Key Points
Question
Can children with developmental and epileptic encephalopathies tolerate adjunctive cannabidiol (CBD) transdermal gel, and is CBD gel associated with reduced seizure frequency?
Findings
In this nonrandomized controlled trial, CBD transdermal gel was well tolerated over 6.5 months of treatment, with 60% of patients experiencing a treatment-related adverse event, 96% of which were mild or moderate. A 43.5% reduction in seizure frequency was observed in focal impaired awareness seizures and tonic-clonic seizures.
Meaning
These results suggest that CBD transdermal gel is well tolerated in children with developmental and epileptic encephalopathies and may be associated with a reduction in epileptic seizures.
This nonrandomized controlled trial examines the safety and tolerability of transdermal cannabidiol (CBD) gel among children with developmental and epileptic encephalopathies and assesses the association between treatment with CBD gel and seizure frequency.
Abstract
Importance
Developmental and epileptic encephalopathies (DEEs) are the most severe group of drug-resistant epilepsies. Alternatives to oral therapies are urgently needed to reduce seizures and improve developmental outcomes and comorbidities in this medically complex population.
Objective
To assess the safety and tolerability of cannabidiol (CBD) transdermal gel in children with DEEs and to evaluate seizure frequency, sleep, and quality of life.
Design, Setting, and Participants
This nonrandomized controlled trial was conducted in 2 centers in Australia and New Zealand from April 2018 to July 2019. Children and adolescents aged 3 to 18 years with DEEs who were receiving a stable regimen of 1 to 4 antiseizure medications were eligible for this study. After 1-month baseline and titration periods, patients entered a 5.5-month flexible-dosing maintenance period for a total of 6.5 months of treatment. Data were analyzed throughout the 6.5-month treatment period.
Interventions
Twice-daily applications of CBD transdermal gel at doses of 125 to 500 mg for 6.5 months.
Main Outcomes and Measures
Safety and tolerability assessments included adverse events (AEs) and examination of skin. The outcome for seizures was the median percentage change from baseline in monthly (28-day) seizure frequency of focal impaired awareness seizures (FIAS) and tonic-clonic seizures (TCS) over 6.5 months.
Results
Of 48 patients (mean [SD] age, 10.5 [3.8] years; 26 [54%] boys), 29 (60%) had at least 1 treatment-related AE over 6.5 months; 44 of 46 treatment-related AEs (96%) were mild or moderate. Treatment-related AEs that occurred in at least 5% of patients were application-site dryness, application-site pain, and somnolence (each reported by 4 patients [8%]). The only treatment-related gastrointestinal AE was diarrhea, reported in a single patient. CBD treatment was associated with reductions in FIAS and TCS frequency. Analysis of the 33 patients with FIAS and TCS showed a median (interquartile range) monthly reduction in seizures of 58% (−5.3% to 81.8%) at 5 months and 43.5% (−23.8% to 57.5%) over the entire 6.5-month study period. Parents and caregivers noted improvements in social or interpersonal engagement and irritability (33 of 43 [77%] participants); alertness, energy, and sleep (23 of 43 [53%]); and cognition or concentration (20 of 43 [47%]).
Conclusions and Relevance
In this study, CBD transdermal gel was safe, well tolerated, and was associated with reductions in FIAS and TCS frequency and disease burden.
Trial Registration
ClinicalTrials.gov Identifier: ACTRN12618000516280
Introduction
Developmental and epileptic encephalopathies (DEEs) are the most severe group of epilepsies and typically begin in infancy and childhood. They include, but are not limited to, many well-known epilepsy syndromes, such as Dravet, Lennox-Gastaut syndrome (LGS), West, and myoclonic-atonic epilepsy. The incidence of DEEs with onset under 18 months is at least 1 in 2000 live births,1 which does not include Dravet syndrome (because of methodology) and syndromes beginning at an older age, such as LGS.1,2
DEEs are defined by drug-resistant seizures, frequent epileptiform activity, and developmental slowing.3,4,5 Patients with DEEs usually have intellectual disabilities, often coupled with behavioral problems. Comorbidities contribute to their clinical instability and include motor disorders (up to 75%), autism spectrum disorder (47%), sleep disturbance (58%), chronic respiratory tract infections (33%), need for a percutaneous endoscopic gastrostomy (PEG) tube (15%), and an increased mortality risk.6,7,8,9,10,11,12 DEEs are usually refractory to antiseizure medicines (ASMs), and ameliorating epileptiform activity may improve developmental outcome.13
ASMs are usually administered orally, which is often extremely challenging in children with behavioral and cognitive problems. Restraint, or even insertion of a PEG tube, may be required for therapy to be given in consistent doses. A safe and effective nonoral alternative could facilitate adherence to prescribed therapeutic regimens and thereby improve outcomes.
Cannabidiol (CBD), the primary nonpsychoactive cannabinoid of the cannabis plant, reduces neuronal excitability and limits seizures through effects on multiple targets.14 An open-label trial of oral CBD suggested it was safe and well tolerated in patients with drug-resistant epilepsy.15 This was followed by randomized clinical trials enrolling patients with Dravet syndrome16,17 and LGS.18,19 Orally administered treatments in patients with DEEs remains a challenge, and alternative methods of drug delivery deserve consideration.
We performed an open-label study of a transdermal gel formulation of CBD20 (Zynerba Pharmaceuticals) in children with DEEs. The primary objective of the study was to evaluate the safety and tolerability of CBD transdermal gel in children with DEEs. Secondary objectives were to evaluate associated changes in seizure frequency according to seizure type, sleep disturbance, caregiver burden, and quality of life.
Methods
Ethics
This study was conducted in accordance with the principles of the Guidelines for Good Clinical Practice, the Declaration of Helsinki,21 the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline, and all applicable local regulations. The protocol was approved by the human research ethics committee at Austin Health and the New Zealand Health and Disability ethics committee (Supplement 1). Patients, parents, or legal guardians provided written informed consent at screening.
Study Design and Patients
This open-label, 2-center study (conducted in health centers in Melbourne, Australia, and Wellington, New Zealand) assessed the long-term safety and tolerability of CBD transdermal gel in children with DEEs.13 Eligible patients included children aged 3 to 18 years with 1 or more years since DEE diagnosis. Eligible patients had 5 or more countable seizures during the 28-day baseline period. Countable seizure types required clear observable signs and included focal impaired awareness seizures (FIAS, previously called complex partial seizures), tonic-clonic seizures (TCS), atonic, tonic, clonic, focal aware motor seizures, and epileptic spasms. Categorization of TCS included both generalized TCS and focal to bilateral TCS, which can be difficult to distinguish clinically.
Patients were excluded if they currently or recently (within 3 months) received cannabis-based therapy. Patients were disqualified if their levels of alanine aminotransferase, aspartate aminotransferase, or bilirubin were more than 3 times the upper limit of normal. Other exclusion criteria were a change in epilepsy dietary therapy in the 1 month prior to screening or current use of a strong inhibitor or inducer of CYP3A4 or a sensitive substrate for CYP3A4.
This study included a baseline period of 1 month followed by treatment with CBD transdermal gel for a total of 6.5 months, which consisted of a 1-month titration period and a 5.5-month flexible-dosing maintenance period. Parents and caregivers were taught how to apply CBD transdermal gel. The first patient was enrolled in April 2018, and the last patient completed 6.5 months of treatment in July 2019. As this was a nonrandomized, controlled (ie, before and after) study, treatment assignment was neither randomized nor masked.
Parents or caregivers recorded daily seizure frequency in a paper diary and provided a skin check score using a 5-point scale (0 = no erythema, 1 = minimal erythema, 2 = moderate erythema with sharply defined borders, 3 = intense erythema with or without edema, and 4 = intense erythema with edema and blistering/erosion).22 Investigators reviewed the diaries at each visit.
Study visits occurred at weeks 2, 4, 6, 14, and 26 (defined as 6.5 months based on 4-week months). A 6-month extension study is ongoing. Patients not continuing into the 6-month extension study entered a 1- to 3-week taper period, with the duration depending on the CBD dose. Telephone review occurred 1 month after the taper finished.
Outcomes
Safety
Safety assessments included adverse event (AE) review, physical and neurologic examinations, vital signs, electrocardiogram, skin check examination (by an investigator) and diary (completed by a parent or caregiver), and clinical laboratory tests (ie, hematology, chemistry, urinalysis, urine drug screen, testosterone [for male participants]).
Seizures
Seizures were captured daily and analyzed by monthly period. Seizure outcome was assessed by change in monthly (ie, 28-day) mean seizure frequency. Seizure frequency was captured for each seizure type. As the best response was observed in FIAS and TCS, these seizure types were subjected to further analysis.
Secondary outcomes included the scores on the University of Washington Caregiver Stress Scale,23 the modified Epilepsy and Learning Disabilities Quality of Life (ELDQOL-modified) scale24 (modified to eliminate the side effect profile), and the Sleep Disturbance Scale for Children25 (SDSC). These were completed by caregivers at baseline, 3.5 months, and end of study (eTable 1 in Supplement 2). At 6.5 months, a qualitative assessment interview of caregivers ascertained any changes related to the patient’s daily activities, school attendance, behaviors, seizures, and other relevant matters. For patients who discontinued early, assessments at 6.5 months were completed at the early termination visit.
Treatments
Study treatment was a 4.2% topical CBD transdermal gel supplied in sachets containing 2.98 g and delivering 125 mg CBD per sachet. Doses were administered twice daily. Initial total daily doses were 250 mg/d for patients weighing 25 kg or less and 500 mg/d for patients weighing more than 25 kg. At 1 month, further increases to 500 mg/d for patients weighing 25 kg or less and 750 mg/d for patients weighing more than 25 kg were permissible. At 2.5 months, increases to 750 mg/d for patients weighing 25 kg or less and 1000 mg/d for those weighing more than 25 kg were permissible.
Statistical Analysis
The safety analysis included all patients who received at least 1 dose of the study drug (Supplement 3). All AEs were classified by preferred term using the Medical Dictionary for Regulatory Activities version 21.0 and summarized using descriptive statistics by therapeutic class and preferred term. Application site erythema ratings were summarized at all visits.
The number and percentage of patients with treatment-emergent AEs (TEAEs) and serious AEs (SAEs) are presented with 95% CIs based on a binomial distribution. Seizure frequency analysis included all patients who were administered 80 days of study medication and completed 80% of seizure diaries. Reductions in seizure frequency from baseline were calculated for each period as frequency(baseline) − frequency(period x). The percentage reduction from baseline in seizure frequency was calculated as 100 × [frequency(baseline) − frequency(period x)] / frequency(baseline). In addition, a patient was categorized as a 35%, 50%, or 90% responder if their reduction in seizure frequency was equal to or greater than 35%, 50%, or 90%, respectively.
Seizure frequency was assessed by the median percentage change in monthly (ie, 28-day) seizure frequency according to seizure type (first and third quartile [Q1, Q3] and interquartile range [IQR]). The percentage of responders is presented with 95% CIs and standard errors (SEs) based on the binomial distribution. For sleep and quality of life outcomes, mean changes from baseline are reported and the P value is based on a paired t test. Two independent reviewers categorized the end of study qualitative statements from the caregivers and results are summarized descriptively.
As this study was exploratory, a formal sample size was not determined. A total of 54 patients were screened with the aim of enrolling 50 patients in open-label treatment. The cohort was chosen to be representative of a broad range of DEEs, with the number of patients with Dravet syndrome and LGS limited to approximately a quarter of the cohort. The threshold for significance was P < .05 in 2-sided tests.
Results
Patients
Of 48 patients (26 [54.2%] boys), the mean (SD) age at enrollment was 10.5 (3.8) years. Patients had a wide range of DEE syndromes and etiologies (Table 1). Intellectual disability was present in 47 patients, ranging from profound (11 of 48 patients [23%]), severe (20 of 48 [42%]), and moderate (8 of 48 [17%]) to mild (8 of 48 [17%]). Patients had a range of comorbidities: 14 (29%) had autism spectrum disorder, 18 (38%) had chronic respiratory tract conditions or infections, 22 (46%) had gait and movement disorders, 7 (15%) had a PEG tube, and 19 (40%) had sleep disturbances. Patients were taking a mean (SD) of 2.7 (1.1) concomitant ASMs, the most common of which were sodium valproate (34 patients [71%]), clobazam (25 patients [52%]), levetiracetam (17 patients [35%]), lamotrigine (16 patients [33%]), and topiramate (13 patients [27%]).
Table 1. Demographic and Baseline Characteristics.
| Characteristic | Participants, No. (%) |
|---|---|
| Safety cohort (48 participants) | |
| Age, mean (SD), y | 10.5 (3.8) |
| Sex | |
| Boys | 26 (54.2) |
| Girls | 22 (45.8) |
| Weight, mean (SD), kg | 39.3 (20.0) |
| Height, mean (SD), cm | 139.0 (20.4) |
| BMI, mean (SD) | 19.2 (4.9) |
| Etiology | |
| Genetic or presumed genetic | 43 (89.6) |
| Nonstructural | 38 (79.2) |
| Structural malformationa | 5 (10.4) |
| Brain injuryb | 5 (10.3) |
| Syndrome | |
| Dravet syndrome | 8 (16.7) |
| Lennox-Gastaut syndrome | 5 (10.4) |
| Myoclonic-atonic epilepsy | 6 (12.5) |
| West syndrome | 3 (6.3) |
| Otherc | 26 (54) |
| Receiving prior medications | |
| Sodium valproate | 34 (70.8) |
| Clobazam | 25 (52.1) |
| Levetiracetam | 17 (35.4) |
| Lamotrigine | 16 (33.3) |
| Topiramate | 13 (27.1) |
| Focal impaired awareness/tonic-clonic seizure cohort (33 participants) | |
| Seizure types analyzedd | |
| Focal impaired awareness seizures | 26 (79) |
| Focal to bilateral tonic-clonic seizures | 7 (21) |
| Generalized tonic-clonic seizures | 14 (42) |
| Median baseline seizure frequency (range) | |
| Focal impaired awareness seizures | 6.22 (0-712.6) |
| Focal to bilateral tonic-clonic seizures | 0 (0-236.4) |
| Generalized tonic-clonic seizures | 0 (0-24) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DEE, developmental and epileptic encephalopathies.
Includes polymicrogyria, hypothalamic hamartoma, cortical dysplasia, and absence of septum pellucidum.
Includes ischemic stroke, hypoxic-ischemic encephalopathy, and traumatic brain injury.
Includes generalized epileptic encephalopathy, focal DEE, multifocal DEE, late-onset infantile spasms, and DEE unclassified.
During the 1-month baseline period. Patients can appear in more than 1 seizure type and therefore will not sum to 33.
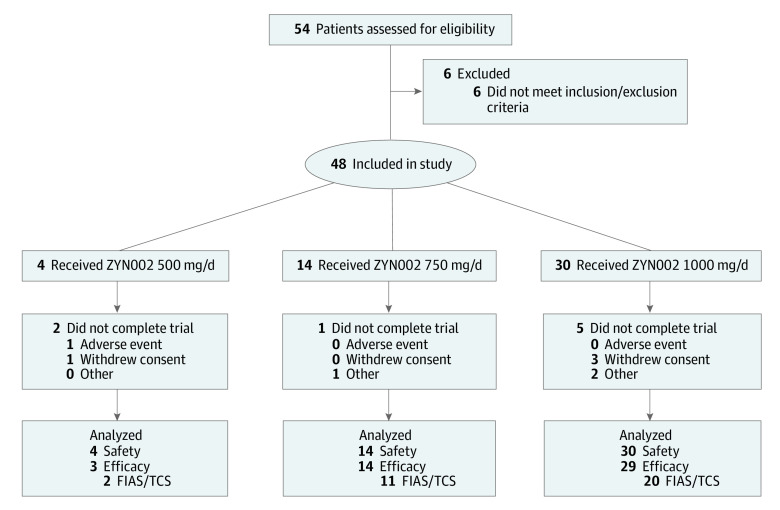
Of the 54 screened patients with DEEs, 48 were enrolled and 46 patients (treatment cohort) were included in the seizure frequency analysis (Figure 1). Two patients were excluded from this analysis: 1 did not complete 80% of seizure diaries, and 1 did not use CBD transdermal gel for the minimum duration of 80 days. A total of 8 (17%) patients discontinued CBD: 1 because of an application site reaction, and 7 because of lack of effect.
Figure 1. Flow Diagram of CBD Transdermal Gel in DEE Trial.
CBD, indicates cannabidiol; DEE, developmental and epileptic encephalopathies; FIAS, focal impaired awareness seizures; TCS, tonic-clonic seizues; and ZYN002, transdermal cannabidiol (Zynerba Pharmaceuticals).
Safety
In the 1-month baseline period, 29% patients reported at least 1 AE. Over the 6.5-month treatment period, 46 of 48 patients (96%; 95% CI, 85.8%-99.5%) reported at least 1 TEAE. The incidence ratios of AEs compared with baseline decreased from 2.5 at 1 month to 1.0 at 6 months. The most common TEAEs were upper respiratory tract infection, nasopharyngitis, somnolence, and vomiting (Table 2).
Table 2. Treatment-Emergent Adverse Events Reported in 3 or More Patients.
| Adverse events | Patients, No. (%) (n = 48) |
|---|---|
| Upper respiratory tract infection | 20 (42) |
| Nasopharyngitis | 10 (21) |
| Somnolence | 6 (13) |
| Vomiting | 5 (10) |
| Application site dryness | 4 (8) |
| Application site pain | 4 (8) |
| Decreased appetite | 4 (8) |
| Diarrhea | 4 (8) |
| Gastroenteritis | 4 (8) |
| Pyrexia | 4 (8) |
| Viral infection | 4 (8) |
| Viral upper respiratory tract infection | 4 (8) |
| Contusion | 3 (6) |
| Cough | 3 (6) |
| Ear infection | 3 (6) |
| Fall | 3 (6) |
| Fatigue | 3 (6) |
| Pneumonia | 3 (6) |
| Scratch | 3 (6) |
| Seizure | 3 (6) |
Sixty percent of patients (29 of 48) experienced a treatment-related AE. Most treatment-related AEs were mild (32 of 46 [70%]) or moderate (12 of 46 [26%]). Treatment-related AEs that occurred in at least 5% of patients were application site dryness, application site pain, and somnolence (each reported by 4 patients [8%]). All 4 patients with somnolence were taking concomitant clobazam. The only treatment-related gastrointestinal AE was diarrhea, which was reported by 1 patient (2%) for days 14 to 23. Upon resolution, this patient subsequently titrated to a higher dose (1000 mg daily) of CBD transdermal gel without recurrence of diarrhea.
During the treatment period, 10 of 48 patients (21%; 95% CI, 10.5%-35.0%) patients had 15 SAEs—10 (67%) were infections and 4 (27%) were seizure-related. SAEs considered to be possibly treatment related included 1 case of nonconvulsive status epilepticus and 1 lower respiratory tract infection, experienced in separate patients. All SAEs resolved, and none resulted in alteration of study medication.
By month 6.5 of treatment, 7255 of 7846 parental/caregiver daily skin scores (92.5%) indicated no or minimal erythema (ie, score 0 or 1). One patient with a history of keratosis pilaris had application site reaction with intense erythema and discontinued the study medication. Dermatologic patch testing suggested that this was due to an irritant contact dermatitis complicated by a secondary bacterial infection, rather than allergic contact dermatitis from CBD gel. At no time at a study visit did the investigators’ skin rating scores exceed an application site reaction of moderate erythema.
There were no clinically significant changes in vital signs or electrocardiograms. Laboratory findings were unremarkable, except for 1 patient with a transient, benign, isolated elevation of alkaline phosphatase (507 U/L) at 6.5 months (approximately 1.69 × upper limit of normal), which dropped to 402 U/L by 9 months after the patient exited the study. This was not considered related to the study drug.
Seizure Response
Across all countable seizures in the treatment cohort (46 patients) from baseline through the treatment period (month 6.5), the median (IQR) seizure reduction was 12.3% (−18.6% to 46.3%). The seizure types that showed the greatest median response to CBD transdermal gel were FIAS (reduction, 44.5% [−17.8% to 59.1%]) and TCS (reduction, 22.7% [−6.8% to 62.3%]) (eFigure 1 in Supplement 2).
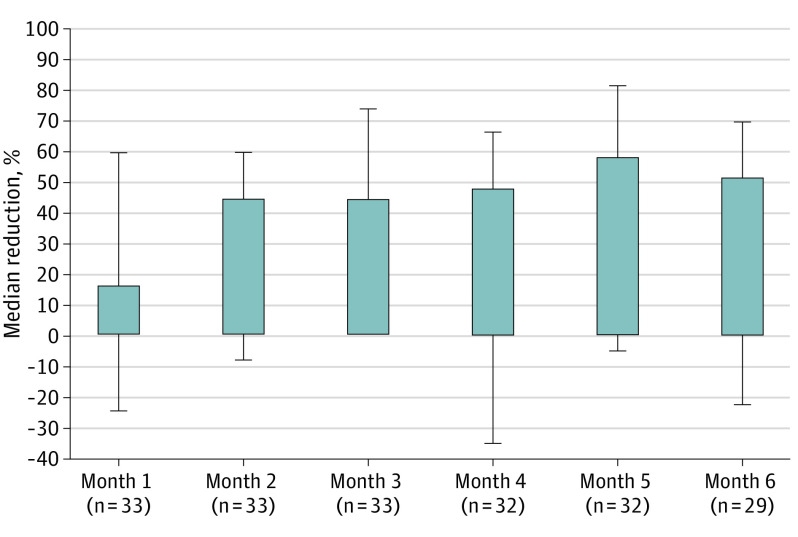
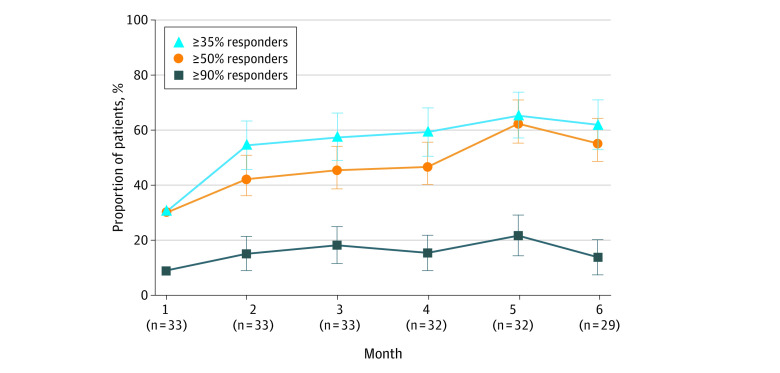
At baseline, 33 of 46 patients had FIAS and TCS. Box plots of 28-day seizure frequency values at baseline and months 1 through 6 indicated a reduction in mean and median seizure frequency after the start of CBD transdermal gel treatment (eFigures 2 and 3 in Supplement 2). The median (IQR) reduction from baseline in FIAS and TCS (33 patients) over the 6.5-month treatment period was 43.5% (−23.8% to 57.5%), and with reductions at 2 months of 44.4% (−8.0% to 60.2%; 33 patients) and 5 months of 57.7% (−5.3% to 81.8%; 32 patients) (Figure 2). The percentage of patients with a 50% or above response in the FIAS and TCS group ranged from 42.4% (95% CI, 25.5%-60.8%; 33 patients) at 2 months and peaked at 62.5% (95% CI, 43.7%-78.9%; 32 patients) at 5 months (Figure 3). The 20 patients with FIAS and TCS prescribed concomitant clobazam had less seizure improvement compared with the 13 not on clobazam. The median (IQR) reduction from baseline in the 20 individuals on clobazam over 6.5 months was 26.8% (−24.9% to 63.2%) compared with 45.5% (−2.6% to 48.9%) in the 13 patients not on clobazam.
Figure 2. Percentage Reduction in Focal Impaired Awareness Seizures and Tonic-Clonic Seizures.
Error bars indicate interquartile ranges.
Figure 3. Responder Rates for Focal Impaired Awareness Seizures and Tonic-Clonic Seizures.
Error bars indicate SE.
There were significant improvements in median vs baseline scores at months 3.5 and 6.5 on the ELDQOL-modified subscales of seizure severity, behavior, and mood (eTable 1 in Supplement 2). On the SDSC, patients had a significantly improved mean total score at 3.5 months (total score difference, −3.7; range, −50 to 27; P = .047) and 6.5 months (total score difference, −5.1; range, −33 to 24; P = .01) compared with the baseline score. This was also seen with the subscore for disorders of initiating and maintaining sleep at 3.5 months (score difference, −3.2; range, −32 to 13; P = .04) and 6.5 months (score difference, −5.1; range, −29 to 12; P = .006). At 6.5 months, there were improvements on subscores for disorders of arousal or nightmares (score difference, −1.7; range, −12 to 11; P = .03) and sleep-wake transitions (score difference, −4.6; range, −38 to 25; P = .03). There were no significant changes from baseline scores on the University of Washington Caregiver Stress Scale.
On qualitative assessments of change associated with treatment (eTable 2 in Supplement 2), 43 parents or caregivers provided reports; 36 (84%) provided at least 1 statement about improvement, and 26 (60%) provided at least 1 statement about worsening. The most frequently cited improvements were in alertness (17 of 43 patients [40%]), reduction in seizure frequency (16 of 43 [37%]), engagement or participation (15 of 43 [35%]), cognition (14 of 43 [33%]), attending school on time and/or more often (12 of 43, 28%), and more energy and less fatigue (12 of 43 [28%]). The most common negative statements were related to difficulty in applying the study medication (11 of 43 [26%]) and reactions at the application site (8 of 43 [19%]). On summary qualitative measures, parents and caregivers noted improvements related to social, interpersonal engagement, and irritability (33 of 43 [77%]); the frequency, duration, and intensity of postictal symptoms of seizures (22 of 43 [51%]); alertness, energy, improved sleep (23 of 43 [53%]); and cognition or concentration (20 of 43 [47%]).
Discussion
This is the first trial of a nonoral formulation of CBD in a cohort of children and adolescents with DEEs. CBD gel was generally well tolerated. The most frequently reported AEs were related to the application site, and most AEs were mild. The only patient to withdraw because of an AE developed an irritant dermatitis complicated by secondary bacterial skin infection that was not due to hypersensitivity to CBD transdermal gel. There were no clinically significant changes in vital signs or laboratory tests, with the exception of a single child who had an elevated alkaline phosphatase that was not associated with the study drug.
The safety and tolerability profiles observed in this trial were consistent with a 2019 trial using CBD transdermal gel to assess psychological and behavioral change in fragile X syndrome.22 The high incidence of AEs in this study, particularly those related to infections, was likely due to the high rate of complex comorbidities and seizure severity of these patients, and to the relatively long, 6.5-month duration of the trial.
The low rate of gastrointestinal AEs highlights a potential significant benefit of transdermal delivery of CBD. In this study, diarrhea occurred in 8% patients over 6.5 months, a similar frequency to placebo in randomized controlled trials of oral CBD in Dravet syndrome (31% in CBD group vs 10% in placebo group) and LGS (15% CBD group vs 8% placebo group) in 14-week studies.16,18
CBD transdermal gel added to a stable regimen of ASMs in children with DEEs may reduce FIAS and TCS frequency. The median reduction in FIAS and TCS was 43.5%, and the 50% responder rate peaked at 62.5% at month 5. These findings are similar to a large open-label oral CBD study that reported a median reduction in monthly motor seizures of 36.5% and a 50% responder rate of 39%.15 Randomized clinical trials of oral CBD found 50% responder rates of 43% for convulsive seizures in Dravet syndrome16 and 39% for drop attacks in LGS.18
CBD transdermal gel was associated with additional benefits beyond seizure reduction according to parents and caregivers. The ELDQOL scores showed improvement in behavior and mood, which was supported by parents’ reports of improvements in behavior, irritability, vitality, alertness, school attendance, mood, and sleep. Sleep assessments showed improvements in initiating and maintaining sleep and in overall sleep score. Together, these subjective findings of improved behavior with CBD transdermal gel reflect similar findings of the fragile X CBD gel study.22 They highlight important benefits beyond seizure reduction that improve overall quality of life for patients and families.
Limitations
This study had several limitations. As a study with a nonrandomized and open-label design, the consistent response rate over time partly reflects that the patients who remain in the trial are those who respond, and that the denominator reduces over time because of the withdrawal of nonresponders. With no control group, it is not possible to know how the placebo effect contributed to the observed primary and secondary findings. As the public has high expectations of medicinal cannabis, this may lead to higher response rates in both open-label and randomized double-masked clinical trials than seen with other compounds. Moreover, it is not known how long a placebo response may persist. In addition, given the requirement of 5 seizures in the baseline period, we cannot discount the effect of regression toward the mean accounting for some of the observed reduction in seizure frequency.
Conclusions
In children and adolescents with DEEs, CBD transdermal gel was safe and well tolerated. Treatment was associated with a reduction in FIAS and TCS frequency, as well as with caregiver-reported improvements in behavior, sleep, cognition, and quality of life. These findings highlight the need for a double-masked randomized clinical trial of CBD transdermal gel.
Study Protocol Amendment
eTable 1. Secondary Outcome Endpoints: Changes from Baseline Assessment
eTable 2. Qualitative Evaluation of Improvement or Worsening Associated With CBD Transdermal Gel
eFigure 1. Median Percentage Reduction by Seizure Type From Baseline to Week 26
eFigure 2. Box Plots of FIAS and TCS for Each 28-day Period
eFigure 3. Box Plots (Without Outliers) of FIAS and TCS for Each 28-day Period
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Howell KB, Eggers S, Dalziel K, et al. ; Victorian Severe Epilepsy of Infancy Study Group . A population-based cost-effectiveness study of early genetic testing in severe epilepsies of infancy. Epilepsia. 2018;59(6):1177-1187. doi: 10.1111/epi.14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symonds JD, Zuberi SM, Stewart K, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303-2318. doi: 10.1093/brain/awz195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel J Jr; International League Against Epilepsy (ILAE) . A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42(6):796-803. doi: 10.1046/j.1528-1157.2001.10401.x [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. doi: 10.1111/j.1528-1167.2010.02522.x [DOI] [PubMed] [Google Scholar]
- 5.Engel J Jr. Report of the ILAE classification core group. Epilepsia. 2006;47(9):1558-1568. doi: 10.1111/j.1528-1167.2006.00215.x [DOI] [PubMed] [Google Scholar]
- 6.Besag FM. Cognitive and behavioral outcomes of epileptic syndromes: implications for education and clinical practice. Epilepsia. 2006;47(suppl 2):119-125. doi: 10.1111/j.1528-1167.2006.00709.x [DOI] [PubMed] [Google Scholar]
- 7.Knupp KG, Scarbro S, Wilkening G, Juarez-Colunga E, Kempe A, Dempsey A. Parental perception of comorbidities in children with Dravet syndrome. Pediatr Neurol. 2017;76:60-65. doi: 10.1016/j.pediatrneurol.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 8.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12(8):465-476. doi: 10.1038/nrneurol.2016.98 [DOI] [PubMed] [Google Scholar]
- 9.Rodda JM, Scheffer IE, McMahon JM, Berkovic SF, Graham HK. Progressive gait deterioration in adolescents with Dravet syndrome. Arch Neurol. 2012;69(7):873-878. doi: 10.1001/archneurol.2011.3275 [DOI] [PubMed] [Google Scholar]
- 10.Turner SJ, Brown A, Arpone M, Anderson V, Morgan AT, Scheffer IE. Dysarthria and broader motor speech deficits in Dravet syndrome. Neurology. 2017;88(8):743-749. doi: 10.1212/WNL.0000000000003635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villas N, Meskis MA, Goodliffe S. Dravet syndrome: characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017;74:81-86. doi: 10.1016/j.yebeh.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 12.Strasser L, Downes M, Kung J, Cross JH, De Haan M. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60(1):19-29. doi: 10.1111/dmcn.13598 [DOI] [PubMed] [Google Scholar]
- 13.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512-521. doi: 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699-730. doi: 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278. doi: 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011-2020. doi: 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 17.Devinsky O, Patel AD, Thiele EA, et al. ; GWPCARE1 Part A Study Group . Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204-e1211. doi: 10.1212/WNL.0000000000005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group . Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897. doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 19.Thiele EA, Marsh ED, French JA, et al. ; GWPCARE4 Study Group . Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096. doi: 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 20.Paudel KS, Hammell DC, Agu RU, Valiveti S, Stinchcomb AL. Cannabidiol bioavailability after nasal and transdermal application: effect of permeation enhancers. Drug Dev Ind Pharm. 2010;36(9):1088-1097. doi: 10.3109/03639041003657295 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Heussler H, Cohen J, Silove N, et al. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J Neurodev Disord. 2019;11(1):16. doi: 10.1186/s11689-019-9277-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amtmann D, Jensen MP, Salem R, et al. University of Washington Caregiver Stress Scale—Users Guide, Version 2.0. Updated May 13, 2020. Accessed October 1, 2020. https://uwcorr.washington.edu/wp-content/uploads/2020/06/uw-css-user-guide-v2.pdf
- 24.Buck D, Smith M, Appleton R, Baker GA, Jacoby A. The development and validation of the Epilepsy and Learning Disabilities Quality of Life (ELDQOL) scale. Epilepsy Behav. 2007;10(1):38-43. doi: 10.1016/j.yebeh.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 25.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC): construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251-261. doi: 10.1111/j.1365-2869.1996.00251.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol Amendment
eTable 1. Secondary Outcome Endpoints: Changes from Baseline Assessment
eTable 2. Qualitative Evaluation of Improvement or Worsening Associated With CBD Transdermal Gel
eFigure 1. Median Percentage Reduction by Seizure Type From Baseline to Week 26
eFigure 2. Box Plots of FIAS and TCS for Each 28-day Period
eFigure 3. Box Plots (Without Outliers) of FIAS and TCS for Each 28-day Period
Statistical Analysis Plan
Data Sharing Statement