Abstract
Lessons Learned
Cemiplimab in combination with radiation therapy, cyclophosphamide, and granulocyte macrophage colony‐stimulating factor did not demonstrate efficacy above what can be achieved with other PD‐1 inhibitor monotherapies in patients with refractory and metastatic head and neck squamous cell carcinoma.
The safety profile of cemiplimab combination therapy was consistent with previously reported safety profiles of cemiplimab monotherapy. No new safety signal was observed.
Background
Refractory and metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) generally does not respond to PD‐1 inhibitor monotherapy. Cemiplimab is a human anti–PD‐1 monoclonal antibody. An expansion cohort enrolled patients with R/M HNSCC in a phase I study combining cemiplimab plus radiation therapy (RT), cyclophosphamide, and granulocyte macrophage colony‐stimulating factor (GM‐CSF).
Methods
Patients with R/M HNSCC refractory to at least first‐line therapy and for whom palliative RT is clinically indicated received cemiplimab plus RT, cyclophosphamide, and GM‐CSF. The co‐primary objectives were the safety, tolerability, and efficacy of cemiplimab plus RT, cyclophosphamide, and GM‐CSF in 15 patients with R/M HNSCC.
Results
Fifteen patients were enrolled. Patients discontinued treatment due to progression of disease. The most common treatment‐emergent adverse events (TEAEs) of any grade were fatigue (40.0%), constipation (26.7%), and asthenia, dyspnea, maculo‐papular rash, and pneumonia (each 20%). The only grade ≥3 TEAE that occurred in two patients was pneumonia (13.3%). By investigator assessment, there was one partial response (6.7%); disease control rate was 40.0% (95% confidence interval [CI], 16.3–67.7; five patients with stable disease); seven patients had progressive disease, and two were not evaluable. Median progression‐free survival by investigator assessment was 1.8 months (95% CI, 1.7–4.7).
Conclusion
The regimen demonstrated tolerability but not efficacy above that which can be achieved with anti–PD‐1 inhibitor monotherapy for R/M HNSCC.
Keywords: Cemiplimab, Head and neck, Radiotherapy
Discussion
Treatment with anti–PD‐1 inhibitor in R/M HNSCC has demonstrated modest efficacy with only a slight improvement in median overall survival and overall response rate (ORR) when compared with chemotherapy alone [1]. Hence, combinations with adjunct immunostimulatory drugs and RT to achieve higher responses are strategies currently under investigation. We sought to investigate the combination of cemiplimab plus RT, cyclophosphamide, and GM‐CSF in this cohort of patients. This was one expansion cohort of a dose‐escalation phase I trial of REGN2810 (cemiplimab) in patients with advanced malignancies (Figs. 1 and 2). There was a total of 26 cohorts. RT augments immunotherapy drugs via the release of soluble tumor cell antigens from killed cells, increases tumor cell surface expression of antigens that mediate T‐cell recognition, and increases the efficiency of antigen‐presenting cells [2, 3]. However, regulatory T cell–mediated immunosuppression caused by RT can be an obstacle for a proimmune tumor microenvironment. Thus cyclophosphamide, which has been shown to abrogate this effect, might improve the efficacy of the anti–PD‐1 plus RT combination [4]. The addition of GM‐CSF improves dendritic cell maturation and antigen presentation of released cancer antigens and has demonstrated efficacy in advanced refractory solid tumors [5, 6]. These observations generated the hypothesis for the combination outlined in this clinical trial. Our trial demonstrated the tolerability and the safety of the combination with the most common adverse events being fatigue, constipation, and grade 3 pneumonia and dehydration. The ORR was 6.7%, and the DCR was 40%, which is similar to what is achieved with single agent anti–PD‐1 monotherapy [1]. One explanation for the failure of this combination to exceed single agent data may be the enrollment of patients who were heavily pretreated, with 100% of patients receiving platinum‐based chemotherapy, 60% monoclonal antibodies, 60% pyrimidine analogue, 53% taxanes, and 93.3% prior radiotherapy. This can lead to a general immunosuppressive state, potentially abrogating the immunostimulatory effect of the regimen. Moreover, patients received only 7 days of radiation as opposed to longer courses of radiation administered in previous trials demonstrating some efficacy [6]. A future potential avenue to consider is studying this regimen in earlier lines of therapy with a longer duration of RT as opposed to a shorter duration aimed at palliative treatment and potentially using a different schedule of the drugs.
Figure 1.
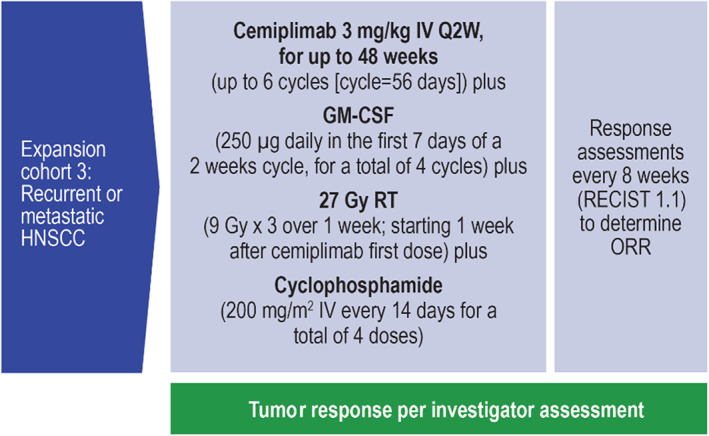
Study design: HNSCC expansion cohort. Abbreviations: HNSCC, head and neck squamous cell carcinoma; ORR, overall response rate; Q2W, every 2 weeks; RT, radiation therapy.
Figure 2.
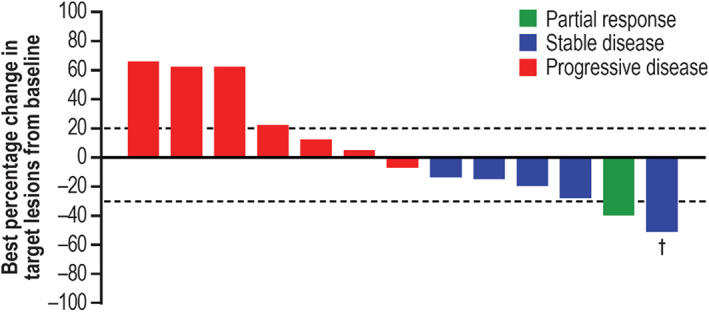
Clinical activity of tumor response to cemiplimab combination therapy per investigator assessment. Plot shows the best percentage change in the sum of target lesion diameters from baseline for the 13 patients who had at least one response evaluation. Lesion measurements after progression were excluded. The horizontal lines indicate criteria for partial response (≥30% decrease in the sum of target lesion diameters) and progressive disease (≥20% increase in the target lesion diameters), respectively. †, Partial response was not confirmed in this patient; therefore, the tumor response was downgraded to stable disease.
Trial Information
| Disease | Head and neck cancers |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | One prior regimen |
| Type of Study | Phase I, expansion cohort |
| Primary Endpoint | Safety |
| Additional Details of Endpoints or Study Design | Co‐primary objectives of the HNSCC expansion cohort were to (a) characterize the safety, tolerability, and dose‐limiting toxicities (DLTs) of cemiplimab in combination with RT, cyclophosphamide, and GM‐CSF and (b) evaluate the efficacy of cemiplimab in combination with RT, cyclophosphamide, and GM‐CSF by measuring ORR. |
| Investigator's Analysis | Level of activity did not meet planned endpoint |
Drug Information
| Cemiplimab | |
| Generic Name | Cemiplimab |
| Trade Name | Libtayo |
| Company Name | Cemiplimab (REGN2810) |
| Drug Type | Immunotherapy (PD‐1 inhibitor) |
| Drug Class | Immune therapy |
| Dose | 3 milligrams (mg) per kilogram (kg) |
| Route | i.v. |
| Schedule of Administration | Every 2 weeks for up to 48 weeks (up to six cycles [cycle = 56 days]). |
| GM‐CSF | |
| Generic Name | GM‐CSF |
| Company Name | GM‐CSF |
| Drug Type | Immunostimulatory |
| Drug Class | Immune therapy |
| Dose | 250 mcg per m2 |
| Route | i.v. |
| Schedule of Administration | Daily in the first 7 days of a 2‐week cycle, for a total of four cycles. |
| Cyclophosphamide | |
| Generic Name | Cyclophosphamide |
| Trade Name | Cyclophosphamide |
| Company Name | |
| Drug Type | Alkylating chemotherapy |
| Drug Class | Alkylating agent |
| Dose | 200 milligrams (mg) per squared meter (m2) |
| Route | i.v. |
| Schedule of Administration |
Every 14 days for a total of four doses. In addition to cemiplimab, GM‐CSF, and cyclophosphamide treatment included palliative radiation (27 Gy: 9 Gy × 3 over 1 week, starting 1 week after first dose of cemiplimab). |
Patient Characteristics
| Number of Patients, Male | 9 | |
| Number of Patients, Female | 6 | |
| Stage | Refractory/advanced/metastatic | |
| Age | Median (range): 62 (45–78) years | |
| Performance Status: ECOG |
0 — 20% 1 — 80% 2 — 0 3 — 0 Unknown — 0 |
|
| Other | Median age, years (range) | 62.0 (45–78) |
| Male, n (%) | 9 (60.0) | |
| ECOG performance status score, n (%) | ||
| 0 | 3 (20.0) | |
| 1 | 12 (80.0) | |
| Any prior cancer‐related systemic therapy, n (%) | 15 (100.0) | |
| Antineoplastic agents | 15 (100.0) | |
| Platinum compounds | 15 (100.0) | |
| Monoclonal antibodies | 9 (60.0) | |
| Pyrimidine analogues | 9 (60.0) | |
| Taxanes | 8 (53.3) | |
| Combination of antineoplastic agents | 1 (6.7) | |
| Other | 2 (13.3) | |
| Immunosuppressants | 2 (13.3) | |
| Any prior cancer‐related radiotherapy, n (%) | 14 (93.3) |
Primary Assessment Method
| Title | Efficacy |
| Number of Patients Screened | 15 |
| Number of Patients Enrolled | 15 |
| Number of Patients Evaluable for Toxicity | 15 |
| Number of Patients Evaluated for Efficacy | 13 |
| Evaluation Method | RECIST version 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 1 (6.7%) |
| Response Assessment SD | n = 5 (33.3%) |
| Response Assessment PD | n = 7 (46.7%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 1.8 months; 95% CI, 1.7–4.7. |
Adverse Events
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Fatigue | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Constipation | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Dyspnea | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Rash maculo‐papular | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Lung infection | 0 | 60 | 0 | 40 | 0 | 0 | 100 |
| Arthralgia | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Dehydration | 0 | 67 | 0 | 33 | 0 | 0 | 100 |
| Hypokalemia | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Nausea | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Weight loss | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
All cycles. TEAEs of any grade were reported in 14 patients (93.3%). TEAEs regardless of attribution are summarized. The most frequently experienced grade ≥ 3 TEAE was pneumonia (n = 2; 13.3%). One patient discontinued study treatment owing to a TEAE of community‐acquired pneumonia. No DLTs were observed. No grade 4 or 5 events were reported.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Level of activity did not meet planned endpoint |
Head and neck squamous cell carcinoma (HNSCC) is associated with significant morbidity and mortality worldwide with more than 600,000 cases diagnosed annually [7]. Most patients present with locoregionally advanced disease, and many patients develop recurrence [8, 9]. Patients who develop disease progression within 6 months of platinum‐based chemotherapy have a poor prognosis [10]. Nivolumab demonstrated improved clinical outcomes when compared with standard of care chemotherapy (SOC) in refractory and metastatic (R/M) HNSCC after platinum‐based chemotherapy in a randomized phase III clinical [1]. This clinical trial demonstrated a median overall survival of 7.5 months versus 5.1 months, overall response rate (ORR) of 13.3% versus 5.8%, 6‐month progression‐free survival (PFS) rate of 19.7% versus 9.9%, and 1‐year survival rate of 36% versus 16.6% favoring the anti–PD‐1 inhibitor. However, PFS was 2 months for nivolumab compared with 2.3 months for SOC, hinting at late efficacy in this cohort of patients. Moreover, a large randomized clinical trial demonstrated an efficacy of the pembrolizumab plus chemotherapy and pembrolizumab alone (PD‐L1–positive patients) in the frontline treatment of patients with HNSCC [11]. However, given the modest efficacy of single agent anti–PD‐1 inhibitor in R/M HNSCC, a search for adjunct immunostimulatory regimens to improve efficacy is underway. Cyclophosphamide, when administered in low dose, has been shown to improve the immunologic and clinical responses of anticancer vaccines [12]. This immunologic reaction is achieved by increasing expression of class I human leukocyte antigen in the tumor microenvironment or on cancer cells as well as depleting regulatory T (Treg) cells, which can be increased after radiation treatment, as radiation therapy (RT) can increase Treg cells relative to cytotoxic T cells [13]. By reducing Treg cells, antitumor CD8+ cytotoxic effector T cells can be activated and expanded [12]. Low dose cyclophosphamide depleted Treg cells in patients treated with oncolytic viruses without compromising antitumor or antiviral T‐cell responses in a clinical study [14]. Radiation therapy sensitizes cancer cells to immune‐mediated attack via release of tumor antigens from killed cells, increasing tumor cell expression of antigens and receptor‐mediated T‐cell recognition and killing, and enhanced activity of antigen‐presenting cells [2]. In addition, RT induces expression of chemokines needed for T‐cell trafficking, and preclinical data demonstrated that tumor cells may counterbalance the aforementioned effect by upregulating the expression of PD‐L1 [15]. Thus, these observations suggested a strong rationale for combining an anti–PD‐1 inhibitor with RT and cyclophosphamide. Granulocyte macrophage colony‐stimulating factor plays a role in dendritic cell maturation and proliferation in addition to activation of T cells [5]. Proliferation of dendritic cells enhances cancer cell antigen presentation produced by RT and thus can ameliorate the activity of the studied regimen and was thus studied in combination [16]. Our clinical trial demonstrated tolerability of the regimen with the most common treatment‐emergent adverse events (TEAEs) of fatigue (40%) and constipation (26.7%) and asthenia, dyspnea, maculo‐papular rash, and pneumonia (20% each). The most common grade 3 TEAE was pneumonia, which occurred in two patients (13.3%), and dehydration, which occurred in one patient (6.7%). One patient discontinued treatment due to TEAE of community acquired pneumonia. Serious TEAE of any grade occurred in 40% of patients, and serious TEAE of grade 3 occurred in 26.7% of patients. All patients discontinued therapy due to progression of disease. The median number of cemiplimab doses administered was 6 (range 1–19), and the median duration of exposure was 12.3 weeks (range 2.0–41.7). The median duration of follow‐up was 3.3 months (range 0.5–10.2). The median PFS was 1.8 months (95% confidence interval, 1.7–4.7). The ORR was 6.7% (RECIST version 1.1) with one patient achieving a partial response (PR) and a second patient with unconfirmed PR, who hence was downgraded to stable disease, and the stable disease rate was 33.3%. The data demonstrate that this regimen is similar to anti–PD‐1 monotherapy treatment in patients with R/M HNSCC. This potentially may be secondary to the fact that enrolled patients received multiple lines of therapy including RT; hence it difficult to elicit an appropriate proimmune tumor microenvironment. In a small randomized trial of nivolumab with or without a similar radiation regimen, no benefit was seen with the addition of radiation [17]. The protocol allowed for the enrollment of patients regardless of PD‐L1 expression or human papillomavirus positivity, and hence it is difficult to correlate responses with those biomarkers. The trial/cohort enrolled a low number of patients, and hence it is difficult to establish efficacy. This regimen can be further investigated in earlier lines of therapy; however, in patients with R/M HNSCC the combination failed to reveal higher efficacy compared with single agent anti–PD‐1.
Disclosures
Hani Babiker: Celgene, Endocyte (C/A), Regeneron (RF), Bayer, Sirtex (H); Irene Brana: AstraZeneca, Bristol‐Meyer Squibb, Merck Serono, Merck Sharp & Dohme, Orion Pharma, Rakuten Aspyrian, Roche, Celgene, GlaxoSmithKline, Incyte, Janssen, Kura, Novartis, Pfizer, Shattuck Lab, VCN Biosciences (RF), AstraZeneca, Bristol‐Meyer Squibb, Merck Serono, Merck Sharp & Dohme, Orion Pharma, Rakuten Aspyrian, Roche (personal fees); Daruka Mahadevan: Abbvie (speakers' bureau, travel, accommodation expenses); Taofeek Owonikoko: Novartis, Celgene, Lilly, Sandoz, Abbvie, Eisai, G1 Therapeutics, Takeda, Seattle Genetics, Bristol‐Myers Squibb, MedImmune (C/A); Emiliano Calvo: Boehringer Ingelheim, Roche/Genentech, Bristol‐Myers Squibb, Novartis, PsiOxus, Nanobiotix, Janssen, Abbvie, PharmaMar, PUMA, Sanofi, Lilly, Pfizer, Merck, Nektar, Amcure, Amgen, AstraZeneca, Principia, Bayer, CytomX, H3, Incyte, Kura, LOXO, Macrogenics, Menarini, Merck, Serono, Merus, Millenium, Rigontec, Tahio, TesaroReceipt (RF), Novartis, Nanobiotix, Janssen‐Cilag, PsiOxus Therapeutics, Seattle Genetics, Pierre Fabre, Boehringer Ingelheim, Cerulean Pharma, EUSA, Abbvie, Celgene (C/A, H), Novartis (speaker's bureau fees); Danny Rischin: Genentech, Kura Oncology, Regeneron Pharmaceuticals, Inc., Bristol Myers‐Squibb, GSK, Merck (RF), Regeneron Pharmaceuticals, Inc., Bristol Myers‐Squibb, GSK,Merck (SAB), Merck (travel support); Victor Moreno: Bayer, Basilea Pharmaceuticals, Bristol‐Myers Squibb, Janssen, Pieris (C/A); Kyriakos P. Papadopoulos: Regeneron Pharmaceuticals, Inc., ARMO Biosciences, Amgen, Incyte, Merck Serono, MedImmune, Mabspace Biosciences, ADC Therapeutics, 3D Medicines, Jounce Therapeutics, F‐Start, Bayer (RF–institutional), Bayer (SAB, Other [personal fees]; Marka Crittenden: Regeneron Pharmaceuticals Inc, Bristol‐Myers Squibb, Mavu Pharma, Nanobiotix Jounce (RF), Regeneron Pharmaceuticals Inc., Celldex (SAB); Pilar Garrido: Roche, Merck Sharp & Dohme, Bristol‐Myers Squibb, Boehringer Ingelheim, Pfizer, Abbvie, Guardant Health, Novartis, Eli Lilly, AstraZeneca, Jansen, Sysmex, Blueprint Medicines, Takeda (C/A), Roche, Merck Sharp & Dohme, Bristol‐Myers Squibb, Pfizer, Novartis, Boehringer Ingelheim, Rovi (speaker fees); Kosalai Kal Mohan: Regeneron Pharmaceuticals (E, OI); Matthew Fury: Regeneron Pharmaceuticals (E, OI); Israel Lowy: Regeneron Pharmaceuticals (E, OI, IP); Melissa Mathias: Regeneron Pharmaceuticals (E, OI); Minjie Feng: Regeneron Pharmaceuticals (E–previously, OI), Bristol‐Myers Squibb (E–currently); Jingjin Li: Regeneron Pharmaceuticals (E); Elizabeth Stankevich: Regeneron Pharmaceuticals (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank the patients, their families, and all investigators involved in this study. The study was funded by Regeneron Pharmaceuticals, Inc., and Sanofi. Hani Babiker is currently affiliated with Mayo Clinic, Jacksonville, FL.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.govIdentifier: NCT02383212
- Sponsor: Regeneron Pharmaceuticals and Sanofi
- Principal Investigator: Hani Babiker
- IRB Approved: Yes
References
- 1.Ferris RL, Blumenschein G, Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara TA, Hodge JW et al. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther 2009;11:37–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Kershaw MH, Devaud C, John LB et al. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology 2013;2:e25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewan MZ, Vanpouille‐Box C, Kawashima N et al. Synergy of topical toll‐like receptor 7 agonist with radiation and low‐dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res 2012;18:6668–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hercus TR, Thomas D, Guthridge MA et al. The granulocyte‐macrophage colony‐stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood 2009;114:1289–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden EB, Chhabra A, Chachoua A et al. Local radiotherapy and granulocyte‐macrophage colony‐stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof‐of‐principle trial. Lancet Oncol 2015;16:795–803. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP, le Maître A, Maillard E et al.; MACH‐NC Collaborative Group . Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol J 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Domenge C, Ozsahin M et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 10.Saloura V, Cohen EEW, Licitra L et al. An open‐label single‐arm, phase II trial of zalutumumab, a human monoclonal anti‐EGFR antibody, in patients with platinum‐refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2014;73:1227–1239. [DOI] [PubMed] [Google Scholar]
- 11.Burtness B, Harrington KJ, Greil R et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): A randomised, open‐label, phase 3 study. Lancet 2019;394:1915–1928. [DOI] [PubMed] [Google Scholar]
- 12.Le DT, Jaffee EM. Regulatory T cell modulation using cyclophosphamide in vaccine approaches: A current perspective. Cancer Res 2012;72:3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiringhelli F, Larmonier N, Schmitt E et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336–344. [DOI] [PubMed] [Google Scholar]
- 14.Cerullo V, Vähä‐Koskela M, Hemminki A. Oncolytic adenoviruses. Oncoimmunology 2012;1:979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng L, Liang H, Burnette B et al. Irradiation and anti–PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vatner RE, Janssen EM. STING, DCs and the link between innate and adaptive tumor immunity. Mol Immunol 2019;110:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride S, Sherman E, Tsai CJ et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol 2021;39:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


