Abstract
A series of neurological manifestations such as intellectual disability and epilepsy are closely related to hypomagnesemia. Cyclin M2 (CNNM2) proteins, as a member of magnesium (Mg2+) transporters, were found along the basolateral membrane of distal renal tubules and involved in the reabsorption of Mg2+. Homozygous and heterozygous variants in CNNM2 reported so far were responsible for a variable degree of hypomagnesemia, several of which also showed varying degrees of neurological phenotypes such as intellectual disability and epilepsy. Here, we report a de novo heterozygous CNNM2 variant (c.2228C > T, p.Ser743Phe) in a Chinese patient, which is the variant located in the cyclic nucleotide monophosphate-binding homology (CNBH) domain of CNNM2 proteins. The patient presented with mild intellectual disability and refractory epilepsy but without hypomagnesemia. Thus, we reviewed the literature and analyzed the phenotypes related to CNNM2 variants, and then concluded that the number of variant alleles and the changed protein domains correlates with the severity of the disease, and speculated that the CNBH domain of CNNM2 possibly plays a limited role in Mg2+ transport but a significant role in brain development. Furthermore, it can be speculated that neurological phenotypes such as intellectual disability and seizures can be purely caused by CNNM2 variants.
Keywords: CNNM2, CNBH domain, hypomagnesemia, intellectual disability, intractable epilepsy
Introduction
As an abundant intracellular divalent cation in human body, magnesium (Mg2+) plays an important role in numerous biological processes such as the synthesis of RNA, DNA and protein, and the production and storage of cellular energy (Volpe, 2013). Transmembrane transport of Mg2+ requires the action of specialized proteins known as Mg2+ transporters. In mammals, eight diverse sorts of Mg2+ transporters have been identified so far, many of which are related to numerous congenital diseases such as neural tube defects (Walder et al., 2009) and spastic paraplegia (Rainier et al., 2003), involving a diversity of tissues, most prominently in intestine, kidney, brain and skin (Quamme, 2010). Cyclin M2 (CNNM2) is one of the members of the Mg2+ transporters, participating in Mg2+ reabsorption in kidney tubules.
Variants in CNNM2 gene (MIM 607803) had been related to multiple phenotypes, ranging from single hypomagnesemia to severe intellectual disability and intractable epilepsy. Evidence has been provided that heterozygous variants in CNNM2 gene can cause renal hypomagnesemia (HOMG6 [MIM 613882]) (Stuiver et al., 2011), seizures, and intellectual disability (HOMGSMR1 [MIM 616418]). Among them, HOMGSMR1 is characterized by onset of seizures related to hypomagnesemia in the first year of life and affected individuals show delayed psychomotor development in variable degrees (Arjona et al., 2014). Heterozygous variants in CNNM2 gene were described for the first time in two unrelated families with autosomal dominant renal hypomagnesemia, displaying severely lowered serum Mg2+ levels and a defect in tubular Mg2+ reabsorption, without other electrolyte disturbances or neurological function impairment (Stuiver et al., 2011). Subsequently, the other four heterozygous and two homozygous variants in CNNM2 gene were reported to cause patients variable degrees of intellectual disability except for autosomal dominant or recessive renal hypomagnesemia, and most of whom had seizures(Arjona et al., 2014; Accogli et al., 2019; Bamhraz et al., 2020). What is noteworthy is that all reported cases showed the varying degrees of hypomagnesemia up to now, with or without neurological impairment.
Here, we present a de novo heterozygous variant in CNNM2 in a Chinese girl, presenting with intellectual disability and intractable epilepsy, but no hypomagnesemia.
Case Description
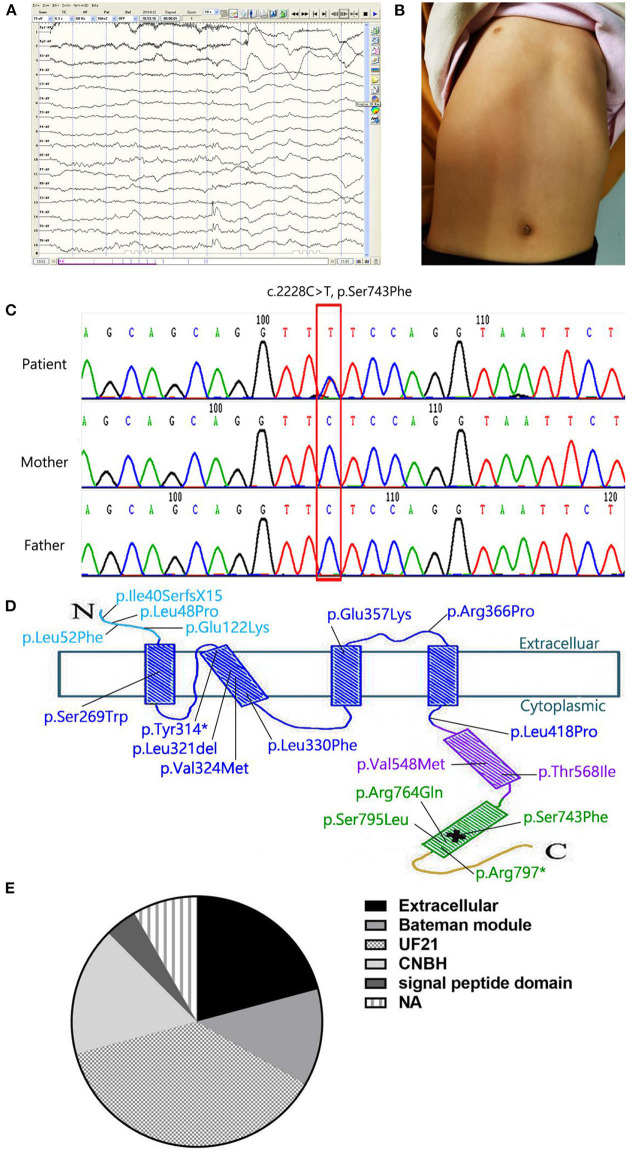
A 4-year-old girl attracted our attention with the symptoms of intractable epilepsy and mild intellectual disability. She was found to suffer cerebral convulsions during sleep, characterized by focal seizures since she was 3 years old. The EEG monitoring showed the release of sharp-slow and spinous-slow waves in the left posterior temporal region and the right middle posterior temporal regions (Figure 1A). Despite three kinds of antiepileptic medications (Oxcarbazepine, Valproic acid, Topiramate) were used in proper sequence, the seizures were not controlled, once every two days to half a month. When Lacosamide, the fourth antiepileptic drug, was used, the patient still had focal seizures once every month. She was assessed to be well below age expectations in verbal and social communication skills, and obtained an intelligence quotient score of 68. In physical examination, she had a funnel-shaped chest (Figure 1B). Her head circumference was measured 48 cm, below age expectations, but not diagnosed microcephaly. Other physical examinations were normal. Laboratory investigations demonstrated serum magnesium (0.74 mmol/l, range 0.67 to 1.04 mmol/L) is in the normal range. Declined calcitonin (1.82 pg/ml, range 5.17 to 9.82 pg/ml), and calcium (2.16 mmol/l, range 2.23 to 2.80 mmol/L) were found, and serum phosphorus, parathyroid hormone, and bone specific alkaline phosphatase were normal. We asked the medical history carefully, found that children with respiratory tract infection disease almost every month, resulting in poor appetite, less nutrients intake. She was the only patient in her family. Both parents are healthy, without family history of seizures. Umbilical cord around the neck and meconium stained amniotic fluid occurred when she was in perinatal period, delayed crying and lack of oxygen at birth. Soon afterwards she was found to be delayed in athletic and intellectual development, characterized by a delay in walking and speaking. A cranial magnetic resonance imaging (MRI) at 2 and 5 years of age showed no obvious abnormality.
Figure 1.
(A) The EEG monitoring showed the release of sharp-slow and spinous-slow waves in the left posterior temporal region and the right middle posterior temporal regions. (B) Patient's picture showing a funnel-shaped chest. (C) Partial CNNM2 electropherograms of the patient and her parents. In the electropherograms, the variant is indicated by a red box and the changes in nucleotide and resulting effects on the protein are shown. (D) Localization of the variant in the secondary structure of CNNM2. The N-terminal extracellular domain and the transmembrane domain are in light blue and dark blue respectively. The CBS domain is in purple, the CNBH domain is in green, and the unstructured C-terminus is yellow. *means stop codon. The location of pathological variant is indicated by a cross. (E) variant domains of cases listed in Table 1. In 24 cases, we found 5 domains: extracellular (5/24), bateman module (3/24), UF21(9/24), CNBH(4/24), and signal peptide(1/24). The other two cases were not available (2/24).
During follow-up, the patient had focal seizures once every month treated by Oxcarbazepine, Valproic acid, Topiramate and Lacosamide at 7 years old with weight was 14 kg (< −3 SD), height of 105 cm (< −3 SD) and BMI of 12.7 kg/cm2. She showed poor social interaction and verbal communication skills. Laboratory investigations demonstrated serum magnesium (0.78 mmol/l, range 0.75 to 1.02 mmol/L) was still in the normal range. Her father and mother had been tested for serum magnesium in our hospital, and serum magnesium was normal, respectively 0.95 mmol/L and 1.03 mmol/L. Uric calcium (3.2 mmol/24 h, range from 2.5 to 7.5 mmol/24 h) in the patient was normal. Urine sodium, potassium and chloride for 24 h were also normal, and 25 hydroxyvitamin D was low (21.04 ng/ml, normal range > = 30). Hypocalcemia may be caused by a lack of nutrients intake.
Genetic Testing
As an etiological investigation, whole Exome Sequencing was performed for the patient and generated about 10 Gb high-quality raw sequencing data. The average sequencing depth for the sample was 106.98-fold, with 94.48% of coverage of the targeted regions at a 20-fold sequencing depth and 78.95% at 50-fold depth. After removal of sequencing adapters, low-quality reads and duplicated reads, we identified more than 40,000 single nucleotide variations (SNVs) and indels using the GATK tool. Afterwards a series of bioinformatics filtering strategy, including variant filtration against multiple databases, functional prediction by multiple in silico tools and gene function, were carried out as described previously (PMID: 30488659, 28386848, 22595939). Finally, three candidate variants were submitted for validation of sanger sequencing from the proband and parents, and only one rare and novel missense variant (c.2228C>T, p.Ser743Phe) in CNNM2 was retain because of the other two variants (KIF1A, c.4682G>A, p.Arg1561His; DYNC1H1, c.6343A>G, P.Lys2115Glu) inherited from the healthy one of the parents (Figure 1C).
The variant resulting in Ser743Phe occurred in the cyclic nucleotide monophosphate-binding homology (CNBH) domain adjacent to the C terminus of the putative protein product (Figure 1D). We also found that c.2228C>T in CNNM2 was a rare variant and not present in any publicly available databases, including the Exome Aggregation Consortium database, ESP6500 database, 1,000 Genomes Project, cg69 database and Genome Aggregation database. In addition, the novel variant has not been reported in the published literature previously and were clearly predicted to be functionally deleterious by the prediction tool ClinPred with a score of 0.741, SIFT with a score of 0.007 and CADD with a score of 23.1, although PolyPhen-2 tool showed benign prediction with a score of 0.013. Therefore, based on the information above and the international guidelines of the American College of Medical Genetics (ACMG) Laboratory Practice Committee Working Group, the variant was classified as a likely pathogenic variant and confirmed as a de novo variant by parental samples.
Discussion
Up to now, 99 types of variants in CNNM2 have been reported (Table 1). Two heterozygous variants in CNNM2 (c.117delG, p.Ile40SerfsX15; c.1703C>T, p.Thr568Ile) occurred in four patients of two unrelated families (Stuiver et al., 2011). One variant in CNNM2 located in extracellular domains, the other located in Bateman modules. And all four patients showed hypomagnesemia with a renal defect in Mg2+ reabsorption. The other two homozygous variants (c.364G>A, p.Glu122Lys; c.1642G>A, p.Val548Met) (Arjona et al., 2014; Accogli et al., 2019) and 13 heterozygous in CNNM2 occurred in unrelated families (Kosmicki et al., 2017; Snoeijen-Schouwenaars et al., 2019; Bamhraz et al., 2020; Franken et al., 2021). In 24 cases, different variant domains were listed in Figure 1E. We found DUF21 was the dominant domain (9/24) from the figure. Almost all patients showed hypomagnesemia (except our case and F8 in reference Franken et al., 2021) and varying degrees of intellectual disability. Patients with heterozygous variants showed mild to moderate intellectual disability with impaired motor and language skills, whereas patients with homozygous variants showed severe intellectual disability with very limited motor skills and no verbal. Besides, 16 patients showed a seizure as the symptom at onset, two patients were diagnosed with myoclonus or anorexia nervosa. Epilepsy in patients with heterozygous variants can be well controlled with antiepileptic drugs such as phenobarbital, valproate and clobazam. On the contrary, patients with homozygous variants showed refractory epilepsy, only one of whom responded to valproate and lamotrigine. Furthermore, patients with homozygous variants presented with brain anomalies and microcephaly. Thus, it can be seen that the severity of the neurological impairment seems to be related to the pattern of inheritance. In the 16 patients, Mg2+ supplementation therapy failed to completely correct the hypomagnesemia, meanwhile it cannot control the seizures. So we speculated that hypomagnesemia is not the possible cause of epileptic seizures by the variant of CNNM2.
Table 1.
Clinical, biochemical and neuroradiological data of patients with CNNM2 variants.
| Stuiver et al. ( 2011 ) | Arjona et al. ( 2014 ) | Accogli et al. (2019) | Bamhraz et al. (2020) | Our case | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | II.3 | III.2 | I.2 | II.1 | F1.1 | F1.2 | F2.1 | F3.1 | F4.1 | F5.1 | III.1 | NA | proband |
| Gender | M | F | F | M | M | F | F | F | M | F | M | F | F |
| Ethnicity | Dutch | Dutch | Czech Republic | Czech Republic | Serbian | Serbian | German | German | German | Polish | Moroccan | NA | Chinese |
| Age onset | 15 years | 1 year | NA | 16 years | 1 day | 6 days | 7 months | 1 year | 4 months | 1 6years | 1 day | 15 years | 3 years |
| Symptoms at onset | Muscle spasms Headache, palpitations |
Muscle spasms, stuttering, loss of consciousness |
Symptomless | Weakness, Vertigo, headache |
Seizure | Seizure | Seizure | Seizure, paresthesia |
Seizure | Myoclonus, paresthesia |
Seizure | Anorexia nervosa | Seizure |
| Initial serum Mg2+(mmol/L) | 0.46 | 0.51 | 0.52 | 0.36 | 0.5 | 0.5 | 0.56 | 0.44 | 0.5 | 0.66 | 0.38 | 0.53 | 0.74 |
| Mg2+ after supplementation (mmol/L) | NA | 0.58 | NA* | 0.61 | 0.66 | 0.54 | 0.56 | 0.53 | 0.68 | NA | 0.49 | 0.56 | 0.78* |
| Epilepsy treatment | N | N | N | N | Response to valproate and Lamotrigine | Resistance to Valproate and Levertiracetam | Response to Phenobarbital | Response to Valproate | Response to Clobazam | NA | Drug-resistant | N | Drug-resistant |
| Intellectual disability | N | N | N | N | Severe | Severe | Moderate | Moderate | Moderate | Mild | Severe | Mild | Mild |
| Language impairment | N | N | N | N | N | N | ELD | ELD | ELD | NA | N | NA | ELD |
| Motor skills impairment | N | N | N | N | Very limited | Very limited | Impaired | Impaired | Impaired | NA | Very limited | NA | Delayed walking |
| malformation | N | N | N | N | Microcephaly | Microcephaly | NA | NA | NA | NA | wide mouth, pectus xcavatum, Microcephaly |
N | pectus excavatum |
| Abnormal brain development | NA | NA | NA | NA | Widened outer cerebrospinal liquor space, myelination defects, opercularization defect |
NA | N | N | N | NA | Global reduction of white matter, cerebral cortical atrophy | - | NA |
| variant (DNA level) |
c.117delG | c.117delG | c.1703C > T | c.1703C > T | c.364G > A | c.364G > A | c.1069G > A | c.806C > G | c.1069G > A | c.988C > T | c.1642G > A | NA | c.2228C > T |
| variant (Protein level) |
p.Ile40SerfsX15 | p.Ile40SerfsX15 | p.Thr568Ile | p.Thr568Ile | p.Glu122Lys | p.Glu122Lys | p.Glu357Lys | p.Ser269Trp | p.Glu357Lys | p.Leu330Phe | p.Val548Met | p.Arg366Pro | p.Ser743Phe |
| variant domain | Extracellular | Extracellular | Bateman module | Bateman module | Extracellular | Extracellular | DUF21 | DUF21 | DUF21 | DUF21 | Bateman module |
DUF21 | CNBH |
| Zygosity | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Homozygous | Homozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Homozygous | Heterozygous | Heterozygous |
| Kosmicki et al. ( 2017 ) | Snoeijen-Schouwenaars et al. ( 2019 )3 | Franken et al. ( 2021 ) | |||||||||||
| Patient | NA | NA | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | ||
| Gender | NA | NA | F | M | M | M | M | M | F | F | F | ||
| Ethnicity | NA | Netherlands | Caucasian | Sub-Saharan African | Caucasian | Caucasian | Sub-Saharan African | Caucasian | Caucasian | Caucasian | Caucasian | ||
| Age onset | NA | NA | 6 years | 3 months | 2 years | 3 years | 8 months | 13 months | 16 years | 8 months | 1–1.5 year | ||
| Symptoms at onset | Epilepsy, autism spectrum disorder | Autism spectrum disorder, Intellectual disability, seizures and epilepsy |
seizures | seizures | seizures | N | seizures | seizures | seizures | N | seizures, autism spectrum disorder | ||
| Initial serum Mg2+(mmol/L) | NA | NA | 0.63 | 0.57 | 0.45 | 0.48 | 0.5 | 0.54 | 0.49 | 0.72 | 0.57 | ||
| Serum Mg2+ after supplementation (mmol/L) |
NA | NA | 0.65 | NA | 0.53-0.66 | NA | 0.51 | 0.52 | 0.58 | 0.7 | 0.69 | ||
| Epilepsy treatment | NA | NA | NA | NA | NA | N | NA | NA | NA | N | NA | ||
| Intellectual disability | NA | NA | Y | Y | N | Y | Y | Y | Y | Y | Y | ||
| Language impairment |
NA | NA | ELD | N | ELD | N | ELD | ELD | NA | ELD | ELD | ||
| Motor skills impairment | NA | NA | Y | Y | Y | N | Y | Y | N | Y | N | ||
| Malformation | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Abnormal brain development | NA | NA | N | N | N | N | N | N | N | N | slightly hyperintense white matter | ||
| Mutation (DNA level) |
c.154C>T | c.2291G>A | Del ex1-4 | Del ex3-8 | c.143T>C | c.942C>G | c.961_963del | c.970G>A | c.1253T>C | c.2384C>T | c.2389C>T | ||
| Mutation (Protein level) |
p.Leu52Phe | p.Arg764Gln | NA | NA | p.Leu48Pro | p.Tyr314* | p.Leu321del | p.Val324Met | p.Leu418Pro | p.Ser795Leu | p.Arg797* | ||
| Mutational domain | Extracellular | CNBH | NA | NA | signal peptide domain | DUF21 | DUF21 | DUF21 | DUF21 | CNBH | CNBH | ||
| Zygosity | NA | NA | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | Heterozygous | ||
NA, not available; ELD, expressive language disorder; F, female; M, male.
no Mg2+ supplementation; Y, yes; N, no.
Our patient showed an intractable epilepsy, characterized by focal seizures. Seizure activity can not be completely controlled despite antiepileptic treatment with oxcarbazepine, valproic acid, topiramate and lacosamide. She was also diagnosed with a mild intellectual disability and a delay in walking and speaking, poor verbal and reading skills and a head circumference below age expectations. Interestingly, she showed no hypomagnesemia, but the serum magnesium was low, which can not be excluded from deficient nutrient intake. In reference Franken et al. (2021), F8 also presented with normal serum magnesium via taking Mg2+ supplementation. The two patients had something in common: a de novo mutation in CNBH domain, manifested as seizures, ID and different degrees of language expression disorder. The phenotype of all CNNM2 cases and our patient is summarized in Table 1.
Mg2+ is mostly absorbed in the intestinal epithelia, and complemented by a reabsorption in the kidney. Serum Mg2+ levels are fine-tuned by transcellular Mg2+ reabsorption which occurs in the distal renal tubule (Corral-Rodriguez et al., 2014). As a transporter of Mg2+, CNNM2 is highly expressed in the basolateral membrane of the distal renal tubule, mediating the reabsorption of Mg2+. CNNMs show a modular structure, which contains an extracellular N-terminal domain, a DUF21 domain that is composed of four transmembrane α-helices, a CNBH domain, a Bateman module including two consecutive cystathionine β-synthase (CBS) motifs (CBS1 and CBS2) and linkers of different length for connection (Corral-Rodriguez et al., 2014; Gimenez-Mascarell et al., 2019). The Bateman module of CNNMs forms a disc-like symmetric dimer called the CBS module (Mahmood et al., 2009), which represents the most conserved region and the most extensively studied region of CNNM proteins. The CBS module interacts with nucleotides in a Mg2+ dependent manner and results in its own change in conformation from a “twisted” structure toward a “flat” disc-like state, which might account for the rapid transport of Mg2+ by CNNMs. Interestingly, the pathological variant T568I in CNNM2, found in patients who suffer from familial dominant hypomagnesaemia, locks the CBS modules in the “flat” conformation and might hinder it from returning to the “twisted” conformation, makes the CBS modules nonfunctional (Hirata et al., 2014; Gimenez-Mascarell et al., 2019). The failure of the CBS modules may account for the impaired transport of Mg2+, leading to hypomagnesemia.
Compared with the CBS domain, the CNBH domain is less well known. Except for a large variable loop, CNBH domains of CNNMs show a great conservation among isoforms (Chen et al., 2018). The CNBH domain of CNNMs resembles numerous cyclic nucleotide-binding domains of cyclic nucleotide-gated channels structurally. The role and exact mechanism of the CNBH domain of CNNMs in Mg2+ transport remain uncertain. Similar to the CBS domains, the cyclic nucleotide-binding domains of cyclic nucleotide monophosphate (cNMP)-dependent kinases and ion channels are known to be capable of interacting with cyclic nucleotides and then having structural modifications (Shabb and Corbin, 1992). Interestingly, the nuclear magnetic resonance (NMR) and thermal shift assays (TSA) have confirmed that the CNBH domain of CNNMs lacks the ability of binding cyclic nucleotides such as cAMP and cGMP. Instead, the function of CNBH domains may be dimerization, regulating the activity of Mg2+ efflux in the basolateral membrane of epithelial cells and mediating the absorption of Mg2+ in a cyclic nucleotide-independent manner. Nevertheless, variants that prevent CNBH dimerization had divergent influences in the cellular Mg2+ efflux, one showed obviously efflux impairment whereas another manifested close to efflux of wild-type (Chen et al., 2018). It can be speculated that the dimerization is crucial but not necessary for Mg2+ efflux and some variants in CNBH domain may not impair the Mg2+ efflux activity of CNNMs significantly. The pathogenic variant of our case is located in CNBH domain, which is the rarely variant site in the CNBH domain of CNNM2.
In CNNM2, the CNBH module exists as homodimers and associates each other in dimers. Gehring et al. elucidated the integral fold of the CNBH module of CNNM2 at 2.6 Å resolution, which showed a central eight-stranded antiparallel β roll that has two α-helixes in the N-terminal and an α-helix in the C-terminal. Thereinto, residues 724 to 767 of CNNM2 were deleted because they were hardly conserved between isoforms and were not predicted to form a regular secondary structure (Chen et al., 2018). However, the pathogenic variant Ser743Phe of our case is located in residues 724 to 767 of CNNM2. And it is reasonable to conjecture that the special phenotype (no hypomagnesemia) in our case results from the special location of the variant. Of course, the presence of a innocuous polymorphism can not be absolutely ruled out. But the clinical phenotype with mild intellectual disability and intractable epilepsy supports a partial loss of CNNM2 function due to the p.Ser743Phe variant.
For patients with heterozygous or homozygous variant in CNNM2, seizures and hypomagnesemia constituted the major symptom. Low serum Mg2+ is related to a series of neurological conditions, including epilepsy. In the nervous system, N-methyl-D-aspartate (NMDA) receptors, which play an important role in excitatory synaptic transmission, are directly inhibited by the extracellular Mg2+concentration. Whereas GABAA receptors, whose function is directly excited by Mg2+concentration, have an inhibitory function (Nowak et al., 1984; Paoletti et al., 2013). When the concentration of Mg2+ is low, NMDA receptors are overexcited and GABAA receptors become less excited, making neurons hyperexcitability and leading to epileptic activity (Moykkynen et al., 2001). Therefore, it is undeniable that severely Mg2+ deficiency plays a stimulative role in the seizures development. However, some authors thought that variants in CNNM2 were the reason why intellectual disability and seizures occurred in patients with hypomagnesemia. Arjona et al. injected morpholino (MO) into zebrafish embryos to block the translation of early expressed zebrafish CNNM2 paralogues. At non-lethal doses of MO, knockdown of CNNM2 paralogues lead to morphological phenotypes characterized by accumulation of cerebrospinal fluid, motor neuronal phenotype characterized by increased embryonic spontaneous contractions and cerebral development defect. All phenotypes were rescued by co-injection with wild-type Cnnm2 cRNA of the mouse rather than co-injection with mutant CNNM2 cRNA or exposure to the media with high Mg2+ concentrations. Interestingly, “co-injection with mutant CNNM2 cRNA even worsened motor neuronal phenotype by increasing the number of embryonic spontaneous contractions significantly” (Arjona et al., 2014). It can be proven that CNNM2 gene is crucial for cerebral development and neurological function. And in patients, seizure activity continued despite normal serum Mg2+ levels, which prompted that it might be a genuine brain function disturbance caused by impaired CNNM2 but not hypomagnesemia. This is the reason that oral Mg2+ therapy failed to control seizures.
Our patient showed a mild intellectual disability, with a de novo heterozygous missense CNNM2 variant. Reviewing the literature (Stuiver et al., 2011; Arjona et al., 2014; Accogli et al., 2019; Bamhraz et al., 2020; Franken et al., 2021), we found that severe intellectual disability all occurred in patients with homozygous missense variants. Hence, it can be speculated that homozygous variants significantly affect the function of CNNM2, leading to severe phenotypes. In contrast, the heterozygous state of their parents may not so sufficient to cause functional loss, so the Mg2+ homeostasis was maintained. Compared to patients with homozygous variants in the extracellular domain, those with homozygous variants in the CBS domain had more severe hypomagnesemia and more intractable epilepsy. In summary, these results support the viewpoint that the severity of the disease correlates with the number of variant alleles and the damaged protein domains.
Concluding Remarks
In summary, we have presented a de novo heterozygous CNNM2 variant, and the CNBH domain of CNNM2 possibly plays a limited role in Mg2+ transport but a significant role in neural development. So we speculated that neurological phenotypes such as intellectual disability and seizures may be purely caused by CNNM2 variants, and the number of variant alleles and the changed protein domains correlates with the severity of the disease. CNNM2 variants should also be considered in patients with epilepsy and intellectual disability, even no hypomagnesemia.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
XL, SB and WW collected data and wrote the manuscript. XS, YH, and FL reviewed the article. QZ and FZ draw the table and the graph. ZL directed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Accogli A., Scala M., Calcagno A., Napoli F., Di Iorgi N., Arrigo S., et al. (2019). CNNM2 homozygous mutations cause severe refractory hypomagnesemia, epileptic encephalopathy and brain malformations. Eur. J. Med. Genet. 62, 198–203. 10.1016/j.ejmg.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Arjona F. J., de Baaij J. H., Schlingmann K. P., Lameris A. L., van Wijk E., Flik G., et al. (2014). CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet. 10:e1004267. 10.1371/journal.pgen.1004267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamhraz A., Rodrigues A., Grady R., Deveau S., deBaaij J., Bindels R., et al. (2020). SAT-072 a novel mutation in the CNNM2 gene causing hypomagnesaemia and neurological impairment in an adolescent. Kidney Int. Rep. 5(Suppl. 3), S32–S33. 10.1016/j.ekir.2020.02.078 [DOI] [Google Scholar]
- Chen Y. S., Kozlov G., Fakih R., Funato Y., Miki H., Gehring K. (2018). The cyclic nucleotide-binding homology domain of the integral membrane protein CNNM mediates dimerization and is required for Mg(2+) efflux activity. J. Biol. Chem. 293, 19998–20007. 10.1074/jbc.RA118.005672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Rodriguez M. A., Stuiver M., Abascal-Palacios G., Diercks T., Oyenarte I., Ereno-Orbea J., et al. (2014). Nucleotide binding triggers a conformational change of the CBS module of the magnesium transporter CNNM2 from a twisted towards a flat structure. Biochem. J. 464, 23–34. 10.1042/BJ20140409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken G. A. C., Muller D., Mignot C., Keren B., Levy J., Tabet A. C., et al. (2021). The phenotypic and genetic spectrum of patients with heterozygous mutations in cyclin M2 (CNNM2). Hum. Mutat. 42, 473–486. 10.1002/humu.24182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Mascarell P., Gonzalez-Recio I., Fernandez-Rodriguez C., Oyenarte I., Muller D., Martinez-Chantar M. L., et al. (2019). Current structural knowledge on the CNNM family of magnesium transport mediators. Int. J. Mol. Sci. 20:1135. 10.3390/ijms20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y., Funato Y., Takano Y., Miki H. (2014). Mg2+-dependent interactions of ATP with the cystathionine-beta-synthase (CBS) domains of a magnesium transporter. J. Biol. Chem. 289, 14731–14739. 10.1074/jbc.M114.551176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmicki J. A., Samocha K. E., Howrigan D. P., Sanders S. J., Slowikowski K., Lek M., et al. (2017). Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat. Genet. 49, 504–510. 10.1038/ng.3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N. A., Biemans-Oldehinkel E., Poolman B. (2009). Engineering of ion sensing by the cystathionine beta-synthase module of the ABC transporter OpuA. J. Biol. Chem. 284, 14368–14376. 10.1074/jbc.M901238200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moykkynen T., Uusi-Oukari M., Heikkila J., Lovinger D. M., Luddens H., Korpi E. R. (2001). Magnesium potentiation of the function of native and recombinant GABA(A) receptors. Neuroreport 12, 2175–2179. 10.1097/00001756-200107200-00026 [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. (1984). Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462–465. 10.1038/307462a0 [DOI] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- Quamme G. A. (2010). Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol. 298, C407–429. 10.1152/ajpcell.00124.2009 [DOI] [PubMed] [Google Scholar]
- Rainier S., Chai J. H., Tokarz D., Nicholls R. D., Fink J. K. (2003). NIPA1 gene mutations cause autosomal dominant hereditary spastic paraplegia (SPG6). Am. J. Hum. Genet. 73, 967–971. 10.1086/378817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabb J. B., Corbin J. D. (1992). Cyclic nucleotide-binding domains in proteins having diverse functions. J. Biol. Chem. 267, 5723–5726. 10.1016/S0021-9258(18)42609-9 [DOI] [PubMed] [Google Scholar]
- Snoeijen-Schouwenaars F. M., van Ool J. S., Verhoeven J. S., van Mierlo P., Braakman H. M. H., Smeets E. E., et al. (2019). Diagnostic exome sequencing in 100 consecutive patients with both epilepsy and intellectual disability. Epilepsia 60, 155–164. 10.1111/epi.14618 [DOI] [PubMed] [Google Scholar]
- Stuiver M., Lainez S., Will C., Terryn S., Gunzel D., Debaix H., et al. (2011). CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am. J. Hum. Genet. 88, 333–343. 10.1016/j.ajhg.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe S. L. (2013). Magnesium in disease prevention and overall health. Adv. Nutr. 4, 378S−383S. 10.3945/an.112.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Yang B., Stokes J. B., Kirby P. A., Cao X., Shi P., et al. (2009). Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum. Mol. Genet. 18, 4367–4375. 10.1093/hmg/ddp392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.