Abstract
Background
Immunotherapy (IO) has been associated with improved outcomes in patients with locally advanced Merkel cell carcinoma (laMCC) and metastatic Merkel cell carcinoma (mMCC). The primary objective of SPEAR‐Merkel was to explore treatment patterns, clinical outcomes, and health care resource utilization (HCRU) in patients with laMCC or mMCC initiating first‐line (1L) treatment with avelumab, non‐avelumab IO, or chemotherapy in a U.S. community oncology setting.
Methods
Adult patients with laMCC or mMCC initiating 1L avelumab, non‐avelumab IO, or chemotherapy from January 1, 2015, to March 31, 2019, were identified from the U.S. Oncology Network electronic health care record database and followed up through September 30, 2019. Baseline characteristics and HCRU were analyzed descriptively, including physician‐stated overall response rate in the real‐world clinical setting. Kaplan‐Meier methods were used to measure duration of response, real‐world progression‐free survival (rwPFS), and overall survival (OS).
Results
Among the overall population (n = 94), 28 received 1L avelumab (9 laMCC, 19 mMCC), 26 received 1L non‐avelumab IO (8 laMCC, 18 mMCC), and 40 received 1L chemotherapy (10 laMCC, 30 mMCC). The real‐world overall response rate was 64.3%, 61.5%, and 42.5%, respectively. From 1L treatment initiation, median rwPFS was 11.4, 8.1, and 6.1 months, and median OS was 20.2 months, not reached, and 14.7 months for the respective cohorts.
Conclusion
SPEAR‐Merkel showed that patients with laMCC or mMCC treated with IO had improved outcomes compared with chemotherapy in clinical practice. The study provides insight on utilization and clinical outcomes associated with newer, more innovative therapies in clinical practice, which may help clinicians understand the variety of newer treatment options for both laMCC and mMCC.
Implications for Practice
To the authors’ knowledge, SPEAR‐Merkel is the first study to evaluate real‐world clinical outcomes in patients with locally advanced Merkel cell carcinoma (laMCC) and metastatic Merkel cell carcinoma (mMCC) receiving first‐line (1L) avelumab, non‐avelumab immuno‐oncology therapies, or chemotherapy in a real‐world setting. SPEAR‐Merkel showed clinical benefit for immuno‐oncology therapies compared with chemotherapy. The study provides insight on uses and clinical outcomes associated with innovative therapies in clinical practice, which may help clinicians understand the variety of newer treatment options for both laMCC and mMCC. The study is of particular importance as it shows that chemotherapy is still being used as 1L treatment despite its inferior clinical and safety profile.
Keywords: Merkel cell carcinoma, Avelumab, Real‐world outcomes, Immunotherapy, Community oncology
Short abstract
The SPEAR‐Merkel study provides on current treatment patterns, real‐world clinical outcomes, and healthcare resource utilization in patients with locally advanced/metastatic Merkel cell carcinoma initiating first‐line treatment with avelumab, non‐avelumab immunotherapies, or chemotherapy within a large network of community‐based oncology practices in the U.S. Results are reported here.
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine cutaneous neoplasm [1]. MCC is an aggressive cancer that is associated with a poor prognosis, as it can grow rapidly and metastasize early in the course of disease [2, 3, 4]. MCC has a recurrence rate of 43%–48% [5, 6] and a 5‐year survival rate of 78% for localized disease, 51% for regional disease, and 17% for advanced/metastatic disease [7]. MCC is more common in elderly, White men [8, 9]. The disease is also commonly found in patients who are immunocompromised, namely those who have undergone organ transplant, have HIV infection or lymphoproliferative malignancies, or have had treatment with immunosuppressant medications [3, 4, 10].
Until avelumab received U.S. Food and Drug Administration (FDA) approval in March 2017 for the treatment of metastatic MCC (mMCC), the main first‐line (1L) therapy for mMCC was chemotherapy. Although the chemotherapy‐associated objective response is favorable (objective response rate of 52%–61% for 1L therapy in patients with mMCC), the duration of response is short (2–4 months), and toxicities, such as myelosuppression and neutropenia, have been observed [11, 12, 13, 14]. These toxicities pose a challenge in the MCC population, which includes considerable proportions of patients aged 65 years and older and immunocompromised patients [11, 15].
Immunosuppression mediated by immune‐inhibitory mechanisms, such as the programmed death ligand 1 (PD‐L1) and receptor (PD‐1) pathway, is involved in MCC pathogenesis. PD‐L1 upregulation appears to help tumors evade the host immune response; thus, therapies that stimulate antitumor immune responses are possible treatment options [13]. The FDA approval of avelumab, an anti–PD‐L1 immunotherapy, was based on the JAVELIN Merkel 200 trial (NCT02155647) part A, which studied 88 patients who had experienced disease progression with prior chemotherapy for mMCC. After 1 year of follow‐up, the objective response rate was 33.0%, and 74% of the responses lasted over a year; the median overall survival (OS) was 12.9 months [16, 17]. JAVELIN Merkel 200 part B investigated patients who had not received any prior systemic therapy for mMCC. In 116 patients with ≥15 months of follow‐up, the objective response rate was 39.7%. The median OS was 20.3 months, and the 12‐month OS rate was 60%. Grade ≥ 3 adverse events (AEs) were seen in 18.1% of patients, and no treatment‐related deaths occurred [18].
Pembrolizumab received FDA approval in 2018 for patients with recurrent locally advanced MCC (laMCC) or mMCC, based on KEYNOTE‐017 data [19]. In 50 patients with recurrent laMCC or distant mMCC treated with 1L pembrolizumab and a median follow‐up of 14.9 months, response durability at 24 months was 79.1%, median progression‐free survival (PFS) was 16.8 months, median OS was not reached (NR), and the objective response rate was 56% [19].
Nivolumab, although not FDA approved for MCC, was studied in the neoadjuvant setting in CheckMate 358 and was associated with a favorable objective response rate in 39 patients with stage IIA–IV resectable MCC and had a favorable safety profile [20, 21].
Since 2018, 1L avelumab, pembrolizumab, and nivolumab have been included in the National Comprehensive Cancer Network Guidelines as preferred interventions for disseminated disease, based on similarity of response rates and superior durability of response compared with cytotoxic chemotherapy [22, 23]. However, aside from clinical trials, limited studies have examined treatment patterns and clinical outcomes in MCC in the real world because of the rarity of the disease. After data became available from the JAVELIN Merkel 200 trial, an expanded access program (EAP) was initiated in 38 countries to provide avelumab on a compassionate‐use basis to patients with mMCC. The response rate was 46.7% in 240 patients evaluable for response, 46.7% in 15 patients receiving only 1L avelumab, and 37.5% in 16 immunocompromised patients. The median duration of treatment was 7.9, 4.5, and 5.2 months in the respective groups [15].
The objective of the Study informing treatment Pathway dEcisions in Merkel cell cARcinoma (SPEAR‐Merkel) was to obtain insight into contemporary treatment patterns, outcomes, and healthcare resource utilization (HCRU) in patients with laMCC and mMCC who initiate 1L treatment with avelumab, non‐avelumab immunotherapy (IO), or chemotherapy within a large network of community‐based oncology practices.
Materials and Methods
Study Design and Data Sources
This was a retrospective, observational, descriptive study of U.S. adult patients with a diagnosis of laMCC or mMCC who initiated 1L systemic treatment with avelumab, non‐avelumab IO (pembrolizumab or nivolumab), or chemotherapy between January 1, 2015, and March 31, 2019, and were followed up through September 30, 2019.
Patients were from practices in the U.S. Oncology Network (USON), a division of McKesson Corporation that uses the iKnowMed (iKM) electronic health record (EHR). The USON is a community‐based network of oncology practices comprising over 470 cancer treatment centers and 1,200 physicians in 25 states treating over 1 million patients each year [24].
Data were obtained from iKM EHR via programmatic database abstraction and chart review, with supplemental vital status data from the Social Security Administration's Limited Access Death Master File (LADMF). For demographic and clinical characteristics and treatment patterns, data were abstracted from EHR structured fields. For clinical outcomes and HCRU, data were abstracted from EHR unstructured fields (e.g., providers’ free‐text notes and imaging reports), which could only be captured through chart review. All data were handled in compliance with the Health Insurance Portability and Accountability Act and the Health Information Technology for Economic and Clinical Health Act. The study protocol was granted an exception and waiver of informed consent by the U.S. Oncology Institutional Review Board (no. 19‐024E).
Patient Population
Eligible patients received a diagnosis of MCC before March 31, 2019. They were aged ≥18 years at initial diagnosis of MCC and had at least one visit after the index event during the study period at a USON site using the full EHR capabilities of iKM. Patients were required to have a minimum of 6 months of follow‐up, unless a death was recorded in this period.
Patients had to have initiated systemic immunotherapy (i.e., avelumab, nivolumab, pembrolizumab) or chemotherapy in the 1L setting (first date of treatment = index event) for laMCC or mMCC during the study period. laMCC was defined as lack of evidence of distant metastatic disease and with evidence of nodal involvement in the same region as the primary tumor; locally recurrent disease; or unresectable or high‐risk locally advanced disease. mMCC was defined as having evidence of distant metastases or disseminated disease to the initial/primary site of the diagnosis.
Patients were considered immunocompromised if they had a CD4 count of <500 cells/mm3 in the year prior to index or were diagnosed with HIV before study entry; were diagnosed with select hematologic diseases (chronic lymphocytic leukemia, multiple myeloma, or hypogammaglobulinemia) in the 5 years prior to study entry; received a documented organ or allogeneic stem cell transplant prior to study entry or during follow‐up; or received select immunosuppressive treatment within 28 days prior to the initiation of 1L treatment or during follow‐up (supplemental online Table 1).
Statistical Analysis
Demographic and clinical characteristics at baseline, treatment patterns, and HCRU were assessed descriptively. Clinical outcomes were physician assessed and obtained directly from patient charts and physician progress notes. Patients’ real‐world, physician‐assessed best overall response (rwBOR) was classified as complete response (CR; documentation as “complete response” to therapy, indicating that the patient was in “remission,” “all lesions” had disappeared, or “no evidence of disease”), response not otherwise specified (R‐NOS; documentation of improved or responding disease), stable disease (documented as disease was stable, not progressed, or not improved), progressive disease (documented as disease had “progressed,” or worsening of disease), mixed response (improved and worsened disease), or not evaluated. Real‐world overall response rate (rwORR) was assessed based on patients’ rwBOR, specifically, the proportion of patients who achieved a CR or R‐NOS among the total number of patients in each of the cohorts. Individual treatment groups with n < 11 were not reportable; thus non‐avelumab IO of pembrolizumab (n = 19) and nivolumab (n = 7), both PD‐1 inhibitors, were combined as a single cohort.
Time‐to‐event outcomes were assessed using the Kaplan‐Meier method. Patients who did not experience the event during the study observation period were censored on the study end date or the last visit date available in the data set, whichever occurred first. Real‐world duration of response (rwDOR) was defined as duration of time from first documented response of CR or R‐NOS to the earliest date of first progression (as defined by physician progress note or imaging report), recurrent disease or death, or initiation of next treatment. Real‐world PFS (rwPFS) was measured from the index date to the date of progression (as defined by physician progress note or imaging report) or to the date of death due to any cause. Real‐world OS was defined as the interval between the index date and the date of death or the end of follow‐up as documented in the LADMF and iKM EHR database and evaluated by treatment group.
Hospitalizations and emergency department (ED) visits documented in patient charts during and within 30 days after 1L treatment were summarized. Reasons for hospitalization and ED visits, including treatment related, were summarized. Results are presented by treatment group: 1L avelumab, 1L non‐avelumab IO, and 1L chemotherapy.
Results
Patient Population and Characteristics
In the 94 patients who met eligibility criteria, 27 (28.7%) had laMCC, and 67 (71.3%) had mMCC at 1L initiation. Twenty‐eight patients received 1L avelumab (9 laMCC, 19 mMCC), 26 received 1L non‐avelumab IO (8 laMCC, 18 mMCC), and 40 received 1L chemotherapy (10 laMCC, 30 mMCC) (Fig. 1).
Figure 1.
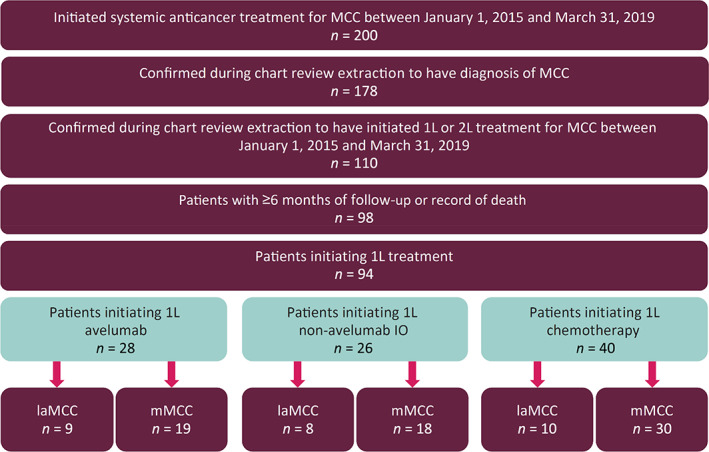
Study attrition.
Abbreviations: 1L, first line; 2L, second line; IO, immunotherapy; laMCC, locally advanced MCC; MCC, Merkel cell carcinoma; mMCC, metastatic MCC.
Demographic and clinical characteristics of the study cohort, stratified by treatment group, are presented in Table 1. The median age of the overall population at the time of initiating 1L treatment was 73 years (range, 21–90+), 68.1% were male, and 83.0% were White. Two‐thirds (64.3%) of patients receiving 1L avelumab were 75 years or older; 85.7% were male, and 82.1% were White. Half of patients (50.0%) who received 1L non‐avelumab IO were 75 years or older; 57.7% were male, and 84.6% were White. In the 40 patients who received 1L chemotherapy, 22.5% were 75 years or older, 62.5% were male, and 82.5% were White.
Table 1.
Baseline demographic and clinical characteristics of patients who received 1L treatment for locally advanced or metastatic MCC
| Demographic or clinical characteristic | Overall (n = 94) | Avelumab (n = 28) | Non‐avelumab IO (n = 26) | Chemotherapy (n = 40) |
|---|---|---|---|---|
| Age at 1L treatment initiation, median (range), years | 73 (21–90+) | 78 (52–90+) | 74 (43–90+) | 69 (21–90+) |
| Age group, n (%) | ||||
| <65 years | 20 (21.3) | 3 (10.7) | 4 (15.4) | 13 (32.5) |
| 65–74 years | 34 (36.2) | 7 (25.0) | 9 (34.6) | 18 (45.0) |
| 75+ years | 40 (42.6) | 18 (64.3) | 13 (50.0) | 9 (22.5) |
| Male, n (%) | 64 (68.1) | 24 (85.7) | 15 (57.7) | 25 (62.5) |
| Race, n (%) | ||||
| White | 78 (83.0) | 23 (82.1) | 22 (84.6) | 33 (82.5) |
| Not documented/Not reporteda | 16 (17.0) | 5 (17.9) | 4 (15.4) | 7 (17.5) |
| U.S. practice region, n (%) | ||||
| South | 40 (42.6) | 16 (57.1) | 12 (46.2) | 12 (30.0) |
| West | 42 (44.7) | 11 (39.3) | 10 (38.5) | 21 (52.5) |
| Northeast | 2 (2.1) | 1 (3.6) | 0 | 1 (2.5) |
| Midwest | 10 (10.6) | 0 | 4 (15.4) | 6 (15.0) |
| Stage at initial MCC diagnosis, n (%) | ||||
| IA–IIIA | 17 (18.1) | 4 (14.3) | 5 (19.2) | 8 (20.0) |
| IIIB | 12 (12.8) | 4 (14.3) | 3 (11.5) | 5 (12.5) |
| IV | 22 (23.4) | 6 (21.4) | 5 (19.2) | 11 (27.5) |
| Not documented | 43 (45.7) | 14 (50.0) | 13 (50.0) | 16 (40.0) |
| Disease status at 1L treatment initiation, n (%) | ||||
| Locally advanced | 27 (28.7) | 9 (32.1) | 8 (30.8) | 10 (25.0) |
| Metastatic | 67 (71.3) | 19 (67.9) | 18 (69.2) | 30 (75.0) |
| Primary tumor location, n (%) | ||||
| Face | 25 (26.6) | 5 (17.9) | 9 (34.6) | 11 (27.5) |
| Lower limb | 19 (20.2) | 6 (21.4) | 5 (19.2) | 8 (20.0) |
| Upper limb | 17 (18.1) | 8 (28.6) | 4 (15.4) | 5 (12.5) |
| Trunk | 10 (10.6) | 4 (14.3) | 3 (11.5) | 3 (7.5) |
| Scalp and neck | 8 (8.5) | 4 (14.3) | 1 (3.8) | 3 (7.5) |
| Unknown primary | 4 (4.3) | 0 | 2 (7.7) | 2 (5.0) |
| Face, other | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Not documented | 9 (9.6) | 1 (3.6) | 2 (7.7) | 6 (15.0) |
| Immunocompromised, n (%) | 7 (7.4) | 1 (3.6) | 3 (11.5) | 3 (7.5) |
| ECOG performance score, n (%) | ||||
| 0 | 15 (16.0) | 2 (7.1) | 4 (15.4) | 9 (22.5) |
| 1 | 37 (39.4) | 14 (50.0) | 9 (34.6) | 14 (35.0) |
| 2 | 17 (18.1) | 3 (10.7) | 4 (15.4) | 10 (25.0) |
| 3 | 1 (1.1) | 0 | 1 (3.8) | 0 |
| Not documented | 24 (25.5) | 9 (32.1) | 8 (30.8) | 7 (17.5) |
| Lactate dehydrogenase, median (range), U/L | 205.5 (87.0–1,815.0) | 206.0 (87.0–854.0) | 217.5 (131.0–552.0) | 205.0 (119.0–1,815.0) |
| Metastatic sites recorded prior to 1L treatment initiation, n (%) | ||||
| Patients with at least 1 recorded metastatic site | 66 (70.2) | 19 (67.8) | 18 (69.2) | 29 (72.5) |
| Lymph nodeb | 38 (57.6) | 10 (55.6) | 11 (57.9) | 17 (58.6) |
| Liverb | 17 (25.4) | 8 (42.1) | 5 (27.8) | 4 (13.3) |
| Boneb | 16 (23.9) | 3 (15.8) | 4 (22.2) | 9 (30.0) |
| Lungb | 3 (4.5) | 2 (10.5) | 0 | 1 (3.3) |
| Brainb | 3 (4.5) | 0 | 1 (5.6) | 2 (6.7) |
| Otherb | 53 (79.1) | 14 (73.7) | 15 (83.3) | 24 (80.0) |
| Time from initial MCC diagnosis to laMCC/mMCC status, median (range), weeks | 5.3 (0.1–239.7) | 5.1 (0.1–239.7) | 6.1 (0.1–155.8) | 4.0 (0.1–190.3) |
| Follow‐up from 1L treatment initiation, median (range), months | 11.0 (0.3–53.8) | 11.2 (0.5–27.2) | 9.8 (0.3–35.9) | 10.7 (0.7–53.8) |
Not documented combined with Not reportable because of cell count <5 as per McKesson Life Sciences best practices on reporting race.
Percentage among all patients with metastases in treatment cohort.
Abbreviations: 1L, first‐line; ECOG, Eastern Cooperative Oncology Group; IO, immunotherapy; laMCC, locally advanced MCC; MCC, Merkel cell carcinoma; mMCC, metastatic MCC.
In the overall study population, the median follow‐up from initiation of 1L treatment was 11.0 months (range, 0.3–53.8). More than half (55.3%) had favorable (0 or 1) baseline Eastern Cooperative Oncology Group performance status (ECOG PS), although it was not documented for 25.5%. The most common tumor sites were the face (26.6%), lower limbs (20.2%), and upper limbs (18.1%). Although 36.2% had stage IIIB–IV disease at initial MCC diagnosis, the stage was not documented for 45.7%. Most patients (92.6%) were immunocompetent at index.
In patients receiving 1L avelumab, the median follow‐up from initiation of 1L avelumab was 11.2 months (range, 0.5–27.2). Approximately one‐third (32.1%) had laMCC prior to 1L treatment, and the most common tumor sites were the upper (28.6%) and lower (21.4%) limbs. Patients receiving 1L non‐avelumab IO had a median follow‐up of 9.8 months (range, 0.3–35.9). Approximately one‐third (30.8%) had laMCC prior to 1L treatment, and the most common tumor site at index was the face (34.6%), followed by the lower limbs (19.2%). Patients receiving 1L chemotherapy had a median follow‐up of 10.7 months (range, 0.7–53.8). One‐quarter (25%) had laMCC prior to 1L treatment, and the most common tumor site was the face (27.5%), followed by the lower limbs (20.0%).
The median time from initial MCC diagnosis to laMCC or mMCC was 5.3 weeks (range, 0.1–239.7) in the overall study population, 5.1 weeks (range, 0.1–239.7) in the 1L avelumab cohort, 6.1 weeks (range, 0.1–155.8) in the 1L non‐avelumab IO cohort, and 4.0 weeks (range, 0.1–190.3) in the 1L chemotherapy cohort. Prior to 1L initiation, the most common sites of metastases were the lymph nodes and the liver in the overall study population (57.6% and 25.4%, respectively), the 1L avelumab cohort (55.6% and 42.1%, respectively), and the 1L non‐avelumab IO cohort (57.9% and 27.8%, respectively) and the lymph nodes and bone (58.6% and 30.0%, respectively) in the 1L chemotherapy cohort.
Treatment Patterns
Table 2 presents treatment patterns for the overall study population, stratified by 1L treatment cohort. The median time from diagnosis of laMCC or mMCC to 1L treatment initiation was 7.7 weeks (range, 0–164.5) in the overall study population, 6.0 weeks (range, 0.1–161.7) in the 1L avelumab cohort, 15.3 weeks (range, 0–165.4) in the 1L non‐avelumab IO cohort, and 5.9 weeks (range, 0.3–164.0) in the 1L chemotherapy cohort. Prior to 1L initiation, approximately one‐fifth (21.3%) of the overall population underwent surgical resection and 11.7% underwent radiation treatment; prior surgery and radiation, respectively, were observed in 21.4% and 7.1% of the 1L avelumab cohort, 15.4% and 19.2% of the 1L non‐avelumab IO cohort, and 25.0% and 10.0% of the 1L chemotherapy cohort. The most common 1L chemotherapy regimens were carboplatin + etoposide (52.5%), cisplatin + etoposide (25.0%), and carboplatin + paclitaxel (5.0%), and 90% received platinum‐based chemotherapy.
Table 2.
Treatment characteristics of patients initiating 1L treatment
| Treatment characteristic | Overall (n = 94) | Avelumab (n = 28) | Non‐avelumab IO (n = 26) | Chemotherapya (n = 40) |
|---|---|---|---|---|
| Time from laMCC/mMCC diagnosis to 1L treatment initiation, median (range), weeks | 7.7 (0.0–165.4) | 6.0 (0.1–161.7) | 15.3 (0.0–165.4) | 5.9 (0.3–164.0) |
| Patients with surgical resections prior to 1L treatment, n (%) | 20 (21.3) | 6 (21.4) | 4 (15.4) | 10 (25.0) |
| Patients with radiation treatments prior to 1L treatment, n (%) | 11 (11.7) | 2 (7.1) | 5 (19.2) | 4 (10.0) |
| Patients with 1L concurrentb surgical resections, n (%) | 7 (7.4) | 3 (10.7) | 1 (3.8) | 3(7.5) |
| Patients with 1L concurrentb radiation treatments, n (%) | 23 (24.5) | 6 (21.4) | 6 (23.1) | 11 (27.5) |
| Patients who discontinued treatment, n (%)c | 78 (83.0) | 18 (64.3) | 21(80.8) | 39 (97.5) |
| Reasons for treatment discontinuation, n (%)d | ||||
| Disease progression | 20 (25.6) | 6 (33.3) | 6 (28.6) | 8 (20.5) |
| Toxicity | 14 (17.9) | 2 (11.1) | 7 (33.3) | 5 (12.8) |
| Death | 11 (14.1) | 3 (16.7) | 4 (19.0) | 5 (12.8) |
| Physician preference | 9 (11.5) | 5 (27.8) | 1 (4.8) | 2 (5.1) |
| Patient choice | 2 (2.6) | 0 | 1 (4.8) | 1 (2.6) |
| Decline in performance status | 1 (1.3) | 0 | 1 (4.8) | 0 |
| Completed treatmente | 20 (25.6) | 1 (5.6) | 1 (4.8) | 18 (46.2) |
| Not documented | 3 (3.8) | 2 (11.1) | 1 (4.8) | 1 (2.6) |
| TTD, median (95% CI), months | 3.8 (3.0–5.6) | 10.5 (5.3–14.3) | 7.3 (2.5–18.2) | 2.2 (1.9–3.5) |
Most common 1L treatments among chemotherapy patients were carboplatin + etoposide (n = 22) and cisplatin + etoposide (n = 10).
Concurrent treatment occurs during 1L therapy (between start and end dates).
Discontinuation before the end of the follow‐up period.
Patients could have multiple reasons for treatment discontinuation.
Physician notes were used to find out if the patient completed treatment.
Abbreviations: 1L, first‐line; CI, confidence interval; IO, immunotherapy; laMCC, locally advanced MCC; mMCC, metastatic Merkel cell carcinoma; TTD, time to discontinuation.
The median time to discontinuation of 1L treatment was 3.8 months (95% confidence interval [CI], 3.0–5.6) overall, 10.5 months (95% CI, 5.3–14.3) in the 1L avelumab cohort, 7.3 months (95% CI, 2.5–18.2) in the 1L non‐avelumab IO cohort, and 2.2 months (95% CI, 1.9–3.5) in the 1L chemotherapy cohort. Most of the overall study population (83.0%) discontinued 1L treatment during the study period. The most common reasons for discontinuation were disease progression (25.6%), toxicity (17.9%), and death (14.1%). A quarter (25.6%) completed treatment. In the 1L avelumab cohort, two‐thirds (64.3%) discontinued treatment during the study period, with the most common reasons being disease progression (33.3%), physician preference (27.8%), and toxicity (11.1%). Three (16.7%) of the 1L avelumab cohort had death recorded as the reason for treatment discontinuation. Most (80.8%) of the 1L non‐avelumab IO cohort discontinued treatment during the study period: the most common reasons were toxicity (33.3%), disease progression (28.6%), and death (19.0%). Nearly all (97.5%) of the 1L chemotherapy cohort discontinued treatment during the study period; approximately half (46.2%) had completed treatment. The most common reasons for discontinuation in the 1L chemotherapy cohort were disease progression (20.5%), toxicity (12.8%), and death (12.8%).
Clinical Outcomes
In the overall study population, 51 patients (18 1L avelumab, 16 1L non‐avelumab IO, and 17 1L chemotherapy) achieved a rwBOR of CR or R‐NOS to 1L treatment. The rwORR in the overall study population was 54.3% (n = 51/94; 95% CI, 43.7–64.6) (Table 3). The rwORR was 64.3% (n = 18/28; 95% CI, 44.1–81.4) in the 1L avelumab cohort, 61.5% (n = 16/26; 95% CI, 40.6–79.8) in the 1L non‐avelumab IO cohort, and 42.5% (n = 17/40; 95% CI, 27.0–59.1) in the 1L chemotherapy cohort. From 1L treatment initiation, the median rwDOR was 44.5 months (95% CI, 8.3–NR) in the overall study population, 15.5 months (95% CI, 7.5–NR) in the 1L avelumab cohort, NR in the 1L non‐avelumab IO cohort, and 44.5 months (95% CI, 2.5–NR) in the 1L chemotherapy cohort (Fig. 2).
Table 3.
Summary of responses to 1L treatment
| Response | Overall (n = 94) | Avelumab (n = 28) | Non‐avelumab IO (n = 26) | Chemotherapy (n = 40) |
|---|---|---|---|---|
| rwORR, % (95% CI) | 54.3 (43.7–64.6) | 64.3 (44.1–81.4) | 61.5 (40.6–79.8) | 42.5 (27.0–59.1) |
| Best overall response, n (%) | ||||
| Complete response | 14 (14.9) | 9 (32.1) | 3 (11.5) | 2 (5.0) |
| Response (NOS) | 37 (39.4) | 9 (32.1) | 13 (50.0) | 15 (37.5) |
| Stable disease | 4 (4.3) | 2 (7.1) | 1 (3.8) | 1 (2.5) |
| Mixed response | 3 (3.2) | 2 (7.1) | 0 | 1 (2.5) |
| Progressive disease | 13 (13.8) | 2 (7.1) | 5 (19.2) | 6 (15.0) |
| Not evaluated | 23 (24.5) | 4 (14.3) | 4 (15.4) | 15 (37.5) |
| TTD, median (95% CI),a months | 3.8 (3.0–5.6) | 10.5 (5.3–14.3) | 7.3 (2.5–18.2) | 2.2 (1.9–3.5) |
Unadjusted Kaplan‐Meier estimate.
Abbreviations: 1L, first‐line; CI, confidence interval; IO, immunotherapy; NOS, not otherwise specified; rwORR, real‐world overall response rate; TTD, time to discontinuation.
Figure 2.
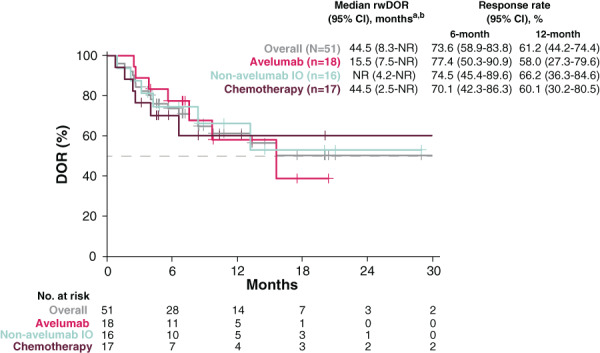
Kaplan‐Meier plot for rwDOR to 1L treatment. This figure presents the unadjusted Kaplan‐Meier curves of rwDOR from first documented response of CR or R‐NOS to the earliest date of first progression (as defined by physician progress note or imaging report), recurrent disease or death, or initiation of next treatment. aUnadjusted Kaplan‐Meier estimate. brwDOR in chemotherapy patients is influenced by two long‐term responders with 40+ months of rwDOR. Median rwDOR for chemotherapy is 6.5 months without these two patients. Abbreviations: 1L, first line; CI, confidence interval; CR, complete response; DOR, duration of response; IO, immunotherapy; NR, not reached; R‐NOS, response not otherwise specified; rwDOR, real‐world duration of response.
Median OS from the initiation of 1L treatment was 33.0 months (95% CI, 11.6–NR) in the overall study population, 20.2 months (95% CI, 11.1–NR) in the 1L avelumab cohort, NR in the 1L non‐avelumab IO cohort, and 14.7 months (95% CI, 8.8–NR) in the 1L chemotherapy cohort (Fig. 3). Median rwPFS from 1L treatment initiation was 7.5 months (95% CI, 5.3–11.4) in the overall study population, 11.4 months (95% CI, 5.3–NR) in the 1L avelumab cohort, 8.1 months (95% CI, 3.0–NR) in the 1L non‐avelumab IO cohort, and 6.1 months (95% CI, 3.6–10.6) in the 1L chemotherapy cohort.
Figure 3.
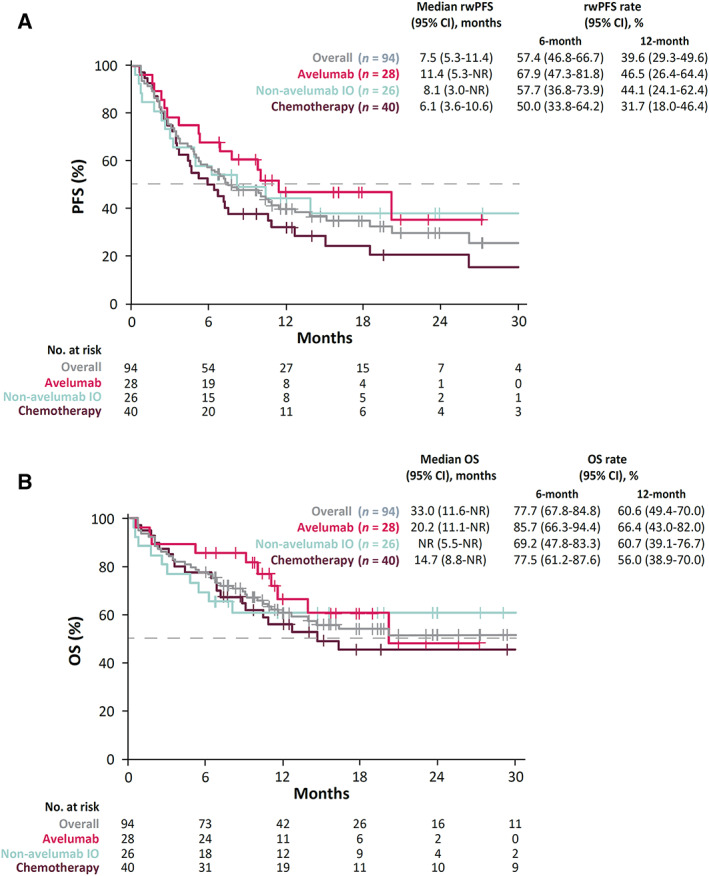
Kaplan‐Meier plots for rwPFS from 1L treatment initiation and OS from 1L treatment initiation. (A): Unadjusted Kaplan‐Meier curves of rwPFS from 1L treatment initiation to earliest date of first progression (as defined by physician progress note or imaging report), recurrent disease, or death, censoring patients who were progression‐free or alive at last contact. (B): Unadjusted Kaplan‐Meier curves of OS from 1L treatment initiation to death, censoring patients who were alive at last contact. Abbreviations: 1L, first line; CI, confidence interval; IO, immunotherapy; NR, not reached; OS, overall survival; PFS, progression‐free survival; rwPFS, real‐world progression‐free survival.
Hospitalizations and ED Visits
During 1L treatment, 24.5% of the overall study population had at least one ED visit (Table 4). In the respective 1L avelumab, 1L non‐avelumab IO, and 1L chemotherapy cohorts, the proportions of patients with at least one ED visit were 32.1%, 26.9%, and 17.5%. Approximately one‐third (37.2%) of the overall study population, 42.9% of the 1L avelumab cohort, 46.2% of the 1L non‐avelumab IO cohort, and 27.5% of the 1L chemotherapy cohort had at least one hospitalization while on treatment. In the 1L avelumab cohort, the most common reasons for ED visits were pain (14.3%), gastrointestinal (10.7%), and respiratory (10.7%), and the most common reasons for hospitalizations were infection (17.9%), gastrointestinal (10.7%), and cardiovascular (7.1%). In the 1L non‐avelumab IO cohort, the most common reasons for ED visits were gastrointestinal (7.7%), pain (7.7%), and respiratory (3.8%), and the most common reasons for hospitalizations were gastrointestinal (15.4%), cardiovascular (7.7%), pain (7.7%), and respiratory (7.7%). In the 1L chemotherapy cohort, the most common reasons for ED visits during 1L treatment were pain (10.0%), gastrointestinal (5.0%), and infection (5.0%), and the most common reasons for hospitalizations were infection (7.5%), cardiovascular (5.0%), gastrointestinal (5.0%), renal (5.0%), and pain (5.0%). Death was the reason for treatment discontinuation in 16.7% of the 1L avelumab cohort, 19.0% of the 1L non‐avelumab IO cohort, and 12.8% of the 1L chemotherapy cohort.
Table 4.
Emergency department visits and hospitalizations during 1L treatment
| Emergency department visit or hospitalization, n (%) | Overall (n = 94) | Avelumab (n = 28) | Non‐avelumab IO (n = 26) | Chemotherapy (n = 40) |
|---|---|---|---|---|
| Patients with at least one ED visit during 1L treatment and ≤30 days after continuation | 23 (24.5) | 9 (32.1) | 7 (26.9) | 7 (17.5) |
| Reasons for ED visitsa | ||||
| Cardiovascular – arrhythmia | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Cardiac – hypotension | 1 (1.1) | 0 | 0 | 0 |
| GI – diarrhea | 4 (4.3) | 2 (7.1) | 2 (7.7) | 0 |
| GI – nausea | 4 (4.3) | 1 (3.6) | 1 (3.8) | 2 (5.0) |
| GI – vomiting | 3 (3.2) | 1 (3.6) | 1 (3.8) | 1 (2.5) |
| Hematology – anemia | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Hematology – bleeding | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Infection – fever | 3 (3.2) | 2 (7.1) | 0 | 1 (2.5) |
| Infection – pneumonia | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Infection – urinary | 2 (2.1) | 1 (3.6) | 0 | 1 (2.5) |
| Neurologic | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Pain – abdominal | 6 (6.4) | 3 (10.7) | 0 | 3 (7.5) |
| Pain – back | 2 (2.1) | 0 | 1 (3.8) | 1 (2.5) |
| Pain – generalized | 2 (2.1) | 1 (3.6) | 1 (3.8) | 0 |
| Renal – acute kidney injury | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Renal – elevated serum creatinine | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Respiratory – dyspnea | 4 (4.3) | 3 (10.7) | 1 (3.8) | 0 |
| Other | 15 (16.0) | 5 (17.9) | 6 (23.1) | 4 (10.0) |
| Patients with at least one hospitalization during treatment and ≤30 days after continuation | 35 (37.2) | 12 (42.9) | 12 (46.2) | 11 (27.5) |
| Reasons for hospitalizationb | ||||
| Cardiovascular – arrhythmia | 2 (2.1) | 0 | 1 (3.8) | 1 (2.5) |
| Cardiovascular – congestive heart failure | 1 (1.1) | 0 | 1 (3.8) | 0 |
| Cardiovascular – hypertension | 0 | 2 (7.1) | 0 | 0 |
| Cardiovascular – hypotension | 3 (3.2) | 1 (3.6) | 1 (3.8) | 1 (2.5) |
| Dehydration | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Endocrinopathies – diabetes | 1 (1.1) | 1 (3.6) | 0 | 0 |
| GI – colitis | 1 (1.1) | 0 | 1 (3.8) | 0 |
| GI – diarrhea | 5 (5.3) | 1 (3.6) | 3 (11.5) | 1 (2.5) |
| GI – nausea | 4 (4.3) | 2 (7.1) | 1 (3.8) | 1 (2.5) |
| GI – vomiting | 3 (3.2) | 1 (3.6) | 2 (7.7) | 0 |
| Hematology – anemia | 2 (2.1) | 1 (3.6) | 0 | 1 (2.5) |
| Hematology – bleeding | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Infection – fever | 5 (5.3) | 3 (10.7) | 0 | 2 (5.0) |
| Infection – neutropenia | 2 (2.1) | 0 | 0 | 2 (5.0) |
| Infection – pneumonia | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Infection – urinary | 3 (3.2) | 2 (7.1) | 0 | 1 (2.5) |
| Neurologic – confusion | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Pain – abdominal | 2 (2.1) | 1 (3.6) | 0 | 1 (2.5) |
| Pain – back | 1 (1.1) | 0 | 0 | 1 (2.5) |
| Pain – generalized | 3 (3.2) | 1 (3.6) | 2 (7.7) | 0 |
| Renal – acute kidney injury | 3 (3.2) | 0 | 1 (3.8) | 2 (5.0) |
| Renal – elevated serum creatinine | 1 (1.1) | 1 (3.6) | 0 | 0 |
| Respiratory – dyspnea | 2 (2.1) | 1 (3.6) | 1 (3.8) | 0 |
| Respiratory – other | 1 (1.1) | 0 | 1 (3.8) | 0 |
| Other | 25 (26.6) | 10 (35.7) | 7 (26.9) | 8 (20.0) |
Patients could have more than one reason for an ED visit.
Patients could have more than one reason for a hospitalization.
Abbreviations: ED, emergency department; GI, gastrointestinal.
Discussion
This retrospective study presents data on treatment patterns, response and survival outcomes, and HCRU in a longitudinal cohort of real‐world patients initiating 1L treatment for laMCC or mMCC in a U.S. community oncology setting. The results of SPEAR‐Merkel indicate that patients with laMCC or mMCC treated with IO had clinical benefits compared with chemotherapy in real‐world community oncology practices.
The patient populations in JAVELIN Merkel 200 part B, KEYNOTE‐017, and the EAP and other real‐world studies had some similarities in baseline demographic characteristics to our study. Their populations tended to be older, with median ages from late 60s to late 70s, and most patients were male (64%–77%) and/or White (85%–92%) [15, 18, 19, 25].
Some clinical outcomes in SPEAR‐Merkel are consistent with the results of the pivotal clinical trials of IO in MCC [18, 25, 26] as well as real‐world trials [11, 15]. In the SPEAR‐Merkel 1L avelumab cohort versus phase II JAVELIN Merkel 200 part B, the median OS was 20.2 versus 20.3 months, and the rate of survival at 12 months was 66.4% versus 60%. In JAVELIN Merkel 200 part B, PFS was 7.5 months versus 11.4 months for the rwPFS in the SPEAR‐Merkel 1L avelumab cohort. In SPEAR‐Merkel, the rwORR was 64.3% at a median follow‐up from 1L avelumab initiation of 11.2 months, and in JAVELIN Merkel 200 part B, the objective response rate was 39.7% at a median follow‐up of 21.2 months. However, there are two caveats when comparing SPEAR‐Merkel with JAVELIN Merkel 200 part B. First, in the SPEAR‐Merkel 1L avelumab cohort, 32.1% had laMCC and the remainder had mMCC at initiation of 1L treatment, whereas the JAVELIN Merkel 200 part B study population all had mMCC. Second, rwPFS and response outcomes in SPEAR‐Merkel were not assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), unlike in JAVELIN Merkel 200.
When comparing the SPEAR‐Merkel 1L avelumab cohort with real‐world data (EAP), the rwORR in the avelumab cohort in SPEAR‐Merkel was 64.3% (n = 28 for the 1L avelumab cohort), whereas the objective response rate was 46.7% in evaluable patients receiving only 1L in the EAP (n = 15) [15]. However, any comparison of SPEAR‐Merkel with the EAP is limited. The study populations were small in both the EAP and SPEAR‐Merkel, which is an expected limitation given the rarity of MCC. Also, only three patients receiving treatment in the EAP were from the U.S., and none of them had response data; treatment patterns can vary across countries, limiting comparisons of SPEAR‐Merkel and EAP outcomes.
KEYNOTE‐017 examined patients with recurrent locoregional MCC or distant mMCC treated with 1L pembrolizumab (43 patients with stage IV disease, 7 with unresectable stage IIIB disease) [26]. In the SPEAR‐Merkel 1L non‐avelumab cohort versus the KEYNOTE‐017 study population, the median OS was NR in both, and the median rwPFS in SPEAR‐Merkel was 8.1 months versus the KEYNOTE‐17 PFS of 16.8 months. In SPEAR‐Merkel, the rate of response at 12 months was 66.2%, whereas in KEYNOTE‐017, the objective response rate was 56% at a median follow‐up of 14.9 months.
In a real‐world study of patients receiving 1L chemotherapy for distant mMCC, Iyer et al. found a response rate of 55%, median PFS of 94 days (approximately 3 months), and median OS of 9.5 months from chemotherapy initiation [11]. In SPEAR‐Merkel in the 1L chemotherapy cohort, the rwORR was 42.5%, the median rwPFS was 6.1 months, and the median OS was 14.7 months.
The SPEAR‐Merkel results for the 1L chemotherapy cohort must be interpreted with caution. rwDOR in the 1L chemotherapy cohort was longer than that for the 1L avelumab cohort (44.5 and 15.5 months, respectively), but this result was based on limited data: two patients in the 1L chemotherapy cohort had rwDOR of 40+ months. When these two outliers were removed, median rwDOR for the chemotherapy group was only 6.5 months. In addition, 17 patients in the 1L chemotherapy cohort had a response, and the small sample size limits conclusions about rwDOR.
Toxicity data from SPEAR‐Merkel also show similarities to those observed in clinical trials. In the 1L avelumab cohort in SPEAR‐Merkel, a low proportion of patients (11.1%) discontinued treatment because of toxicity. In JAVELIN Merkel 200 part B, 12.1% of patients discontinued treatment because of toxicity [18]. However, comparison with other real‐world studies of avelumab is limited. In the EAP study, AEs were reported at the discretion of the treating physician at the time of resupply of avelumab; therefore, many patients lacked safety data beyond 3 months [15].
In the SPEAR‐Merkel 1L non‐avelumab IO cohort, 33.3% discontinued because of toxicity, 26.9% had at least one ED visit during 1L treatment because of toxicity, and 46.2% had at least one hospitalization during 1L treatment because of toxicity. In KEYNOTE‐017, 77% of patients had treatment‐related AEs of any grade, and 15% had grade 3 or 4 treatment‐related AEs [26]. In the SPEAR‐Merkel 1L chemotherapy cohort, 17.5% had at least one ED visit, 27.5% had at least one hospitalization, and 12.8% discontinued because of toxicity. However, comparisons of SPEAR‐Merkel safety data with other studies must be considered cautiously, as data on AE severity were not collected.
The SPEAR‐Merkel study has several limitations that must be noted. Study cohorts had small sample sizes, which were likely due to the rarity of the disease, that limit the drawing of conclusions. As the study data were from community‐based oncology practices, they were originally collected for clinical purposes as opposed to research purposes. Certain variables of interest may not have been captured as completely across the study population as they would have been in a clinical trial (e.g., stage of disease and ECOG PS data were not documented for sizable proportions of our study cohorts, whereas these variables are captured for all patients in clinical trials). Physicians are not typically required to record treatment responses consistent with RECIST in clinical trial research; and thus, response assessments in the real‐world setting can be more subjective than assessments in a controlled clinical trial. Furthermore, treatment decisions regarding clinical response and therapy continuation may include non‐RECIST symptomatic criteria. Finally, the USON encourages the use of evidence‐based guidelines, so some USON patients may have received different treatments than patients treated at other community oncology practices or in academic centers. Therefore, the results of this study would be most generalizable to community‐based oncology practices that likewise adhere to best practice guidelines.
Despite these limitations, by sourcing data from a large network of community‐based oncology clinics, this study provides valuable insights into real‐world patient profiles, treatment patterns, and clinical outcomes in a small population of patients with laMCC or mMCC initiating treatment with avelumab. As this was a retrospective observational study, patients were not randomized to the treatment cohorts, and assessment schedules in SPEAR‐Merkel were typical of those in clinical practice. Also, patients who have generally not been enrolled in oncology clinical trials (e.g., patients with ECOG PS of 2+) were included in the analysis. Therefore, the results of SPEAR‐Merkel supplement findings from clinical trials.
Conclusion
To our knowledge, this is the first study to examine patients with laMCC or mMCC treated with avelumab or non‐avelumab IO in a real‐world setting and the first study to assess them along with those treated with chemotherapy. The study also demonstrates the continued use of 1L chemotherapy in clinical practice despite its less favorable outcomes and safety profile. Characterizing uses and clinical outcomes in patients with laMCC or mMCC in community‐based oncology practices, as opposed to the clinical trial setting, may expand knowledge about the real‐world effectiveness of innovative therapies beyond the pivotal clinical studies and provide further practical information to help clinicians who are actively treating laMCC and mMCC.
Author Contributions
Conception/design: Abhijeet Bhanegaonkar, Frank X. Liu, Marley Boyd, and C. Lance Cowey
Provision of study material or patients: Marley Boyd and Nicole Fulcher
Collection and/or assembly of data: Marley Boyd
Data analysis and interpretation: Abhijeet Bhanegaonkar, Frank X. Liu, Marley Boyd, Nicole Fulcher, Ruth Kim, Stan Krulewicz, Jodi Smith, C. Lance Cowey
Manuscript writing: Abhijeet Bhanegaonkar, Frank X. Liu, Marley Boyd, Nicole Fulcher, Ruth Kim, Stan Krulewicz, Jodi Smith, C. Lance Cowey
Final approval of manuscript: Abhijeet Bhanegaonkar, Frank X. Liu, Marley Boyd, Nicole Fulcher, Ruth Kim, Stan Krulewicz, Jodi Smith, C. Lance Cowey
Disclosures
Abhijeet Bhanegaonkar: EMD Serono, Inc; an affiliate of Merck KGaA, Darmstadt, Germany (E, OI); Frank X. Liu: EMD Serono, Inc; an affiliate of Merck KGaA, Darmstadt, Germany (E, OI); Marley Boyd: McKesson Life Sciences (E); Nicole Fulcher: McKesson Life Sciences (E); Ruth Kim: Pfizer (E), Bristol Myers Squibb, Exelixis (OI); Stan Krulewicz: Pfizer (E, OI), Abbott Laboratories, Amgen, Bristol Myers Squibb, CVS Health, GlaxoSmithKline, Gilead Sciences, and Medtronic (OI); Jodi Smith: EMD Serono, Inc; an affiliate of Merck KGaA, Darmstadt, Germany (E, OI at the time the study was conducted); C. Lance Cowey: EMD Serono, Inc. (C/A, RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1: Immunosuppressive medications within 28 days prior to the initiation of 1L treatment or during follow‐up
Acknowledgments
This study was financially supported by EMD Serono, Inc. (an affiliate of Merck KGaA, Darmstadt, Germany) as part of an alliance between Merck KGaA and Pfizer. Editorial support was provided by Lisa Kaspin‐Powell, McKesson Life Sciences, The Woodlands, TX, and funded by EMD Serono, Inc. J.S. is currently affiliated with Mirati Therapeutics, Inc., San Diego, CA.
Disclosures of potential conflicts of interest may be found at the end of this article.
[Correction added on 24 July 2021, after first online publication: The affiliation of Dr. Bhanegaonkar, Dr. Liu and Dr. Smith was modified.]
References
- 1.Toker C.Trabecular carcinoma of the skin. Arch Dermatol 1972;105:107–110. [PubMed] [Google Scholar]
- 2.Heath M, Jaimes N, Lemos B et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J Am Acad Dermatol 2008;58:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koljonen V.Merkel cell carcinoma. World J Surg Oncol 2006;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saini AT, Miles BA. Merkel cell carcinoma of the head and neck: Pathogenesis, current and emerging treatment options. Onco Targets Ther 2015;8:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen PJ, Bowne WB, Jaques DP et al. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol 2005;23:2300–2309. [DOI] [PubMed] [Google Scholar]
- 6.Santamaria‐Barria JA, Boland GM, Yeap BY et al. Merkel cell carcinoma: 30‐year experience from a single institution. Ann Surg Oncol 2013;20:1365–1373. [DOI] [PubMed] [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M. SEER Cancer Statistics Review, 1975‐2016, National Cancer Institute, Bethesda, MD, based on November 2018 SEER data submission. 2019. Available at https://www.cancer.org/cancer/merkel-cell-skin-cancer/detection-diagnosis-staging/survival-rates.html. Accessed May 10, 2020.
- 8.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 2003;49:832–841. [DOI] [PubMed] [Google Scholar]
- 9.Albores‐Saavedra J, Batich K, Chable‐Montero F et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J Cutan Pathol 2010;37:20–27. [DOI] [PubMed] [Google Scholar]
- 10.Lemos BD, Storer BE, Iyer JG et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer JG, Blom A, Doumani R et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 2016;5:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowey CL, Mahnke L, Espirito J et al. Real‐world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol 2017;13:1699–1710. [DOI] [PubMed] [Google Scholar]
- 13.Nghiem P, Kaufman HL, Bharmal M et al. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol 2017;13:1263–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JC, Lorenz E, Ugurel S et al. Evaluation of real‐world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second‐line chemotherapy in Europe. Oncotarget 2017;8:79731–79741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker JW, Lebbe C, Grignani G et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: Experience from a global expanded access program. J Immunother Cancer 2020;8:e000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman HL, Russell JS, Hamid O et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow‐up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration . Avelumab (BAVENCIO). Available at https://www.fda.gov/drugs/resources-information-approved-drugs/avelumab-bavencio. Accessed May 15, 2020.
- 18.D'Angelo SP, Lebbe C, Mortier L et al. First‐line avelumab treatment in patients with metastatic Merkel cell carcinoma: Primary analysis after ≥15 months of follow‐up from JAVELIN Merkel 200, a registrational phase 2 trial. Poster presented at: SITC 2019 Congress; November 6–10, 2019; National Harbor, MD.
- 19.Nghiem P, Bhatia S, Lipson EJ et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first‐line therapy. J Clin Oncol 2019;37:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Bhatia S, Amin A et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 trial. J Clin Oncol 2020;38:2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian S, Bhatia S, Hollebecque A et al. Non‐comparative, open label, multiple cohort, phase 1/2 study to evaluate nivolumab in patients with virus‐associated tumors (CheckMate 358): Efficacy and safety in Merkel cell carcinoma. Cancer Res 2017;77(suppl 13):CT074a. [Google Scholar]
- 22.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Merkel cell carcinoma. V1 (2020). Available at https://merkelcell.org/wp-content/uploads/2017/10/MCC_v.2.2019-2.pdf. Accessed March 20, 2020.
- 23.Bichakjian CK, Olencki T, Aasi SZ et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:742–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Oncology Network . Available at https://www.usoncology.com/our-company. Accessed July 1, 2020.
- 25.D'Angelo SP, Russell J, Lebbe C et al. Efficacy and safety of first‐line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol 2018;4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 blockade with pembrolizumab in advanced Merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1: Immunosuppressive medications within 28 days prior to the initiation of 1L treatment or during follow‐up


