortality, ICU admission/mechanical ventilation (MV) necessity, ICH/gastrointestinal bleeding, and the composite of any arterial or venous thrombotic event (any of the following: myocardial infarction, other arterial thrombosis, VTE, or ischemic stroke/transient ischemic attack [TIA]/SE). The secondary outcomes were hospital admission, myocardial infarction, VTE, ischemic stroke/TIA/SE, and all bleeding. Further details about the ICD-10-CM codes used for the identification of every outcome are included in Additional file 1: Table S2.
Ethical issues
As a federated network, research studies using TriNetX do not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating HCOs and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health information or personal data are made available to the users of the platform.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD), and tested for differences with independent-sample t tests. Categorical variables were expressed as absolute frequencies and percentages, and tested for differences with chi-squared test.
The TriNetX platform was used to run 1:1 PSM using logistic regression. The platform uses ‘greedy nearest-neighbour matching’ with a caliper of 0.1 pooled standard deviations and difference between propensity scores ≤ 0.1. We assessed covariate balance between groups using standardised mean differences (SMDs). Any baseline characteristic with a SMD between cohorts lower than 0.1 is considered well matched [28].
Cox proportional Hazard Ratios (HRs) with 95% confidence intervals (CI) for 30-days outcomes were calculated following PSM. Kaplan–Meier survival curves were also produced with Log-Rank tests after PSM. No imputations were made for missing data. Two-sided p-values < 0.05 were accepted as statistically significant. Statistical analysis was performed using the TriNetX Analytics function in the online research platform.
Results
Overall, 2275 patients (mean age 67.7 ± 12.8 years, 1222 [53.7%] males) with COVID-19 and cardiometabolic disease were included. Of these, 648 (363 [56.0%] males, mean age of 67.9 ± 12.9 years) were on VKA therapy at the time of COVID-19 diagnosis, and 1627 (859 [52.8%] males, mean age 67.6 ± 12.8 years) were on DOACs.
There were no differences between cohorts regarding the main reasons for OAC (i.e. AF or previous pulmonary embolism). Other VTEs were more common in patients taking VKA therapy. In addition, patients on VKA were in general more comorbid, as demonstrated by the higher prevalence of hypertension, heart failure, ischemic heart disease, hyperlipidaemia, overweight/obesity, diseases of the nervous and digestive systems, acute kidney failure/chronic kidney disease, and neoplasms (Table 1). After PSM, 1270 remained in the study, 635 individuals on VKA therapy and 635 on DOACs (i.e. proportion of 1:1), well balanced on age, gender, ethnicity, and comorbidities (Table 1).
Table 1.
Comparison of clinical characteristics of the study cohort before and after propensity score matching
| Initial populations | Propensity score matched populations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 patients on prior VKA N = 648 |
COVID-19 patients on prior DOAC N = 1627 |
p-value | SMD | COVID-19 patients on prior VKA N = 635 |
COVID-19 patients on prior DOAC N = 635 |
p-value | SMD | |||||
| Age (years), mean (SD) | 67.90 | 12.88 | 67.61 | 12.79 | 0.636 | 0.022 | 67.80 | 12.90 | 68.29 | 12.26 | 0.492 | 0.039 |
| Male sex | 363 | 56.02% | 859 | 52.80% | 0.164 | 0.065 | 353 | 55.59% | 371 | 58.43% | 0.308 | 0.057 |
| Ethnicity | ||||||||||||
| Not Hispanic or Latino | 494 | 76.24% | 1133 | 69.64% | 0.002 | 0.149 | 482 | 75.91% | 481 | 75.75% | 0.948 | 0.004 |
| Hispanic or Latino | 65 | 10.03% | 117 | 7.19% | 0.024 | 0.101 | 64 | 10.08% | 59 | 9.29% | 0.635 | 0.027 |
| Unknown Ethnicity | 89 | 13.74% | 377 | 23.17% | 0.481 | 0.245 | 89 | 14.02% | 95 | 14.96% | 0.632 | 0.027 |
| Comorbidities | ||||||||||||
| Diabetes mellitus | 648 | 100% | 1627 | 100% | 1.000 | 0.000 | 635 | 100% | 635 | 100% | 1.000 | < 0.001 |
| Hypertension | 613 | 94.60% | 1479 | 90.90% | 0.003 | 0.143 | 600 | 94.49% | 602 | 94.80% | 0.803 | 0.014 |
| Heart failure | 270 | 41.67% | 574 | 35.28% | 0.004 | 0.132 | 265 | 41.73% | 249 | 39.21% | 0.360 | 0.051 |
| Ischemic heart disease | 322 | 49.69% | 721 | 44.32% | 0.020 | 0.108 | 314 | 49.45% | 311 | 48.98% | 0.866 | 0.009 |
| Atrial fibrillation | 393 | 60.65% | 984 | 60.48% | 0.941 | 0.003 | 386 | 60.79% | 389 | 61.26% | 0.863 | 0.010 |
| Peripheral vascular disease | 105 | 16.20% | 239 | 14.69% | 0.363 | 0.042 | 103 | 16.22% | 96 | 15.12% | 0.589 | 0.030 |
| Hyperlipidemia | 570 | 87.96% | 1322 | 81.25% | < 0.001 | 0.187 | 557 | 87.72% | 553 | 87.09% | 0.735 | 0.019 |
| Cerebrovascular disease | 170 | 26.24% | 367 | 22.56% | 0.062 | 0.086 | 166 | 26.14% | 160 | 25.20% | 0.700 | 0.022 |
| Cerebral infarction | 81 | 12.50% | 176 | 10.82% | 0.253 | 0.052 | 81 | 12.76% | 85 | 13.39% | 0.739 | 0.019 |
| Pulmonary embolism | 124 | 19.14% | 266 | 16.35% | 0.111 | 0.073 | 121 | 19.06% | 121 | 19.06% | 1.000 | 0.000 |
| Other venous embolism and thrombosis | 195 | 30.09% | 391 | 24.03% | 0.003 | 0.137 | 187 | 29.45% | 189 | 29.76% | 0.902 | 0.007 |
| Overweight/obesity | 397 | 61.27% | 913 | 56.12% | 0.025 | 0.105 | 387 | 60.94% | 389 | 61.26% | 0.908 | 0.006 |
| Diseases of the respiratory system | 543 | 83.80% | 1307 | 80.33% | 0.056 | 0.090 | 532 | 83.78% | 523 | 82.36% | 0.501 | 0.038 |
| Diseases of the nervous system | 528 | 81.48% | 1264 | 77.69% | 0.046 | 0.094 | 517 | 81.42% | 518 | 81.58% | 0.942 | 0.004 |
| Diseases of the digestive system | 526 | 81.17% | 1230 | 75.60% | 0.004 | 0.136 | 514 | 80.94% | 508 | 80.00% | 0.671 | 0.024 |
| Acute kidney failure and chronic kidney disease | 287 | 44.29% | 582 | 35.77% | < 0.001 | 0.175 | 277 | 43.62% | 272 | 42.84% | 0.777 | 0.016 |
| Diseases of liver | 130 | 20.06% | 290 | 17.82% | 0.214 | 0.057 | 129 | 20.32% | 136 | 21.42% | 0.629 | 0.027 |
| Neoplasms | 351 | 54.17% | 766 | 47.08% | 0.002 | 0.142 | 341 | 53.70% | 345 | 54.33% | 0.822 | 0.013 |
| Pharmacological therapy | ||||||||||||
| Beta blockers | 495 | 76.39% | 1228 | 75.48% | 0.647 | 0.021 | 485 | 76.38% | 485 | 76.38% | 1.000 | < 0.001 |
| ACE inhibitors | 396 | 61.11% | 836 | 51.38% | < 0.001 | 0.197 | 385 | 60.63% | 385 | 60.63% | 1.000 | < 0.001 |
| Angiotensin II inhibitors | 237 | 36.57% | 621 | 38.17% | 0.479 | 0.033 | 236 | 37.17% | 231 | 36.38% | 0.771 | 0.016 |
| Alpha blockers | 549 | 84.72% | 1282 | 78.80% | 0.001 | 0.154 | 537 | 84.57% | 542 | 85.35% | 0.695 | 0.022 |
| Antilipemic agents | 366 | 56.48% | 863 | 53.04% | 0.137 | 0.069 | 359 | 56.54% | 349 | 54.96% | 0.572 | 0.032 |
| Calcium channel blockers | 477 | 73.61% | 1091 | 67.06% | 0.002 | 0.144 | 466 | 73.39% | 463 | 72.91% | 0.849 | 0.011 |
| Diuretics | 455 | 70.22% | 1065 | 65.46% | 0.030 | 0.102 | 445 | 70.08% | 438 | 68.98% | 0.670 | 0.024 |
| Antiarrhythmics | 401 | 61.88% | 988 | 60.73% | 0.609 | 0.024 | 396 | 62.36% | 387 | 60.95% | 0.603 | 0.029 |
| Antiplatelets | 495 | 76.39% | 1228 | 75.48% | 0.647 | 0.021 | 485 | 76.38% | 485 | 76.38% | 1.000 | < 0.001 |
| Blood glucose regulation agents (including oral antidiabetics and insulin) | 554 | 85.50% | 1389 | 85.37% | 0.947 | 0.003 | 545 | 85.83% | 545 | 85.83% | 1.000 | < 0.001 |
ACE: Angiotensin-converting enzyme; DOAC: direct-acting oral anticoagulant; SD: standard deviation; SMD: standardized mean difference; VKA: vitamin K antagonist
Comparisons of clinical outcomes
In the initial populations, all event rates were numerically higher in the COVID-19 patients with cardiometabolic disease on prior VKA, which were significant for any arterial or venous thrombotic event (14.04% vs. 8.54%, p < 0.001), VTE (9.88% vs. 6.82%, p = 0.007), and ischemic stroke/TIA/SE (4.17% vs. 1.72%, p < 0.001). After PSM, the rate of any arterial or venous thrombotic event (14.02% vs. 9.61%, p = 0.015) and ischemic stroke/TIA/SE (4.25% vs. 1.73%, p = 0.008) remained higher in users of VKA compared to users of DOACs. Further details are shown in Table 2.
Table 2.
Primary and secondary outcomes in patients on VKAs or DOAC at COVID-19 diagnosis, before (unmatched) and after propensity score matching
| Outcomes | Initial populations | Propensity score matched populations | ||||
|---|---|---|---|---|---|---|
| COVID-19 patients on prior VKA (N = 648) |
COVID-19 patients on prior DOAC (N = 1627) |
p-value | COVID-19 patients on prior VKA (N = 635) |
COVID-19 patients on prior DOAC (N = 635) |
p-value | |
| All-cause mortality | 10 (1.54%) | 22 (1.35%) | 0.913 | 10 (1.57%) | 11 (1.73%) | 0.826 |
| ICU admission/MV necessity | 12 (1.85%) | 21 (1.29%) | 0.378 | 12 (1.89%) | 10 (1.57%) | 0.667 |
| ICH/gastrointestinal bleeding | 10 (1.54%) | 12 (0.74%) | 0.145 | 10 (1.57%) | 10 (1.57%) | 1.000 |
| Any arterial or venous thrombotic event | 91 (14.04%) | 139 (8.54%) | < 0.001 | 89 (14.02%) | 61 (9.61%) | 0.015 |
| Hospital admission | 17 (2.62%) | 42 (2.58%) | 0.891 | 17 (2.68%) | 17 (2.68%) | 1.000 |
| Myocardial infarction | 10 (1.54%) | 13 (0.80%) | 0.190 | 10 (1.57%) | 10 (1.57%) | 1.000 |
| Venous thromboembolism | 64 (9.88%) | 111 (6.82%) | 0.007 | 62 (9.76%) | 50 (7.87%) | 0.235 |
| All bleeding events | 16 (2.45%) | 18 (1.11%) | 0.084 | 16 (2.52%) | 10 (1.57%) | 0.235 |
| Ischemic stroke/TIA/SE | 27 (4.17%) | 28 (1.72%) | < 0.001 | 27 (4.25%) | 11 (1.73%) | 0.008 |
DOAC: direct-acting oral anticoagulant; ICH: intracranial haemorrhage; ICU: intensive care unit; MV: mechanical ventilation; SE: systemic embolism; TIA: transient ischemic attack; VKA: vitamin K antagonist
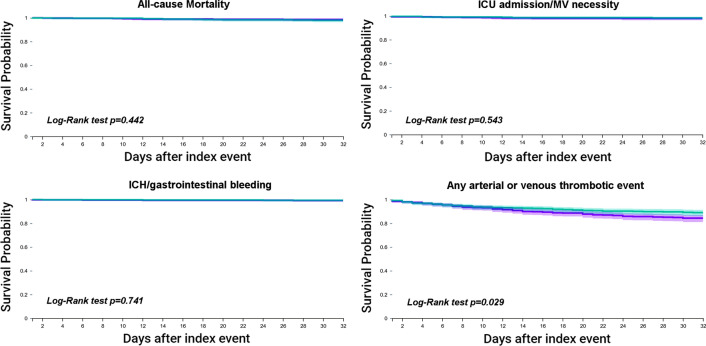
In terms of the primary outcomes after PSM, there were no significant differences in the risks of all-cause mortality, ICU admission/MV necessity, or ICH/gastrointestinal bleeding between patients on VKA or DOACs. Thus, the risk for all-cause mortality was similar comparing the VKA cohort and DOAC cohort (HR 0.70, 95% 0.28–1.74). The risk of ICU admission/MV necessity in the VKA cohort was not significantly different compared to the DOAC cohort (HR 1.31, 95% CI 0.55–3.10), and the risk of ICH/gastrointestinal bleeding was similar (HR 1.29, 95% CI 0.29–5.75). Event-free survival for these three outcomes was not different between cohorts, as assessed by the Kaplan–Meier analyses (Log-Rank tests: p = 0.442 for mortality; p = 0.543 for ICU admission/MV necessity; and p = 0.741 for ICH/gastrointestinal bleeding) (Fig. 1).
Fig. 1.
Comparison of survival curves for the primary outcomes between patients on VKAs or DOACs at COVID-19 diagnosis after propensity score matching. Purple line: Prior VKA use; Green line: Prior DOAC use
The composite outcome of any arterial or venous thrombotic event was inferior in the DOAC cohort in comparison to the VKA cohort, as demonstrated by the 43% higher risk in VKA users (HR 1.43, 95% CI 1.03–1.98) and the lower event-free survival (Log-Rank test p = 0.029) (Fig. 1).
Secondary outcomes
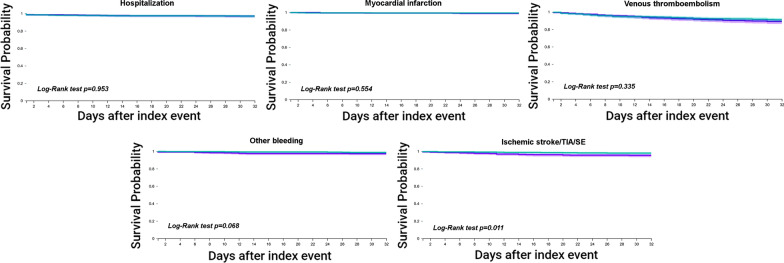
Hospital admission was similar in VKA and DOAC patients (HR 0.98, 95% CI 0.50–1.92). Likewise, there were no significant differences in the risk of myocardial infarction between both groups (HR 1.46, 95% CI 0.41–5.18), nor in the risk of VTE (HR 1.20, 95% CI 0.83–1.74). The risk of all bleeding events was not significantly different in patients previously taking VKA (HR 2.24, 95% CI 0.92–5.44) (Fig. 2).
Fig. 2.
Comparison of survival curves for the secondary outcomes between patients on VKAs or DOACs at COVID-19 diagnosis after propensity score matching. Purple line: Prior VKA use; Green line: Prior DOAC use
The risk of suffering an ischemic stroke/TIA/SE at 30-days after COVID-19 diagnosis was 2.42-fold higher in users of VKA compared to DOAC users (HR 2.42, 95% CI 1.20–4.88; Log-Rank test p = 0.011) (Fig. 2).
Sensitivity analysis
A sensitivity analysis was performed including only those patients fulfilling the initial inclusion/exclusion criteria and AF, as this is the most common indication for OAC use. After PSM on a 1:1 proportion, 866 patients (mean age 71.2 ± 11.6 years, 508 [61%] males) with COVID-19, AF and other cardiometabolic disease were included, of which 433 (254 [58.7%] males, mean age of 71.1 ± 12.2 years) were on VKA therapy at the time of COVID-19 diagnosis, and 433 (254 [58.7%] males, mean age 71.3 ± 11.0 years) were on DOACs.
Overall, the risk for the primary outcomes in this sensitivity analysis between patients on VKA or DOACs was similar compared to the main analysis. Thus, there were no significant differences in VKA or DOAC-treated patients regarding all-cause mortality (HR 1.36, 95% CI 0.47–3.91; Log-Rank test p = 0.570), ICU admission/MV necessity (HR 1.35, 95% CI 0.57–3.19; Log-Rank test p = 0.499), and ICH/ gastrointestinal bleeding (HR 0.34, 95% CI 0.07–1.67; Log-Rank test p = 0.162). The risk of composite outcome of any arterial or venous thrombotic event was higher in the VKA cohort compared to the DOAC cohort (HR 1.80, 95% CI 1.04–3.12), with a significantly lower event-free survival (Log-Rank test p = 0.033).
Concerning the secondary outcomes, the risks of hospital admission (HR 0.94, 95% CI 0.46–1.90; Log-Rank test p = 0.859), myocardial infarction (OR 0.58, 95% CI 0.17–1.97; Log-Rank test p = 0.372), VTE (HR 1.48, 95% CI 0.69–3.19; Log-Rank test p = 0.315), and all bleeding events (HR 1.14, 95% CI 0.44–2.96; Log-Rank test p = 0.782), was also similar in patients previously taking VKA or DOACs. The risk of ischemic stroke/TIA/SE was increased in VKA users (HR 3.69, 95% CI 1.37–9.93), and Kaplan–Meier analysis demonstrated a significantly lower event-free survival in the VKA-treated cohort (Log-Rank test p = 0.006).
Discussion
In this study including COVID-19 outpatients with cardiometabolic disease, patients taking VKA before COVID-19 diagnosis showed a 43% higher 30-day risk of any arterial/venous thrombotic event and ischemic stroke/TIA/SE, compared to patients taking DOAC, adjusting for comorbidities using PSM. These results were consistent also in COVID-19 patients with AF and other cardiometabolic disease.
Previous studies have shown that cardiometabolic multimorbidity is common among patients with COVID-19, and it is associated with a higher risk of hospitalization and a worsened prognosis [29–32]. In particular, diabetes is frequent in these patients [33, 34], and associated with an increased risk of complications, likely due to clustering with other conditions [29]. Simultaneously, several cardiovascular diseases, including hypertension, cerebrovascular disease and coronary artery disease, are prevalent in COVID-19 patients, and are associated with a higher risk of adverse outcomes [35]. Given that the presence of cardiometabolic disease is not uncommon in patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, this might have major implications for prognosis in this condition.
It is well established that COVID-19 increases the risk of arterial and venous thrombosis [20, 21], leading to the research focus on thromboinflammation and antithrombotic therapy, particularly in anticoagulation therapy [23, 25, 36, 37]. Many patients had preexisting cardiovascular diseases and were already on OAC therapy when they were diagnosed of COVID-19 [38]. Hence, the role of prior OAC therapy in the context of COVID-19 is gaining interest. For example, a recent study concluded that prior use of therapeutic anticoagulation was not associated with improved survival in hospitalized COVID-19 patients [39]. Similarly, a small study found that regular VKA use in hospitalized frail older patients with COVID-19 was associated with increased mortality during the first week [40]. On the contrary, a retrospective study concluded that COVID-19 patients on OAC at the time of infection and throughout their disease course had significantly lower risk of all-cause mortality at 21 days [41]. Indeed, OAC therapy was associated with lower risk of all-cause mortality in elderly AF patients with COVID-19 [42], and more recently, the ACTION trial showed that among patients admitted with COVID-19 and elevated D-dimer, therapeutic anticoagulation was not superior to prophylactic anticoagulation; and rivaroxaban for stable patients and enoxaparin for unstable patients increased major bleeding without improving clinical outcomes [43, 44].
Nevertheless, a common limitation of most studies (both, those with positive and negative results in favour of OACs) is the hospitalization setting. Such patients have already suffered deterioration of their baseline status, which has led to hospitalization and may be a manifestation of a more severe state of the SARS-CoV-2 infection, with an increased risk of thrombosis, mortality, ICU admission, and MV. In these more severe patients, several confounding factors may be acting when evaluating the potential role of previous OAC, and the differences between VKA and DOAC may be non-existent.
As a result, COVID-19 outpatients with cardiometabolic diseases under anticoagulation therapy are a population with scarce data, and their management could be particularly complex. A recent study concluded that OAC therapy in high-risk AF patients was associated with a lower risk of receiving a positive COVID-19 test and severe COVID-19 outcomes [45]. In turn, pre-admission and in-hospital OAC therapy (either VKA or DOAC) were positively associated with higher survival in a study including elderly AF patients with COVID-19 [46]. Although these prior studies did not observe differences between VKA and DOACs, we found that VKA users had a higher risk of thromboembolism, which supported several previous studies demonstrating that DOAC therapy is more effective and even as safe as VKA therapy [47, 48].
DOACs have recently demonstrated to be superior in comparison to VKAs among patients with different conditions. For example, DOACs was associated with lower long-term all-cause mortality than VKAs in AF patients who were successfully discharged after transcatheter aortic valve replacement [49]. In addition, in patients with left ventricular thrombi, there is a significant reduction in stroke with the use of DOACs, without an increase in bleeding [50], and among diabetic AF patients, DOACs are associated with a lower risk of thromboembolism, major bleeding, and major adverse limb events than VKAs [51].
Nonetheless, the published data for DOACs in the field of COVID-19 is conflicting. In the context of hospitalization, many of the COVID-19 patients with prior DOAC therapy will be switched to heparins because they are expected to receive medications interacting with DOACs, or have coagulation system and homeostasis disorders [52, 53]. For this reason, it is necessary to act before hospitalization in these especially vulnerable patients. Since our results show that within 30 days of COVID-19 diagnosis there is an increased risk of complications in patients on prior VKA, whenever possible, a change to a DOAC should be considered. This may not only reduce the risk of adverse events in the case of COVID-19, but also the need for monitoring and therefore the number of visits to health care centers and the social contact that this implies, which can contribute to a lower risk of infection [54]. Some societies consider that DOAC could be maintained even during hospitalization, based on clinical conditions and assessment for drug–drug interactions [21, 55, 56].
However, there are some indications such as antiphospholipid syndrome or prosthetic heart valves for which DOACs are not approved. In such patients, a switch from VKA to DOACs is not possible, and therefore other interventions are required to achieve and maintain the highest quality of anticoagulation in order to reduce the risk of worse clinical outcomes when on VKAs. These include the identification and modification of causes of poor anticoagulation control with VKAs (eg. potential pharmacologic interactions), routine assessment of adherence to treatment, patient-centred education/counselling and educational programs for healthcare professionals, the use of multidose drug dispensing systems, self-management of VKA therapy, specialized anticoagulation clinics to improve adherence and a more careful follow-up [57, 58]. Due to the COVID-19 pandemic, telemedicine visits and telehealth programs may help to achieve these objectives while minimize exposure risks for both patients and healthcare professionals [59].
Limitations
There are some limitations that need to be acknowledged. First, the data were collected from the HCO EMRs and some health conditions may be underreported. Recording of ICD codes in EMR may vary by factors including age, comorbidities, severity of illness, length of in-hospital stay, and in-hospital mortality. Further residual confounding may include lifestyle factors such as alcohol consumption and physical activity, which were not available.
Propensity scores are a method used to balance covariates, but in observational studies propensity scores are estimated and therefore there is no certainty that the propensity score was 100% accurate. We also could not determine if there was any impact of attending different HCOs because of data privacy restrictions. We examined all deaths of the included patients captured within the TriNetX network; however, deaths outside of the participating HCOs are not well captured. It should also be noted that we had not access to time in therapeutic range of International Normalized Ratio (INR) for VKA-treated patients, so there are uncertainties about the quality of anticoagulation therapy in these patients. Finally, our main objective was to investigate the association of prior VKA or DOAC therapy with short-term prognosis after outpatient COVID-19 diagnosis. For this reason we did not take into account the anticoagulation therapy once patients were diagnosed of COVID-19, since our interest was on the previous use of VKAs or DOACs. For the same reason, we have not analyzed the potential role of other pharmacological therapies such as antidiabetics agents or insulin, although we recognize influence of such therapies on outcomes.
Conclusion
In COVID-19 outpatients with cardiometabolic diseases, prior use of DOAC therapy compared to VKA therapy at the time of COVID-19 diagnosis might reduce the risk of composite arterial or venous thrombotic outcomes, without increasing the risk of bleeding.
Supplementary Information
Additional file 1: Table S1. ICD-10-CM codes and LOINCs for the identification of COVID-19. Table S2. Clinical outcomes and ICD-10-CM codes.
Acknowledgements
None.
Abbreviations
- AF
Atrial fibrillation
- CDC
Centres for Disease Control and Prevention
- CI
Confidence interval
- COVID-19
Coronavirus Disease 2019
- DOAC
Direct-acting oral anticoagulant
- EMR
Electronic medical record
- HCO
Healthcare organisation
- HR
Hazard ratio
- ICH
Intracranial haemorrhage
- ICU
Intensive care unit
- INR
International normalized ratio
- MV
Mechanical ventilation
- OAC
Oral anticoagulant
- PSM
Propensity score matching
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SD
Standard deviation
- SE
Systemic embolism
- SMD
Standardised mean difference
- TIA
Transient ischemic attack
- VKA
Vitamin K antagonist
- VTE
Venous thromboembolism
Authors' contributions
JMR-C and GYHL designed the study. EF-E and PU provided TriNetX access and made a critical revision. JMR-C and GYHL acquired data, performed statistical analyses and drafted the manuscript. SLH, BJRB and FM made a critical revision. All authors approved the final content for journal submission and publication. All authors read and approved the final manuscript.
Funding
The authors received no specific funding for this work.
Availability of data and materials
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because some materials are used for other unpublished projects.
Declarations
Ethics approval and consent to participate
As a federated network, research studies using TriNetX do not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating HCOs and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health information or personal data are made available to the users of the platform.
Consent for publication
Not applicable.
Competing interests
JMR-C has received a grant from Sociedad Española de Trombosis y Hemostasia (grant for short international training stays 2020), and the First Contact Initiative Grant 2020 from the European Society of Cardiology Council on Basic Cardiovascular Science. SLH has received a grant from Bristol Myers Squibb (BMS). FM: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and AstraZeneca; research grants from Bayer. GYHL: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. Other authors report no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 5.Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 6.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 7.Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 9.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Liu Y, Zhang Y, Guo X, Xu Y. Differences in safety and efficacy of oral anticoagulants in patients with non-valvular atrial fibrillation: a Bayesian analysis. Int J Clin Pract. 2018;74:e13308. doi: 10.1111/ijcp.13308. [DOI] [PubMed] [Google Scholar]
- 11.Graham DJ, Baro E, Zhang R, Liao J, Wernecke M, Reichman ME, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604. doi: 10.1016/j.amjmed.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE, et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018;319(5):463–473. doi: 10.1001/jama.2017.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Caterina R, Ageno W, Agnelli G, Chan NC, Diener HC, Hylek E, et al. The non-vitamin K antagonist oral anticoagulants in heart disease: section V-special situations. Thromb Haemost. 2019;119(1):14–38. doi: 10.1055/s-0038-1675816. [DOI] [PubMed] [Google Scholar]
- 14.Campello E, Spiezia L, Simion C, Tormene D, Camporese G, Dalla Valle F, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9(23):e018917. doi: 10.1161/JAHA.120.018917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsebaie MAT, van Es N, Langston A, Büller HR, Gaddh M. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17(4):645–656. doi: 10.1111/jth.14398. [DOI] [PubMed] [Google Scholar]
- 16.Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(6):949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10):e13378. doi: 10.1111/eci.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjöström A, Wersäll J, Warnqvist A, Farm M, Magnusson M, Oldner A, et al. Platelet count rose while d-dimer levels dropped as deaths and thrombosis declined, an observational study on anticoagulation shift in COVID-19. Thromb Haemost. 2021 doi: 10.1055/a-1477-3829. [DOI] [PubMed] [Google Scholar]
- 19.Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Matin S, et al. Intermediate vs standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to ICU: ninety-day results from the INSPIRATION Trial. Thromb Haemost. 2021 doi: 10.1055/a-1485-2372. [DOI] [PubMed] [Google Scholar]
- 20.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–73. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the Multicenter Italian CORIST Study. Thromb Haemost. 2021;121(8):1054–1065. doi: 10.1055/a-1347-6070. [DOI] [PubMed] [Google Scholar]
- 23.Talasaz AH, Sadeghipour P, Kakavand H, Aghakouchakzadeh M, Kordzadeh-Kermani E, Van Tassell BW, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(7):1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers-for-Disease-Control-and-Prevention. ICD-10-CM official coding guidelines - supplement coding encounters related to COVID-19 coronavirus outbreak 2020 [02-03-2021. https://www.cdc.gov/nchs/data/icd/ICD-10-CM-Official-Coding-Gudance-Interim-Advice-coronavirus-feb-20-2020.pdf.
- 27.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddaloni E, D'Onofrio L, Alessandri F, Mignogna C, Leto G, Pascarella G, et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II) Cardiovasc Diabetol. 2020;19(1):164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellicori P, Doolub G, Wong CM, Lee KS, Mangion K, Ahmad M, et al. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev. 2021;3:CD013879. doi: 10.1002/14651858.CD013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svensson P, Hofmann R, Häbel H, Jernberg T, Nordberg P. Association between cardiometabolic disease and severe COVID-19: a nationwide case-control study of patients requiring invasive mechanical ventilation. BMJ Open. 2021;11(2):e044486. doi: 10.1136/bmjopen-2020-044486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10(5):e019259. doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scalsky RJ, Chen YJ, Desai K, O’Connell JR, Perry JA, Hong CC. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK Biobank. PLoS ONE. 2021;16(4):e0248602. doi: 10.1371/journal.pone.0248602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison SL, Buckley BJR, Rivera-Caravaca JM, Zhang J, Lip GYH. Cardiovascular risk factors, cardiovascular disease and COVID-19: An umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes. 2021;7(4):330–339. doi: 10.1093/ehjqcco/qcab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBane RD, 2nd, Torres Roldan VD, Niven AS, Pruthi RK, Franco PM, Linderbaum JA, et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patell R, Chiasakul T, Bauer E, Zwicker JI. Pharmacologic thromboprophylaxis and thrombosis in hospitalized patients with COVID-19: a pooled analysis. Thromb Haemost. 2021;121(1):76–85. doi: 10.1055/s-0040-1721664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerotziafas GT, Catalano M, Colgan MP, Pecsvarady Z, Wautrecht JC, Fazeli B, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European Independent Foundation in angiology/vascular medicine. Thromb Haemost. 2020;120(1):1597–1628. doi: 10.1055/s-0040-1715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelenberg JP, van Gelder M, Maas ML, Hovens MMC, Esselink A, Dofferhoff ASM, et al. Prior use of therapeutic anticoagulation does not protect against COVID-19 related clinical outcomes in hospitalized patients: a propensity score-matched cohort study. Br J Clin Pharmacol. 2021 doi: 10.1111/bcp.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ménager P, Brière O, Gautier J, Riou J, Sacco G, Brangier A, et al. Regular use of VKA prior to COVID-19 associated with lower 7-day survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID cohort study. Nutrients. 2020;13(1):39. doi: 10.3390/nu13010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison RF, Forte K, Buscher MG, Jr, Chess A, Patel A, Moylan T, et al. The association of preinfection daily oral anticoagulation use and all-cause in hospital mortality from novel coronavirus 2019 at 21 days: a retrospective cohort study. Crit Care Explor. 2021;3(1):e0324. doi: 10.1097/CCE.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denas G, Gennaro N, Ferroni E, Fedeli U, Lorenzoni G, Gregori D, et al. Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: a population-based propensity score matched study. Int J Cardiol. 2021;329:266–269. doi: 10.1016/j.ijcard.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopes RD, de Barros ESPGM, Furtado RHM, Macedo AVS, Ramacciotti E, Damini LP, et al. Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus infection (SARS-CoV2) admitted to hospital: rationale and design of the ACTION (AntiCoagulaTlon cOroNavirus)-Coalition IV Trial. Am Heart J. 2021;238:1–11. doi: 10.1016/j.ahj.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopes RD, Alexander JH. Coalition ACTION Trial: Randomized Clinical Trial To Evaluate A Routine Full Anticoagulation Strategy In Patients With Coronavirus Infection (SARS-CoV-2) Admitted To Hospital. American College of Cardiology (ACC) meeting - ACC212021. [DOI] [PMC free article] [PubMed]
- 45.Wong AY, Tomlinson L, Brown JP, Elson W, Walker AJ, Schultze A, et al. Association between oral anticoagulants and COVID-19 related outcomes: two cohort studies. 2021:2021.04.30.21256119.
- 46.Fumagalli S, Trevisan C, Del Signore S, Pelagalli G, Volpato S, Gareri P, et al. COVID-19 and atrial fibrillation in older patients. Does oral anticoagulant therapy provide a survival benefit? An insight from the GeroCovid Registry. Thromb Haemost. 2021 doi: 10.1055/a-1503-3875. [DOI] [PubMed] [Google Scholar]
- 47.Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STROKEAHA.118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastori D, Menichelli D, Del Sole F, Pignatelli P, Violi F. Long-term risk of major adverse cardiac events in atrial fibrillation patients on direct oral anticoagulants. Mayo Clin Proc. 2021;96(3):658–665. doi: 10.1016/j.mayocp.2020.06.057. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima H, Watanabe Y, Hioki H, Kozuma K, Kataoka A, Nakashima M, et al. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation after TAVR. JACC Cardiovasc Interv. 2020;13(22):2587–2597. doi: 10.1016/j.jcin.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Michael F, Natt N, Shurrab M. Direct oral anticoagulants vs vitamin K antagonists in left ventricular thrombi: a systematic review and meta-analysis. CJC Open. 2021. [DOI] [PMC free article] [PubMed]
- 51.Chan YH, Lee HF, Li PR, Liu JR, Chao TF, Wu LS, et al. Effectiveness, safety, and major adverse limb events in atrial fibrillation patients with concomitant diabetes mellitus treated with non-vitamin K antagonist oral anticoagulants. Cardiovasc Diabetol. 2020;19(1):63. doi: 10.1186/s12933-020-01043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aly R, Gupta S, Singh B, Kaur P, Kim K, Gupta S. The use of direct acting oral anticoagulants in patients with COVID-19 infection. J Commun Hosp Intern Med Perspect. 2021;11(2):184–186. doi: 10.1080/20009666.2020.1867295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Testa S, Prandoni P, Paoletti O, Morandini R, Tala M, Dellanoce C, et al. Direct oral anticoagulant plasma levels' striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18(6):1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vivas D, Roldán V, Esteve-Pastor MA, Roldán I, Tello-Montoliu A, Ruiz-Nodar JM, et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73(9):749–57. doi: 10.1016/j.recesp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. EP Europace. 2021 doi: 10.1093/europace/euab065. [DOI] [PubMed] [Google Scholar]
- 57.Clarkesmith DE, Pattison HM, Lip GYH, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PloS ONE. 2013;8(9):e74037. doi: 10.1371/journal.pone.0074037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prochaska JH, Hausner C, Nagler M, Göbel S, Eggebrecht L, Panova-Noeva M, et al. Subtherapeutic anticoagulation control under treatment with vitamin K-antagonists-data from a specialized coagulation service. Thromb Haemost. 2019;119(8):1347–1357. doi: 10.1055/s-0039-1692175. [DOI] [PubMed] [Google Scholar]
- 59.Linz D, Pluymaekers N, Hendriks JM. TeleCheck-AF for COVID-19. Eur Heart J. 2020;41(21):1954–1955. doi: 10.1093/eurheartj/ehaa404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. ICD-10-CM codes and LOINCs for the identification of COVID-19. Table S2. Clinical outcomes and ICD-10-CM codes.
Data Availability Statement
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because some materials are used for other unpublished projects.