Abstract
Backgrounds
Risk factors related to the built environment have been associated with women’s mental health and preventive care. This study sought to identify built environment factors that are associated with variations in prenatal care and subsequent pregnancy-related outcomes in an urban setting.
Methods
In a retrospective observational study, we characterized the types and frequency of prenatal care events that are associated with the various built environment factors of the patients’ residing neighborhoods. In comparison to women living in higher-quality built environments, we hypothesize that women who reside in lower-quality built environments experience different patterns of clinical events that may increase the risk for adverse outcomes. Using machine learning, we performed pattern detection to characterize the variability in prenatal care concerning encounter types, clinical problems, and medication prescriptions. Structural equation modeling was used to test the associations among built environment, prenatal care variation, and pregnancy outcome. The main outcome is postpartum depression (PPD) diagnosis within 1 year following childbirth. The exposures were the quality of the built environment in the patients’ residing neighborhoods. Electronic health records (EHR) data of pregnant women (n = 8,949) who had live delivery at an urban academic medical center from 2015 to 2017 were included in the study.
Results
We discovered prenatal care patterns that were summarized into three common types. Women who experienced the prenatal care pattern with the highest rates of PPD were more likely to reside in neighborhoods with homogeneous land use, lower walkability, lower air pollutant concentration, and lower retail floor ratios after adjusting for age, neighborhood average education level, marital status, and income inequality.
Conclusions
In an urban setting, multi-purpose and walkable communities were found to be associated with a lower risk of PPD. Findings may inform urban design policies and provide awareness for care providers on the association of patients’ residing neighborhoods and healthy pregnancy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-021-04056-1.
Keywords: Pregnancy care, Postpartum depression, Built environment
Background
The built environment, referring to the surroundings and physical artifacts of where humans live, is considered to be one of the five major social determinants of health (SDoH) [1]. The built environment is strongly associated with our way of life through determining the housing quality, mode of transportation, and exposure to pollutants, among others. Poor built environment has been reported to lead to adverse effects on physical and mental health by disrupting sleep, hindering healthy lifestyles, and lowering access to healthcare [2–5]. There is a gender difference in the association between the built environment and health. For example, an increased risk of depression among female was reported by Mulling et al. to be associated with living in an underdeveloped neighborhood characterized by inadequate sewer treatment, water supply, and dependable supply of electricity [6]. In addition, the Chicago Community Adult Health Study found the women’s use of preventive care to be associated with objective and perceived neighborhood support and stressors such as odors, presence of trees, and noise levels [7].
The existing literature motivated this study to examine the impact of the built environment on health and healthcare utilization among women, and particularly, pregnant women as a population to further investigate [8–10]. Levels of prenatal care vary across the United States [11–13]. A substantial proportion of pregnant women, especially those with a higher comorbidity burden or low health literacy, seek and depend on care provided by emergency departments (ED) rather than primary and obstetric care [13–15]. The lack of adequate prenatal care is considered a risk factor for poor pregnancy outcomes and lack of proper postpartum care for mothers and infants [16]. Previous studies have studied the built environment on maternal health and birth outcomes including birth weight, gestational age, Apgar score, and newborn intensive care unit admission rates [5, 17]. Yet, evidence is still accumulating on how the built environment affects the variability in prenatal care and maternal mental health outcomes. In particular, few studied the concurrent impacts of prenatal care and built environment on mental health outcomes [18–22]. Existing studies have also commonly relied on the subjective perceived measures obtained from interviews and questionnaires to define postpartum depression (PPD), thus limiting larger-scale analysis [7, 18, 19, 23].
A study conducted in Mexico found that an increase in average particulate matter ≤ 2.5 μm in diameter (PM2.5) exposure during pregnancy was statistically associated with an increased risk of PPD at six months and also for the late-onset PPD [18]. Likewise, in a US-based cohort, Sheffield et al. discovered that increased PM2.5 exposure in mid-pregnancy was associated with higher anhedonia and depressive symptoms specifically in Black women [19]. He et al. found that pregnant women with no prior history of mental illness, exposed to noise, specifically night-time noise, have a higher risk of hospitalization for depression and other mental health disorders later in life [20]. In addition, Crockett et al. showed that the lack of public transportation acted as a barrier in accessing care for rural low-income African American pregnant women at risk for PPD [21]. Similarly, a study involving a home-based intervention for depression in low-income mothers noted that residing in neighborhoods with poor housing, higher crime rates, lack of essential resources increased the notion of uncertainty in life and participants required more encouragement to remain in the study [22].
In this study, based on existing evidence above, we hypothesize that the built environment, through a wide range of measures influencing the accessibility to the transportation system and infrastructure elements, green space, and other urban structure, is associated with variability in prenatal care and subsequent maternal mental health outcomes. Given findings from previous literature on the impact of the built environment on women’s mental health and use of healthcare, we defined PPD as our primary outcome [24]. PPD has been associated with increased infant mortality, higher rates of hospitalizations, impaired mother-child attachment, developmental problems in children, and increased stress within families [25–28]. The plethora of physical and psychological effects of PPD reported in previous studies include postpartum weight retention, reduced physical health, bodily pain, anxiety, low self-esteem, risky addictive behavior of substances, and suicide ideation [29]. The biological risk factors of PPD include genetic factors, age, pregnancy complications, medical illness, and smoking during pregnancy [4, 30–32]. The social, cultural, and environmental risk factors include income status, domestic violence, lack of social support, quantity and quality of green spaces, and residential noise pollution [31, 33–37].
We tested our hypotheses by linking patients’ health data extracted from de-identified electronic health records (EHR) with publicly available census-tract level data on the built environment. Routinely collected from clinical encounters, EHR data capture detailed longitudinal health data on health and health service utilization. Increasingly, EHR data have been used as a source of longitudinal data in population health studies for its ability to provide detailed and rich health information within patient cohorts [38]. Leveraging a large cohort of nearly 9,000 women in New York City from 2015 to 2017, we applied machine learning algorithms to EHR data to identify patterns in prenatal care [39]. We then evaluated the relationships among prenatal care patterns, PPD incidence, and the built environment using structural equation modeling [40]. The association found may inform patients, care providers, and public health policymakers in supporting a healthy pregnancy and new motherhood through a better understanding of the built environment as a modifiable social determinant of health.
Methods
Study setting
EHR data
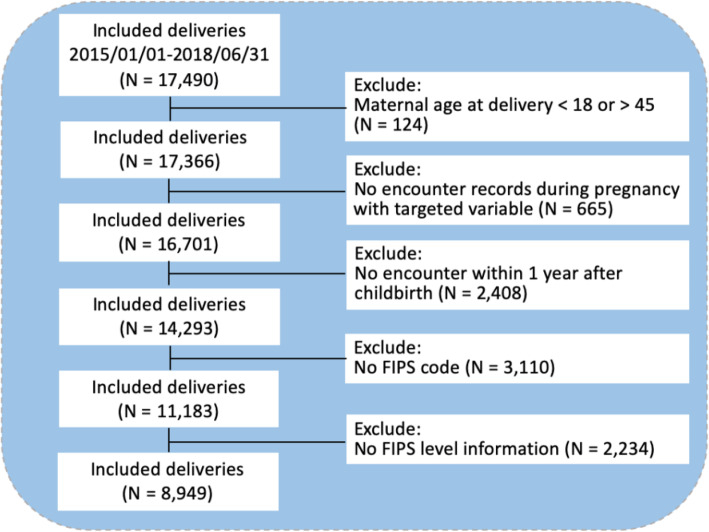
EHR data on 8,949 pregnant women from an urban academic medical center from 2015 to 2017 were extracted. The cohort inclusion and exclusion criteria are described in Fig. 1. We excluded patients whose ages were below 18 or above 45, had no encounter recorded in the EHR from 1 year prior to pregnancy to 1 year after delivery, or missing home locations information. We extracted patient information including gender, age, race, ethnicity, body mass index (BMI), marital status, outpatient and inpatient diagnoses, outpatient and inpatient prescription medication orders, and corresponding encounter dates from the EHR data. Patient age was calculated as the time difference between the birth date and first prenatal checkup date. The gestational week was calculated using the date of delivery and the specific gestational age at prenatal checkups. Marital status was defined as single (single, divorced, widowed, unknown), and married, as extracted from unstructured clinical notes using regular expression. The trimester of each event was determined using the difference in time between each event and delivery. All diagnoses were represented as Systematized Nomenclature of Medicine-Clinical Terms (SNOMED-CT) codes [41]. Anatomical Therapeutic Chemical (ATC) Classification System was used to standardize the specific drug prescription and dosage information [42]. The primary outcome of PPD was defined as having at least one diagnosis of depression within 1 year after childbirth based on SNOMED codes (see Additional file 1).
Fig. 1.
Study cohort inclusion and exclusion criteria
Built environment data
Accessibility to public and active transportation, and green spaces
Three indicators were defined to measure the accessibility to public and active transportation facilities [43–46] within a 500-meter radius [47]: the number of bus stops, the number of subway stations within, and the length of bike paths within. The spatial data on public transportation and bike facilities were obtained in shapefile formats from New York State [48]. We used ArcGIS 10.6 spatial analysis tools to count the number of bus stops and subway stations within each 500-meter radius around each patients’ home location and also to measure the length of bike paths within the 500-meter radius. Access to green spaces, as defined by the City of New York under recreation land use, were calculated using the green areas that fall within the 500-meter buffer.
Exposure to traffic
We obtained traffic data from the New York activity-based travel demand model referred to as “New York Best Practice Model (NYBPM)” [49]. The model predicts daily traffic volume in each roadway link for the different types of vehicles by two categories: light- (passenger vehicles and taxis) and heavy-duty (buses and trucks) vehicles for their different levels of health impacts [50]. The vehicle kilometer traveled (VKT) as an indicator for travel activity within the 500-meter radius was then calculated. The buffer was chosen since both monitoring [51] and simulation [52] studies have shown that vehicle pollution concentration reaches the background level. VKT is calculated by multiplying traffic volume by the distance of travel, representing the amount of traffic activity.
Land use
Four indicators were defined to measure the role of land use: entropy-based land use mix (LUM) index, retail floor area ratio (RetFAR), street connectivity, and sidewalk availability. The variables measure the availability and variety of land use types (i.e., type of activity) within 500 m of the subject’s home location. The land use data including information about land use class and parcel area at the parcel level were extracted from the parcel shapefile obtained from New York State [48]. The LUM index within a 500-meter radius measures the heterogeneity of land use, such as residential, commercial, retail, and industrial, within the radius [53]. The LUM index ranges between 0 and 1, where 0 represents homogeneity and 1 represents maximum heterogeneity [53]. Higher LUM values indicate higher walkability of the area. The RetFAR is the retail building floor area divided by the retail land area within the 250-m radius [53]. RetFAR is indicative of pedestrian-orientated design and higher walkability. Examples with higher and lower RetFAR are multi-floor departmental stores and open-style outlets, respectively. The number of intersections within the 500-meter radius is another land use indicator used to measure the walkability of the neighborhood [54]. The number of intersections was extracted from the transportation network developed for the NYBPM travel demand model. To calculate the sidewalk area, as a measure of access to walking facilities, within the 500-meter radius, we used the sidewalk shapefiles [49].
Air pollution
PM2.5 and ozone (O3) concentrations at the census tract level for the period of 2015–2017 were obtained from the Center for Air, Climate and Energy Solutions which applied Land Use Regression (LUR) models to estimate every subject’s exposure to air pollution [55]. PM2.5 and O3 together could represent both regional background and hotspot air pollution levels.
Other SDoH
Lastly, SDoH information at the census-tract (11-digit Federal Information Processing Standard code) level was extracted using the FACETS dataset [56]. Variables used in the analysis included census-tract level average percent of college degree education, GINI index, and uninsured percentage from American Community Survey, a binary indicator of low access to healthy food within half-mile from the Food Access Research Atlas, United States Department of Agriculture, the population-weighted distance to closest 7 parks from the Centers for Disease Control and Prevention, and lastly walk score scales the from Rundle-Columbia Built Environment and Health Research Group.
Patterns of prenatal care
We extracted the health and healthcare utilization information during the prenatal period for each patient from the EHR data. Patients who had similar overall prenatal care patterns were categorized into clusters as having experienced generally similar prenatal events. The similarity between pairs of patients was measured using the longest common subsequence (LCS) distance. LCS measures the longest overlap that 2 sequences have in common; thus, larger LCS indicates a more similar course of the clinical events. In this study, we compared the sequence of each patient’s clinical events (e.g., encounters, diagnoses, prescription medications) to others in the cohort to generate pairs of LCS distances. Based on the similarity, the categorization of patients was performed using the hierarchical clustering algorithm, a well-established machine learning method for detecting underlying clusters in a population [39]. The final number and size of the clusters were determined using the Silhouette value [39]. This method was previously used to mine EHR data to identify health and healthcare utilization patterns among patients with chronic kidney disease, heart failure, and undifferentiated abdominal pain [39, 57, 58]. Because of the large number (n > 6,000) of unique clinical events recorded in the EHR data, we limited the pattern mining to focus on variables that were found to be most predictive of PPD in a related work preparatory to this study [59]. The list of variables, including complications during pregnancy and medication usage, are shown in Additional file 2. The cluster analysis was done in Python 3.6.5 and R 4.0.0.
Within each cluster, we applied a well-established sequence mining algorithm, Sequential Pattern Discovery using Equivalent Classes (SPADE) algorithm, [60] to discover and visualize common patterns within each cluster identified above. These patterns include sequential events that are shared by a large enough portion of the patients. For the implementation of SPADE, we used the “arulesSequences package” in R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Statistical analysis
The distribution of study variables described in sections EHR Data and Built Environment Data (Table 1) were assessed within each identified cluster. Multivariate Imputation by Chained Equations (MICE) was used to address the missing value issue [61]. We further studied the relationship between prenatal care, as reflected by the cluster membership, the built environment characteristics, and incidence of PPD using structural equation models (SEMs) [40]. Two SEMs were constructed for the primary and secondary outcomes separately. All independent variables were considered, but removed if there was multicollinearity as determined by variable inflation factor larger than 10. Statistical analysis was done using Stata/IC 16.0 and R 4.0.0. We applied Chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables to compare the differences across clusters. P-value of 0.05 was used as the significance threshold.
Table 1.
Descriptive statistics of the study cohort
| Variables | Values |
|---|---|
| Demographics | |
| Age, mean (SD), year | 33.69 (4.59) |
| Pre-pregnancy BMI, mean (SD), kg/m2 | 23.77 (4.31) |
| Gestational Week, mean (SD), week | 38.69 (2.09) |
| Race, No. (%) | |
| White | 4409 (49.27) |
| Asian | 1689 (18.87) |
| Black or African American | 560 (6.26) |
| Other | 976 (10.91) |
| Unknown | 1315 (14.69) |
| Marital Status, No. (%) | |
| Single | 1193 (13.33) |
| Married | 7756 (86.67) |
| Cesarean Section, No. (%) | |
| Yes | 1878 (20.99) |
| No | 7071 (79.01) |
| Insurance, No. (%) | |
| Commercial | 7519 (84.02) |
| Medicaid | 1226 (13.70) |
| Other | 204 (2.28) |
| Built Environment | |
| Number of bus stops within 500 m radius, mean (SD) | 25.26 (10.0) |
| Number of subway stations within 500 m radius, mean (SD) | 1.81 (1.83) |
| Parks Area within 500 m radius, mean (SD), m2 | 463112.43 (660506.3) |
| Bike Path Length within 500 m radius, mean (SD), m | 29070.94 (15172.89) |
| VKT of light vehicles within 500 m radius, mean (SD), 100,000 units | 3283.87 (2242.98) |
| VKT of heavy vehicles within 500 m radius, mean (SD), 10,000 units | 3608.43 (2516.02) |
| LUM index within 500 m radius, mean (SD) | 0.64 (0.17) |
| RetFar within 500 m radius, mean (SD) | 0.24 (0.23) |
| Number of Intersections within 500 m radius, mean (SD) | 12.06 (7.76) |
| Sidewalk Area within 500 m radius, mean (SD), 1000 m2 | 907.77 (208.53) |
| Ozone Concentration, mean (SD), µg/m3 | 46.56 (0.50) |
| PM2.5 Concentration, mean (SD), µg/m3 | 9.28 (0.47) |
| Percent of Colleges Degree, mean (SD), % | 35.79 (11.49) |
| Average Poverty Rate, mean (SD), % | 1.62 (2.15) |
| Average Respiratory Hazard Index, mean (SD) | 4.51 (1.16) |
| Low Access to Healthy Food, No. (%) | 297 (3.32) |
| Uninsured Percentage, mean (SD), % | 8.26 (5.60) |
| Postpartum Depression | |
| Yes, No. (%) | 273 (3.05) |
| Average number of ED visits per patient | |
| Pre-delivery (N = 3900, 43.58 %), mean (SD) | 0.74 (1.16) |
| Post-delivery (N = 482, 5.39 %), mean (SD) | 0.07 (0.31) |
Results
Table 1 shows the descriptive statistics of the study cohort where continuous variables are presented as mean (standard deviation (SD)), and categorical variables are presented as N (% in total cohort). The average age of our patient population was 33.7 years (SD = 4.59). Nearly half (49.27 %) of the patients were White, and the majority were married (86.7 %) and had Commercial insurances (84.0 %). Over 3 % of the cohort were diagnosed with PPD. A total of 3,900 (43.6 %) and 482 (5.4 %) patients had at least one ED visit pre- and post-delivery.
We identified 3 clusters with 1,934 (cluster 1), 4,129 (cluster 2), and 2,886 (cluster 3) patients, respectively, based on their clinical event sequences. For the primary outcome of PPD, 6.72 % of the women in cluster 1 had a diagnosis of PPD within 1 year after childbirth, which was higher than clusters 2 (2.66 %) and 3 (1.14 %) (P < .001). Table 2 displays the distributions of variables used to determine the clusters. The distributions of variables were all statistically different across clusters except for antidepressant prescriptions and the diastolic blood pressure in the third trimester. Table 3 presents a post-hoc analysis of the distribution of demographics, medications, diagnoses, and built environment factors that were significantly different across the three clusters. The mean (SD) age across three clusters were 35.01 (4.73) years, 33.78 (4.29) years and 32.68 (4.66) years, respectively (P < .001). There were more unmarried patients in cluster 1 than the other two clusters (P < .001). In addition, the number of ED visits in both the pre- and post-delivery periods in cluster 1 was higher (P < .001) than the other clusters. We observed higher rates of prescription medications in cluster 1, such as analgesics, antipyretics and opioids (P < .001). Also, more patients in cluster 1 had complications during pregnancy, unplanned pregnancies, high-risk pregnancy, abnormal glucose level, elderly primigravida and advanced maternal age gravidas than the other two clusters (P < .001).
Table 2.
Distribution of clinical pathway elements across clusters
| Variables | Cluster 1 (N = 1934) |
Cluster 2 (N = 4129) |
Cluster 3 (N = 2886) |
P-value |
|---|---|---|---|---|
| Diagnosis | ||||
| Anxiety history, no. (%) | 111 (5.74) | 86 (2.08) | 26 (0.90) | < 0.001 |
| Other disorder history, no. (%) | 83 (4.29) | 89 (2.16) | 35 (1.21) | < 0.001 |
| Mood disorder history, no. (%) | 75 (3.88) | 61 (1.48) | 32 (1.11) | < 0.001 |
| Depression in pregnancy, no. (%) | 28 (1.45) | 24 (0.58) | 12 (0.42) | < 0.001 |
| Anxiety in pregnancy, no. (%) | 41 (2.12) | 24 (0.58) | 9 (0.31) | < 0.001 |
| Mental disorder in pregnancy, no. (%) | 21 (1.09) | 22 (0.53) | 13 (0.45) | 0.014 |
| Palpitations, no. (%) | 57 (2.95) | 56 (1.36) | 19 (0.66) | < 0.001 |
| Diarrhea, no. (%) | 48 (2.48) | 52 (1.26) | 27 (0.94) | < 0.001 |
| Vomiting in pregnancy, no. (%) | 50 (2.59) | 85 (2.06) | 44 (1.52) | 0.034 |
| Hypertensive disorder, no. (%) | 28 (1.45) | 43 (1.04) | 12 (0.42) | 0.001 |
| Acute pharyngitis, no. (%) | 40 (2.07) | 31 (0.75) | 12 (0.42) | < 0.001 |
| Hemorrhage in early pregnancy antepartum, no. (%) | 29 (1.50) | 35 (0.85) | 15 (0.52) | 0.002 |
| Threatened miscarriage, no. (%) | 170 (8.79) | 164 (3.97) | 54 (1.87) | < 0.001 |
| Abdominal pain, no. (%) | 195 (10.08) | 241 (5.84) | 112 (3.88) | < 0.001 |
| Migraine, no. (%) | 28 (1.45) | 25 (0.61) | 8 (0.28) | < 0.001 |
| Hypothyroidism, no. (%) | 337 (17.43) | 342 (8.28) | 148 (5.13) | < 0.001 |
| Placental infarct, no. (%) | 77 (3.98) | 82 (1.99) | 71 (2.46) | < 0.001 |
| Deliveries by cesarean, no. (%) | 510 (26.37) | 833 (20.17) | 535 (18.54) | < 0.001 |
| Primigravida, no. (%) | 1206 (62.36) | 2453 (59.41) | 1024 (35.48) | < 0.001 |
| Pre-eclampsia, no. (%) | 23 (1.19) | 25 (0.61) | 13 (0.45) | 0.007 |
| Abnormality of organs and/or soft tissues of pelvis affecting pregnancy, no. (%) | 169 (8.74) | 225 (5.45) | 103 (3.57) | < 0.001 |
| False labor at or after 37 completed weeks of gestation, no. (%) | 31 (1.60) | 93 (2.25) | 101 (3.50) | < 0.001 |
| Medications | ||||
| Antidepressants, no. (%) | 12 (0.62) | 14 (0.34) | 6 (0.21) | 0.061 |
| Beta blocking agents, no. (%) | 55 (2.84) | 53 (1.28) | 33 (1.14) | < 0.001 |
| Antihistamines for systemic use, no. (%) | 185 (9.57) | 234 (5.67) | 83 (2.88) | < 0.001 |
| Direct acting antivirals, no. (%) | 143 (7.39) | 187 (4.53) | 70 (2.43) | < 0.001 |
| Other antibacterials, no. (%) | 119 (6.15) | 205 (4.96) | 54 (1.87) | < 0.001 |
| Health Services | ||||
| Pre-delivery ED visits (within 1-year), mean (SD) | 1.12 (1.54) | 0.68 (1.01) | 0.56 (0.97) | < 0.001 |
| Vitals | ||||
| Diastolic blood pressure in the third trimester | 69.35 (6.22) | 69.28 (5.86) | 69.12 (4.76) | 0.321 |
| Marital Status | ||||
| Single (vs. Married), no. (%) | 348 (17.99) | 578 (14.0) | 267 (9.25) | < 0.001 |
| Race , no. (%) | ||||
| Asian | 280 (14.48) | 679 (16.44) | 730 (25.29) | < 0.001 |
| Black | 145 (7.50) | 260 (6.30) | 155 (5.37) | |
| Other | 229 (11.84) | 477 (11.55) | 270 (9.36) | |
| Unknown | 202 (10.44) | 564 (13.66) | 549 (19.02) | |
| White | 1078 (55.74) | 2149 (52.05) | 1182 (40.96) |
Table 3.
Post-hoc analysis of other demographic and clinical characteristics across clusters
| Variables | Cluster | P-value | ||
|---|---|---|---|---|
| 1 (N = 1934) | 2 (N = 4129) | 3 (N = 2886) | ||
| Sociodemographic | ||||
| Age, mean (SD), year | 35.01 (4.73) | 33.78 (4.29) | 32.68 (4.66) | < 0.001 |
| Average Poverty Rate, mean (SD), % | 1.35 (1.83) | 1.42 (1.87) | 2.07 (2.61) | < 0.001 |
| Cesarean Section, no. (%) | ||||
| Yes | 510 (26.37) | 833 (20.17) | 535 (18.54) | < 0.001 |
| No | 1424 (73.63) | 3296 (79.83) | 2351 (81.46) | |
| Insurance, no. (%) | ||||
| Commercial | 1603 (82.89) | 3492 (84.57) | 2424 (83.99) | 0.45 |
| Medicaid | 283 (14.63) | 552 (13.37) | 391 (13.55) | |
| Other (Medicare, Self-pay, Unknown) | 48 (2.48) | 85 (2.06) | 71 (2.46) | |
| Other Medication Prescriptions | ||||
| Other Analgesics and Antipyretics, no. (%) | 324 (16.75) | 534 (12.93) | 324 (11.23) | < 0.001 |
| Opioids, no. (%) | 285 (14.74) | 323 (7.82) | 243 (8.42) | < 0.001 |
| Thyroid Preparations, no. (%) | 291 (15.05) | 273 (6.61) | 84 (2.91) | < 0.001 |
| Drugs for Functional Gastrointestinal Disorders, no. (%) | 171 (8.84) | 235 (5.69) | 150 (5.2) | < 0.001 |
| Antiemetics and Antinauseants, no. (%) | 170 (8.79) | 242 (5.86) | 145 (5.02) | < 0.001 |
| Other Plain Vitamin Preparations, no. (%) | 172 (8.89) | 252 (6.10) | 83 (2.88) | < 0.001 |
| Beta-lactam Antibacterials, Penicillins, no. (%) | 175 (9.05) | 245 (5.93) | 81 (2.81) | < 0.001 |
| Progestogens, no. (%) | 284 (14.68) | 156 (3.78) | 42 (1.46) | < 0.001 |
| Other Clinical Characteristics | ||||
| Normal Delivery, no. (%) | 1435 (74.2) | 3346 (81.04) | 2310 (80.04) | < 0.001 |
| Complication Occurring During Pregnancy, no. (%) | 887 (45.86) | 1439 (34.85) | 605 (20.96) | < 0.001 |
| Unplanned Pregnancy, no. (%) | 641 (33.14) | 1178 (28.53) | 742 (25.71) | < 0.001 |
| Post-term Pregnancy, no. (%) | 465 (24.04) | 1116 (27.03) | 532 (18.43) | < 0.001 |
| Elderly Primigravida, no. (%) | 674 (34.85) | 935 (22.64) | 360 (12.47) | < 0.001 |
| High Risk Pregnancy, no. (%) | 536 (27.71) | 662 (16.03) | 297 (10.29) | < 0.001 |
| Abnormal Glucose Level, no. (%) | 479 (24.77) | 757 (18.33) | 163 (5.65) | < 0.001 |
| Advanced Maternal Age Gravida, no. (%) | 416 (21.51) | 675 (16.35) | 222 (7.69) | < 0.001 |
| Disorder of Pregnancy, no. (%) | 342 (17.68) | 499 (12.09) | 276 (9.56) | < 0.001 |
| Pre-pregnancy BMI, mean (SD), kg/m2 | 24.24 (5.19) | 23.55 (4.32) | 23.77 (3.54) | < 0.001 |
| Gestational Week, mean (SD), week | 38.58 (2.12) | 38.83 (1.92) | 38.55 (2.26) | < 0.001 |
| Post-delivery ED visits (within 6-months), mean (SD) | 0.10 (0.37) | 0.06 (0.29) | 0.05 (0.28) | < 0.001 |
| Postpartum Depression | ||||
| Yes, no. (%) | 130 (6.72) | 110 (2.66) | 33 (1.14) | < 0.001 |
| No, no. (%) | 1804 (93.28) | 4019 (97.34) | 2853 (98.86) | |
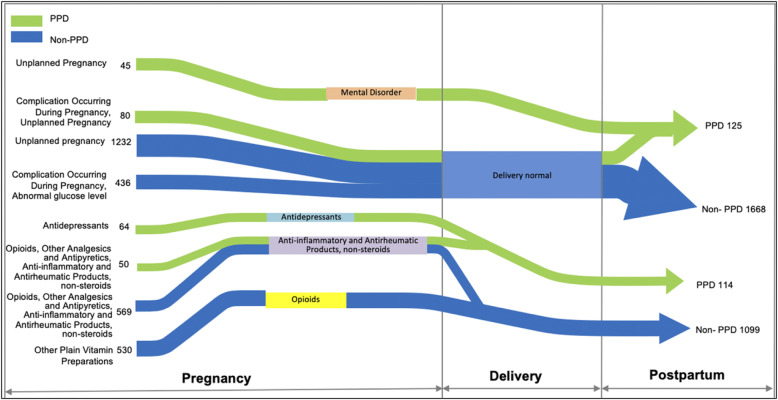
Figure 2 showcases sequential patterns in the prenatal care identified from the study data. Green pathways signify pathways leading to PPD diagnoses, in contrast to blue pathways that do not. The pathways are made up of events (left-hand side) which converge or diverge to other events (right-hand side) before the development, or the absence, of PPD. For example, we found that 45 mothers with PPD had unplanned pregnancies followed by diagnoses of mental health disorders during pregnancy. Also, we observed that 64 mothers who developed PPD had multiple prescriptions of antidepressants during pregnancy. Another pattern indicated 50 mothers who developed, and 569 mothers who did not develop, PPD were prescribed Opioids, Other Analgesics and Antipyretics, and Anti-inflammatory and Antirheumatic Products, non-steroids, before the refill of Anti-inflammatory and Antirheumatic Products, non-steroids.
Fig. 2.
Selected patterns in prenatal care identified from the EHR
Table 4 displays the results from the SEM for the outcome of PPD. Regarding the primary outcome, patients in clusters 1 (odds ratio = 6.3, P < .001) and 2 (odds ratio = 2.43, P < .001) are more likely to have a diagnosis PPD within 12 months after childbirth than women in cluster 3. Relative to cluster 3, patients in cluster 1 are more likely to have patients living in census tracts that have lower PM 2.5 (odds ratio = 0.858, P = .02), lower retail floor area ratio (odds ratio = 0.882, P = .03), lower LUM (odds ratio = 0.508, P < .001), higher GINI (odds ratio = 4.317, P = .002), and higher college degree percentage (odds ratio = 4.401, P < .001). Patients are also more likely to be older (odds ratio = 1.115, P < .001) and not married (odds ratio = 0.404, P < .001). Relative to cluster 3, patients in cluster 2 are more likely to have patients living in census tracts that have lower PM 2.5 (odds ratio = 0.890, P = .03), lower retail floor area ratio (odds ratio = 0.867, P = .001), lower GINI (odds ratio = 0.412, P = .02), and higher college degree percentage (odds ratio = 4.996, P < .001). Patients are also moderately more likely to be older (odds ratio = 1.046, P < .001) and not married (odds ratio = 0.560, P < .001). Race and insurance types (commercial, Medicaid, Other including Medicare) were not significantly associated with the cluster membership in the models although the unadjusted association was significant.
Table 4.
Structural equation model results. OR: odds ratio
| Variable | OR | P-value | |
|---|---|---|---|
| PPD | Cluster 1 | 6.3 | <.001 |
| Cluster 2 | 2.43 | <.001 | |
| Cluster 1 (vs. Cluster 3 as reference) | Retail | 0.882 | .03 |
| PM2.5 | 0.858 | .02 | |
| Age | 1.115 | <.001 | |
| Married | 0.404 | <.001 | |
| LUM | 0.508 | <.001 | |
| GINI | 4.317 | .002 | |
| College | 4.401 | <.001 | |
| Cluster 2 (vs. Cluster 3 as reference) | Retail | 0.867 | .001 |
| PM2.5 | 0.890 | .03 | |
| Age | 1.046 | <.001 | |
| Married | 0.560 | <.001 | |
| LUM | 0.749 | .06 | |
| GINI | 0.412 | .02 | |
| College | 4.996 | <.001 |
PPD postpartum depression, PM2.5 particulate matter ≤ 2.5 μm in diameter, LUM land use mix, GINI GINI inequality index
We further contrasted the characteristics of PPD cases across clusters as shown in Additional file 3. The association between PPD and the built environment factors was examined and shown in Additional file 4. The factors that were significantly associated with increased risk for PPD were the number of intersections within a 500-meter radius, the number of bus stops within a 500-meter radius, and retail floor area ratio, while adjusting for GINI index for income inequality which were also significant in the model.
Discussion
There were two major findings in this study. Three clusters of prenatal health and healthcare utilization patterns were discovered from a cohort of women whose pregnancies were managed entirely or partially in an urban academic medical center from 2015 to 2017. The distribution of the primary outcome, PPD, was significantly different across the clusters. Clinically, the clusters differed in maternal age, BMI, marital status, medication use, chronic conditions, and complications during pregnancy. In addition, we found that the cluster membership was associated with built environment factors related to walkability, access to retail resources, air quality, and neighborhood income equality. These findings contribute to the growing body of evidence that the built environment in the community confers an impact on the trajectories of health and health service utilization during pregnancy.
The associations found between retail, land use and the study outcomes among the pregnant cohort are novel and important contributions to the literature. The mixed land use and more retail access may be a proxy for the connectedness of the neighborhood in providing community support to women. These community resources potentially lead to increased opportunities for social contact, lower stress levels, and higher physical activity levels, which is consistent with previous literature tying maternal mental health to green space [9, 10]. Air quality has been linked with adverse birth outcomes including preterm birth and miscarriages in previous literature [9]. However, we found that lower PM2.5 concentration to be associated with clusters with higher PPD incidences in contrary to previous literature. In our urban study setting, PM2.5 concentration is highest in the most affluent area and becomes lower as we move out to other parts of the study setting. Therefore, our findings on the association of poor air quality with higher incidence of PPD potentially reflect patient cohorts who are predominantly in or outside the most affluent part of the city who have better access to mental health reporting and care. Patterns learned from this study may inform expecting and new mothers, their care providers, as well as guideline and policymakers, to better prepare and navigate pregnancy and postpartum care. Additionally, our findings may have implications for policies during the current COVID-19 pandemic as our communities and their stores face significant changes.
There are limitations to the study. All diagnoses in the study were defined using diagnostic codes. Therefore, missed and under-diagnosis of health conditions during pregnancy, including PPD, is a crucial limitation. It is possible that this study missed PPD patients who did not disclose symptoms due to stigma against mental health, and patients who were diagnosed outside of our health system. The underdiagnosis and misdiagnosis may be more prevalent among women who live in low-income neighborhoods. Some of these limitations may be addressed in future work by patient interviews and questionnaires, and prospective cohort studies. Additionally, the application of natural language processing on unstructured clinical notes may allow us to elicit underdiagnosed and missed PPD as well as other conditions. Moreover, we were not able to address the possible reporting bias in our study population with respect to information such as race and marital status. Nearly 15 % of the racial information was unknown from the EHR data. Future studies may explore the leveraging of patient-reported outcome data in overcoming this limitation. Furthermore, in analyzing the medication data, we did not consider the dose-response relationship between medications and the outcome as prescription fill information was not available. Detailed medication dose and frequency information can be analyzed in future work if pharmacy claims data become available. PPD cases across the clusters did not differ significantly in our analysis, but this may be due to the small sample size. Applying our methods to a larger patient cohort may allow us to further delineate the association of the built environment and potentially different PPD patient types. Lastly, while this study used data from a single health system in NYC, further work will aim to validate our findings using EHR data from other institutions and across different cities in the US.
Conclusions
We found that the built environment quality is associated with variability in prenatal care and maternal mental health outcomes in a large retrospective cohort study using EHR data. Built environment qualities that were identified in a structural equation model include LUM and RetFAR as indicators for walkability and street connectivity, and air quality. Findings from this study may inform healthcare providers and public health policymakers in understanding modifiable risk factors such as isolation that are associated with poor pregnancy care and outcomes.
Supplementary information
Definition of PPD based on SNOMED codes
Variables used in the construction of the clinical pathways
Characteristics of PPD cases across clusters
Associations between PPD and the built environment variables in the study cohort
Acknowledgements
Weill Cornell Medicine Information Technology Services, Research Informatics.
Abbreviations
- SDoH
social determinants of health
- ED
emergency departments
- PPD
postpartum depression
- PM2.5
particulate matter ≤ 2.5 μm in diameter
- EHR
electronic health records
- BMI
body mass index
- SNOMED-CT
Systematized Nomenclature of Medicine-Clinical Terms
- ATC
Anatomical Therapeutic Chemical
- NYBPM
New York Best Practice Model
- VKT
vehicle kilometer traveled
- LUM
land use mix
- RetFAR
retail floor area ratio
- O3
ozone
- LUR
Land Use Regression
- LCS
longest common subsequence
- SPADE
Sequential Pattern Discovery using Equivalent Classes
- MICE
Multivariate Imputation by Chained Equations
- SEM
structural equation model
- ANOVA
analysis of variance
- SD
standard deviation
Authors’ contributions
YZ designed, analyzed, interpreted, and drafted the manuscript. MT, SW, and YL conducted the data analysis. MS conducted literature search. AH and RJ provided clinical interpretation of the results. AR provided statistical support. OG and JP provided guidance on study design. The author(s) all drafted, read and approved the final manuscript.
Funding
This study was funded by the Center for Transportation, Environment, and Community Health New Research Initiatives Fund.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to its inclusion of patient health information protected by the Health Insurance Portability and Accountability Act but are available from the corresponding author on reasonable request. Analysis was conducted in StataIC 16.1.
Declarations
Ethics approval and consent to participate
This study was in accordance to guidelines of Weill Cornell Medicine, and was approved by the research ethics committee Weill Cornell Medicine Internal Review Board (protocol number: 1711018789). Weill Cornell Medicine Internal Review Board has waived the need of individual informed consent for this study.
Consent for publication
Not applicable.
Competing interests
YZ, AH, RJ, and JP have equity ownership at Iris OB Health, Inc.
MT, SW, MS, AR, YL, OG have no conflicts to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koh HK. A 2020 Vision for Healthy People. New England Journal of Medicine. 2010;362(18):1653–6. doi: 10.1056/NEJMp1001601. [DOI] [PubMed] [Google Scholar]
- 2.Chaiyachati KH, Hom JK, Hubbard RA, Wong C, Grande D. Evaluating the association between the built environment and primary care access for new Medicaid enrollees in an urban environment using Walk and Transit Scores. Prev Med Rep. 2018;9:24–8. doi: 10.1016/j.pmedr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutel ME, Brahler E, Ernst M, Klein E, Reiner I, Wiltink J, et al. Noise annoyance predicts symptoms of depression, anxiety and sleep disturbance 5 years later. Findings from the Gutenberg Health Study. Eur J Public Health. 2020;30(3):516–21. doi: 10.1093/eurpub/ckaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galea S, Ahern J, Rudenstine S, Wallace Z, Vlahov D. Urban built environment and depression: a multilevel analysis. J Epidemiol Community Health. 2005;59(10):822–7. doi: 10.1136/jech.2005.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emeruwa UN, Ona S, Shaman JL, Turitz A, Wright JD, Gyamfi-Bannerman C, Melamed A. Associations Between Built Environment, Neighborhood Socioeconomic Status, and SARS-CoV-2 Infection Among Pregnant Women in New York City. JAMA. 2020;324(4):390-2. 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed]
- 6.Mullings JA, McCaw-Binns AM, Archer C, Wilks R. Gender differences in the effects of urban neighborhood on depressive symptoms in Jamaica. Revista Panamericana De Salud Publica-Pan American. Journal of Public Health. 2013;34(6):385–92. [PubMed] [Google Scholar]
- 7.Veldhuis CB, Maki P, Molina K. Psychological and neighborhood factors associated with urban women’s preventive care use. J Behav Med. 2020;43(3):346–64. doi: 10.1007/s10865-019-00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielminotti J, Landau R, Wong CA, Li G, Patient- Hospital-, and Neighborhood-Level Factors Associated with Severe Maternal Morbidity During Childbirth: A Cross-Sectional Study in New York State 2013–2014. Matern Child Health J. 2019;23(1):82–91. doi: 10.1007/s10995-018-2596-9. [DOI] [PubMed] [Google Scholar]
- 9.McEachan RRC, Prady SL, Smith G, Fairley L, Cabieses B, Gidlow C, et al. The association between green space and depressive symptoms in pregnant women: moderating roles of socioeconomic status and physical activity. Journal of Epidemiology and Community Health. 2016;70(3):253–9. doi: 10.1136/jech-2015-205954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichani V, Dirks K, Burns B, Bird A, Grant C. Green Space and Depression during Pregnancy: Results from the Growing Up in New Zealand Study. Int J Environ Res Public Health. 2017;14(9):1083. 10.3390/ijerph14091083. [DOI] [PMC free article] [PubMed]
- 11.Glance LG, Dick AW, Glantz JC, Wissler RN, Qian F, Marroquin BM, et al. Rates Of Major Obstetrical Complications Vary Almost Fivefold Among US Hospitals. Health Affairs. 2014;33(8):1330–6. doi: 10.1377/hlthaff.2013.1359. [DOI] [PubMed] [Google Scholar]
- 12.Grobman WA, Bailit JL, Rice MM, Wapner RJ, Varner MW, Thorp JM, et al. Can differences in obstetric outcomes be explained by differences in the care provided? The MFMU Network APEX study. American Journal of Obstetrics and Gynecology. 2014;211(2). [DOI] [PMC free article] [PubMed]
- 13.Farr SL, Dietz PM, Rizzo JH, Vesco KK, Callaghan WM, Bruce FC, et al. Health Care Utilisation in the First Year of Life Among Infants of Mothers With Perinatal Depression or Anxiety. Paediatric and Perinatal Epidemiology. 2013;27(1):81–8. doi: 10.1111/ppe.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham SD, Magriples U, Thomas JL, Kozhimannil KB, Herrera C, Barrette E, et al. Association Between Maternal Comorbidities and Emergency Department Use Among a National Sample of Commercially Insured Pregnant Women. Academic Emergency Medicine. 2017;24(8):940–7. doi: 10.1111/acem.13215. [DOI] [PubMed] [Google Scholar]
- 15.Kilfoyle KA, Vrees R, Raker CA, Matteson KA. Nonurgent and urgent emergency department use during pregnancy: an observational study. American journal of obstetrics and gynecology. 2017;216(2):181. doi: 10.1016/j.ajog.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Ascoli PT, Alexander GR, Petersen DJ, Kogan MD. Parental factors influencing patterns of prenatal care utilization. J Perinatol. 1997;17(4):283–7. [PubMed] [Google Scholar]
- 17.Akaraci S, Feng XQ, Suesse T, Jalaludin B, Astell-Burt T. A Systematic Review and Meta-Analysis of Associations between Green and Blue Spaces and Birth Outcomes. International Journal of Environmental Research and Public Health. 2020;17(8). [DOI] [PMC free article] [PubMed]
- 18.Niedzwiecki MM, Rosa MJ, Solano-Gonzalez M, Kloog I, Just AC, Martinez-Medina S, et al. Particulate air pollution exposure during pregnancy and postpartum depression symptoms in women in Mexico City. Environ Int. 2020;134:105325. doi: 10.1016/j.envint.2019.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffield PE, Speranza R, Chiu YM, Hsu HL, Curtin PC, Renzetti S, et al. Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One. 2018;13(4):e0195267. doi: 10.1371/journal.pone.0195267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Smargiassi A, Low N, Bilodeau-Bertrand M, Ayoub A, Auger N. Residential noise exposure and the longitudinal risk of hospitalization for depression after pregnancy: Postpartum and beyond. Environ Res. 2019;170:26–32. doi: 10.1016/j.envres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Crockett K, Zlotnick C, Davis M, Payne N, Washington R. A depression preventive intervention for rural low-income African-American pregnant women at risk for postpartum depression. Arch Womens Ment Health. 2008;11(5–6):319–25. doi: 10.1007/s00737-008-0036-3. [DOI] [PubMed] [Google Scholar]
- 22.Beeber LS, Holditch-Davis D, Belyea MJ, Funk SG, Canuso R. In-home intervention for depressive symptoms with low-income mothers of infants and toddlers in the United States. Health Care Women Int. 2004;25(6):561–80. doi: 10.1080/07399330490444830. [DOI] [PubMed] [Google Scholar]
- 23.Giurgescu C, Zenk SN, Templin TN, Engeland CG, Dancy BL, Park CG, et al. The Impact of Neighborhood Environment, Social Support, and Avoidance Coping on Depressive Symptoms of Pregnant African-American Women. Womens Health Issues. 2015;25(3):294–302. doi: 10.1016/j.whi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn-Holbrook J, Cornwell-Hinrichs T, Anaya I. Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Front Psychiatry. 2017;8:248. doi: 10.3389/fpsyt.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacques N, de Mola CL, Josephc G, Mesenburg MA, da Silveira MF. Prenatal and postnatal maternal depression and infant hospitalization and mortality in the first year of life: A systematic review and meta-analysis. Journal of Affective Disorders. 2019;243:201–8. doi: 10.1016/j.jad.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 26.Weobong B, ten Asbroek AHA, Soremekun S, Gram L, Amenga-Etego S, Danso S, et al. Association between probable postnatal depression and increased infant mortality and morbidity: findings from the DON population-based cohort study in rural Ghana. Bmj Open. 2015;5(8). [DOI] [PMC free article] [PubMed]
- 27.Field T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behavior & Development. 2010;33(1):1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–19. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- 29.Moore Simas TA, Huang MY, Packnett ER, Zimmerman NM, Moynihan M, Eldar-Lissai A. Matched cohort study of healthcare resource utilization and costs in young children of mothers with postpartum depression in the United States. J Med Econ. 2020;23(2):174–83. doi: 10.1080/13696998.2019.1679157. [DOI] [PubMed] [Google Scholar]
- 30.Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, et al. The risk factors for postpartum depression: A population-based study. Depress Anxiety. 2017;34(2):178–87. doi: 10.1002/da.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384(9956):1775–88. doi: 10.1016/S0140-6736(14)61276-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen HL, Cai JY, Zha ML, Shen WQ. Prenatal smoking and postpartum depression: a meta-analysis. J Psychosom Obstet Gynaecol. 2019;40(2):97–105. doi: 10.1080/0167482X.2017.1415881. [DOI] [PubMed] [Google Scholar]
- 33.OHara MW, Swain AM. Rates and risk of postpartum depression - A meta-analysis. International Review of Psychiatry. 1996;8(1):37–54. doi: 10.3109/09540269609037816. [DOI] [Google Scholar]
- 34.Zhang SM, Wang LS, Yang TB, Chen LZ, Qiu X, Wang TT, et al. Maternal violence experiences and risk of postpartum depression: A meta-analysis of cohort studies. European Psychiatry. 2019;55:90–101. doi: 10.1016/j.eurpsy.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Norhayati MN, Hazlina NHN, Asrenee AR, Emilin WMAW. Magnitude and risk factors for postpartum symptoms: A literature review. Journal of Affective Disorders. 2015;175:34–52. doi: 10.1016/j.jad.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 36.Feng XQ, Astell-Burt T. Residential green space quantity and quality and symptoms of psychological distress: a 15-year longitudinal study of 3897 women in postpartum. Bmc Psychiatry. 2018;18. [DOI] [PMC free article] [PubMed]
- 37.He SY, Smargiassi A, Low N, Bilodeau-Bertrand M, Ayoub A, Auger N. Residential noise exposure and the longitudinal risk of hospitalization for depression after pregnancy: Postpartum and beyond. Environmental Research. 2019;170:26–32. doi: 10.1016/j.envres.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Schinasi LH, Auchincloss AH, Forrest CB, Roux AVD. Using electronic health record data for environmental and place based population health research: a systematic review. Annals of Epidemiology. 2018;28(7):493–502. doi: 10.1016/j.annepidem.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Padman R, Patel N. Paving the COWpath: Learning and visualizing clinical pathways from electronic health record data. J Biomed Inform. 2015. [DOI] [PubMed]
- 40.Huber C. Introduction to Structural Equation Modeling Using Stata. California Association for Institutional Research. 2014.
- 41.Odigie E, Lacson R, Raja A, Osterbur D, Ip I, Schneider L, et al. Fast Healthcare Interoperability Resources, Clinical Quality Language, and Systematized Nomenclature of Medicine-Clinical Terms in Representing Clinical Evidence Logic Statements for the Use of Imaging Procedures: Descriptive Study. JMIR Med Inform. 2019;7(2):e13590. doi: 10.2196/13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronning M, Blix HS, Harbo BT, Strom H. Different versions of the anatomical therapeutic chemical classification system and the defined daily dose - are drug utilisation data comparable? European Journal of Clinical Pharmacology. 2000;56(9–10):723–7. doi: 10.1007/s002280000200. [DOI] [PubMed] [Google Scholar]
- 43.Frank LD, Engelke PO. The built environment and human activity patterns: exploring the impacts of urban form on public health. Journal of planning literature. 2001;16(2):202–18. doi: 10.1177/08854120122093339. [DOI] [Google Scholar]
- 44.Jiang B, Liang S, Peng Z-R, Cong H, Levy M, Cheng Q, et al. Transport and public health in China: the road to a healthy future. The Lancet. 2017;390(10104):1781–91. doi: 10.1016/S0140-6736(17)31958-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pucher J, Buehler R, Bassett DR, Dannenberg AL. Walking and cycling to health: a comparative analysis of city, state, and international data. American journal of public health. 2010;100(10):1986–92. doi: 10.2105/AJPH.2009.189324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Besser LM, Dannenberg AL. Walking to public transit: steps to help meet physical activity recommendations. American journal of preventive medicine. 2005;29(4):273–80. doi: 10.1016/j.amepre.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Berke EM, Koepsell TD, Moudon AV, Hoskins RE, Larson EB. Association of the built environment with physical activity and obesity in older persons. American journal of public health. 2007;97(3):486–92. doi: 10.2105/AJPH.2006.085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.State NY. The Official Website of New York State [Available from: https://www.ny.gov/.
- 49.Vovsha P, Petersen E, Donnelly R. Microsimulation in travel demand modeling: Lessons learned from the New York best practice model. Transportation Research Record: Journal of the Transportation Research Board. 2002(1805):68–77.
- 50.Pollution HEIPotHEoT-RA. Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects: Health Effects Institute; 2010.
- 51.Karner AA, Eisinger DS, Niemeier DA. Near-Roadway Air Quality: Synthesizing the Findings from Real-World Data. Environmental Science & Technology. 2010;44(14):5334–44. doi: 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- 52.Tayarani M, Poorfakhraei A, Nadafianshahamabadi R, Rowangould GM. Evaluating unintended outcomes of regional smart-growth strategies: Environmental justice and public health concerns. Transportation research part D: transport and environment. 2016;49:280 – 90.
- 53.Frank LD, Sallis JF, Conway TL, Chapman JE, Saelens BE, Bachman W. Many pathways from land use to health: associations between neighborhood walkability and active transportation, body mass index, and air quality. Journal of the American planning Association. 2006;72(1):75–87. doi: 10.1080/01944360608976725. [DOI] [Google Scholar]
- 54.Sugiyama T, Leslie E, Giles-Corti B, Owen N. Associations of neighbourhood greenness with physical and mental health: do walking, social coherence and local social interaction explain the relationships? Journal of Epidemiology and Community Health. 2008;62(5). [DOI] [PubMed]
- 55.Kim S-Y, Bechle M, Hankey S, Sheppard L, Szpiro A, Marshall J, editors. A Parsimonious Approach to National Prediction: Criteria Pollutants in the Contiguous US, 1979–2015. ISEE Conference Abstracts; 2018.
- 56.Cantor MN, Chandras R, Pulgarin C. FACETS: using open data to measure community social determinants of health. Journal of the American Medical Informatics Association. 2017;0:0. doi: 10.1093/jamia/ocx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Padman R, Epner P, Bauer V, Solomonides A, Rao G. Identifying Diagnostic Paths for Undifferentiated Abdominal Pain from Electronic Health Record Data. AMIA Jt Summits Transl Sci Proc. 2018;2017:290-9. [PMC free article] [PubMed]
- 58.Movahedi F, Kormos RL, Lohmueller L, Seese L, Kanwar M, Murali S, et al. Sequential pattern mining of longitudinal adverse events after Left Ventricular Assist Device implant. IEEE J Biomed Health Inform. 2019. [DOI] [PMC free article] [PubMed]
- 59.Wang S, Pathak J, Zhang Y. Using Electronic Health Records and Machine Learning to Predict Postpartum Depression. Studies in health technology and informatics. 2019;264:888 – 92. [DOI] [PubMed]
- 60.Zaki MJ. Spade: an efficient algorithm for mining frequent sequences. Mach Learn. 2001;42(1–2):31–60. doi: 10.1023/A:1007652502315. [DOI] [Google Scholar]
- 61.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? International Journal of Methods in Psychiatric Research. 2011;20(1):40–9. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of PPD based on SNOMED codes
Variables used in the construction of the clinical pathways
Characteristics of PPD cases across clusters
Associations between PPD and the built environment variables in the study cohort
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to its inclusion of patient health information protected by the Health Insurance Portability and Accountability Act but are available from the corresponding author on reasonable request. Analysis was conducted in StataIC 16.1.