Abstract
Coronavirus disease 2019 (Covid-19) is a novel worldwide pandemic caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). During Covid-19 pandemic, socioeconomic deprivation, social isolation, and reduced physical activities may induce heart failure (HF), destabilization, and cause more complications. HF appears as a potential hazard due to SARS-CoV-2 infection, chiefly in elderly patients with underlying comorbidities. In reality, the expression of cardiac ACE2 is implicated as a target point for SARS-CoV-2-induced acute cardiac injury. In SARS-CoV-2 infection, like other febrile illnesses, high blood viscosity, exaggerated pro-inflammatory response, multisystem inflammatory syndrome, and endothelial dysfunction-induced coagulation disorders may increase risk of HF development. Hypoxic respiratory failure, as in pulmonary edema, severe acute lung injury (ALI), and acute respiratory distress syndrome (ARDS) may affect heart hemodynamic stability due to the development of pulmonary hypertension. Indeed, Covid-19-induced HF could be through the development of cytokine storm, characterized by high proliferation pro-inflammatory cytokines. In cytokine storm-mediated cardiac dysfunction, there is a positive correlation between levels of pro-inflammatory cytokine and myocarditis-induced acute cardiac injury biomarkers. Therefore, Covid-19-induced HF is more complex and related from a molecular background in releasing pro-inflammatory cytokines to the neuro-metabolic derangements that together affect cardiomyocyte functions and development of HF. Anti-heart failure medications, mainly digoxin and carvedilol, have potent anti-SARS-CoV-2 and anti-inflammatory properties that may mitigate Covid-19 severity and development of HF. In conclusion, SARS-CoV-2 infection may lead to the development of HF due to direct acute cardiac injury or through the development of cytokine storms, which depress cardiomyocyte function and cardiac contractility. Anti-heart failure drugs, mainly digoxin and carvedilol, may attenuate severity of HF by reducing the infectivity of SARS-CoV-2 and prevent the development of cytokine storms in severely affected Covid-19 patients.
Keywords: Covid-19, SARS-CoV-2, Development, Heart failure
Background
Coronavirus disease 2019 (Covid-19) is a novel worldwide pandemic caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was primarily recognized in Wuhan, China, according to the initial epidemiological finding (Lugnier et al. 2021). Covid-19 affects millions of people, and up to late May 2021, the number of infected people reaches more than 160 million. Though greatest of affected people are asymptomatic or presented with mild symptoms in 80%, however, 15-20% of affected patients require hospitalization due to development of acute lung injury (ALI) and/or respiratory distress syndrome (ARDS) (Al-kuraishy et al. 2021b). Severe Covid-19 cases are linked to hyper-inflammations and the development of cytokine storms (Al-kuraishy et al. 2021a).
The leading causes of inflammatory and immunological disorders in patients with critical Covid-19 are related to the overactivation of T cells and macrophages following the release of massive pro-inflammatory cytokines such as interleukins and chemokines (Al-kuraishy et al. 2021e). Mainly, IL-6, IL1β, IL-8, and tumor necrosis factor-alpha (TNF-α) via severe SARS-CoV-2 infections are linked with the development of ALI/ARDS and multi-organ failure (MOF) (Al-Kuraishy et al. 2020). One of the most significant probable entry-point of SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2). The interaction between SARS-CoV-2 and ACE2 leads to noteworthy lessening of this anti-inflammatory receptor. ACE2 is involved in the control of the renin-angiotensin system (RAS) via conversion of vasoconstrictor angiotensin II (Ang II) into vasodilator anti-inflammatory Ang 1-7 and Ang 1-9 (Al-Kuraishy et al. 2020). Therefore, downregulation of ACE2 and advancement of circulating AngII during SARS-CoV-2 infection could be the possible mechanism overdue initiation of inflammatory instabilities (Moubarak et al. 2021).
Heart failure (HF) is defined as failure of heart to maintain sufficient blood flow for body tissue. Patients with HF experience exertional dyspnea, leg edema, and excessive tiredness. HF is divided to right side HF, left side HF, and congestive HF. The leading causes of HF are hypertension, ischemic heart disease, arrhythmia, cardiomyopathy, and myocarditis (Rossignol et al. 2019). HF represents one of the common cardiovascular disorders in occidental counties; it affects 2% of the adult population that increases to 10% over the age of 65 years; this rate may increase due to expanding life span and improvement of survival rates from associated risk factors of HF (Farré et al. 2017).
During the Covid-19 pandemic, socioeconomic deprivation, social isolation, and reduced physical activities may induce HF destabilization and cause more complications. Besides, SARS-CoV-2 infection and related inflammatory burden could aggravate preexistent HF or induces new-onset HF (Reza et al. 2020). In this sense, this mini-review aimed to highlighted and shed light on the pragmatic association between SARS-CoV-2 infection and development of HF in Covid-19 patients regarding metabolic and inflammatory points.
Viral infections and risk of heart failure
It has been reported that different viral infections may induce development of HF; Butt et al. (2011) illustrated that human immune deficiency virus (HIV) infection is regarded as a risk factor for induction of HF due to immunological reaction-induced myocarditis, secondary infections, nutritional deficiency, and negative impact of antiretroviral drugs on the heart (Butt et al. 2011). Of note, about 50% of patients with HIV infection have diastolic dysfunction and myocarditis in 50% of HIV infection patients (Nico et al. 2010). In contrast, Kristoffersen et al., radionuclide ventriculography study of HIV infection patients, did not find any evidence of ventricular dysfunction (Kristoffersen et al. 2008).
Hepatitis C virus (HCV) could prompt progression of HF by causing myocarditis and acute cardiac injury, as evident by a positive correlation between biomarkers of cardiac injury and anti-HCV antibodies (Matsumori et al. 2006). Similarly, there is a strong association between anti-HCV antibodies and HF-associated inflammatory biomarkers in seropositive patients for HCV (Tsui et al. 2009). Adinolfi et al. explore that chronic HCV infection can cause HF through the progression of coronary atherosclerotic plaque (Adinolfi et al. 2014). Moreover, chronic HCV infection and used antiviral agents may increase the risk of hospitalization of patients with complicated HF due to the development of dilated cardiomyopathy (Lin et al. 2018).
Furthermore, infection with a respiratory syncytial virus (RSV) often leads to development of HF due to underlying cardiopulmonary and immunoprophylaxis disorders (Falsey et al. 2019). RSV also led to various cardiovascular disorders such as ischemic changes due to coronary plaque destabilization and HF through induction of different pro-inflammatory cytokines, adversely affecting heart function (Falsey et al. 2019).
During the influenza epidemic, the hospitalization rate for patients with HF is dramatically increased, suggesting a temporal association between viral infection and cardiomyocyte injury (Vardeny and Solomon 2019). The potential interplay between influenza infection and the development of HF is related to different pathophysiological disturbances, including hypoxemia, neuroendocrine and sympathetic activation, cardio-renal injury-induced volume overload, direct cardiomyocyte injury, and associated hyperinflammatory that interacted mutually in the progress of HF (Panhwar et al. 2019).
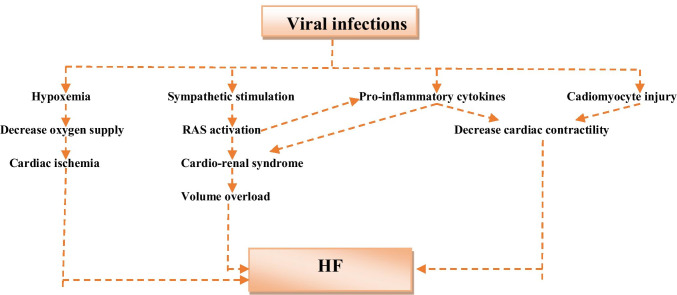
In consideration of these findings, different viral infections in early and late stages may cause progression of HF through the direct viral effect or indirectly through activated pro-inflammatory cytokines. Viral infections induce hypoxemia, sympathetic activation, release of pro-inflammatory cytokines, and cardiomyocyte injury (Chen and Hao 2020). Sympathetic activation activates the renin-angiotensin system (RAS) with induction of cardio-renal syndrome and volume overload. In addition, pro-inflammatory cytokines and RAS are interacted together in the development of the cardio-renal syndrome and reduction of cardiac contractility. These pathophysiological changes promote the development of HF (Soto et al. 2017) (Fig. 1).
Fig. 1.
Role viral infections in the development of heart failure (HF): viral infections induce hypoxemia, sympathetic activation, release of pro-inflammatory cytokines, and cardiomyocyte injury. Sympathetic activation activates renin-angiotensin system (RAS) with induction of cardio-renal syndrome and volume overload. In addition, pro-inflammatory cytokines and RAS interacted together in the development of cardio-renal syndrome and reduction of cardiac contractility. These pathophysiological changes promote development of HF
Covid-19 and risk of heart failure
With the progress of Covid-19 menacing, HF appears as a potential hazard due to SARS-CoV-2 infection chiefly in the elderly patients with underlying comorbidities such as hypertension, ischemic heart disease, and diabetes mellitus (Bader et al. 2021). It has been shown that cardiac injury biomarkers are increased in patients with severe Covid-19 suggesting that development of direct acute cardiac injury and/or myocarditis or indirect due to pulmonary complications such as ALI and ARDS (Zhou et al. 2020). It has been hypothesized that early SARS-CoV-2 infection leads to a classic form of HF with preserved ejection fraction due to acute cardiac injury, whereas in the later phase of SARS-CoV-2 infection, HF with systolic dysfunction is developed due to progression of cytokine storm (Peng et al. 2021).
In reality, the expression of cardiac ACE2 is implicated as a target point for SARS-CoV-2-induced acute cardiac injury. Expression of cardiac ACE2 is at pericytes, mainly in patients with HF that increase the risk of a heart attack during SARS-CoV-2 infection (Chen et al. 2020b). Different interwoven and contested articles implicate angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in the enhancement of SARS-CoV-2 infection through overexpression of cardiac ACE2 (Patel and Verma 2020; Sanchis-Gomar et al. 2020). Previously, different studies highlighted the protective role of ACE2 against the development of HF, atherosclerosis, and endothelial dysfunction (Lovren et al. 2008; Wang et al. 2013). Ohtsuki et al. (2010) confirmed that expression of ACE2 improves cardiomyocytes remodeling in patients with end-stage left ventricular HF (Ohtsuki et al. 2010). Therefore, cardiac ACE2 is regarded as an adaptive mechanism retarding the progression of left ventricular remodeling. Likewise, Keidar et al. (2005) illustrated that aldosterone antagonist eplerenone improves cardiac contractility through augmentation expression of ACE2, which increase the protective Ang1-7 and reduces harmful AngII (Keidar et al. 2005). These findings may explain the protective role of ACEIs and ARBs against SARS-CoV-2 infection-induced acute cardiac injury through overexpression of cardiac ACE2 (Al-kuraishy et al. 2021d). Moreover, in HF, chymase activity is increased, leading to elevation of AngII and reduction of Ang 1-7. As well, administration of recombinant ACE2 will normalize AngII/Ang1-7 ratio and could be a potential therapeutic modality for HF (Al-kuraishy et al. 2021c). Herein, cessation of ACEIs and ARBs in an early phase of Covid-19 may destabilize cardio-metabolic response and aggravate HF and cardiovascular complications (Bozkurt et al. 2020). In the SARS epidemic, viral RNA of SARS-CoV was observed in 35% of autopsy from human heart SARS epidemic in Toronto; these autopsies characterized by myocyte necrosis, macrophage infiltration, and reduction of ACE2 expression (Oudit et al. 2009).
In SARS-CoV-2 infection, like other febrile illnesses, high blood viscosity, exaggerated pro-inflammatory response, multisystem inflammatory syndrome, and endothelial dysfunction-induced coagulation disorders may increase risk of HF (Belhadjer et al. 2020). Hypoxic respiratory failure, as in pulmonary edema, severe ALI, and ARDS may affect heart hemodynamic stability due to the development of pulmonary hypertension (Taz et al. 2021). Indeed, Covid-19-induced HF might be through the development of cytokine storm, which is characterized by high pro-inflammatory cytokines such as IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1) that lead to fulminant myocarditis (Chen et al. 2020a). There is a positive correlation between pro-inflammatory cytokine levels and biomarkers of myocarditis-induced acute cardiac injury (Saed Aldien et al. 2021). As well, SARS-CoV-2 infection-induced hyperferritinemia may lead to acute cardiac dysfunction, HF, and cardiac arrest through augmentation of inflammatory-mediated cardiomyocyte injury (VasanthiDharmalingam et al. 2021). Ashraf et al., a case-report study, illustrated that high ferritin serum level in women with Hodgkin lymphoma is associated with development of HF (Ashraf et al. 2011).
In line with the large body of literature that confirmed the association between cytokine storm and risk of HF, IL-6 is regarded as a prototype of pro-inflammatory cytokine linked with the progression of ventricular dysfunction and congestive HF (Al-Kuraishy and Al-Gareeb 2017). A retrospective cohort study involving 2329 patients with HF showed that IL-6 serum is increased in 50% of patients and predicts mortality (Markousis-Mavrogenis et al. 2019). Therefore, IL-6 could be a potential link between SARS-CoV-2 infection and development of HF since IL-6 serum level is associated with Covid-19 severity and impairment of T cell cytotoxic activity (Han et al. 2020). In addition, high TNF-α level in severe Covid-19 may be associated with HF development since TNF-α level is correlated with biomarkers of cardiomyocyte injury (Al-kuraishy et al. 2018; Noroozi et al. 2020).
During early SARS-CoV-2 infection, both nods like receptor pyrin 3 (NLRP3) inflammasome and NF-κB signaling pathway are activated directly by SARS-CoV-2 viral proteins leading to the bursting of pro-inflammatory cytokines (Hemmat et al. 2021). It has been reported that NLRP3 inflammasome has a vital role in the progression of HF through activation of systemic inflammatory milieu (Butts et al. 2015). Also, hyperactivity of the NF-κB signaling pathway is associated with the development of cardiac dysfunction and progression of HF (Al-Kuraishy and Al-Gareeb 2016). Therefore, stimulation of NLRP3 inflammasome and NF-κB signaling pathway by SARS-CoV-2 viral proteins could be the causal relationship between Covid-19 severity and progression of HF. In addition, p38 mitogen-activated protein kinase (MAPK) is highly activated by SARS-CoV-2 and linked with Covid-19 severity and systemic complications such as HF and cytokine storm (Meijles et al. 2020; Asiedu et al. 2021).
Taken together, these inflammatory mediators may cause sequential impeding of cardiac contractility during early and late phases of SARS-CoV-2 infection. Besides, hyperinflammation and hypercytokinemia during cytokine storm may depress cardiomyocyte functions through the development of severe myocarditis (Imazio et al. 2020).
It has been shown in experimental studies that high circulating AngII may lead to HF due to direct cardiomyocyte injury through AT1R and upregulation of distinct stretch-activated channels (Piezol 1 receptor), which involved in ventricular remodeling (Liang et al. 2017). High AngII in SARS-CoV-2 infection due to downregulation of ACE2 could be a proposed mechanism of HF in severe Covid-19. Thus, attenuation of AngII by ARBs or ACEIs may reduce Covid-19 severity and development of HF (Matsoukas et al. 2021). Indeed, RAS is also involved in metabolic derangement and inflammatory disorders through prorenin receptors, which activate p38 MAPK and p42/p44 MAPK. Prorenin activation is completed by both cathepsin B and cathepsin D (Sun et al. 2017). Padmanabhan et al. illustrated that cathepsin B is regarded as a critical cellular protease that activates trimming of SARS-CoV-2 SP and enhances viral entry. Therefore, cathepsin B inhibitors such as hydroxychloroquine may attenuate SARS-CoV-2 infection and RAS activation (Padmanabhan et al. 2020).
Regarding clinical viewpoints, it has been shown that hospitalized Covid-19 patients with HF are at higher risk for development of complications, poor clinical outcomes, and high mortality. Nearly 1 in 4 four hospitalized Covid-19 patients with HF died during hospitalization (Bhatt et al. 2021). However, later in pandemic, the risk of mortality for Covid-19 patients with HF are reduced, which could be due to improvement of health system efficiencies and expanded testing capabilities (Horby et al. 2020). Moreover, associated comorbidities such as diabetes mellitus, hypertension, and ischemic heart diseases may augment risk for development of HF during SARS-CoV-2 infection (Phelps et al. 2021). However, a cohort-observational study revealed that hospitalized Covid-19 patients with HF had similar clinical outcomes compared with Covid-19 patients without HF. Though, during intensive care unit stay, Covid-19 patients with HF had more in hospital death (Badreldin et al. 2021). The susceptibility of patients with HF for more pronounced complications during development of Covid-19 could be related to injured heart-blood barrier. Leakage of heart-blood barrier is induced by matrix metalloproteinase 9 (MMP-9), which provoked by SARS-CoV-2 causing more cardiomyocyte injury (Tyagi and Singh 2021).
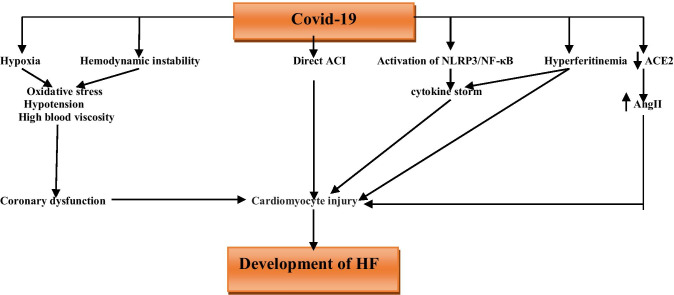
Therefore, Covid-19-induced HF is more complex and related to the pro-inflammatory cytokines and neuro-metabolic imbalances that together affect cardiomyocyte functions and development of HF (Fig. 2).
Fig. 2.
The association between Covid-19 and development of heart failure (HF): in Covid-19 hypoxia and hemodynamic instability lead to hypotension, oxidative stress and high blood viscosity may cardiomyocyte injury through induction of coronary dysfunction. As well, SARS-CoV-2 may cause direct acute myocardial injury (ACI) and development of HF. In addition, hyperferritinemia and activation of NLRP3/NF-κB signaling pathway contribute into ACI through induction of cytokine storm. Besides, downregulation of ACE2 by SARS-CoV-2 upregulates circulating AngII, which involved in cardiomyocyte injury and development of HF
Anti-heart failure drugs and Covid-19
Digoxin is a common drug used in managing HF and atrial fibrillation; it inhibits Na2+/K+ ATPase in the heart, with subsequent increase cardiomyocyte Ca2+, which increases cardiac contractility (Lopes et al. 2018). It has been reported that Na2+/K+ ATPase is involved in different RNA and DNA viral infections such as influenza H1N1, which reduce expression of Na2+/K+ ATPase in the alveolar epithelial cells (Ulug et al. 1996). However, enterovirus 71 (EV71) increases the expression of Na2+/K+ ATPase through interaction with the β3 subunit of this enzyme (Ulug et al. 1996).
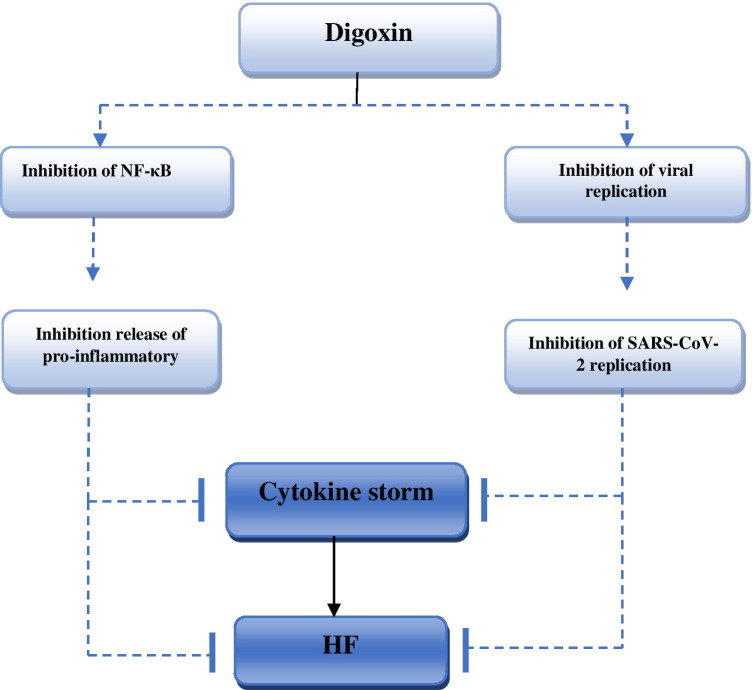
The antiviral mechanisms of digoxin and other cardiac glycosides are through inhibiting the synthesis of viral proteins, blocking the viral life cycle, inhibiting pre-mRNA splicing, inhibiting the synthesis of structural proteins, and inhibiting entry of coronaviruses by blocking endocytosis (Lu et al. 2016; Amarelle and Lecuona 2018). In SARS-CoV-2 infection, cardiac glycosides including digoxin and ouabain have potent in vitro killing of SARS-CoV-2 more than 99% compared to negative control and chloroquine (Cho et al. 2020). Digoxin had antiviral and anti-inflammatory effects against SARS-CoV and MERS-CoV through inhibition of mRNA transcription, protein translation, and release of viral particles (Burkard et al. 2015). However, Siniorakis et al. proposed that use of digoxin in Covid-19 may be associated with high mortality due to complicated electrolyte disturbance and acute renal failure (Siniorakis et al. 2021). Therefore, the anti-inflammatory effects of digoxin may inhibit the development of cytokine storms in SARS-CoV-2 infection since Pollard et al. showed that digoxin attenuates cytokine storms in the influenza epidemic (Pollard et al. 2020). Herein, digoxin is recommended to manage atrial fibrillation in patients with severe Covid-19 (Hu et al. 2020). Of note, a combination of digoxin with doxycycline and melatonin might be a promising combination against Covid-19 (Athanasios et al. 2020). The primary mechanism of digoxin anti-inflammatory effect is the suppression of pro-inflammatory cytokines through the NF-κB inhibition-dependent pathway (Ihenetu et al. 2008). Further, digoxin regulates the unregulated production of immunoglobulins and T cell activations via induction of regulatory T cells (Lee et al. 2015). Thus, digoxin may reduce autoimmune myocarditis-induced cardiomyocyte injury and the development of HF. Nevertheless, a high dose of digoxin may induce local myocardium release of pro-inflammatory cytokine and aggravate myocardial injury in viral myocarditis (Matsumori et al. 1999). The potential role of digoxin in Covid-19 is mainly related to the antiviral and anti-inflammatory effects (Fig. 3).
Fig. 3.
Role of digoxin in the attenuation Covid-19 induced-heart failure. Digoxin inhibits replication of SARS-CoV-2 and NF-κB, thereby inhibiting cytokine storm-induced heart failure
Indeed, carvedilol, a non-selective β adrenoceptor antagonist, and α-1 adrenoceptor antagonist, which used to manage congestive HF and left ventricle dysfunction, have a recent activity against SARS-CoV-2 infection (Skayem and Ayoub 2020). Carvedilol downregulates cardiac ACE2 and inhibits SARS-CoV-2-induced acute cardiac injury (Skayem and Ayoub 2020). In addition, carvedilol blocks release and action of IL-6 (Kurum et al. 2007), which involved in tissue injury and the development of cytokine storms. Amirshahrokhi et al. illustrated that carvedilol can attenuate the development of paraquat-induced ALI through suppression of oxidative stress and NF-κB signaling pathway (Amirshahrokhi and Khalili 2016). Also, carvedilol effectively manages Covid-19 complications such as esophageal varices (Congly et al. 2020) and post-Covid-19 sinus tachycardia (Kartik Pandurang and Pankaj 2020). Therefore, through its antiviral and anti-inflammatory properties, carvedilol may have dual protective effects in Covid-19 by mitigation development of HF and ALI (Servato et al. 2021).
Moreover, mineralocorticoid receptor antagonists, like eplerenone and spironolactone that are used in the management of HF, have anti-SARS-CoV-2 effects and prevent acute complications of Covid-19, including HF and ALI (Kotfis et al. 2021). Likewise, mineralocorticoid antagonists attenuate the development of ALI, ARDS, and pulmonary hypertension in severe Covid-19 through regulation of RAS (Dumanlı et al. 2020).
Furthermore, vasopressin receptor antagonists such as conivaptan have in silico anti-SARS-CoV-2 to inhibit RNA-dependent polymerase and non-structural protein 9 (Gul et al. 2020). Besides, conivaptan inhibits release of pro-inflammatory cytokines (Can et al. 2018); thus, it can be a potential candidate against SARS-CoV-2 infection and linked inflammatory disorders.
Taken together, medications that are used in the management of HF like digoxin and adrenoceptor antagonists during the development of Covid-19 should be revised as some drugs could have dual properties against HF and SARS-CoV-2 infection in patients with severe Covid-19.
Conclusion
SARS-CoV-2 infection may lead to the development of HF due to direct acute cardiac injury or through progression of cytokine storms, which depress cardiomyocyte function and cardiac contractility. Anti-heart failure drugs, mainly digoxin and carvedilol, may attenuate development of HF by reducing the infectivity of SARS-CoV-2 and prevent the development of cytokine storms in severely affected Covid-19 patients.
Author contribution
HMA, HO, AIA, SQ, EMA, and GEB conceived and designed research. HMA, HO, and AIA conducted experiments. HMA, HO, AIA, SQ, EMA, and GEB AM and GR analyzed the graphical illustrations data. HMA, HO, AIA, and GEB wrote the manuscript. All authors read and approved the manuscript and all data were generated in-house and that no paper mill was used.
Data availability
The datasets/information used for this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All the authors have read and agreed to the final copy of the finding as contained in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hope Onohuean, Email: hope.onohuean1@kiu.ac.ug, Email: onohuean@gmail.com.
Hayder M. Al-kuraishy, Email: Haydermutter@uomustansiriyah.edu.iq
Ali I. Al-Gareeb, Email: dr.alialgareeb@uomustansiriyah.edu.iq
Safaa Qusti, Email: squsti@kau.edu.sa.
Eida M. Alshammari, Email: eida.alshammari@uoh.edu.sa
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com, Email: dr_gaber_batiha@vetmed.dmu.edu.eg.
References
- Adinolfi LE, Zampino R, Restivo L, et al. (2014) Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol10.3748/wjg.v20.i13.3410 [DOI] [PMC free article] [PubMed]
- Al-kuraishy H, Al-Gareeb A, Al-Buhadilly A (2018) Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases10.3390/diseases6010009 [DOI] [PMC free article] [PubMed]
- Al-Kuraishy H, Hussien N, Al-Naimi M, et al. (2020) Is ivermectin-azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J10.4103/bbrj.bbrj_109_20
- Al-Kuraishy HM, Al-Gareeb AI (2017) Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: role of statins. J Lab Physicians10.4103/0974-2727.208263 [DOI] [PMC free article] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI (2016) Potential effects of pomegranate on lipid peroxidation and pro-inflammatory changes in daunorubicin-induced cardiotoxicity in rats. Int J Prev Med10.4103/2008-7802.184314 [DOI] [PMC free article] [PubMed]
- Al-kuraishy HM, Al-Gareeb AI, Abdullah SM, et al. Case report: Hyperbilirubinemia in Gilbert syndrome attenuates Covid-19-induced metabolic disturbances. Front Cardiovasc Med. 2021 doi: 10.3389/fcvm.2021.642181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-kuraishy HM, Al-Gareeb AI, Alblihed M et al (2021) COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: the anti-inflammatory role of metformin. Front Med. 10.3389/fmed.2021.644295 [DOI] [PMC free article] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, & Batiha GES (2021) Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: The enigmatic entity. European journal of pharmacology, 174196 [DOI] [PMC free article] [PubMed]
- Al-kuraishy HM, Al-Gareeb AI, Alzahrani KJ, et al. The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem. 2021 doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-kuraishy HM, Al-Gareeb AI, Faidah H, et al. (2021e) The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr [DOI] [PMC free article] [PubMed]
- Amarelle L, Lecuona E (2018) The antiviral effects of Na,K-ATPase inhibition: a minireview. Int J Mol Sci [DOI] [PMC free article] [PubMed]
- Amirshahrokhi K, Khalili AR (2016) Carvedilol attenuates paraquat-induced lung injury by inhibition of proinflammatory cytokines, chemokine MCP-1, NF-κB activation and oxidative stress mediators. Cytokine 10.1016/j.cyto.2016.09.004 [DOI] [PubMed]
- Ashraf H, Norouzi P, Jafari S (2011) Hodgkin’s disease with hyperferritinemia, hepatic and heart failure. Govaresh
- Asiedu SO, Kwofie SK, Broni E, Wilson MD. Computational identification of potential anti-inflammatory natural compounds targeting the p38 mitogen-activated protein kinase (Mapk): implications for covid-19-induced cytokine storm. Biomolecules. 2021 doi: 10.3390/biom11050653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasios T, Psychos C, Domenikos S (2020) Communication: Novel drug combination doxycycline-melatonin-digoxin(D-M-D) for possible Covid-19 treatment. J Mod Med Chem 10.12970/2308-8044.2020.08.01
- Bader F, Manla Y, Atallah B, Starling RC (2021) Heart failure and COVID-19. Heart Fail. Rev [DOI] [PMC free article] [PubMed]
- Badreldin H, Hafidh D, Bin Saleh D, et al. Clinical characteristics and outcomes of patients with heart failure admitted to the intensive care unit with coronavirus disease 2019 (COVID19): a multicenter cohort study. Eur J Cardiovasc Nurs. 2021 doi: 10.1093/eurjcn/zvab060.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical outcomes in patients with heart failure hospitalized with COVID-19. JACC Hear Fail. 2021 doi: 10.1016/j.jchf.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt B, Kovacs R, Harrington B (2020) Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail [DOI] [PMC free article] [PubMed]
- Burkard C, Verheije MH, Haagmans BL, et al. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J Virol. 2015 doi: 10.1128/jvi.03274-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011 doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts B, Gary RA, Dunbar SB, Butler J (2015) The importance of NLRP3 inflammasome in heart failure. J Card Fail [DOI] [PMC free article] [PubMed]
- Can B, Öz S, Muşmul A, et al (2018) Effects of conivaptan and mannitol on serum cytokine levels (TNF-α, IL-15 and IL-35) following bilateral carotid artery occlusion. undefined
- Chen C, Zhou Y, Wang DW (2020a) SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz [DOI] [PMC free article] [PubMed]
- Chen L, Hao G (2020) The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovasc Res [DOI] [PMC free article] [PubMed]
- Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020 doi: 10.1093/CVR/CVAA078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Lee YJ, Kim JH, et al. Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19. Sci Rep. 2020 doi: 10.1038/s41598-020-72879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congly SE, Sadler MD, Abraldes JG, et al. Practical management of esophageal varices in the context of SARS-CoV-2 (COVID-19): the Alberta protocol. Can Liver J. 2020 doi: 10.3138/canlivj-2020-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanlı GY, Dilken O, Ürkmez S (2020) Use of spironolactone in SARS-CoV-2 ARDS patients. Turk J Anaesthesiol Reanim [DOI] [PMC free article] [PubMed]
- Falsey AR, Walsh EE, Esser MT, et al. Respiratory syncytial virus–associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J Med Virol. 2019 doi: 10.1002/jmv.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré N, Vela E, Clèries M, et al. Real world heart failure epidemiology and outcome: a population-based analysis of 88,195 patients. PLoS One. 2017 doi: 10.1371/journal.pone.0172745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul S, Ozcan O, Asar S, et al. In silico identification of widely used and well-tolerated drugs as potential SARS-CoV-2 3C-like protease and viral RNA-dependent RNA polymerase inhibitors for direct use in clinical trials. J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1802346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020 doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmat N, Asadzadeh Z, Ahangar NK, et al (2021) The roles of signaling pathways in SARS-CoV-2 infection; lessons learned from SARS-CoV and MERS-CoV. Arch Virol [DOI] [PMC free article] [PubMed]
- Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19–preliminary report. N Engl J Med. 2020 doi: 10.1101/2020.06.22.20137273. [DOI] [Google Scholar]
- Hu YF, Cheng WH, Hung Y, et al. (2020) Management of atrial fibrillation in COVID-19 pandemic. Circ J [DOI] [PubMed]
- Ihenetu K, Espinosa R, De Leon R, et al. Digoxin and digoxin-like immunoreactive factors (DLIF) modulate the release of pro-inflammatory cytokines. Inflamm Res. 2008 doi: 10.1007/s00011-008-7249-9. [DOI] [PubMed] [Google Scholar]
- Imazio M, Klingel K, Kindermann I, et al. (2020) COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart [DOI] [PubMed]
- Kartik Pandurang J, Pankaj V J (2020) Ivabradine versus carvedilol in the management of palpitation with sinus tachycardia among recovered COVID-19 patients. J Cardiol Cardiovasc Med 10.29328/journal.jccm.1001107
- Keidar S, Gamliel-Lazarovich A, Kaplan M, et al. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005 doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- Kotfis K, Lechowicz K, Drożdżal S, et al (2021) COVID-19—the potential beneficial therapeutic effects of spironolactone during SARS-CoV-2 infection. Pharmaceuticals [DOI] [PMC free article] [PubMed]
- Kristoffersen US, Lebech AM, Gerstoft J, et al. Right and left cardiac function in HIV-infected patients investigated using radionuclide ventriculography and brain natriuretic peptide: a 5-year follow-up study. HIV Med. 2008 doi: 10.1111/j.1468-1293.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Kurum T, Tatli E, Yuksel M (2007) Effects of carvedilol on plasma levels of pro-inflammatory cytokines in patients with ischemic and nonischemic dilated cardiomyopathy. Texas Hear Inst J [PMC free article] [PubMed]
- Lee J, Baek S, Lee J, et al. Digoxin ameliorates autoimmune arthritis via suppression of Th17 differentiation. Int Immunopharmacol. 2015 doi: 10.1016/j.intimp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Liang J, Huang B, Yuan G, et al (2017) Stretch-activated channel Piezo1 is up-regulated in failure heart and cardiomyocyte stimulated by Angii. Am J Transl Res [PMC free article] [PubMed]
- Lin MS, Chung CM, Chang ML, et al. The unraveled link between antiviral therapy and heart failure hospitalization in chronic hepatitis c virus infection—a nationwide cohort study. Circ J. 2018 doi: 10.1253/circj.CJ-17-1118. [DOI] [PubMed] [Google Scholar]
- Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 doi: 10.1016/j.jacc.2017.12.060. [DOI] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol - Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hou H, Wang F, et al. ATP1B3: a virus-induced host factor against EV71 replication by up-regulating the production of type-I interferons. Virology. 2016 doi: 10.1016/j.virol.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C, Al-Kuraishy HM, Rousseau E (2021) PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem Pharmacol [DOI] [PMC free article] [PubMed]
- M. Al-Kuraishy H, S. Al-Niemi M, R. Hussain N, et al (2020) The potential role of renin angiotensin system (RAS) and dipeptidyl peptidase-4 (DPP-4) in COVID-19: navigating the uncharted. In: Selected chapters from the renin-angiotensin system
- Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019 doi: 10.1002/ejhf.1482. [DOI] [PubMed] [Google Scholar]
- Matsoukas J, Apostolopoulos V, Zulli A, et al (2021) From angiotensin II to cyclic peptides and angiotensin receptor blockers (Arbs): perspectives of arbs in covid-19 therapy. Molecules [DOI] [PMC free article] [PubMed]
- Matsumori A, Igata H, Ono K, et al. High doses of digitalis increase the myocardial production of proinflammatory cytokines and worsen myocardial injury in viral myocarditis: a possible mechanism of digitalis toxicity. Jpn Circ J. 1999 doi: 10.1253/jcj.63.934. [DOI] [PubMed] [Google Scholar]
- Matsumori A, Shimada T, Chapman NM, et al. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006 doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Meijles DN, Cull JJ, Markou T, et al. Redox regulation of cardiac ASK1 (apoptosis signal-regulating kinase 1) controls p38-MAPK (mitogen-activated protein kinase) and orchestrates cardiac remodeling to hypertension. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.119.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubarak M, Kasozi KI, Hetta HF, et al. The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life. 2021 doi: 10.3390/life11080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nico Reinsch, Neuhaus Kathrin, Esser Stefan, Potthoff Anja, Hower Martin, Brockmeyer Norbert H, Raimund Erbel TN. Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2010;11:156–162. doi: 10.1310/HCT1103-156. [DOI] [PubMed] [Google Scholar]
- Noroozi R, Branicki W, Pyrc K, et al (2020) Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine [DOI] [PMC free article] [PubMed]
- Ohtsuki M, Morimoto SI, Izawa H, et al. Angiotensin converting enzyme 2 gene expression increased compensatory for left ventricular remodeling in patients with end-stage heart failure. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2009.11.057. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009 doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan P, Desikan R, Dixit NM (2020) Targeting TMPRSS2 and cathepsin B/L together may be synergistic against SARS-CoV-2 infection. ChemRxiv. 10.26434/chemrxiv.12213125.v2 [DOI] [PMC free article] [PubMed]
- Panhwar MS, Kalra A, Gupta T, et al. Effect of influenza on outcomes in patients with heart failure. JACC Hear Fail. 2019 doi: 10.1016/j.jchf.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Patel AB, Verma A (2020) COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA - J Am Med Assoc [DOI] [PubMed]
- Peng X, Wang Y, Xi X, et al. (2021) Promising therapy for heart failure in patients with severe COVID-19: calming the cytokine storm. Cardiovasc Drugs Ther [DOI] [PMC free article] [PubMed]
- Phelps M, Christensen DM, Gerds T, et al. Cardiovascular comorbidities as predictors for severe COVID-19 infection or death. Eur Hear J - Qual Care Clin Outcomes. 2021 doi: 10.1093/ehjqcco/qcaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard BS, Blancol JC, Pollard JR (2020) Classical drug digitoxin inhibits influenza cytokine storm, with implications for Covid-19 therapy. In Vivo (Brooklyn). 10.21873/invivo.12221 [DOI] [PMC free article] [PubMed]
- Reza N, DeFilippis EM, Jessup M (2020) Secondary impact of the COVID-19 pandemic on patients with heart failure. Circ Hear Fail [DOI] [PubMed]
- Rossignol P, Hernandez AF, Solomon SD, Zannad F (2019) Heart failure drug treatment. Lancet [DOI] [PubMed]
- Saed Aldien A, Ganesan GS, Wahbeh F, et al (2021) Systemic inflammation may induce cardiac injury in COVID-19 patients including children and adolescents without underlying cardiovascular diseases: a systematic review. Cardiovasc. Revascularization Med. [DOI] [PMC free article] [PubMed]
- Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, et al (2020) Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc [DOI] [PMC free article] [PubMed]
- Servato ML, Valente FX, García-Moreno LG, et al. Intraventricular conundrum in a SARS-CoV-2–positive patient with elevated biomarkers of myocardial injury. JACC Case Rep. 2021 doi: 10.1016/j.jaccas.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniorakis E, Arvanitakis S, Katsianis A, Elkouris M (2021) Atrial fibrillation and flutter in patients hospitalized for COVID-19: the challenging role of digoxin. J Cardiovasc Electrophysiol [DOI] [PMC free article] [PubMed]
- Skayem C, Ayoub N (2020) Carvedilol and COVID-19: a potential role in reducing infectivity and infection severity of SARS-CoV-2. Am J Med Sci [DOI] [PMC free article] [PubMed]
- Soto M, Bang SI, McCombs J, Rodgers KE. Renin Angiotensin system-modifying therapies are associated with improved pulmonary health. Clin Diabetes Endocrinol. 2017 doi: 10.1186/s40842-017-0044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Danser AHJ, Lu X (2017) (Pro)renin receptor as a therapeutic target for the treatment of cardiovascular diseases? Pharmacol Res [DOI] [PubMed]
- Taz TA, Ahmed K, Paul BK, et al. Identification of biomarkers and pathways for the SARS-CoV-2 infections that make complexities in pulmonary arterial hypertension patients. Brief Bioinform. 2021 doi: 10.1093/bib/bbab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Whooley MA, Monto A, et al. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul Study. J Card Fail. 2009 doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi SC, Singh M (2021) Multi-organ damage by covid-19: congestive (cardio-pulmonary) heart failure, and blood-heart barrier leakage. Mol Cell Biochem [DOI] [PMC free article] [PubMed]
- Ulug ET, Garry RF, Bose HR. Inhibition of Na+K+ATPase activity in membranes of Sindbis virus-infected chick cells. Virology. 1996 doi: 10.1006/viro.1996.0065. [DOI] [PubMed] [Google Scholar]
- Vardeny O, Solomon SD (2019) Influenza and heart failure: a catchy comorbid combination. JACC Hear Fail [DOI] [PubMed]
- VasanthiDharmalingam P, Karuppagounder V, Watanabe K, et al (2021) SARS–CoV-2 mediated hyperferritinemia and cardiac arrest: preliminary insights. Drug Discov Today [DOI] [PMC free article] [PubMed]
- Wang Y, Tikellis C, Thomas MC, Golledge J (2013) Angiotensin converting enzyme 2 and atherosclerosis. Atherosclerosis [DOI] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets/information used for this study are available from the corresponding author on reasonable request.