Abstract
Background
With the implementation of screening programs worldwide, diagnosis of early‐stage colorectal cancer steadily increased, including T1 cancer. Current T1 cancer treatment does not differ according to anatomic location. We therefore compared the disease‐free survival of T1 cancer arising from the rectum versus the colon.
Methods
The hospital‐based study included subjects with T1 cancer at National Taiwan University Hospital from 2005 to 2014. Clinical, colonoscopy, and histopathology were reviewed for patients with a mean follow‐up time of 7.1 (0.7–12.9) years. We conducted Kaplan‐Meier analysis to compare the risk of recurrence by cancer location and Cox regression analysis to identify risk factors for T1 cancer recurrence.
Results
The final cohort included a total of 343 subjects with T1 cancer (mean age, 64.9 ± 11.7 years; 56.1% male), of whom 25 underwent endoscopic resection alone. Of the subjects who underwent surgery, 50 had lymph node metastasis and 268 did not. Kaplan‐Meier analysis showed that the risk of recurrence was higher in T1 rectal cancer than T1 colon cancer (p = .022). Rectal location and larger neoplasm size were independent risk factors for recurrence, with hazard ratios of 4.84 (95% confidence interval, 1.18–19.92), and 1.32 (95% confidence interval, 1.06–1.65), respectively. The occurrence of advanced histology did not differ between T1 rectal and colon cancers (p = .58).
Conclusion
T1 cancers arising from the rectum had less favorable recurrence outcomes than those arising from the colon. Further studies are needed to examine whether adjuvant radiotherapy or chemotherapy can reduce the risk of recurrence in T1 rectal cancer.
Implications for Practice
Current T1 colorectal cancer treatment and surveillance do not differ according to anatomic location. Clinical, colonoscopy, and histopathology were reviewed for 343 patients with T1 cancer with a mean follow‐up time of 7.1 years. Kaplan‐Meier analysis showed that the risk of recurrence was higher in T1 rectal cancer than T1 colon cancer. Moreover, the rectal location was an independent risk factor for recurrence. T1 cancers from the rectum had less favorable recurrence outcomes than those arising from the colon. It is critical to clarify whether adjuvant therapy or more close surveillance can reduce recurrence risk in T1 rectal cancer.
Keywords: T1 cancer, Colorectal cancer, Disease‐free survival, Anatomic location
Short abstract
With the broad implementation of colorectal cancer screening programs worldwide, early stage cancers are increasingly diagnosed. This article compares the disease‐free survival of stage T1 cancer in rectum versus colon cancer.
Introduction
With the broad implementation of colorectal cancer (CRC) screening programs worldwide, early‐stage cancers are increasingly diagnosed [1]. In Taiwan, a nationwide, population‐based screening program has been rolled out since 2004, with up to 50% of all diagnosed cancers being stage 0 or I cancers, including T1 cancers, and an increasing number of patients with CRC at such stages received local therapy [2]. In view of this substantial increase in T1 cancer diagnosis, determining the best therapeutic strategy for such cancers is critical. Because T1 cancers are invasive and growing within the submucosa, the majority of them can be treated by endoscopic resection alone. Lymph node metastasis (LNM) is present in 8%–13% of T1 cancers, which requires additional surgery for tumor eradication [3, 4, 5, 6, 7].
T1 cancer with risk of LNM, also known as “high‐risk T1 cancer,” typically has one or more of the following histologic signs: depth of submucosal invasion >1 mm, poor differentiation, lympho‐vascular invasion, perineural invasion, and high‐grade tumor budding. Growing evidence suggests that the high‐risk T1 cancer has a worse disease‐free survival than low‐risk T1 cancer if treated with endoscopic resection alone [8, 9, 10]. Thus, current guidelines recommend additional surgery with lymph node resection for treating high‐risk T1 cancer [11, 12].
At present, T1 cancer treatment does not differ by anatomic location. However, T1 cancer arising from the rectum is proposed to carry a higher risk of recurrence than that arising from the colon [9, 13, 14]. If this assumption is correct, the criteria for recommending additional surgery should differ between T1 rectal and T1 colon cancers. Moreover, the criteria for adjuvant therapy after surgery also should be tailored according to the anatomic location of T1 cancer. In a multicenter trial of 798 subjects with T1 cancer, Kobayashi et al. observed that the recurrence rate was 25.0% in T1 rectal cancer and 1.1% in T1 colon cancer. Moreover, the rectal location (p = .025), histological grade (p < .0001), LNM (p < .001), and venous invasion status (p = .0013) were significantly associated with recurrence [14]. However, Yoshii et al. found no difference in recurrence rates between T1 rectal and colon cancer [10]. Thus, whether the risk of recurrence differs between these T1 cancers remains uncertain.
The present 10‐year longitudinal study conducted at our institution compares the risk of recurrence between T1 cancers arising from the rectum and colon. We hypothesized that the rate of recurrence is higher in T1 rectal cancer than in T1 colon cancer.
Materials and Methods
Study Participants and Ethical Considerations
We reviewed patient data prospectively collected at National Taiwan University Hospital, between January 2005 and September 2014. Subjects were identified via the cancer registry system of the hospital. The clinical records of all patients with histologically confirmed T1 cancer were reviewed. T1 cancer was defined as invasive cancer with submucosal invasion, in accordance with the American Joint Committee on Cancer, 8th edition [15]. The inclusion criteria included complete treatment for T1 cancer, with endoscopic resection or surgical resection. Patients with hereditary CRC, inflammatory bowel disease, active malignancy in any other organ, synchronous or metachronous advanced CRC, or loss of follow‐up after treatment were excluded. The study received approval (No. 201602062RIND) from the institutional review board and the ethics committee of our institution.
Endoscopic Resection
Details of bowel preparation and the colonoscopy procedure are described elsewhere [16, 17]. We evaluated all colorectal neoplasms under either magnifying chromoendoscopy using Kudo's pit‐pattern classification or narrow band imaging diagnosis [18, 19]. If the colorectal neoplasm was diagnosed as a noninvasive pit‐pattern, endoscopic resection was performed. Endoscopic resection was performed via polypectomy, endoscopic mucosal resection, or endoscopic submucosal dissection.
Surgical Treatment
Patients diagnosed with T1 cancer with an invasive pattern were referred for laparoscopic or open surgery. For patients with T1 cancer presenting with submucosal invasion of more than 1 mm, poorly differentiated adenocarcinoma, evidence of vascular or lymphatic invasion, or margins involved by cancer on histologic diagnosis after endoscopic resection, an additional surgical resection with lymph node dissection was recommended.
Histological Diagnosis
The histological diagnosis of T1 cancer was confirmed by a dedicated gastrointestinal pathologist for all enrolled subjects. The advanced pathologic characteristics indicating high‐risk T1 cancer were reviewed for each case. The advanced pathologic characteristics included grade of differentiation, invasion depth, lymphatic invasion, venous invasion, budding grade, and poor differentiation at the invasive front [5, 20, 21, 22]. The pathologist reviewed the histology blindly to the clinical information, including survival outcome and LNM status.
Posttreatment Surveillance
Subjects with T1 cancer with LNM were followed up as stage III according to the recommendations of the American Society of Clinical Oncology and American Cancer Society [23, 24]. Computed tomography [CT] scan was conducted every 6 to 12 months for 5 years. Colonoscopy was performed at 1 year, and subsequent studies were conducted as dictated by prior findings. No guideline is available to recommend posttreatment surveillance in subjects with stage I CRC. In general, annual CT scan is provided for 5 years for the T1 cancer without LNM. Colonoscopy is performed at 1 year, and subsequent studies are conducted as dictated by prior findings. For those who received endoscopic resection alone, colonoscopy is performed at 6 months, annual CT scans are conducted, and subsequent studies are conducted as dictated by other findings. Recurrence is defined as the cancer recurs at the original resection site, regional lymph node, or distal metastasis.
Statistical Analysis
Demographic data were compared using Student's t test for continuous variables and chi‐square test for categorical variables. The Kaplan‐Meier method was used for constructing cumulative risk curves for cancer recurrence according to LNM status, cancer location, and treatment strategies, and Cox regression analysis was used for multivariate analysis and to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the association between various risk factors and cancer recurrence. Age, gender, LNM status, anatomical site (rectal vs. colon), lesion size, and the number of resected lymph nodes were included in the multivariate analysis for identifying risk factors for recurrence in T1 cancer. The number of resected lymph nodes was analyzed as a continuous variable. p values of <.05 was considered as statistically significant. Statistical analysis was conducted using the SPSS statistical package, version 17.0 (IBM Corp, Armonk, NY).
Results
Patient Demographic and Clinical Information
From January 2005 to September 2014, a total of 6,907 patients at National Taiwan University Hospital were diagnosed with CRC of all stages, including 425 patients with T1 cancer. The final cohort included 343 patients with T1 cancers after exclusion of cases inconsistent with the criteria shown in the study flowchart (Fig. 1).
Figure 1.
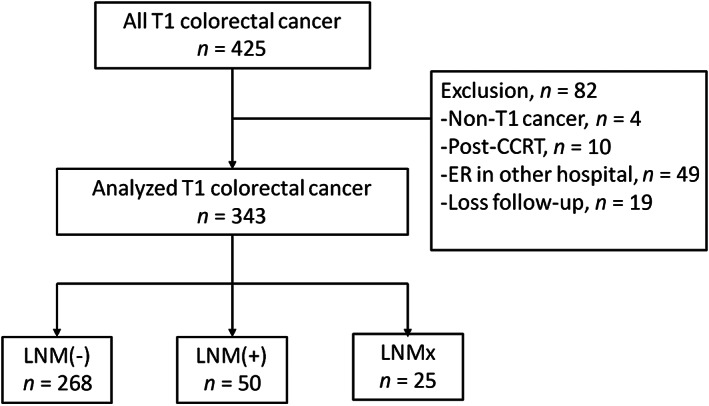
Flow diagram of enrollment.Abbreviations: CCRT, concurrent chemoradial therapy; ER, endoscopic resection; LNM(−), without lymph node metastasis; LNM(+), with lymph node metastasis; LNM(X), undetermined lymph node metastasis.
Of the 318 patients who received surgery, 50 and 268 were with and without LNM, respectively. Thus, the rate of LNM in our T1 cancer cohort was 15.7% (50/318). Twenty‐five patients received endoscopic resection alone. One of the T1 cancers is demonstrated in supplemental online Figure 1, which was treated by endoscopic submucosal dissection followed by subsequent additional surgery because presence of advanced histology.
Patient demographic and clinical characteristics are shown in Table 1. The recurrence rate in subjects with and without LNM was 8.0% (4/50) and 2.6% (7/268), respectively. One recurrence developed in the subject who received endoscopic resection alone, because he refused the recommended additional surgery even with unfavorable histological findings. Unsurprisingly, disease‐free survival was worse in subjects with than without LNM. The subjects who received endoscopic resection alone had a lower recurrence rate than those who received either primary or additional surgery.
Table 1.
Demographic and clinical information
| Demographic information | LNM(−) n = 268 | LNM(+) n = 50 | LNM(x) n = 25 | Overall n = 343 | p value |
|---|---|---|---|---|---|
| Age ± SD, yr | 64.6 ± 11.7 | 65.3 ± 12.2 | 66.9 ± 11.9 | 64.9 ± 11.7 | .63 |
| Male, n (%) | 148 (55.2) | 27 (52.9) | 18 (72.0) | 193 (56.1) | .25 |
| Lymph node resected, n ± SD | 16.9 ± 10.2 | 18.2 ± 9.8 | 15.9 ± 10.7a | <.0001 | |
| Rectal location, n (%) | 91 (34.0) | 18 (36.0) | 10 (40.0) | 119 (34.7) | .81 |
| Disease‐free survival ± SD, yr | 7.00 ± 2.59 | 5.76 ± 2.79 | 9.08 ± 2.02 | 6.97 ± 2.68 | <.0001 |
The denominator excludes the subjects who underwent endoscopic resection alone.
Abbreviations: LNM(−), without lymph node metastasis, LNM(+), with lymph node metastasis; LNM(x), lymph node metastasis undetermined.
Comparison of Rectal and Colon T1 Cancers
The clinical and histologic findings of T1 rectal and colon cancers are summarized in Table 2. Fewer lymph nodes were resected at surgery in patients with T1 rectal than T1 colon cancer (14.3 ± 9.9 vs. 16.8 ± 11.0; p = .03). More patients with T1 rectal cancer received endoscopic resection alone than those with T1 colon cancer (9.9% vs. 6.3%). Presence of unfavorable histology did not differ between T1 rectal and T1 colon cancers (p = .58). T1 rectal cancer had more high‐grade budding than T1 colon cancers, but the difference was not significant (p = .09). Kaplan‐Meier analysis showed that disease‐free survival was worse in T1 rectal cancer than T1 colon cancer (p = .022). Moreover, T1 cancers with LNM had a worse disease‐free survival than those without (p = .011), but the survival was not different between primary and additional surgery (p = .61; Fig. 2).
Table 2.
Comparison of clinical and histologic information between patients with colon and rectal T1 cancers
| Clinical information | Colonn = 224 | Rectumn = 119 | p value |
|---|---|---|---|
| Age ± SD, yr | 65.0 ± 11.8 | 64.7 ± 11.7 | .83 |
| Male, n (%) | 126 (56.3) | 67 (56.3) | .99 |
| Lymph node status | .81 | ||
| LNM(−) | 177 (79.0) | 91 (76.5) | |
| LNM(+) | 32 (14.3) | 18 (15.1) | |
| LNM(x) | 15 (6.7) | 10 (8.4) | |
| Neoplasm size ± SD, cm | 2.2 ± 1.5 | 2.2 ± 1.4 | .94 |
| Lymph node resected, n ± SD | 16.8 ± 11.0 | 14.3 ± 9.9 | .03 |
| Treatment, n (%) | .075 | ||
| Primary surgery | 138 (61.9) | 85 (70.3) | |
| Additional surgery | 71 (31.8) | 24 (19.8) | |
| Endoscopic resection | 15 (6.3) | 10 (9.9) | |
| Morphology, n (%) | .33 | ||
| Polypoid | 87 (38.8) | 52 (43.7) | |
| Nonpolypoid | 136 (60.7) | 65 (54.6) | |
| Undetermined | 1 (0.00) | 2 (0.02) | |
| Advanced histology, n (%) | .58 | ||
| With | 175 (78.1) | 96 (80.7) | |
| Without | 49 (21.9) | 23 (19.3) | |
| Histologic differentiation, n (%) | .42 | ||
| High‐grade | 12 (5.4) | 9 (7.6) | |
| Low‐grade | 212 (94.6) | 110 (92.4) | |
| Invasion depth ± SD, mm | 1.6 ± 1.0 | 1.6 ± 0.9 | |
| Lymphatic invasion, n (%) | 42 (18.8) | 23 (19.3) | .51 |
| Venous invasion, n (%) | 37 (16.5) | 21 (17.6) | .51 |
| High‐grade budding, n (%) | 85 (37.9) | 59 (49.6) | .09 |
| MM pattern, n (%) | .26 | ||
| 1 | 58 (25.9) | 22 (18.5) | |
| 2 | 115 (51.3) | 64 (53.8) | |
| 3 | 51 (22.8) | 33 (27.7) | |
| Poor differentiation at invasive front, n (%) | 55 (24.6) | 36 (30.3) | .29 |
Abbreviations: LNM(−), without lymph node metastasis; LNM(+), with lymph node metastasis; LNM(x), lymph node metastasis undetermined; MM: muscularis mucosae.
Figure 2.
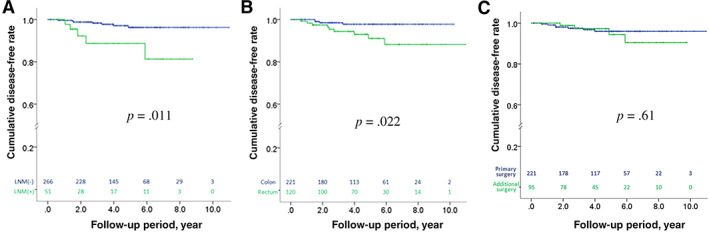
Disease‐free survival in T1 colorectal cancers. Expectedly, the Kaplan‐Meier analysis disclosed that T1 cancers with LNM (green color) had an associated unfavorable disease‐free survival than those without (blue color; p = .011) (A). With regard to the anatomic location of T1 cancers, the T1 cancers arising from the rectum (green color) had an associated unfavorable disease‐free survival in comparison with those arising from the colon (blue color; p = .022) (B). The disease‐free survival was not different between primary surgery (blue color) and additional surgery (green color), and the p value was .61 (C).Abbreviations: LNM(−), without lymph node metastasis; LNM(+), with lymph node metastasis.
Risk Factors Associated with Recurrence in T1 Cancer
The results of Cox regression analysis of risk factors for recurrence in T1 cancer are summarized in Table 3. Rectal location and larger neoplasm size were independent risk factors for recurrence, with aHRs of 4.84 (95% CI, 1.18–19.92), and 1.32 (95% CI, 1.06–1.65), respectively. Subanalysis of T1 rectal cancers showed that the presence of LNM was an independent risk factor for recurrence (aHR, 6.67; 95% CI, 1.41–31.59), whereas the number of resected lymph nodes, neoplasm size, neoplasm morphology, presence of advanced histology, primary or additional surgery, and laparoscopic or open surgery were not.
Table 3.
Risk factors associated with local recurrence of T1 cancer
| Risk factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p value | aHR (95%CI) | p value | |
| Age | 0.97 (0.91–1.02) | .22 | 0.99 (0.94–1.05) | .74 |
| Male gender | 1.03 (0.31–3.36) | .97 | 1.05 (0.30–3.66) | .95 |
| Lymph node metastasis | 4.86 (1.37–17.28) | .015 | 3.92 (0.95–16.18) | .058 |
| Rectal location | 3.32 (0.97–11.3) | .056 | 4.84 (1.18–19.92) | .029 |
| Less lymph node resected | 1.06 (1.01–1.11) | .021 | 1.06 (1.00–1.12) | .061 |
| Neoplasm size | 1.30 (1.06–1.59) | .012 | 1.32 (1.06–1.65) | .013 |
| Additional versus primary surgery | 1.29 (0.38–4.42) | .68 | ||
| Laparoscopic versus open surgery | 0.78 (0.23–2.66) | .69 | ||
| Nonpolypoid morphology | 6.47 (0.82–51.3) | .076 | ||
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio.
Time Course of Prevalence and Recurrence of T1 CRC
The number of cases of overall CRC, T1 CRC, and T1 CRC with recurrence is shown in Figure 3A. We observed an increase in the prevalence of T1 cancer with time, as expected following the implementation of nationwide screening. The initial prevalence was 1.4% in 2005 but has remained above 5% since 2009. Only 11 recurrence events were observed during the study period. The mean time to recurrence was 2.8 (0.7–6.0) years, and 63.6% (7/11) recurrence events developed within 3 years (Fig. 3B).
Figure 3.
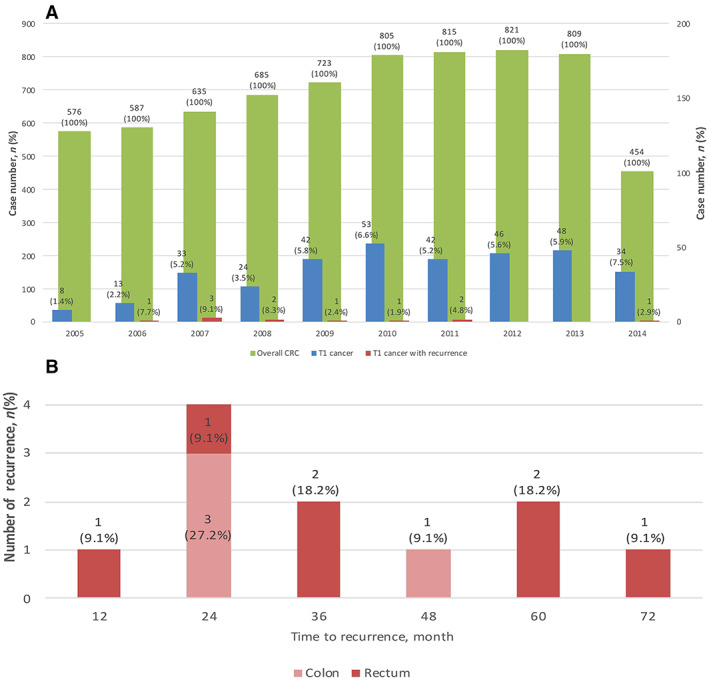
Time trend of prevalence and recurrence of T1 CRC. With the implementation of nationwide screening program since 2004, the diagnosis of T1 CRC increased progressively with time. Eight (1.4%) T1 CRCs were diagnosed in 2005, and the case number increased to 34 (7.5%) cases in 2014 (by September). Moreover, the recurrence of T1 CRC was prone to decrease with the advance of either endoscopic or surgical treatment for T1 cancer (A). Time to recurrence may occur up to 6 years after surgery and more than 60% of them had recurrence within 3 years (B). (T1 cancer: case number of T1 CRC / case number of overall CRC; T1 cancer with recurrence: case number of recurrent T1 CRC / case number of overall T1 CRC).Abbreviation: CRC, colorectal cancer.
Discussion
The present study compared clinical outcomes between patients with T1 rectal and T1 colon cancer. In this hospital‐based cohort with 7.1 years of follow‐up, we found that patients with T1 rectal cancer had a higher risk of recurrence than did those with T1 colon cancer. Our findings together with another study [14] indicate a need for greater individualization of referral criteria for additional surgery and postresection surveillance that take into account the anatomic location of T1 cancer.
The current therapeutic algorithm for treating T1 cancer starts with careful inspection of its surface pattern using a high‐definition magnifying observation. T1 cancer with an invasive pattern on surface should be surgically removed along with lymph node resection [25]. Lesions with a noninvasive pattern could be managed endoscopically first, followed by comprehensive histologic examination. In cases with unfavorable histological findings, additional surgery should be performed. The same therapeutic algorithm is used for all T1 cancers, regardless of anatomic location. A number of studies have shown that the clinical outcomes differ between T1 rectal and T1 colon cancer. Ikematsu et al. reported that high‐risk T1 rectal cancer had a worse 5‐year disease‐free survival than T1 colon cancer if treated with endoscopic resection alone (77.7% vs. 96.5%; p < .01) [26]. Kobayashi et al. reported recurrence rates for T1 rectal and T1 colon cancer of 4.2% and 1.5%, respectively, with a higher risk of recurrence for T1 rectal cancer (p = .025). In our hospital‐based study, statistical analysis showed that rectal location is an independent risk factor for recurrence even after adjusting for the presence of LNM.
Multiple factors contribute to the higher risk of recurrence of T1 rectal cancer. First, the venous and lymphatic return of the rectum differ from those in the colon. Whereas blood return of the rectum is through both the portal and venous systems, that of the colon is primarily through the portal system. The complexity of venous return may increase the likelihood of LNM and subsequent recurrence in T1 rectal cancer. Belderbos et al. found that the risk of LNM is 2.4‐fold higher in T1 rectal than in T1 colon cancer [9]. We observed here that the mean number of resected lymph nodes at surgery was lower in T1 rectal cancer than in T1 colon cancer (14.3 ± 9.9 vs. 16.8 ± 11.0; p = .03). The number of lymph nodes evaluated after surgery is positively associated with survival [27]. It is generally agreed that more lymph nodes are removed during surgery for cancer in the proximal colon than the rectum [28]. The smaller number of resected lymph nodes may contribute to the higher recurrence rate of T1 rectal cancer. Future studies are warranted to ascertain the reason for worse disease‐free survival in T1 rectal cancer.
Most T1 cancers can be treated by endoscopic resection alone without surgery, a substantial benefit in terms of quality of life and lower health care costs. Whether endoscopic resection followed by additional surgery increases the risk of recurrence over that of primary surgery is another question that needs to be addressed. In line with previous findings [9, 13, 29, 30], our current study shows no difference in the risk of recurrence between additional surgery versus primary surgery. In view of these data, endoscopic treatment should be the first step in treating T1 cancer unless the tumor harbors a clearly invasive pattern. Following this protocol, T1 cancer can be effectively treated while avoiding unnecessary surgery. Moreover, growing evidence has demonstrated that adjuvant chemoradiotherapy is an effective alternative treatment instead of additional surgery after endoscopic resection in high‐risk T1 rectal cancers [31, 32, 33]. This is clinically relevant and pertaining to the willingness of the patient to undergo surgery, especially when the lesion is located at distal rectum, and surgical resection may lead to colostomy.
Our study has several strengths. We included a large and well‐characterized cohort with a mean longitudinal observation of 7.1 years. Therefore, the outcomes can be extrapolated to clinical practice. The risk of recurrence in T1 rectal cancer was addressed in previous cross‐sectional studies [14]. Our finding is supported by more robust methodology, including Cox regression analysis. All factors that may contribute to recurrence in T1 cancer (demographic factors, tumor size, macroscopic shape, anatomic location, treatment strategy, and histology) were accommodated in our model. The histology had been reviewed by a single expert pathologist (C.T.S.) who was blinded to the clinical information including recurrence outcome and LNM status.
This study has several limitations. Many factors contribute to the decision whether to treat T1 cancer, either by endoscopic resection or primary surgery. The experience of endoscopists and surgeons is among these factors and could be a possible confounder for recurrence. However, all T1 cancers were treated using the same protocol at our center, thus mitigating such bias [34]. In view of the retrospective design of this study, measured factors such as tumor size, anatomic location, and macroscopic shape may not have been standardized. The further classification of rectal T1 cancer into upper and lower groups to clarify the risk of LNM between them is not feasible. Another limitation is the variation in the number of lymph nodes being resected during surgery in our cohort. Current guidelines recommend the resection of more than 12 lymph nodes during surgery [28, 35]. In the present study, this recommendation was met in only 70% of patients. Thus, the occurrence of LNM may have been underestimated, likely influencing the observed recurrence rate. Moreover, the number of resected lymph nodes was lower in T1 rectal cancer than in T1 colon cancer, which may lead to bias. Last, we did not subdivide patients according to endoscopic resection techniques (endoscopic submucosal dissection, endoscopic mucosal resection, or polypectomy). Therefore, differences in the recurrence outcome between techniques could not be observed in this study.
Conclusion
T1 cancers arising from the rectum carry unfavorable recurrence outcomes compared with those arising from the colon. This finding indicates the need to individualize criteria for determining the need for additional surgery and to personalize the surveillance strategies for T1 cancer according to anatomic location. Further validation of the finding in an independent cohort would be warranted in the future.
Author Contributions
Conception/design: Han‐Mo Chiu, Ming‐Shiang Wu, Chia‐Tung Shun
Provision of study material or patients: Li‐Chun Chang, Han‐Mo Chiu, Been‐Ren Lin, Weng‐Feng Hsu
Collection and/or assembly of data: Li‐Chun Chang, Han‐Mo Chiu, Been‐Ren Lin, Weng‐Feng Hsu
Data analysis and interpretation: Li‐Chun Chang, Han‐Mo Chiu
Manuscript writing: Han‐Mo Chiu, Silvia Sandueanu, Li‐Chun Chang
Final approval of manuscript: Li‐Chun Chang, Chia‐Tung Shun, Been‐Ren Lin, Silvia Sanduleanu, Weng‐Feng Hsu, Ming‐Shiang Wu, Han‐Mo Chiu
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
This study was funded by National Taiwan University Hospital, Taiwan (NTUH‐107‐M003990).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.Rabeneck L, Chiu HM, Senore C. International perspective on the burden of colorectal cancer and public health effects. Gastroenterology 2020;158:447–452. [DOI] [PubMed] [Google Scholar]
- 2.Chiu HM, Chen SL, Yen AM et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the one million Taiwanese screening program. Cancer 2015;121:3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch SL, Teerenstra S, de Wilt JH et al. Predicting lymph node metastasis in pT1 colorectal cancer: A systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013;45:827–834. [DOI] [PubMed] [Google Scholar]
- 4.Bamba Y, Itabashi M, Hirosawa T et al. Follow‐up and recurrence of T1 colorectal cancer. Int Surg 2006;91:12–16. [PubMed] [Google Scholar]
- 5.Okabe S, Shia J, Nash G et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg 2004;8:1032–1039; discussion 1039–1040. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardi R, Madoff RD, Rothenberger DA et al. Population‐based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol 2006;4:1522–1527. [DOI] [PubMed] [Google Scholar]
- 7.Wang HS, Liang WY, Lin TC et al. Curative resection of T1 colorectal carcinoma: Risk of lymph node metastasis and long‐term prognosis. Dis Colon Rectum 2005;48:1182–1192. [DOI] [PubMed] [Google Scholar]
- 8.Yoda Y, Ikematsu H, Matsuda T et al. A large‐scale multicenter study of long‐term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013;45:718–724. [DOI] [PubMed] [Google Scholar]
- 9.Belderbos TD, van Erning FN, de Hingh IH et al. Long‐term recurrence‐free survival after standard endoscopic resection versus surgical resection of submucosal invasive colorectal cancer: A population‐based study. Clin Gastroenterol Hepatol 2017;15:403–411. [DOI] [PubMed] [Google Scholar]
- 10.Yoshii S, Nojima M, Nosho K et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol 2014;12:292–302. [DOI] [PubMed] [Google Scholar]
- 11.Benson AB 3rd, Venook AP, Bekaii‐Saab T et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw 2014;12:1028–1059. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T.Current therapeutic strategies for rectal cancer. Int J Clin Oncol 2015;20:631–632. [DOI] [PubMed] [Google Scholar]
- 13.Backes Y, de Vos Tot Nederveen Cappel WH, van Bergeijk J et al. Risk for incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer: A multicenter cohort study. Am J Gastroenterol 2017;112:785–796. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H, Mochizuki H, Morita T et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol 2011;46:203–211. [DOI] [PubMed] [Google Scholar]
- 15.Weiser MR. AJCC 8th edition: Colorectal cancer. Ann Surg Oncol 2018;25:1454–1455. [DOI] [PubMed] [Google Scholar]
- 16.Chang LC, Shun CT, Hsu WF et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol 2017;15:872–879.e1. [DOI] [PubMed] [Google Scholar]
- 17.Chang LC, Wu MS, Tu CH et al. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc 2014;79:961–969. [DOI] [PubMed] [Google Scholar]
- 18.Kudo S, Rubio CA, Teixeira CR et al. Pit pattern in colorectal neoplasia: Endoscopic magnifying view. Endoscopy 2001;33:367–373. [DOI] [PubMed] [Google Scholar]
- 19.Sano Y, Tanaka S, Kudo SE et al. Narrow‐band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI expert team. Dig Endosc 2016;28:526–533. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima K, Fujimori T, Fujii S et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: A Japanese collaborative study. J Gastroenterol 2004;39:534–543. [DOI] [PubMed] [Google Scholar]
- 21.Nakadoi K, Oka S, Tanaka S et al. Condition of muscularis mucosae is a risk factor for lymph node metastasis in T1 colorectal carcinoma. Surg Endosc 2014;28:1269–1276. [DOI] [PubMed] [Google Scholar]
- 22.Mou S, Soetikno R, Shimoda T et al. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: A systematic review and meta‐analysis. Surg Endosc 2013;27:2692–2703. [DOI] [PubMed] [Google Scholar]
- 23.Meyerhardt JA, Mangu PB, Flynn PJ et al. Follow‐up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013;31:4465–4470. [DOI] [PubMed] [Google Scholar]
- 24.El‐Shami K, Oeffinger KC, Erb NL et al. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin 2015;65:428–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda T, Fujii T, Saito Y et al. Efficacy of the invasive/non‐invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008;103:2700–2706. [DOI] [PubMed] [Google Scholar]
- 26.Ikematsu H, Yoda Y, Matsuda T et al. Long‐term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551–559; quiz e514. [DOI] [PubMed] [Google Scholar]
- 27.Nelson H, Petrelli N, Carlin A et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583–596. [DOI] [PubMed] [Google Scholar]
- 28.Chang GJ, Rodriguez‐Bigas MA, Skibber JM et al. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J Natl Cancer Inst 2007;99:433–441. [DOI] [PubMed] [Google Scholar]
- 29.Overwater A, Kessels K, Elias SG et al. Endoscopic resection of high‐risk T1 colorectal carcinoma prior to surgical resection has no adverse effect on long‐term outcomes. Gut 2018;67:284–290. [DOI] [PubMed] [Google Scholar]
- 30.Rickert A, Aliyev R, Belle S et al. Oncologic colorectal resection after endoscopic treatment of malignant polyps: Does endoscopy have an adverse effect on oncologic and surgical outcomes? Gastrointest Endosc 2014;79:951–960. [DOI] [PubMed] [Google Scholar]
- 31.Jeong JU, Nam TK, Kim HR et al. Adjuvant chemoradiotherapy instead of revision radical resection after local excision for high‐risk early rectal cancer. Radiat Oncol 2016;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki T, Ito Y, Ohue M et al. Postoperative chemoradiotherapy after local resection for high‐risk T1 to T2 low rectal cancer: Results of a single‐arm, multi‐institutional, phase II clinical trial. Dis Colon Rectum 2017;60:914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki T, Sadahiro S, Tanaka A et al. Outcomes of local excision plus chemoradiotherapy in patients with T1 rectal cancer. Oncology 2018;95:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang LC, Chiu HM, Ho BC et al. Copy number alterations of depressed colorectal neoplasm predict the survival and response to oxaliplatin in proximal colon cancer. Cancers (Basel) 2020;12:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compton CC, Fielding LP, Burgart LJ et al. Prognostic factors in colorectal cancer. College of American Pathologists consensus statement 1999. Arch Pathol Lab Med 2000;124:979–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


