Abstract
Background
Circulating tumor cells (CTCs) correlate with adverse prognosis in patients with breast, colorectal, lung, and prostate cancer. Little data are available for renal cell carcinoma (RCC).
Materials and Methods
We designed a multicenter prospective observational study to assess the correlation between CTC counts and progression‐free survival (PFS) in patients with metastatic RCC treated with an antiangiogenic tyrosine kinase inhibitor as a first‐line regimen; overall survival (OS) and response were secondary objectives. CTC counts were enumerated by the CellSearch system at four time points: day 0 of treatment, day 28, day 56 and then at progression, or at 12 months in the absence of progression.
Results
One hundred ninety‐five eligible patients with a median age of 69 years were treated with sunitinib (77.5%) or pazopanib (21%). At baseline, 46.7% of patients had one or more CTCs per milliliter (range, 1 to 263). Thirty patients had at least three CTCs, with a median PFS of 5.8 versus 15 months in the remaining patients (p = .002; hazard ratio [HR], 1.99), independently of the International Metastatic RCC Database Consortium score at multivariate analysis (HR, 1.91; 95% confidence interval [CI], 1.16–3.14). Patients with at least three CTCs had a shorter estimated OS of 13.8 months versus 52.8 months in those with fewer than three CTCs (p = .003; HR, 1.99; multivariate analysis HR, 1.67; 95% CI, 0.95–2.93). Baseline CTC counts did not correlate with response; neither did having CTC sequencing counts greater than or equal to one, two, three, four, or five.
Conclusion
We provide prospective evidence that the presence of three or more CTCs at baseline is associated with a significantly shorter PFS and OS in patients with metastatic RCC.
Implications for Practice
This prospective study evaluated whether the presence of circulating tumor cells (CTCs) in the peripheral blood correlates with activity of first‐line tyrosine kinase inhibitors in metastatic renal cell carcinoma (RCC). This study demonstrated that almost half of patients with metastatic RCC have at least one CTC in their blood and that those patients with at least three CTCs are at increased risk of early progressive disease and early death due to RCC. Studies incorporating CTC counts in the prognostic algorithms of metastatic RCC are warranted.
Keywords: Circulating tumor cells, Renal cell carcinoma, Metastases, Sunitinib, Pazopanib, Biomarker
Short abstract
This article evaluates the distribution and prognostic/predictive role of circulating tumor cells in a wide but homogeneous cohort of patients with metastatic renal cell carcinoma who received tyrosine kinase inhibitor therapy as first‐line treatment for metastatic disease.
Introduction
The treatment of metastatic renal cell carcinoma (mRCC) was revolutionized in the early 2000s by the advent of oral tyrosine kinase inhibitors (TKIs) targeting the vascular endothelial growth factor receptor and platelet‐derived growth factor receptor, with a significant improvement in patients’ progression‐free survival (PFS) and overall survival (OS) [1]. The activity of TKIs can today be significantly increased by the adjunct of anti–programmed cell death‐1 (PD‐1) monoclonal antibodies [2].
However, patients with mRCC are extremely heterogeneous in terms of response to treatment. Prognostic stratification according to 6 independent clinical parameters was validated using the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) in the era of targeted therapies [3], but the IMDC score does not predict radiological response to TKIs. Moreover, the evaluation of radiological response to antiangiogenic agents may be troublesome if we resort to the classic RECIST criteria [4, 5].
Several studies have investigated a wide range of circulating biomarkers in patients with mRCC [6], but so far none of them has been approved for use in clinical practice.
According to the Foundation for the National Institutes of Health Biomarkers Consortium, it is currently accepted that circulating tumor cells (CTCs) may be identified by the expression of markers of epithelial origin (cytokeratins and epithelial cell adhesion molecule [EpCAM]) and the absence of the hematopoietic marker cluster differentiation 45 (CD45) antigen [7], as applied in the CellSearch semiautomated enrichment system. Yet, many alternative tools may be used, such as the more recent biophysical label‐free enrichment strategies [8, 9, 10].
A pivotal study in breast cancer clearly demonstrated that CTC counts above five cells per 7.5 mL predict a dismal prognosis in patients with metastatic disease [11]. Likewise, the prognostic role of CTCs was also confirmed in metastatic colon cancer [12], prostate cancer [13, 14], lung [15] and other solid tumors [16]. Conversely, very little data are available concerning the role of CTCs in mRCC. Using the CellSearch system, 25% of specimens from 11 patients with mRCC showed at least two CTCs in a pilot study [17]; subsequently, 16% of 25 patients with mRCC reported the presence of at least one CTC in another small study [18].
TKIs were developed as antiangiogenic agents, but they also have intrinsic antitumoral and immunomodulatory activity [19, 20]. It may thus be speculated that these agents might modify the release and spread of CTCs in the blood and interfere with their metastatic capability [20, 21], although no clinical evidence is available so far.
We first designed a pilot study to determine the feasibility of CellSearch assay in mRCC [22] and found that CTCs were present in 58.5% of 53 treatment‐naïve patients. Once we were able to identify apoptotic circulating tumor cells with the expression of the M30 neoepitope [23, 24], we found that a higher balance of apoptotic to live CTCs correlated with improved outcome in terms of disease control [22].
We therefore planned a prospective noninterventional multicenter trial designed to evaluate the distribution and prognostic/predictive role of CTCs in a wider, but homogeneous, cohort of patients with mRCC receiving a TKI as their first‐line regimen for metastatic disease.
Materials and Methods
Patients
Eligibility criteria were as follows: histological diagnosis of renal cell carcinoma (RCC; clear cell or non‐clear cell subtype) performed on either a resected kidney tumor or on a biopsy of a metastatic site; any age and Fuhrman grade; the presence of metastatic disease candidate to receive a TKI as a first‐line regimen.
As previously reported, the first 53 patients were enrolled at the Istituto Oncologico Veneto (IOV) and three Italian centers from June 2008 to September 2010 [22]. The remaining patients were enrolled in 15 Italian centers from May 2013 to December 2015 as part of a large multicenter study approved first by the IOV Ethics Committee on April 15, 2013, and then by the Ethics Committee of participating Centers. All patients gave their written consent for the collection of serial blood samples and their clinical data for this observational study. The choice of TKI and dose reductions were left to the treating physician's discretion.
As an observational trial, the study did not involve a predefined schedule of assessments, since each Investigator followed international guidelines for baseline staging and for the monitoring of systemic therapies in mRCC, mainly with contrast‐enhanced CT scan of the chest and abdomen. Blood tests (hematology with renal and liver function, and thyroid function tests) were repeated approximately every month. The status of the disease was mainly reevaluated by means of contrast‐enhanced CT scan performed every 3 to 4 months. Further radiological exams (such as magnetic resonance, ultrasound, or bone scintigraphy) were performed whenever needed. Tumor response and progression were assessed locally according to RECIST, version 1.1 [4].
CTC Count Schedule and Analysis
Blood samples had to be taken at baseline on the day of the first TKI dose, and then on day 28, day 56 and at the time of progression, or after 12 months in the absence of progression.
Whole blood samples were collected into CellSave tubes (Code # 790005, Menarini, Italy). Samples were maintained at room temperature and despatched to IOV for the centralized evaluation of CTCs and Circulating Endothelial Cells (CECs), to be completed within 96 hours. CTCs were enumerated in whole blood through the CellSearch System (Menarini, Italy), according to the manufacturer's instructions for the Circulating Tumor Cell (CTC) Kit (Code # 7900001, Menarini, Italy) and the user guidelines for in vitro diagnostic use [11, 25].
In order to identify nucleated epithelial cells, CTCs were enriched by the automated platform via anti‐EpCAM antibodies and then stained for DAPI and cytokeratin 8, 18, and 19, whereas CD45 staining, which identifies leukocytes, was used as specificity control [7].
An event was classified as CTC when its morphological features were consistent with that of a nucleated cell with round or oval morphology, with a visible nucleus (DAPI‐positive) of at least 4 microns in diameter, and uniform cytoplasmic staining for cytokeratins 8, 18, 19. Moreover, at least 50% of the nucleus and cytoplasm areas had to overlap. CTC numbers were expressed as the number of cells per 7.5 mL of blood.
Objectives
This study's primary objective was to analyze the prognostic role of CTC counts on the PFS of patients with mRCC starting a first‐line regimen with a TKI. Basal values of total CTCs and change of CTC counts over time were assessed. Secondary objectives included correlation between CTC counts and OS, radiological tumor response, clinical parameters and IMDC group stratification.
Statistical Analysis
As an observational prospective trial, this study did not have a target sample of accrual. However, based on the results of our pilot study on 53 patients [21] and given the risk of patient dropout, we planned to enroll at least 150 new evaluable patients in the study's second multicenter cohort.
Analyses of correlation between baseline CTC counts and clinical parameters (gender, age (<70 vs. ≥70 years), Fuhrman grade (1–2 vs. 3–4), previous surgery on primary kidney tumor versus none, time to metastases (less than or more than 1 year), neutrophil‐to‐lymphocyte ratio (<3 or ≥3), site of metastases (bone, liver and brain), histology (clear cell vs. other) and IMDC prognostic group (good, intermediate or poor) were performed using a chi‐squared test and a Fisher's exact test. Several cutoff values for basal CTCs were tested: zero versus at least one CTC, fewer than two versus at least two CTCs, fewer than three versus at least three CTCs, fewer than four versus at least four CTCs, and fewer than five versus at least five CTCs.
PFS was measured from the day of the baseline CTC count (i.e., the first day of treatment) until radiological or clinical progression. Since patients with mRCC may experience long survival after first‐line regimen treatment failure, we decided to censor the PFS of patients lost to follow‐up on the last date they were known to be alive without progression. OS was calculated from the day of the first CTC count to death, whatever the cause.
Cox proportional hazards analysis was used to estimate univariate and multivariate hazard ratios for the whole cohort's PFS and OS. Subgroups were compared according to the two‐tailed log‐rank test. Since the IMDC classification incorporates the most relevant and independent prognostic factors for patients with mRCC [3], we performed the multivariate analysis with the IMDC group and dichotomized CTC levels according to several cutoff values (one, two, three, four, and five).
Subsequently, we stratified patients into four groups according to the values of CTC sequencing obtained in each patient: patients with negative counts at baseline and during treatment (Group 1a), patients with a baseline count of at least one CTC then switching to negative (Group 1b), patients with a baseline negative count switching to at least one CTC (Group 1c) or patients showing CTC count at least one at baseline and during treatment (Group 1d). The same classification could be applied with different cutoff values of two (Groups 2a, 2b, 2c, and 2d), three (Groups 3a, 3b, 3c, and 3d), four (Groups 4a, 4b, 4c, and 4d), and up to five CTCs (Groups 5a, 5b, 5c, and 5d).
Statistical analyses were carried out using SPSS statistical software.
Results
Out of a total of 246 enrolled patients, 51 were excluded due to technical problems during the despatch or analysis of the first blood sample, which was considered to be a mandatory baseline evaluation, or due to early loss to follow‐up. For the present analysis 195 patients were eligible. These patients’ detailed clinical characteristics are reported in Table 1. All patients started at least one cycle of TKI, which was sunitinib (77.5%), pazopanib (21%) or sorafenib (1.5%).
Table 1.
Characteristics of 195 eligible patients
| Characteristics | n (%) | |||||
|---|---|---|---|---|---|---|
| Age, median (range), yr | 69 (27–91) | |||||
| Gender | ||||||
| Male | 140 (71.8) | |||||
| Female | 55 (28.2) | |||||
| Karnofsky performance status at enrollment | ||||||
| 100 | 63 (32.3) | |||||
| 90 | 52 (26.7) | |||||
| 80 | 45 (23.0) | |||||
| 70 | 30 (15.4) | |||||
| 60 | 5 (2.6) | |||||
| Prior surgery | ||||||
| Partial nephrectomy | 10 (5) | |||||
| Radical nephrectomy | 148 (76) | |||||
| Biopsy/not specified | 37 (19) | |||||
| Histology | ||||||
| Clear cell | 160 (82.0) | |||||
| Papillary | 12 (6.2) | |||||
| Chromophobe | 4 (2.0) | |||||
| Undifferentiated/unspecified | 19 (9.8) | |||||
| Fuhrman grade (available in 143 patients) | ||||||
| G1 | 4 (2.7) | |||||
| G2 | 50 (35.0) | |||||
| G3 | 53 (37.1) | |||||
| G4 | 36 (25.2) | |||||
| Site of disease | ||||||
| Lung | 100 (51.3) | |||||
| Lymph nodes | 43 (22) | |||||
| Bone | 39 (20) | |||||
| Kidney | 25 (12.8) | |||||
| Liver | 25 (12.8) | |||||
| Adrenal | 9 (4.6) | |||||
| Pancreas | 7 (3.6) | |||||
| Cerebral | 6 (3%) | |||||
| Soft tissue | 5 (2.6%) | |||||
| IMDC score (available in 151 patients) | ||||||
| Good | 48 (32.0) | |||||
| Intermediate | 77 (51.3) | |||||
| Poor | 25 (16.7) | |||||
| Type of first‐line tyrosine‐kinase inhibitor | ||||||
| Sunitinib | 151 (77.5) | |||||
| Pazopanib | 41 (21) | |||||
| Sorafenib | 3 (1.5) | |||||
| Distribution of baseline CTC counts according to type of TKI, n (%) | ||||||
|---|---|---|---|---|---|---|
| TKI | CTC negative | CTCs ≥1 | CTCs ≥2 | CTCs ≥3 | CTCs ≥4 | CTCs ≥5 |
| Sunitinib | 75 | 76 | 41 | 24 | 15 | 9 |
| Pazopanib | 28 | 13 | 8 | 6 | 3 | 2 |
| Sorafenib | 1 | 2 | 0 | 0 | 0 | 0 |
| Total | 104 (53.3) | 91 (46.7) | 49 (25.1) | 30 (15.4) | 18 (9.2) | 11 (5.6) |
Abbreviations: CTC, circulating tumor cell; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; TKI, tyrosine kinase inhibitor.
CTC Counts
At baseline, 91 of 195 patients (46.7%) had one or more CTCs, median two, range 1 to 263 CTCs. The mean number of CTCs in positive patients was 8.6. Some representative images of CTCs isolated from four patients with mRCC at baseline, before starting therapy (T0) and on day 28 (T1), are shown in supplemental online Figure 1.
Figure 1.
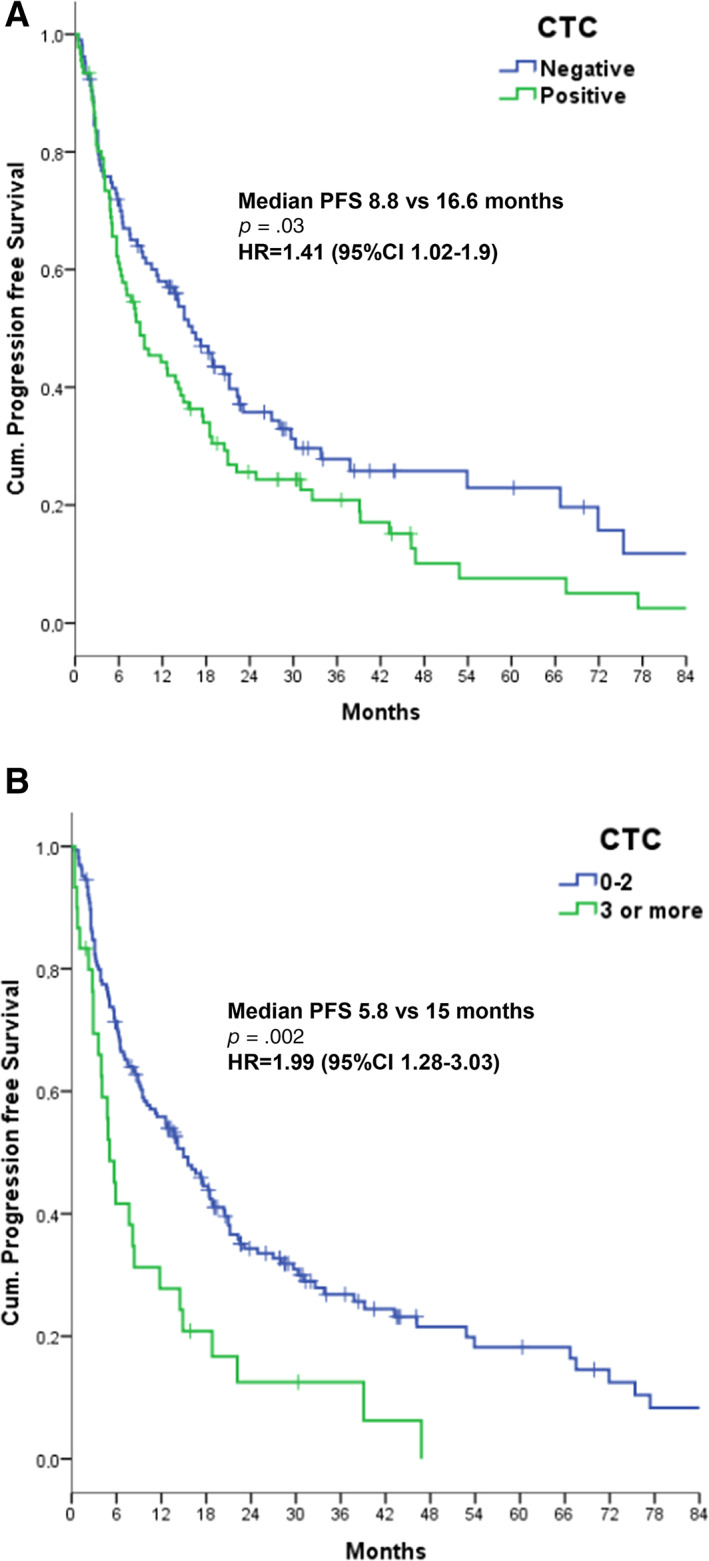
PFS according to negative versus positive baseline CTC counts (two‐tailed log‐rank test, p = .03) (A) and fewer than three versus at least three CTCs (p = .002) (B).
Abbreviations: CI, confidence interval; CTC, circulating tumor cell; HR, hazard ratio; PFS, progression‐free survival.
The probability of having at least one CTC at baseline did not correlate with gender (p = .67), age ≥70 years (p = .12), Fuhrman grade (1–2 vs. 3–4, p = .70), previous removal of primitive tumor (p = .78), time to metastases (less than or more than 1 year, p = .36) or neutrophil‐to‐lymphocyte ratio (<3 or ≥3, p = .27). Interestingly, 56.3% of patients with synchronous metastases (detected within 0 to 3 months from diagnosis) were CTC positive compared with 38.8% of patients with metachronous metastases (p = .015). All the above clinical parameters were not significant with a cutoff value of two, three, four, or five CTCs.
Site of disease did not appear to correlate with the presence of CTCs: 52% positivity in patients with liver, 43.5% in patients with bone and 33% in patients with brain metastases (chi‐squared test, p = .12), although in the bone group the percentage of patients with at least three CTCs almost doubled compared with patients with uninvolved bone (25.6% vs. 12.6%, p = .045).
Interestingly, 15 out of 26 (57.6%) patients with non‐clear cell histology were CTC positive at baseline compared with 43 out of 160 (26.9%) in the clear cell group (p = .002). The difference was also maintained by applying a cutoff of three CTCs (35.4% vs. 16.5%, p = .045). However, when only taking the 160 patients with clear cell histology into consideration, the presence of sarcomatoid features did not appear to correlate with the presence of CTCs according to the different cutoffs of one, two, three, four, and five CTCs tested.
At baseline, the IMDC risk score percentage of patients with at least one CTC increased from 33.3% favorable (16/48 patients), to 45.5% intermediate (35/77 patients) and 64% poor (16/25 patients) (p = .043). Considering the cutoff of at least three CTCs, the IMDC risk score percentage increased from 6.3% favorable (3/48 patients), to 15.6% intermediate (12/77 patients) and 28% poor (7/25 patients) (p = .042).
Response
After a 10.1‐month median duration of treatment, the investigator‐assessed best response in 185 evaluable patients was 3.8% complete response (7 patients), 37.3% partial response (69 patients), 33% stable disease (61 patients), and 25.9% progression (48 patients).
Responses were reported in 38.7% of patients who were CTC‐negative at baseline versus 43.6% of patients with at least one CTC (p = .49) and in 42.3% of patients with fewer than three CTCs versus 34.4% with at least three CTCs (p = .34) (Table 2). Considering only 48 IMDC favorable risk patients, 25 of them achieved a response (52%), but no significant difference was found in terms of the response rate between patients below or above the cutoff values of one CTC (56.2% vs. 43.7%, p = .41, respectively), two CTCs (50.0% vs. 66.7%, p = .44), and three CTCs (53.3% vs. 33.3%, p = .50). The number of favorable risk patients above the cutoff value of four and five CTCs was very low.
Table 2.
Response rates according to cutoff values of baseline CTC counts (185 evaluable patients)
| CTC cutoff value | Responses in patients below cutoff, n (%) | Responses in patients above cutoff, n (%) | p value |
|---|---|---|---|
| One CTC | 38/98 (38.7) | 38/87 (43.6) | .49 |
| Two CTCs | 52/136 (38.2) | 24/49 (48.9) | .19 |
| Three CTCs | 66/156 (42.3) | 10/29 (34.4) | .34 |
| Four CTCs | 72/168 (42.9) | 4/17 (23.5) | .12 |
| Five CTCs | 74/174 (42.5) | 2/11 (18.2) | .16 |
Abbreviation: CTC, circulating tumor cell.
Only the baseline CTC count was available in 43 patients. Table 3 summarizes the distribution of the remaining 152 patients according to the serial variations of CTCs using the different cutoff values of one, two, three, four, and five CTCs, which did not correlate with response. Furthermore, when considering the subgroup of favorable risk patients, responses did not appear to correlate with the pattern distribution of dynamic changes in CTCs.
Table 3.
Best radiological response rate according to distribution of dynamic CTC counts and different CTC cutoff values
| Best response | Group 1a CTCs always negative | Group 1b CTC ≥1 at baseline and then negative | Group 1c CTC negative at baseline and then ≥1 | Group 1d CTC always ≥1 | p = .75 |
|---|---|---|---|---|---|
| Response | 15 | 16 | 22 | 13 | 66 (43.4) |
| No response | 14 | 20 | 33 | 19 | 86 (56.6) |
| Total | 29 (19.1) | 36 (23.7) | 55 (36.2) | 32 (21.0) | Total: 152 (100) |
| Group 2a All CTC values <2 | Group 2b ≥2 CTCs at baseline to <2 | Group 2c <2 CTCs at baseline to ≥2 | Group 2d All CTC values ≥2 | p = .71 | |
|---|---|---|---|---|---|
| Response | 26 | 11 | 21 | 8 | 66 (43.4) |
| No response | 34 | 14 | 32 | 6 | 86 (56.6) |
| Total | 60 (39.5) | 25 (16.4) | 53 (34.9) | 14 (9.2) | Total: 152 (100) |
| Group 3a All CTC values <3 | Group 3b ≥3 CTCs at baseline to <3 | Group 3c <3 CTCs at baseline to ≥3 | Group 3d All CTC values ≥3 | p = .74 | |
|---|---|---|---|---|---|
| Response | 41 | 6 | 17 | 2 | 66 (43.4) |
| No response | 51 | 12 | 19 | 4 | 86 (56.6) |
| Total | 92 (60.6) | 18 (11.8) | 36 (23.7) | 6 (3.9) | Total: 152 (100) |
| Group 4a All CTC values <4 | Group 4b ≥4 CTCs at baseline to <4 | Group 4c <4 CTCs at baseline to ≥4 | Group 4d All CTC values ≥4 | p = .19 | |
|---|---|---|---|---|---|
| Response | 53 | 3 | 8 | 2 | 66 (43.4) |
| No response | 58 | 8 | 19 | 1 | 86 (56.6) |
| Total | 111 (73.0) | 11 (7.3) | 27 (17.8) | 3 (1.9) | Total: 152 (100) |
| Group 5a All CTC values <5 | Group 5b ≥5 CTCs at baseline to <5 | Group 5c <5 CTCs at baseline to ≥5 | Group 5d All CTC values ≥5 | p = .12 | |
|---|---|---|---|---|---|
| Response | 59 | 1 | 5 | 1 | 66 (43.4) |
| No response | 64 | 6 | 13 | 3 | 86 (56.6) |
| Total | 123 (80.9) | 7 (4.6) | 18 (11.9) | 4 (2.6) | Total: 152 (100) |
All values are n or n (%).
Abbreviation: CTC, circulating tumor cell.
Progression‐Free Survival
After a median follow‐up of 31.5 months, the median PFS was calculated as 13.6 months (23% censored; 95% confidence interval [CI], 9.9–17.3 months). The IMDC score confirmed its statistically prognostic role, with a median PFS of 32.6 months in favorable, 11.2 months in intermediate, and 3.1 months in poor risk patients (p < .001).
Patients with at least one CTC had a significantly shorter PFS compared with CTC‐negative patients (8.8 vs. 16.6 months, p = .03; Cox univariate hazard ratio [HR], 1.41; 95% CI, 1.02–1.9) (Fig. 1A).
The difference was lost with the cutoff of at least two CTCs (log‐rank test p = .16; HR, 1.29; 95% CI, 0.90–1.84), but became significant when the cutoff of at least three CTCs was considered: these patients had a median PFS of 5.8 months compared with 15 months for the remaining patients (log‐rank test p = .002; Cox univariate HR, 1.99; 95% CI, 1.28–3.03) (Fig. 1B).
Differences in PFS remained significant with upper cutoff values of at least four CTCs (log‐rank test p = .005; HR, 2.02; 95% CI, 1.21–3.36) and at least five CTCs (p = .003; HR, 2.59; 95% CI, 1.34–4.95). However, since the number of positive patients decreased significantly (Table 1), three CTCs were chosen as the cutoff for multivariate analysis.
With a Cox multivariate analysis taking the IMDC score and CTCs into consideration, having three or more CTCs retained its statistical significance, HR 1.91 (95% CI, 1.16–3.14; p = .011) (supplemental online Table 1).
The median PFS in 32 favorable risk patients with no CTCs was 53.9 months compared with 21.0 months in 16 patients with at least one CTC (33.3%) (p = .06; HR, 1.21; 95% CI, 0.89–2.32) (supplemental online Fig. 2). The median PFS in 45 favorable risk patients with fewer than three CTCs was 32.6 months compared with 8.3 months in three patients with at least three CTCs (p = .17).
Figure 2.
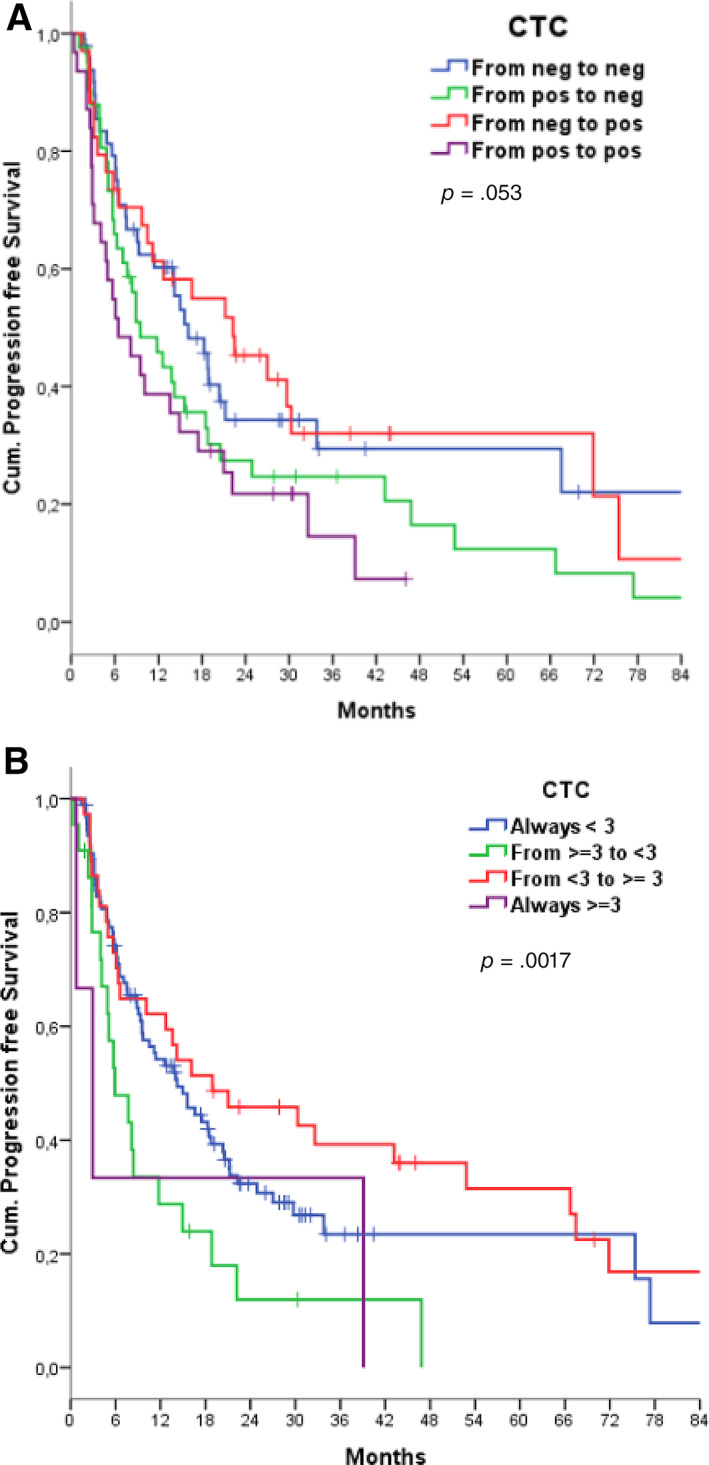
Progression‐free survival according to dynamic changes in CTC counts using the cutoff value of one or more CTCs (up, two‐tailed log‐rank test, p = .053) (A) or three or more CTCs (p = .017) (B).
Abbreviation: CTC, circulating tumor cell.
PFS analysis, according to the dynamic change in CTCs over time (using Group 1a as a reference), showed an unexpected trend in favor of patients categorized as Group 1c (CTC counts negative at baseline and subsequently at least one), with a median PFS of 19.2 months (p = .053) (Fig. 2A). We then repeated the same analysis on serial counts using the cutoff of at least two cells and no difference was found (p = .50). With the cutoff value of three cells (using Group 3a as a reference), we found that Group 3c (CTC counts fewer than three at baseline and subsequently at least three) paradoxically had the longer PFS of 18.9 months (p = .017) (Fig. 2B).
Thereafter, we also tested the correlation of PFS according to the same four different patterns of dynamic changes with cutoff values of four and five CTCs, but no differences were detected (p = .084 and p = .79, respectively).
We repeated the same analysis for the 48 favorable risk patients and again, no differences were found in all the CTC cutoff values tested.
Overall Survival
The estimated OS rate at 24 months was 59.9%. As expected, the IMDC score strongly impacted on OS, with a median OS not reached in favorable patients, 24.9 months reached in intermediate patients and 5.7 months reached in poor risk patients (p < .001).
We only observed a nonsignificant trend in increased OS for CTC‐negative patients when compared with those with one or more CTCs (log‐rank test p = .207; Cox univariate HR, 1.28; 95% CI, 0.86–1.91) (Fig. 3A). Similarly, no difference was found with the cutoff value of at least two CTCs (log‐rank test p = .18; HR, 1.34; 95% CI, 0.87–2.07). However, patients with at least three CTCs had a shorter estimated OS of 13.8 versus 52.8 months in the remaining patients (p = .003; Cox univariate HR, 1.99; CI, 1.17–3.2) (Fig. 3B).
Figure 3.
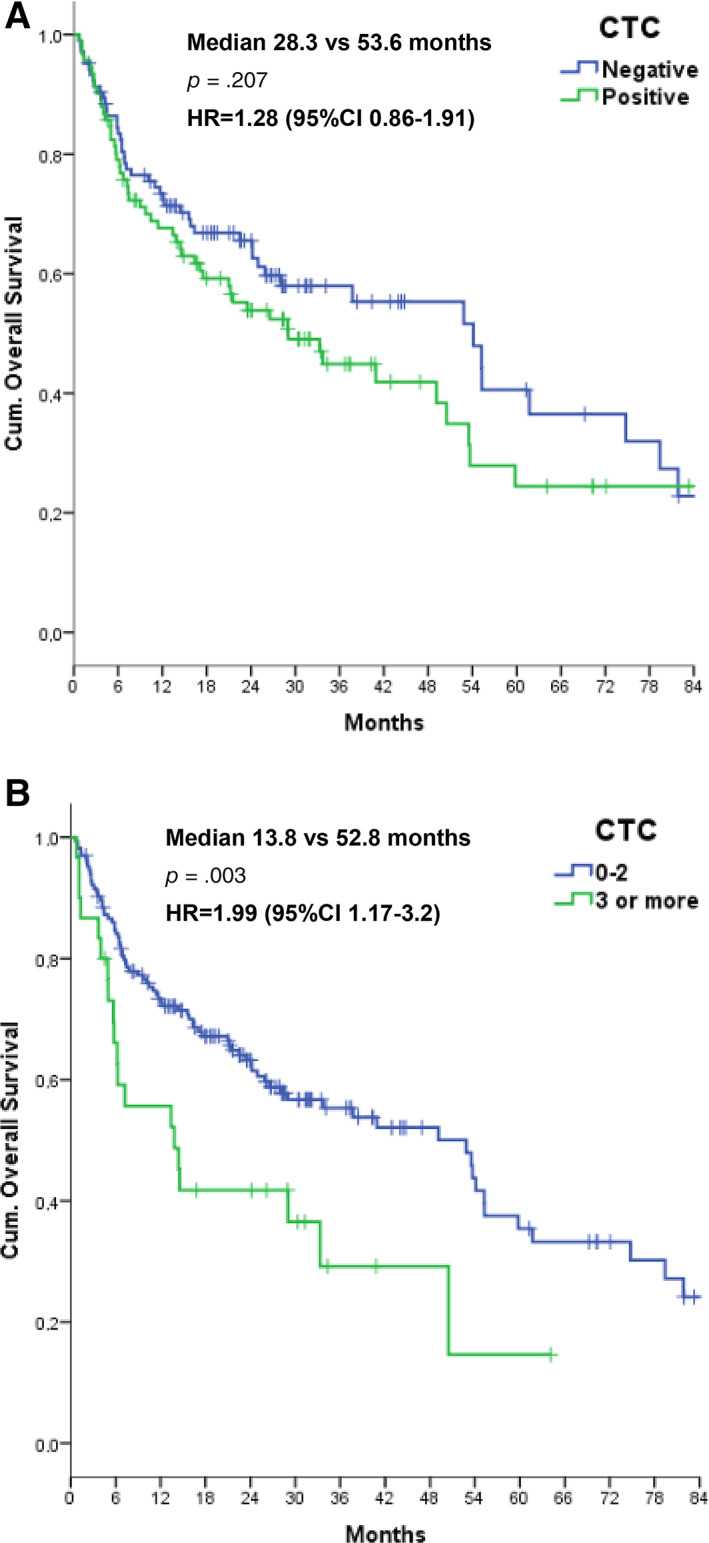
Overall survival according to negative versus positive baseline CTC counts (two‐tailed log‐rank test, p = .207) (A) and fewer than three or at least three CTCs (p = .003) (B).
Abbreviations: CI, confidence interval; CTC, circulating tumor cell; HR, hazard ratio.
Differences in OS remained significant with upper cutoff values of at least four CTCs (p = .005; HR, 2.25; 95% CI, 1.25–4.05) and at least five CTCs (p < .001; HR, 3.89; 95% CI, 1.68–6.82); we consequently also confirmed three CTCs as the cutoff value for multivariate analysis for OS.
Taking the IMDC score into consideration, a CTC count of at least three CTCs at baseline had an HR of 1.67 (95% CI, 0.95–2.93; p = .076) (supplemental online Table 1).
Very few deaths (8 out of 48 patients) were observed in favorable risk patients. At 24 months, 87.5% of patients without CTCs were still alive compared with 87.5% of patients among those with at least one CTC (p = .38), whereas 86.7% of patients with zero to two CTCs were alive compared with 100% of those with at least three CTCs (p = .46).
OS did not differ according to the dynamic change in CTC counts over time during treatment (Group 1a as a reference, log‐rank test p = .433) with a cutoff of one CTC (Fig. 4A). No differences were found in terms of OS when repeating the same analysis on serial CTC counts using the cutoff value of at least three cells, although a small trend for Group 3c appeared (log‐rank test p = .076) (Fig. 4B).
Figure 4.
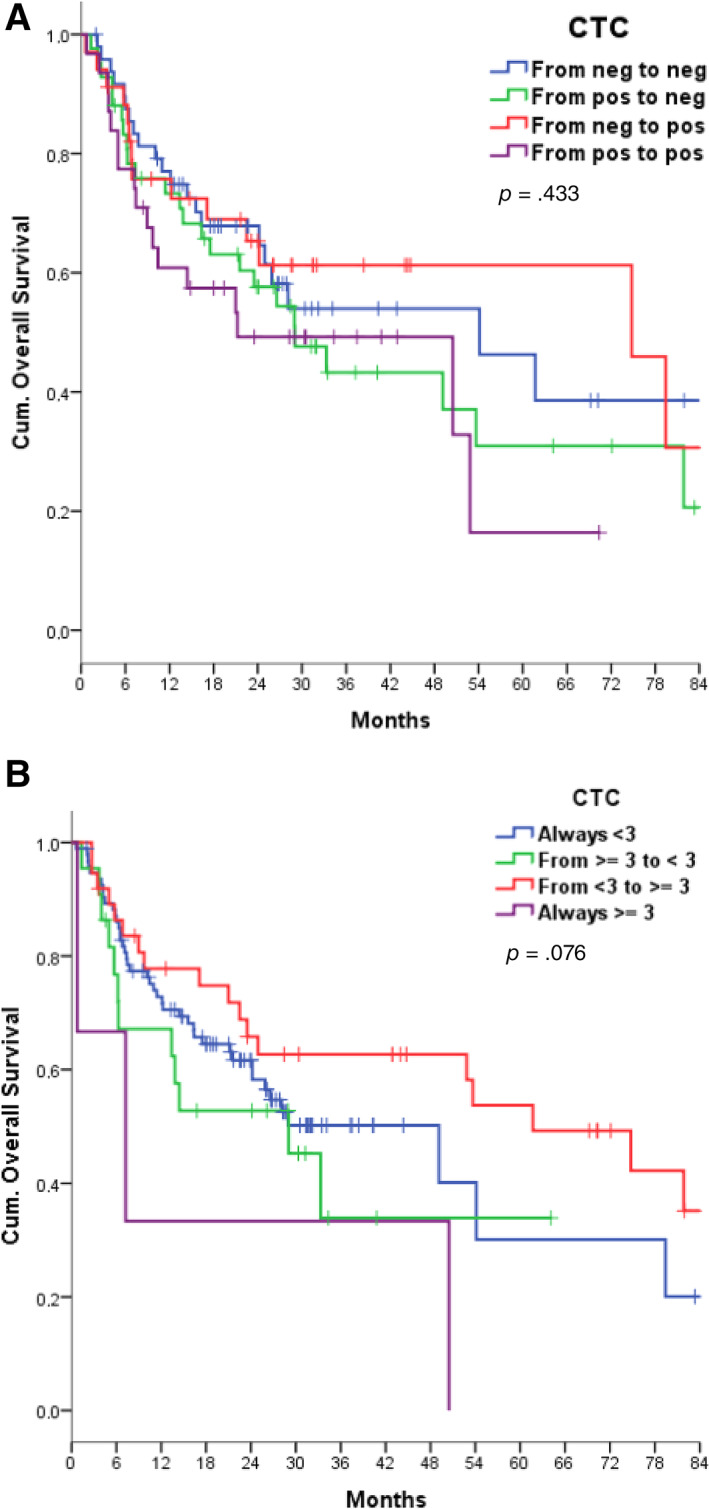
Overall survival according to dynamic changes in CTC counts using the cutoff value of one or more CTCs (two‐tailed log‐rank test, p = .053) (A) or three or more CTCs (p = .076) (B).
Abbreviation: CTC, circulating tumor cell.
We then went on to test the correlation of OS according to the same four different patterns of dynamic changes with cutoff values of four and five cells, but no differences were detected (two‐tailed log‐rank test p = .810 and p = .68, respectively).
We repeated the same analyses for the 48 favorable risk patients, and again no differences were found across all the five cutoff values that were assessed.
Discussion
RCC is a highly vascularized neoplasia resistant to standard chemotherapy, but sensitive to antiangiogenic drugs and immunotherapy. The IMDC algorithm is a mandatory stratification factor for clinical trials and identifies intermediate and poor risk patient candidates who today can receive the most recent immunotherapy combination of Nivolumab plus Ipilimumab [26]. TKIs are synergic with immunotherapy, and combination regimens can be given to patients in all risk groups [2]. There is wide debate on whether monotherapy with TKIs could still be a reasonable and cost‐effective choice for patients with a favorable IMDC risk and a low metastatic burden in the lung and/or lymph nodes [27].
In any case, IMDC criteria do not discriminate between patients responsive to TKIs, and no validated blood biomarker is available to guide the choice between the current first‐line options for patients with mRCC (combined regimens vs. TKI monotherapy) and to predict response during treatment [6].
CTCs appear to be promising for monitoring response to treatment in patients with colorectal cancer [28] and castration‐resistant prostate cancer [29], but limited data are available for mRCC. Allard and colleagues [17] were the first to demonstrate the feasibility of detecting CTCs using the CellSearch platform in 11 patients with RCC: 25% of specimens had at least two CTCs, whereas none of them had at least five CTCs. In 25 patients with mRCC, Gradilone and colleagues [18] reported four CellSearch CTC‐positive patients out of 25 (16%). Other authors reported that 41% of patients with clear cell RCC were EpCAM‐positive (≥5% of staining) [30]. CTCs are a heterogeneous population in patients with mRCC, with different metastatic capabilities, similar to what is reported for other malignancies [31]. Therefore, intensive efforts were focused on non–EpCAM‐based technologies, but few papers addressed this matter in patients with mRCC and even then, with inconclusive results [8, 9, 10].
Broncy and colleagues tried to identify CTCs in the peripheral blood of patients with RCC according to a cytomorphological method termed “isolation by size of epithelial tumor cell technique” (ISET). Twenty out of 30 patients, with mainly localized RCC, were CTC positive with this method (66.6%) [9].
Similarly, Klezl and colleagues [10] used an EpCAM‐independent size‐based enrichment method and reported 86.6% prevalence of CTC‐positive samples in patients with RCC undergoing surgery. Another group recently reported that the positivity of CTCs detected by ISET in 13 out of 36 patients (36.1%) correlated with clinical tumor‐node‐metastasis (TNM) staging, whereas the presence of CTCs detected by CellSearch in the same patients (19.4%) did not. Eventually, CTC counts measured through both methods did not correlate with patients’ PFS and OS [32].
Taking into account the need to exploit standardized, automated assays, suitable for multicenter clinical studies [33], we decided to apply the U.S. Food and Drug Administration–cleared CellSearch platform to patients with RCC.
We demonstrated that CTCs could be isolated in almost 60% of the 53 patients with mRCC before treatment with sunitinib [22], a prevalence that surely exceeds that reported by other small studies conducted in mRCC with CellSearch [17, 18, 32]. This difference could possibly be related to the different sample size as well as to the selection bias, since all our patients with metastatic disease were deemed eligible to receive a systemic treatment with a TKI in view of burden of disease and/or the presence of symptoms.
Most importantly, we obtained the proof of principle that EpCAM expression could be acquired during disease evolution (e.g., negative in the primary tumor and positive in the metastases), supporting the rationale of employing EpCAM‐based technology even if the primary tumor tissue did not express this antigen [22]. Moreover, we know that EpCAM is highly expressed in cancer stem cells and may play a role in cancer migration. We can, therefore, infer that assessment of EpCAM in CTCs may better reflect the dynamic biological changes of invasive and metastatic cell populations, as with other dynamic tumor markers in general. It also has to be remembered that EpCAM expression is higher in papillary and chromophobe histological subtypes of RCC (80%) [34].
We therefore designed a second large multicenter observational trial in order to enroll at least 150 additional evaluable patients with RCC (with either clear or non‐clear histology) treated homogeneously with a first‐line TKI, with the aim of assessing the prognostic as well as the predictive role of CTCs in terms of radiological response.
We confirmed the presence of at least one CTC/7.5 mL of peripheral blood in almost half of the patients (46.7%), and three or more CTCs in 15.4% of cases. Globally, CTC counts in our study appear inferior when compared with other solid tumors. Up to 93.3% of patients with metastatic prostate cancer may be CTC positive [14]. Nevertheless, the number of previous lines of hormonal and radiotherapy treatments in either an adjuvant or metastatic setting may represent an important bias in such comparisons between patients with prostate cancer and our cohort of treatment‐naïve patients with mRCC.
CTC counts at baseline did not correlate with any of the clinical parameters tested, and only the presence of synchronous metastases showed a mild but statistically significant correlation with the presence of at least one CTC. This can be interpreted as a correlation with a more aggressive behavior, although significance was lost with the cutoff value of two or three CTCs, possibly due to lower patient numbers.
Interestingly, non‐clear cell histology correlated with a higher probability of detecting at least one CTC (57.6%) compared with the clear cell phenotype (26.9%, p = .002). The difference was maintained with the cutoff value of two or three CTCs, thus confirming the different biology of the two histological groups. As for the site of metastases, we found a correlation between bone metastases and a higher chance of having a CTC count of at least three, but not with the presence of liver or brain metastases. The correlation between bone metastases and circulating CTC counts has already been reported in breast cancer [35] and other tumors, and several studies are currently focusing on the complex balance of quiescence or proliferation of disseminated tumor cells in the bone metastatic niche.
In our cohort, baseline counts of CTCs became consistently prognostic for PFS and OS, starting from the cutoff value of three CTCs (HR for progression 1.99 and death 1.99). The number of patients with values of at least four CTCs or at least five CTCs was very low (Table 1), and these cutoff values did not achieve predictive significance for radiological response (see below). We thus propose three CTCs as the reference prognostic cutoff value for mRCC when using the CellSearch platform. In a multivariate analysis incorporating the IMDC score and CTC counts, the presence of three or more CTCs maintained an adverse impact on progression (HR, 1.91; p = .011) and a trend towards significance for OS (HR, 1.67; p = .076) (supplemental online Table 1).
Since TKI monotherapy is currently considered to be an option mainly for IMDC favorable risk patients [27], the same analyses for PFS and OS were performed in this subgroup. We obtained comparable results in terms of correlation of baseline CTC counts of at least one with PFS (supplemental online Fig. 2), albeit not statistically significant due to lower patient numbers and fewer events. The number of patients with CTCs above three, four, or five was too small to draw any further comparisons. Very few overall survival events were registered in this subgroup.
The availability of data concerning the correlation between CTC counts and radiological response is limited. Indeed, an early CTC change following therapy did not correlate with the RECIST response in a pooled analysis conducted on patients with various types of advanced cancer treated with chemotherapy and other agents within phase I trials [36]. This underlines the fact that shrinkage of tumor masses is not unequivocally correlated with a reduction in the population of blood CTCs.
Our study failed to demonstrate a correlation between baseline CTC counts and radiological response (as judged by the treating physician) (Table 2). Contrary to what is reported on other solid tumors with the same CellSearch system [11, 12, 14], the pattern of dynamic change of CTC counts over time with all the different cutoff values of CTCs could not even be correlated with response to TKIs (Table 3), PFS (Fig. 2) and OS (Fig. 4). We carried out the same analyses in the subgroup of 48 IMDC favorable risk patients. However, here again, no significant correlation between baseline and dynamic CTC counts and radiological response was registered.
The different mechanisms of action of TKIs, which are mainly antiangiogenic rather than direct cytotoxic drugs, could partly explain such findings. This is corroborated by the fact that roughly one‐third of our patients showed a fluctuation in CTC values over time, for example, increasing and reducing over time in the same patient (or vice versa). Moreover, there have been reports that prolonged inhibition of angiogenesis might paradoxically increase metastasis of tumor cells in animal models [21]. These experimental findings raise the question as to whether CTC spread might even increase in vivo due to an efficacious antiangiogenic pressure induced by TKIs, thus biasing the analysis of correlation between dynamic changes of CTC counts and tumor shrinkage. It would be very interesting to conduct a comparable prospective study on CTCs in patients with mRCC receiving modern PD‐1–based immunotherapy [2, 26], in order to see if PD‐1 blockade can influence the shedding and circulation of CTCs and their correlation with radiological response, PFS and OS. Indeed, a small study conducted on patients with lung cancer recently demonstrated that CTC counts are correlated with the survival of patients with lung cancer treated with nivolumab [37].
This study's limitations are related to the use of an EpCAM‐based automated assay CellSearch system, and the distinctive schedule of blood draws that we applied to patients compared with other studies. The loss of certain time points and the heterogeneity in the timing of the radiological reevaluation of patients might have biased such results, potentially resulting in delayed recognition of progression.
Further analyses are ongoing on apoptotic markers in CTCs and concomitant counts of circulating endothelial cells collected in the same cohort of patients. The aim is to confirm our preliminary observation that the ratio of apoptotic to live CTCs could better reflect the response to treatment and also the duration of disease control [22].
Nevertheless, the results of our study do not provide a rationale for changing treatment on the basis of an unfavorable dynamic change in CellSearch‐detected CTC counts during treatment with first‐line TKIs in mRCC. Moreover, large prospective trials with alternative cytomorphological enrichment strategies are still warranted, especially in patients receiving new immunotherapy regimens.
Conclusion
In this large, homogeneous prospective cohort of patients with metastatic RCC treated in the first line with TKIs, we provide evidence that the presence of three or more CTCs at baseline detected by the EpCAM‐based CellSearch system is associated with a significantly shorter PFS and OS. This impact is independent of the IMDC classification in multivariate analysis for PFS. Baseline and dynamic CTC counts were not predictive of radiological response.
Author Contributions
Conception/design: Umberto Basso, Elisabetta Rossi, Rita Zamarchi, Vittorina Zagonel
Provision of study material or patients: Umberto Basso, Antonella Facchinetti, Elisabetta Rossi, Marco Maruzzo, Vincenza Conteduca, Michele Aieta, Francesco Massari, Anna Paola Fraccon, Claudia Mucciarini, Teodoro Sava, Matteo Santoni, Cristina Pegoraro, Emilia Durante, Maurizio Nicodemo, Alessandra Perin, Alessandra Bearz, Carlo Gatti, Alberto Diminutto, Carmen Barile, Ugo De Giorgi, Rita Zamarchi and Vittorina Zagonel
Collection and/or assembly of data: Elisabetta Rossi, Rita Zamarchi; Antonella Facchinetti, Marco Maruzzo, Umberto Basso, Alberto Diminutto, Pasquale Fiduccia
Data analysis and interpretation: Umberto Basso, Elisabetta Rossi, Rita Zamarchi, Marco Maruzzo and Vittorina Zagonel
Manuscript writing: Umberto Basso, Rita Zamarchi
Final approval of manuscript: Umberto Basso, Antonella Facchinetti, Elisabetta Rossi, Marco Maruzzo, Vincenza Conteduca, Michele Aieta, Francesco Massari, Anna Paola Fraccon, Claudia Mucciarini, Teodoro Sava, Matteo Santoni, Cristina Pegoraro, Emilia Durante, Maurizio Nicodemo, Alessandra Perin, Alessandra Bearz, Carlo Gatti, Pasquale Fiduccia, Alberto Diminutto, Carmen Barile, Ugo De Giorgi, Rita Zamarchi, Vittorina Zagonel
Disclosures
Umberto Basso: Pfizer, Novartis, Bristol‐Myers Squibb, Astellas, Janssen, Ipsen, Sanofi‐Aventis (H), Pfizer, Novartis, Merck Sharp and Dohme, Incyte, Sanofi‐Aventis (C/A); Vincenza Conteduca: Astellas, Janssen, Sanofi‐Aventis (H), Bayer (C/A); Alessandra Bearz: Takeda, Boehringer Ingelheim, Novartis, Pfizer, Merck Sharp and Dohme (C/A, H), Jansen, Roche (other—travel); Ugo De Giorgi: Astellas, Janssen, Bayer, Sanofi Aventis, Pfizer, Novartis, Merck Sharp and Dohme, Ipsen (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 images of CTCs from four patients
Supplemental Figure 2.
Supplementary Table 1 Cox Multivariate analysis for IMDC group and CTC baseline value ≥3 vs 0‐2.
Acknowledgments
This work was supported by Regione Veneto (finalized research project 339/12) as an academic study.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renalcell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 5.Krajewski KM, Nishino M, Ramaiya NH et al. RECIST 1.1 compared with RECIST 1.0 in patients with advanced renal cell carcinoma receiving vascular endothelial growth factor‐targeted therapy. AJR Am J Roentgenol 2015;204:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jafolla MA, Picardo S, Aung K et al. Systematic review and REMARK scoring of renal cell carcinoma prognostic biomarkers manuscripts. PLoS One 2019;14:e0222359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maertens Y, Humberg V, Erlmeier F et al. Comparison of isolation platforms for detection of circulating renal cell carcinoma cells. Oncotarget 2017;8:87710–87717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broncy L, Njima BB, Méjean A et al. Single‐cell genetic analysis validates cytopathological identification of circulating cancer cells in patients with clear cell renal cell carcinoma. Oncotarget 2018;9:20058–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klezl P, Pospisilova E, Kolostova K et al. Detection of circulating tumor cells in renal cell carcinoma: Disease stage correlation and molecular characterization. J Clin Med 2020;9:1372–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristofanilli M, Budd GT, Ellis MJ et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781–791. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SJ, Punt CJ, Iannotti N et al. Relationship of circulating tumor cells to tumor response, progression‐free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–3221. [DOI] [PubMed] [Google Scholar]
- 13.Danila DC, Heller G, Gignac GA et al. Circulating tumor cell number and prognosis in progressive castration‐resistant prostate cancer. Clin Cancer Res 2007;13:7053–7058. [DOI] [PubMed] [Google Scholar]
- 14.De Bono JS, Scher HI, Montgomery RB et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration‐resistant prostate cancer. Clin Cancer Res 2008;14:6302–6309. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay CR, Blackhall FH, Carmel A et al. EPAC‐lung: Pooled analysis of circulating tumour cells in advanced non‐small cell lung cancer. Eur J Cancer 2019;117:60–68. [DOI] [PubMed] [Google Scholar]
- 16.Cabel L, Proudhon C, Gortais H et al. Circulating tumor cells: Clinical validity and utility. Int J Clin Oncol 2017;22:421–430. [DOI] [PubMed] [Google Scholar]
- 17.Allard WJ, Matera J, Miller CM et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897–904. [DOI] [PubMed] [Google Scholar]
- 18.Gradilone A, Iacovelli R, Cortesi E et al. Circulating tumor cells and “suspicious objects” evaluated through CellSearch in metastatic renal cell carcinoma. Anticancer Res 2011;31:4219–4221. [PubMed] [Google Scholar]
- 19.Xin H, Zhang C, Herrmann A et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009;69:2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao Z, Sadek I. Sunitinib: The antiangiogenic effects and beyond. Onco Targets Ther 2016;9:5495–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pàez‐Ribes M, Allen E, Hudock J et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009;15:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi E, Fassan M, Aieta M et al. Dynamic changes of live/apoptotic circulating tumour cells as predictive marker of response to sunitinib in metastatic renal cancer. Br J Cancer 2012;107:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi E, Basso U, Celadin R et al. M30 neoepitope expression in epithelial cancer: Quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin Cancer Res 2010;16:5233–5243. [DOI] [PubMed] [Google Scholar]
- 24.Rossi E, Facchinetti A, Zamarchi R. Notes for developing a molecular test for the full characterization of circulating tumor cells. Chin J Cancer Res 2015;27:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristofanilli M, Pierga JY, Reuben J et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit Rev Oncol Hematol 2019;134:39–45. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Tannir NM, McDermott DF et al. CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aeppli S, Schmaus M, Eisen T et al. First‐line treatment of metastatic clear cell renal cell carcinoma: A decision‐making analysis among experts. ESMO Open. 2021;6:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanduri LK, Hissa B, Scholch S et al. The prognostic role of circulating tumor cells in colorectal cancer. Exp Rev Anticancer Ther 2019;19:1077–1088. [DOI] [PubMed] [Google Scholar]
- 29.Pantel K, Hille C, Cher HI. Circulating tumor cells in prostate cancer: From discovery to clinical utility. Clin Chem 2019;65:87–99. [DOI] [PubMed] [Google Scholar]
- 30.Seligson DB, Pantuck AJ, Liu X et al. Epithelial cell adhesion molecule (KSA) expression: Pathobiology and its role as an independent predictor of survival in renal cell carcinoma. Clin Cancer Res 2004;10:2659–2669. [DOI] [PubMed] [Google Scholar]
- 31.Baccelli I, Schneeweiss A, Riethdorf S et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013;31:539–544. [DOI] [PubMed] [Google Scholar]
- 32.Bai M, Zou B, Wand Z et al. Comparison of two detection systems for circulating tumor cells among patients with renal cell carcinoma. Int Urol Nephrol 2018;10:801–809. [DOI] [PubMed] [Google Scholar]
- 33.Parkinson DR, Dracopoli N, Gumps Petty B et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012;2:10–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimpfer A1, Maruschke M, Rehn S et al. Prognostic and diagnostic implications of epithelial cell adhesion/activating molecule (EpCAM) expression in renal tumours: A retrospective clinicopathological study of 948 cases using tissue microarrays BJU Int 2014;114:296–302. [DOI] [PubMed] [Google Scholar]
- 35.De Giorgi, U, Valero V, Rohren E et al. Circulating tumor cells and bone metastases as detected by FDG‐PET/CT in patients with metastatic breast cancer. Ann Oncol 2010;21:33–39. [DOI] [PubMed] [Google Scholar]
- 36.Massard C, Borget I, Farace F et al. RECIST response and variation of circulating tumour cells in phase 1 trials: A prospective multicentric study. Eur J Cancer 2017;83:185–193. [DOI] [PubMed] [Google Scholar]
- 37.Alama A, Coco S, Genova C et al. Prognostic relevance of circulating tumor cells and circulating cell‐free DNA association in metastatic non‐small cell lung cancer treated with nivolumab. J Clin Med 2019;8:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1 images of CTCs from four patients
Supplemental Figure 2.
Supplementary Table 1 Cox Multivariate analysis for IMDC group and CTC baseline value ≥3 vs 0‐2.


