Abstract
Background
Total neoadjuvant therapy (TNT) is a novel approach for locally advanced rectal cancer (LARC), which attempts to deliver both systemic chemotherapy and neoadjuvant chemoradiotherapy prior to surgery. However, its efficacy and safety remain controversial in randomized controlled trials (RCTs). We conducted this meta‐analysis to assess such concerns.
Materials and Methods
Head‐to‐head phase II/III RCTs were searched in Embase, PubMed, Web of Science, and the Cochrane Library, as well as other sources. The primary endpoint was pathologic complete response (pCR). Secondary endpoints were disease‐free survival (DFS), overall survival (OS), local recurrence‐free survival, distant metastasis‐free survival, and the R0 resection rate.
Results
Eight phase II/III RCTs involving 2,196 patients with LARC were assessed. The primary analysis demonstrated a statistically significant improvement in the pCR rate for TNT treatment (odds ratio, 1.77; 95% confidence interval [CI], 1.28–2.45; p = .0005). TNT treatment also showed improvements in DFS and OS outcomes compared with standard chemoradiotherapy (hazard ratio [HR], 0.83; 95% CI, 0.72–0.96; p = .03 and HR, 0.88; 95% CI, 0.74–1.05; p = .15). In addition, TNT treatment showed significant efficacy in reducing the risk of distant metastasis (HR, 0.81; 95% CI, 0.68–0.95; p = .012).
Conclusion
The overall pCR rate may be improved with TNT compared with standard treatment. The TNT strategy may also improve DFS and OS and reduce the risk of distant metastasis.
Implications for Practice
Locally advanced rectal cancer (LARC) is a relatively common disease, with a poor prognosis because of its high metastatic potential. The role of total neoadjuvant therapy (TNT) has always been controversial. This meta‐analysis found that TNT in LARC is associated with a significant improvement in overall pathologic complete response rate, disease‐free survival, overall survival, and distant metastasis‐free survival compared with standard treatment. TNT is a promising strategy for LARC, especially for patients who have little desire for surgery.
Keywords: Locally advanced rectal cancer (LARC), Pathologic complete response (pCR), Total neoadjuvant therapy (TNT), Meta‐analysis
Short abstract
Total neoadjuvant therapy (TNT) is a novel therapeutic approach for locally advanced rectal cancer that delivers both systemic chemotherapy and neoadjuvant chemoradiotherapy before surgery; however, this approach remains controversial. This article reports the results of a meta‐analysis comparing TNT with standard chemotherapy.
Introduction
Colorectal cancer is the second cause of cancer‐related deaths worldwide and accounts for 10% of all cancer types [1]. For patients with locally advanced rectal cancer (LARC), multimodal treatment consisting of neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) surgery and adjuvant chemotherapy has become the standard of care [2]. Although the standard treatment models have resulted in tumor downstaging, improved quality of life, and lowered rates of local disease recurrence [3], pathologic complete response (pCR) is only achieved in 10%–30% of patients with LARC using neoadjuvant CRT, with a high rate of distant metastasis (DM) also remaining in such patients, seriously threatening their life [4]. As for postoperative chemotherapy, the National Comprehensive Cancer Network guidelines recommend TME should be followed by adjuvant systemic chemotherapy; however, less than 50% of eligible patients receive their scheduled adjuvant chemotherapy due to delays, treatment compliance, and postoperative complications [5, 6].
To overcome such problems, a novel therapeutic approach, total neoadjuvant therapy (TNT), attempts to deliver both systemic chemotherapy and neoadjuvant CRT prior to surgery. Accumulating evidence shows that TNT in patients with LARC is expected to improve survival and reduce DM via systematic chemotherapy to prevent the onset of micrometastases [7]. TNT has also been associated with better compliance, a decrease in toxicity, a reduced need for ileostomy and its duration, and increased complete clinical response, followed by the watch and wait strategy to improve anal sphincter preservation rates [3, 8, 9, 10, 11, 12]. TNT is a promising treatment for with LARC and has been explored in previous single‐arm trials, albeit with small sample sizes. These studies show that the pCR in patients who received TNT treatment was approximately 20%–40% [13, 14]. In a retrospective cohort study, patients with LARC treated with the TNT approach of induction chemotherapy followed by CRT before surgery received a greater percentage of the planned chemotherapy dose than those in the standard CRT group. The TNT group also had a higher pCR rate than the adjuvant chemotherapy group (36% vs. 21%) [15].
Recently, multiple randomized controlled trials (RCTs) evaluated different TNT strategies compared with standard CRT therapy, in terms of CRT sequencing and systematic chemotherapy, radiotherapy modality, and TNT intensity. Yet, the efficacy and safety of TNT remains controversial. This meta‐analysis aims to explore the pCR and survival value of TNT treatment compared with standard CRT. A previous meta‐analysis reported TNT increased the odds of pCR by 39% [8] compared with standard CRT and suggested TNT had better survival than standard CRT. Here, our study builds upon previous understandings by including newer and more reliable research data with larger sample sizes, better quality prospective RCTs, and more detailed subgroup analysis.
Materials and Methods
Study Eligibility and Identification
Embase, PubMed, Web of Science, the Cochrane Library, and other sources were comprehensively searched up to July 15, 2020. Search terms and their combinations included “neoadjuvant therapy,” “rectum tumor,” and “randomized controlled trial.” The detailed search strategy is listed in supplemental online Table 1. Book abstracts and conference proceedings were manually searched to ensure the latest oncological data were considered. After screening titles and abstracts, literature selection was conducted by two independent reviewers (S.L. and L.X.). We also reviewed the reference lists of relevant studies to confirm all eligible studies were included in this meta‐analysis. Our inclusion criteria were (a) prospective phase II or III trials involving rectal cancer patients; (b) patients who received induction (or consolidation) chemotherapy plus neoadjuvant chemoradiotherapy; and (c) at least one available outcome data regarding systemic therapy in patients with LARC. Studies that failed to meet the above criteria were excluded from this meta‐analysis. This systematic review is registered at PROSPERO (registration no. CRD42020187992).
Outcome Measures, Data Extraction, and Quality Assessment
Clinical outcomes were pCR, disease‐free survival (DFS), overall survival (OS), local recurrence‐free survival (LRFS), distant metastasis‐free survival (DMFS), and R0 resection rate. Two investigators (S.L. and T.J.) used a standardized form to independently extract the summaries, clinical outcomes, and study data (the study's authors, publication year, patient categories, age, gender, clinical stage, oncologic performance scale, study design, therapeutic regimens, sample size, and the distance between the tumor and the anal verge). The methodological quality of each study was assessed by two reviewers according to the Cochrane Handbook for Systematic Reviews of Interventions. Seven aspects were assessed: random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other biases. Each included study was assessed for a “high risk of bias,” “low risk of bias,” or “unclear risk of bias.” Any disagreements were resolved by consensus.
Statistical Analysis
The hazard ratios (HRs) for survival outcomes (DFS, OS, LRFS and DMFS), and the odds ratio (OR) for binary outcomes (pCR, R0 resection rate) as well as their 95% confidence intervals (CIs) were used to measure the efficacy and safety of the treatment strategies. For specific comparisons, an agent with HR for DFS, OS, LRFS, and DMFS <1 or OR for pCR and R0 resection rate >1 was deemed preferable to its contrast in efficacy. The statistical significance of the studies was evaluated by the Z test with significance set at a p < .05. Heterogeneity among the studies was quantified using Cochran's Q‐statistic and I2 statistic. Substantial heterogeneity was considered to exist with a p < .1 or I2 > 30%, and the random‐effects method was reported after exploring the causes of heterogeneity. Otherwise, the fixed‐effects method was reported.
For the primary outcomes, we performed subgroup analysis for treatment sequence, TNT intensity and type of radiotherapy. A sensitivity analysis was performed by removing each study in turn to establish the extent to which they contributed to the overall result. All statistical tests were performed by Review Manager version 5.3 and Stata version 12.0.
Results
Characteristics of the Selected Studies
According to the retrieval strategy and other sources, 4,874 related studies were initially identified. In total, 2,881 potential studies were included after removing 1,993 duplicates, 2,578 were excluded from title and abstract screening, 303 studies were considered eligible for full‐text assessment, 289 full‐text articles were excluded, 146 studies were excluded because they were not randomized control trials, 62 lacked relevant intervention or endpoints, 54 studies were excluded as they were reviews, and 12 studies were excluded because of no available results, and our efforts were unsuccessful in contacting the authors for further data. Eight RCTs met the inclusion criteria in this analysis [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]. All eight studies (n = 2,196 patients) were included in this final analysis (n = 1,113 treated with TNT and n = 1,083 treated with standard CRT). This study's flowchart is presented in Figure 1.
Figure 1.
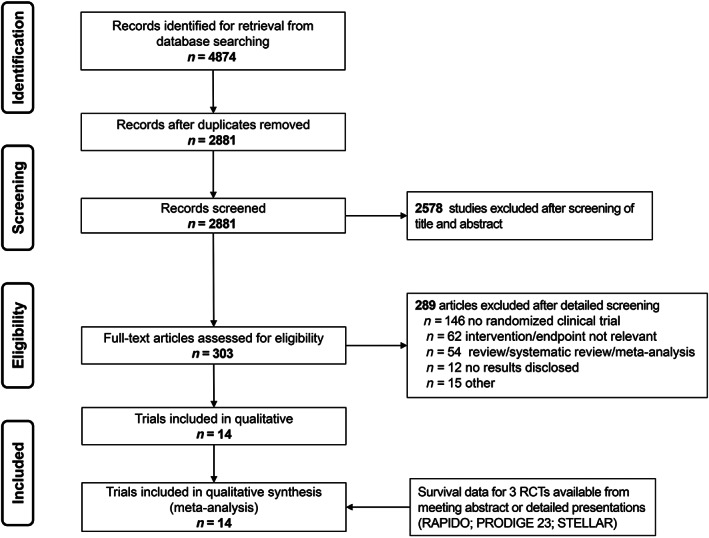
Preferred reporting items for systematic reviews and meta‐analysis flowchart. Abbreviation: RCT, randomized control trial.
Our analysis included four phase II trials and four phase III trials. In most of the included studies, induction or consolidation (neoadjuvant) chemotherapy was multiagent oxaliplatin‐based (n = 6) [17, 18, 20, 25, 26, 29]. One study [24] used 5‐fluorouracil plus folinic acid and another one used mFOLFIRINOX [30] (modify oxaliplatin, irinotecan, leucovorin, 5‐fluorouracil). Three studies [17, 18, 30] used an induction treatment paradigm, and five studies [20, 24, 25, 26, 29] explored the effects of consolidation treatment. In five studies [17, 18, 24, 26, 30], patients were treated with standard, long‐course CRT (~50.4 Gy) in the experimental arm. In three studies [20, 25, 29], short‐course radiotherapy (5 × 5 Gy) was the selected regimen (Table 1). For detailed patient characteristics of each trial, see Table 2.
Table 1.
Trial characteristics
| Study | Author/PI, yr | Study ID Numbers | Primary endpoint | Secondary endpoints | Study design | Study period | No. of trial centers | TNT treatment strategy vs. standard treatment strategy |
|---|---|---|---|---|---|---|---|---|
| Marechal/2011 | Marechal, 2011 | EudraCT: 2006‐006646‐34 | ypT0‐1N0 rate |
1. pCR rate 2. toxic effects 3. sphincter preservation rate |
Phase II RCT | n.i. | Multicenters | mFOLFOX6 × 2 ‐CRT [5FU + 50.4 Gy] ‐ TME vs. CRT [5FU + 50.4 Gy] ‐ TME |
| GCR‐3 | Fernandez‐Martos, 2015 | NA | pCR |
1. Toxicity 2. treatment compliance, downstaging 3. R0 resection rates 4. 30‐d surgical complications 5. cumulative incidence of LR 6. DMs 7. DFS 8. OS |
Phase II RCT | May 2006 to December 2007 | 14 | Capeox × 4 ‐ CRT [Capeox+50.4 Gy] ‐ TME vs. CRT [Capeox+50.4 Gy] ‐ TME ‐ Capeox × 4 |
| POLISH II | Bujko, 2016 | NCT00833131, PGBRJG0109 | The rate of patients with R0 resection [time frame: surrogate endpoint available immediately after surgery] |
1. Overall long‐term survival 2. Progression‐free long‐term survival 3. the rate of local failures 4. the rate of distant metastases 5. the rate of early toxicity of neoadjuvant treatment according to the NCI CTCAE (version 3.0) 6. the rate of postoperative complications 7. the rate of late toxicity according to the RTOG/EORTC scale 8. the rate of complete pathological response |
Phase III RCT | November 2008 to November 2012 | 39 | 5 × 5Gy ‐ FOLFOX4 × 3 ‐TME vs. CRT [50.4 Gy + 5 ‐ FU+ AF + OXA] ‐ TME |
| WAIT | Moore, 2017 | ACTRN12611000339954 | pCR rate | n.i. | Phase II RCT | April 2012 to June 2014 | 3 | CRT [5FU + 50.4 Gy] ‐5FU + LV × 3 ‐TME vs. CRT [5FU + 50.4 Gy] ‐ TME |
| KCSG CO 14‐03 | Kim, 2018 | NCT01952951, KCSG CO14‐03 | Downstaging rate [time frame: expected average of 15 wk after start of study treatment] |
1. Pathologic response 2. radiologic response rate 3. toxicity profile 4. pattern of failure 5. local control rate 6. relapse‐free survival 7. disease‐free survival 8. overall survival 9. quality of life |
Phase II RCT | June 2014 to September 2017 | 7 | CRT [Cape/5FU + 50.4 Gy] ‐Capox × 2 ‐TME vs CRT [Cape/5FU + 50.4 Gy] ‐ TME |
| STELLAR | Jing, 2019 |
XT2015‐03, CH‐GI‐090 |
disease‐free survival rate [time frame: 3 yr] |
1. overall survival rate 2. incidence of surgical complications 3. incidence of acute toxicities during radiation or chemotherapy |
Phase III RCT | August 2015 to August 2023 | 11 | 5 × 5Gy ‐ Capeox × 4 ‐ TME ± Capeox × 2 vs. CRT [25 × 2Gy + Cape] ‐ TME ± Capeox × 6 |
| RAPIDO | Hospers G, 2020 | NCT01558921, NL36315.042.11, 2010‐023957‐12 (EudraCT Number) | Disease‐related treatment failure [time frame: 3 yr follow‐up after surgery] |
1. Overall survival 2. CRM negative rate 3. pCR rate 4. Short and long‐term toxicity 5. Surgical complications 6. Quality of life questionnaires |
Phase III RCT | June 21, 2011, to March 8, 2020 | 56 | 5 × 5Gy ‐ Capeox × 6/FOLFOX4 × 9‐TME vs. CRT [28 × 1.8Gy/25 × 2Gy‐Cape] ‐ TME ‐ Capeox × 8/FOLFOX4 × 12 |
| PRODIGE 23 | T. Conroy, 2020 | NCT01804790, PRODIGE 23 ‐ UCGI 23 | disease‐free survival [time frame: 3 yr] | Overall survival [time frame: 7 yr] | Phase III RCT | January 2012 to September 2019 | 36 | mFOLFIRINOX × 6 ‐ CRT [50.4Gy/25F + Cape] ‐ TME ‐ mFOLFOX6 × 6/Cape × 4 vs. CRT [50.4Gy/25F + Cape] – TME ‐ mFOLFOX6 × 12/Cape × 8 |
Abbreviations: 5FU, 5‐fluorouracil; Cape, capecitabin; Capox, capecitabine + oxaliplatin; CRM, circumferential resection; CRT, chemoradiotherapy; DFS, disease‐free survival; DM, distant metastasis; FOLFOX, oxaliplatin + calcium folinate +5‐fluorouracil; LR, local relapse; mFOLFIRINOX, modify oxaliplatin + irinotecan + calcium folinate +5‐fluorouracil; mFOLFOX, modify oxaliplatin + leucovorin +5‐fluorouracil; n.i., no information; NA, not applicable; OS, overall survival; pCR, pathologic complete response; PI, principal investigator; RCT, randomized clinical trial; TME, total mesorectal excision.
Table 2.
Patients’ characteristics
| Study | Author/PI, yr | Sample size of ITT analysis | Gender, No. | Age, yr | Oncologic performance scale, No. | Clinical stage, No. | Distance between tumor and anal verge (cm) | Circumferential margin, No. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNT treatment | Standard treatment | ||||||||||||||||
| TNT treatment | Standard treatment | Female | Male | Female | Male | TNT treatment | Standard treatment | TNT treatment | Standard treatment | TNT treatment | Standard treatment | TNT treatment | Standard treatment | TNT treatment | Standard treatment | ||
| Marechal/2011 | Marechal, 2011 | 28 | 29 | 7 | 21 | 13 | 16 | 62 (22–80) | 62 (44–79) | ECOG‐0 (21), ECOG‐1 (7) | ECOG‐0 (25), ECOG‐1 (4) | cT2 (1), cT3 (25), cT4 (2), Any cTxN+ (26) | cT2 (3), cT3 (23), cT4 (3), Any cTxN+ (25) | Lower third (11), middle third (13), upper third (4) | Lower third (13), middle third (9), upper third (7) | >5 mm (18), ≤5 mm (7), not carried out (3) | >5 mm (15), ≤5 mm (9), not carried out (5) |
| GCR‐3 | Fernandez‐Martos, 2015 | 56 | 52 | 17 | 19 | 18 | 34 | 60 (38–76) | 62 (42–75) | ECOG‐0 (33), ECOG‐1 (22), ECOG‐2 (1), Unknown (‐) | ECOG‐0 (36), ECOG‐1 (15), ECOG‐2 (‐), Unknown (1) | T4 resectable (7), T3 low third (≤6 cm from pectineal line) (18), threatened or involved MRF (‐), any T3N+ (31), missing data (‐) | T4 resectable (3), T3 low third (≤6 cm from pectineal line) (12), threatened or involved MRF (5), any T3N+ (31), missing data (1) | n.i. | n.i. | n.i. | n.i. |
| POLISH II | Bujko, 2016 | 261 | 254 | 78 | 183 | 85 | 169 | 60 (54–66) | 60 (55–65) | WHO‐0 (129), WHO‐1 (120), WHO‐2 (11), WHO‐3 (1) | WHO‐0 (126), WHO‐1 (115), WHO‐2 (5), WHO‐3 (0) | cT3 (88), cT4 (165), recurrent (8) | cT3 (83), cT4 (163), recurrent (8) | 0–5 (148) >5–10 (106) >10–15 (7) | 0–5 (138) >5–10 (99) >10–15 (16) no data (1) | n.i. | n.i. |
| WAIT | Moore, 2017 | 25 | 24 | 7 | 18 | 6 | 18 | 59.7 ± 9.9 | 60.5 ± 12.6 | n.i. | n.i. | T2 (0), T3 (24), T4 (1), N0 (0), N1 (6), N2 (19) | T2 (1), T3 (18), T4 (5), N0 (2), N1 (7), N2 (15) | 6.6 ± 2.6 | 6.0 ± 2.5 | clear (10), threatened (8), involved (7) | clear (12), threatened (4), involved (8) |
| KCSG CO 14–03 | Kim, 2018 | 53 | 55 | 17 | 36 | 9 | 46 | 56 ± 10 | 55 ± 8 | ECOG‐0 (16), ECOG‐1 (37) | ECOG‐0 (16), ECOG‐1 (39) | T3 (44), T4 (9), N0 (3), N+ (49), unknown (1) | T3 (45), T4 (10), N0 (4), N+ (51), unknown (0) | n.i. | n.i. | Involved (14), not involved (37), unknown (2) | Involved (16), not involved (38), unknown (1) |
| STELLAR | Jing, 2019 | 104 | 105 | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | n.i. | MRF (+) (14) | MRF (+) (15) |
| RAPIDO | Hospers G, 2020 | 462 | 450 | 162 | 300 | 138 | 312 | 62 (31–83) | 62 (23–84) | ECOG‐0 (369), ECOG‐1 (93) | ECOG‐0 (365), ECOG‐1 (85) | cT2‐3N0 (24), cT2‐3N+ (289), cT4N0 (23), cT4N+ (124) | cT2‐3N0 (19), cT2‐3N+ (278), cT4N0 (23), cT4N+ (121) | <5 cm (103), 5–10 cm (180), ≥10 cm (145), unknown (32) | <5 cm (114), 5–10 cm (148), ≥10 cm (148), unknown (21) | MRF+ (284) | MRF+ (263) |
| PRODIGE 23 | T. Conroy, 2020 | 231 | 230 | 81 | 150 | 74 | 156 | 61 (34–77) | 62 (26–75) | WHO‐0 (179), WHO‐1 (52) | WHO‐0 (185), WHO‐1 (45) | T2 (3), T3 (187), T4 (41), N+ (206) | T2 (2), T3 (192), T4 (36), N+ (207) | ≤5 cm (87), 5.1–10 cm (114), 10.1–15 cm (30) | ≤5 cm (83), 5.1–10 cm (118), 10.1–15 cm (29) | ≤1 mm (60) | ≤1 mm (64) |
Abbreviations: CRM, circumferential resection margin; ECOG, Eastern Cooperative Oncology Group; ITT, intention to treat; MRF, mesorectal fascia; n.i., no information; NA, not applicable; pCR, pathologic complete response; RCT, randomized clinical trial; TNT, total neoadjuvant therapy; WHO, World Health Organization.
Risk of Bias
Risk of bias was performed by two investigators (S.L. and T.J.) using the Cochrane risk‐of‐bias tool. All eight trials (100%) described adequate random sequence generation and all eight (100%) had adequate treatment allocation concealment. Patients and care providers were blinded in seven trials (87.5%), and seven trials (87.5%) blinded the outcome assessment process. Detailed risk information is provided in supplemental online Figure 1.
Meta‐Analysis of pCR Rates
For the primary endpoint pCR percentage, eight trials were analyzed with a total of 2,196 patients. All trials reported a pCR rate which demonstrated a statistically significant benefit for TNT treatment (OR, 1.77; 95% CI, 1.28–2.45; p = .0005; Fig. 2A).
Figure 2.
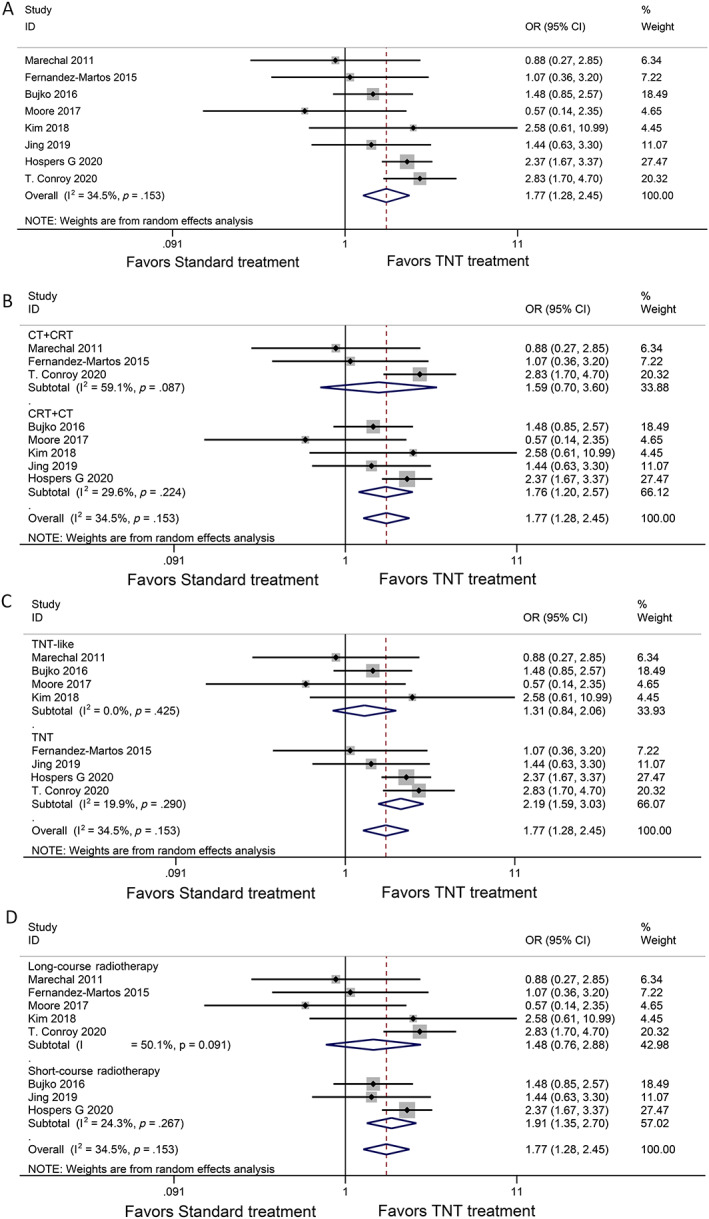
Forest plots for pathologic complete response. (A): Pathologic complete response with total neoadjuvant therapy versus standard chemoradiation, (B) subgroup analysis induction chemotherapy followed by chemoradiotherapy (CRT) vs. CRT followed by consolidation chemotherapy, (C) subgroup analysis TNT‐like vs. TNT, (D) subgroup analysis. Long‐course radiotherapy vs. short‐course radiotherapy are shown. Abbreviations: CI, confidence interval; OR, odds ratio; TNT,
There were different results in the subgroup analysis with CRT followed by consolidation chemotherapy (CRT + CT) versus induction chemotherapy followed by CRT (CT + CRT). Patients treated with CRT + CT (OR, 1.76; 95% CI, 1.20–2.57; p = .004; Fig. 2B) had a higher pCR rate than those who received CT + CRT (OR, 1.59; 95% CI, 0.70–3.60; p = .26; Fig. 2B). We defined two groups according to the total time of neoadjuvant radiotherapy and chemotherapy: less than 12 weeks was considered a TNT‐like strategy, and more than 12 weeks was considered a TNT strategy. In the TNT‐like studies (OR, 1.31; 95% CI, 0.84–2.06; p = .24; Fig. 2C), there was no significant difference in the pCR rate between TNT and the standard treatment arms, although TNT studies (OR, 2.19; 95% CI, 1.59–3.03, p < .00001; Fig. 2C) had a higher pCR compared with standard treatment. The pCR comparisons were also different in the short‐course radiotherapy subgroup (OR, 1.91; 95% CI, 1.35–2.70; p = .0003; Fig. 2D) and the long‐course radiotherapy subgroup (OR, 1.48; 95% CI, 0.76–2.88; p = .25; Fig. 2D).
Meta‐Analysis of Survival Outcomes
For survival outcomes, TNT treatment showed improvements in DFS and DMFS compared with standard CRT (HR, 0.83; 95% CI, 0.72–0.96, p = .03 and HR, 0.81; 95% CI, 0.68–0.95; p = .012; Fig. 3A, D). As for OS and LRFS, no statistically significant differences were observed in the TNT treatment compared with standard treatment (HR, 0.88; 95% CI, 0.74–1.05; p = .15 and HR, 1.19; 95% CI, 0.94–1.51; p = .151; Fig. 3B, C).
Figure 3.
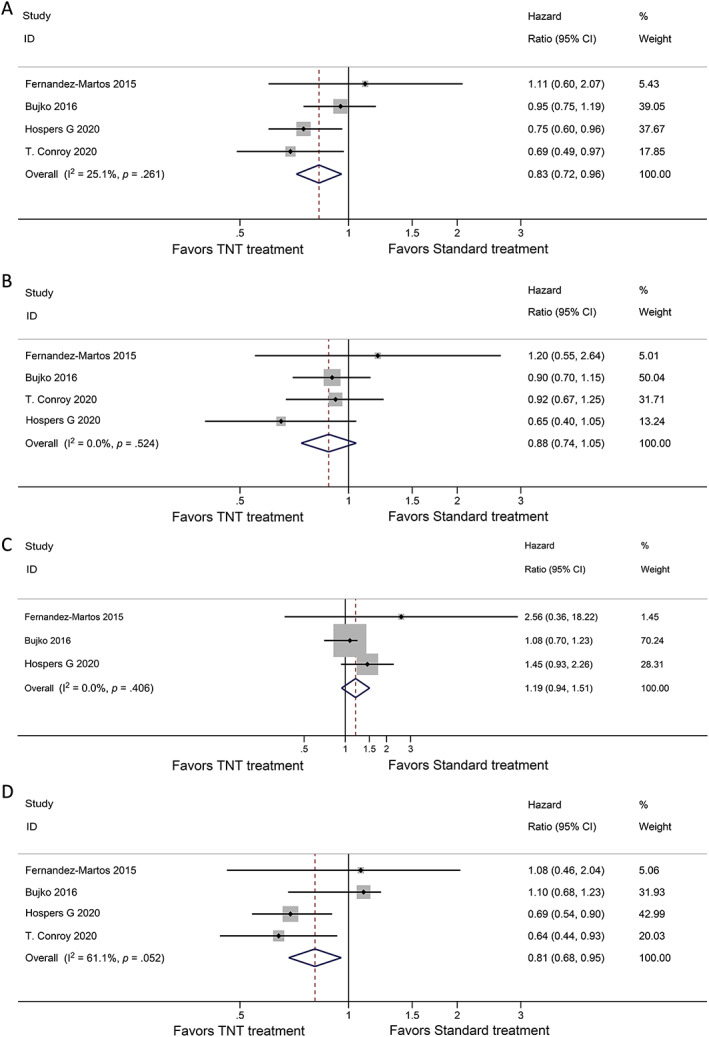
Forest plots for survival endpoints. Analysis for (A) disease‐free survival, (B) overall survival, (C) local recurrence‐free survival, and (D) distant metastasis‐free survival are shown. Abbreviations: CI, confidence interval; TNT, total neoadjuvant therapy.
Meta‐Analysis of the R0 Resection Rate
In comparative studies of the R0 resection rate, the intention‐to‐treat population was the one that could be resected; thus, only four studies were included [18, 24, 25, 31]. No statistically significant findings were observed between patients who received TNT and standard treatment (OR, 1.04; 95% CI, 0.71–1.53; p = .85; Fig. 4).
Figure 4.
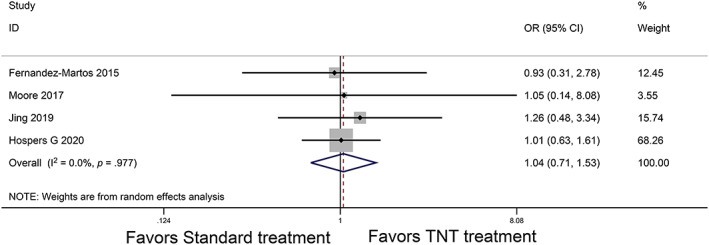
Forest plot for the R0 resection rate. Abbreviations: CI, confidence interval; OR, odds ratio; TNT, total neoadjuvant therapy.
Surgery and safety
Grade 3–4 acute toxicities were more common in the TNT treatment group (9%–41%) than in the standard treatment group (2%–29%). Gastrointestinal (diarrhea, nausea), hematologic (neutropenia), and fatigue adverse events were the most common chemoradiotherapy‐related toxicities. Overall, the surgical methods used and the main postoperative complications of TNT regimens were comparable with standard CRT (Table 3).
Table 3.
Surgery and safety
| Study | Author/PI, yr | Type of surgery, % | Grade 3–4 toxicities, % | Main grade 3–4 acute toxicities of CRT % (≥5%) | Main postoperative complications % (≥5%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| TNT treatment | Standard treatment | TNT treatment | Standard treatment | TNT treatment | Standard treatment | TNT treatment | Standard treatment | ||
| Marechal/2011 | Marechal, 2011 | TME (86), APR (11) | TME (79), APR (17) | Grade 3–4 (26) | Grade 3–4 (7) | gastrointestinal (15), hematological (11) | gastrointestinal (4), hematological (‐) | global postoperative complications (25), pelvic/perineal infections (21) | global postoperative complications (31), pelvic/perineal infections (24) |
| GCR‐3 | Fernandez‐Martos, 2015 | None (4), low anterior (48), abdominoperineal (40), missing (4) | None (11), low anterior (56), abdominoperineal (33), missing (‐) | Grade 3–4 (23) | Grade 3–4 (29) | diarrhea (5) | diarrhea (16) | Infection and wound healing (27), reoperation (8) | Infection and wound healing (20), reoperation (7) |
| POLISH II | Bujko, 2016 | Anterior resection (50), APR (37), Hartmann's procedure (10), resection of locally recurrent tumor (3) | Anterior resection (49), APR (41), Hartmann's procedure (9), resection of locally recurrent tumor (2) | 3 (19), 4 (4), toxic deaths (1) | 3 (16), 4 (5), toxic deaths (3) | n.i. | n.i. | Anastomotic dehiscence requiring reoperation (6). Other complications requiring reoperation (8). Treated conservatively (15) | Anastomotic dehiscence requiring reoperation (4). Other complications requiring reoperation (7). Treated conservatively (12) |
| WAIT | Moore, 2017 | APR/Hartmann (28), Anterior resection (72) | APR/Hartmann (50), Anterior resection (50) | Clavien‐Dindo 3 (12.50), Clavien‐Dindo 4 (20) | Clavien‐Dindo 3 (4.17), Clavien‐Dindo 4 (4) | n.i. | n.i. | n.i. | n.i. |
| KCSG CO 14‐03 | Kim, 2018 | APR (6.8), LAR (77.3), other types of SSS (15.9) | APR (0), LAR (88.5), Other types of SSS (11.5) | Grade 3–4 (9) | Grade 3–4 (4) | NA | NA | NA | NA |
| STELLAR | Jing, 2019 | Dixon (40.4), Mile's (53.8), Hartmann's procedure (5.8) | Dixon (44.8), Mile's (53.3), Hartmann's procedure (1.9) | Grade 3–4 (26) | Grade 3–4 (13) | NA | NA | Grade 3–4 (9) | Grade 3–4 (2) |
| RAPIDO | Hospers G, 2020 | No resection (<1), Hartmann's procedure (5), abdominoperineal resection (34), (low) anterior resection (58), of which without stoma (10), other (2) | No resection (<1), Hartmann's procedure (3), abdominoperineal resection (39), (low) anterior resection (55), of which without stoma (12), other (3) | Grade 3 (41), grade 4 (7) | Grade 3 (23), grade 4 (2) | diarrhea (18), vascular disorders (8) | diarrhea (9), vascular disorders (4) | Pneumonia (5), other infection (7), intra‐abdominal infection (5), reoperations (10) | Pneumonia (3), other infection (5), intra‐abdominal infection (5), reoperations (8) |
| PRODIGE 23 | T. Conroy, 2020 | n.i. | n.i. | n.i. | n.i. | Neutropenia (16.9), G‐CSF use (27.0), diarrhea (11.1), fatigue (7.1), nausea (6.2) | n.i. | postoperative morbidity (29.3) | postoperative morbidity (31.2) |
Abbreviations: APR, abdominoperineal resection; CRT, chemoradiotherapy; G‐CSF, granulocyte colony stimulating factor; LAR, low anterior resection; n.i., no information; NA, not applicable; SSS, sphincter‐saving surgery; TME, total mesorectal excision; TNT, total neoadjuvant therapy.
Publication bias
For the pCR meta‐analysis, the shape of the funnel plots did not show any evidence of asymmetry (supplemental online Fig. 2). There was no obvious evidence of publication bias.
Discussion
To our knowledge, this is the most comprehensive and complete meta‐analysis of RCTs comparing TNT and standard treatment in patients with LARC. Compared with a previously published systematic review, our study includes more up‐to‐date data from the PRODIGE 23 [30], RAPIDO [31], STELLAR, and POLISH II (8‐year survival) trials [27]. We found that the overall pCR rate may be improved with TNT compared with standard treatment (OR, 1.77; 95% CI, 1.28–2.45; p = .0005), which is consistent with Petrelli's study. TNT may also improve OS and DFS and reduce the risk of DM.
Taking a closer look at the treatment strategies reveals the importance of the interval between the completion of CRT and surgery on pCR. The subgroup analysis showed that TNT following CRT, chemotherapy, and then surgery had a higher pCR rate than the standard regimen (OR, 1.76; 95% CI, 1.20–2.57; p = .004). This is consistent with the findings of the subgroup analysis of the CAO/ARO/AIO‐12 [32] trial, which showed that different TNT treatment sequences followed by CRT, chemotherapy, and then surgery would have a higher pCR rate compared with TNT following the sequence of chemotherapy, CRT, and then surgery (25% vs. 17%). The authors of the CAO/ARO/AIO‐12 trial also speculated that the prolonged interval between the completion of CRT and surgery may have contributed to a higher pCR. In fact, Garcia‐Aguilar et al. made similar comparisons in the OPRA trial [33], where they reported that patients treated with CRT followed by systemic chemotherapy increased the chances of sphincter perseveration compared with patients who receive systemic chemotherapy followed by CRT. However, the induction chemotherapy subgroup exhibited no statistical advantage, which might be ascribed to the small sample size.
Another subgroup analysis also revealed the importance of intensity in preoperative chemotherapy conducive to an increased pCR rate. Based on the total time of neoadjuvant radiotherapy and chemotherapy, we divided studies into two groups: a TNT‐like subgroup and a TNT subgroup. The TNT subgroup showed a statistically higher pCR rate compared with standard treatment. The Timing trial also presented similar results in its assessment of whether adding cycles of mFOLFOX6 between chemoradiation and surgery increased the proportion of the pCR rate in a four‐arm design. The pCR rates were 18%, 25%, 30%, and 38% for the four groups, respectively [34, 35]. The pCR rate tended to increase with the number of added mFOLFOX6 cycles during the waiting period. Our results reaffirm the positive value of the TNT strategy when using a full dose of neoadjuvant radiotherapy and chemotherapy.
Relatedly, the radiotherapy dose fraction method also impacts the pCR rate. Over the last few decades, long‐course radiotherapy and short‐course radiotherapy have been developed in parallel, and both are used in neoadjuvant treatment of with LARC. However, which yields superior results is controversial and appears to differ based on certain localities. In general, short‐course radiotherapy is preferred in Northern Europe, whereas long‐course radiotherapy has more support in the U.S. and other European countries [36]. In our study, short‐course preoperative radiotherapy combined with chemotherapy showed a significantly improved pCR rate than the standard CRT regimen (OR, 1.91; 95% CI, 1.35–2.70; p = .0003). Interestingly, all three short‐course radiotherapy subgroup trials were TNT with a consolidation chemotherapy strategy, whereas most of the long‐course radiotherapy subgroup trials were TNT with an induction chemotherapy strategy. The higher pCR rate may be due to the fact that the short‐course radiotherapy subgroup trials had longer intervals between radiotherapy and surgery. Early European studies have reported a higher pCR rate with LARC patients treated with short‐course radiotherapy followed by consolidation chemotherapy, even in the presence of metastatic disease [37, 38], which was the rationale for the POLISH II and RAPIDO studies. One meta‐analysis showed that the pCR rate was higher when surgery was delayed than when it was when performed within 1 week after short‐term radiotherapy, and it was even higher when consolidation chemotherapy was integrated prior to surgery [39]. Short‐course radiotherapy followed by consolidation chemotherapy shows considerable promise, but there is no consensus on the optimal time interval between radiotherapy and chemotherapy, the duration of consolidation chemotherapy, or the ideal modes. Three short‐term radiotherapy TNT studies in our meta‐analysis used three different designs: three cycles of FOLFOX (6 weeks) after 1 week of radiotherapy in the POLISH II trial, four cycles of CAPOX (12 weeks) 11 to 18 days after radiotherapy in the RAPIDO trial, and six cycles of CAPOX (18 weeks) 1 week after radiotherapy in the STELLAR trial. Future studies need to determine the optimal chemotherapy start time after short‐course radiotherapy completion, as well as the ideal regimen and chemotherapy cycle before surgery.
The obvious finding to emerge from this systematic review is that DFS and DMFS may increase with TNT compared with standard CRT (HR, 0.83; 95% CI, 0.72–0.96; p = .03 and HR, 0.81; 95% CI, 0.68–0.95; p = .012). In the Timing study, the four groups showed statistically significant differences in DFS (50%, 81%, 86%, 76%, p = .004) [35]. This is an integral finding supporting the TNT strategy, because DFS and DMFS are long‐term endpoints and may bring about overall survival benefits for some with LARC. As for DFS and DMFS, subgroup analysis was not performed because of the limited availability. Further analysis are needed when the data is available.
One unanticipated finding was that LRFS decreased in the TNT group (HR, 1.19; 95% CI, 0.94–1.51, p = .151). This may be explained by the fact that two of the three included studies used short‐course radiotherapy followed by consolidated chemotherapy. Multiple trials and meta‐analysis appear to demonstrate that short‐course radiotherapy and long‐course radiotherapy are roughly equal in terms of local control [40, 41, 42, 43, 44, 45]. Yet, the interval time between surgery and radiotherapy is an important factor that should be taken into consideration for local recurrence‐free survival. The RCT Lyon R90‐01 indicated that an appropriately prolonged interval time may better tumor regression and improve the pCR rate and sphincter preservation rates. Since then, 6–8 weeks has been considered the most appropriate time for surgery after CRT [46]. Dr. Garcia‐Aguilar et al. found that pelvis fibrosis was more severe with an 11‐week interval time than with a 6‐week interval time after CRT [34]. The results of the GRECCAR‐6 RCT similarly showed that an 11‐week interval after CRT increased the risk of postoperative complications and local recurrence, compared with a 7‐week interval [47]. This may be due to pelvis fibrosis, edema, and accumulating local inflammation making it more difficult to ensure the surgical plane, thereby increasing the difficulty of TME surgery and consequently reducing the R0 resection rate [48, 49, 50, 51].
Grade 3–4 acute toxicities for TNT treatment were generally higher than those from standard CRT treatment. The highest grade 3 acute toxicity was 41% (RAPIDO), but there is no evidence that increased toxicity affects the survival of patients.
Another benefit of the TNT strategy is that it may facilitate greater organ preservation. However, sphincter‐preserving surgery data were too heterogeneous to be pooled in this meta‐analysis. Some studies did not aim to improve anal retention, which may have been one of the sources of heterogeneity. The OPRA study found that the TME‐free rate increased in both TNT treatment groups compared with standard treatment in patients with LARC. An ongoing phase II trial (NCT03840239) in China is evaluating whether two cycles of chemotherapy (CAPOX) before, during, and after long‐course radiotherapy will improve o sphincter preservation rates [52]. The results of this study are eagerly anticipated.
This meta‐analysis has several limitations. First, although eight high‐quality trials were included, only 2,196 patients were analyzed. Second, because of a lack of individual patient data, some analysis were not feasible, including subgroup analysis stratified by clinical stage and the distance between the tumor and the anal verge, and comparison of patients’ compliance to the treatment strategies.
Conclusion
Regardless of the above limitations, this meta‐analysis comprehensively determined the role of TNT. TNT may help improve the pCR rate, increase DFS and OS, and reduce the risk of DM, compared with standard neoadjuvant CRT among patients with LARC. TNT following the sequence of CRT, chemotherapy, and then surgery had a significantly higher pCR rate than the standard regimen. High‐intensity TNT may also be a good choice for patients who are apprehensive or who have little desire for surgery.
Author Contributions
Conception/design: Shuang Liu, GC and YHG, Weiwei Xiao
Provision of study material or patients: Shuang Liu, Lin Xiao
Collection and/or assembly of data: Shuang Liu, Ting Jiang, Shanfei Yang
Data analysis and interpretation: SL Shuang Liu Ting Jiang, Lin Xiao, Qing Liu
Manuscript writing: Shuang Liu, Ting Jiang, Lin Xiao
Final approval of manuscript: Shuang Liu, Ting Jiang, Lin Xiao, Shanfei Yang, Qing Liu, Yuanhong Gao, Gong Chen, Weiwei Xiao
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Supplemental Table 1 MEDLINE search strategy
Acknowledgments
This work was supported by the 5010 Clinical Research Foundation of Sun Yat‐sen University (Grant 5010‐2018‐04).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Yuanhong Gao, Email: gaohy@sysucc.org.cn.
Gong Chen, Email: chengong@sysucc.org.cn.
Weiwei Xiao, Email: xiaoww@sysucc.org.cn.
References
- 1.Siegel RL, Miller KD, Fedewa SA et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 2.Glynne‐Jones R, Wyrwicz L, Tiret E et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28:iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 3.Ludmir EB, Palta M, Willett CG et al. Total neoadjuvant therapy for rectal cancer: An emerging option. Cancer 2017;123:1497–1506. [DOI] [PubMed] [Google Scholar]
- 4.Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF et al. Pathologic complete response in rectal cancer: Can we detect it? Lessons learned from a proposed randomized trial of watch‐and‐wait treatment of rectal cancer. Dis Colon Rectum 2016;59:255–263. [DOI] [PubMed] [Google Scholar]
- 5.Rodel C, Liersch T, Becker H et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol 2012; 13:679–687. [DOI] [PubMed] [Google Scholar]
- 6.Hayden DM, Pinzon MC, Francescatti AB et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: Preventable or unpredictable? J Gastrointest Surg 2013; 17:298–303. [DOI] [PubMed] [Google Scholar]
- 7.Glynne‐Jones R, Grainger J, Harrison M et al. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious? Br J Cancer 2006;94:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrelli F, Trevisan F, Cabiddu M et al. Total neoadjuvant therapy in rectal cancer: A systematic review and meta‐analysis of treatment outcomes. Ann Surg 2020;271:440–448. [DOI] [PubMed] [Google Scholar]
- 9.Azin A, Khorasani M, Quereshy FA. Neoadjuvant chemoradiation in locally advanced rectal cancer: The surgeon's perspective. J Clin Pathol 2019;72:133–134. [DOI] [PubMed] [Google Scholar]
- 10.Goodman KA. Total neoadjuvant therapy for rectal cancer. Cancer Radiother 2018;22:459–465. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Kim NK. Challenges and shifting treatment strategies in the surgical treatment of locally advanced rectal cancer. Ann Gastroenterol Surg 2020;4:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gollins S, Sebag‐Montefiore D.Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (R Coll Radiol) 2016;28:146–151. [DOI] [PubMed] [Google Scholar]
- 13.Chua YJ, Barbachano Y, Cunningham D et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI‐defined poor‐risk rectal cancer: A phase 2 trial. Lancet Oncol 2010;11:241–248. [DOI] [PubMed] [Google Scholar]
- 14.Gao YH, Lin JZ, An X et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: A prospective phase 2 trial. Int J Radiat Oncol Biol Phys 2014;90:1153–1160. [DOI] [PubMed] [Google Scholar]
- 15.Cercek A, Roxburgh CSD, Strombom P et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenz S, Haas CS, Werth SC, Bohnet S, Brabant G.High sensitivity to tolvaptan in paraneoplastic syndrome of inappropriate ADH secretion (SIADH). Ann Oncol 2011;22:2696. [DOI] [PubMed] [Google Scholar]
- 17.Maréchal R, Vos B, Polus M et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: A randomized multicentric phase II study. Ann Oncol 2012;23:1525–1530. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez‐Martos C, Garcia‐Albeniz X, Pericay C et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long‐term results of the Spanish GCR‐3 phase II randomized trial†. Ann Oncol 2015;26:1722–1728. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Jin J, Ling N et al. Phase III study of short‐term radiotherapy followed by neoadjuvant chemotherapy versus preoperative long‐term chemoradiotherapy in locally advanced rectal cancer. Ann Oncol 2015;26(suppl 9):ix70. [Google Scholar]
- 20.Bujko K, Wyrwicz L, Rutkowski A et al. Long‐course oxaliplatin‐based preoperative chemoradiation versus 5 ×5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann Oncol 2016;27:834–842. [DOI] [PubMed] [Google Scholar]
- 21.Jin J, Tang Y, Li S et al. The initial results for a phase III study of short‐term radiotherapy plus chemotherapy vs long‐term chemoradiotherapy in locally advanced rectal cancer (STELLAR trial). J Clin Oncol 2016, 34:e1500a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Jin J, Li S et al. The initial results for a phase 3 study of short‐term versus long‐term chemoradiation therapy in locally advanced rectal cancer (STELLAR trial). Int J Radiat Oncol 2016;96(suppl):S108–S109. [Google Scholar]
- 23.Jin J, Liu S, Zhu Y et al. The updated results for the phase 3 study of 5×5 Gy followed by chemotherapy in locally advanced rectal cancer (stellar trial). Int J Radiat Oncol Biol Phys 2017;99(suppl):E157. [Google Scholar]
- 24.Moore J, Price T, Carruthers S et al. Prospective randomized trial of neoadjuvant chemotherapy during the ‘wait period’ following preoperative chemoradiotherapy for rectal cancer: Results of the WAIT trial. Colorectal Dis 2017;19:973–979. [DOI] [PubMed] [Google Scholar]
- 25.Jin J, Tang Y, Liu S et al. Short‐term radiotherapy plus chemotherapy versus long‐term chemoradiotherapy in locally advanced rectal cancer (STELLAR): A planned interim analysis. Ann Oncol 2018;29(suppl 8):viii167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Joo J, Kim TW et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14‐03. Int J Radiat Oncol Biol Phys 2018;101:889–899. [DOI] [PubMed] [Google Scholar]
- 27.Ciseł B, Pietrzak L, Michalski W et al. Long‐course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long‐term results of the randomized Polish II study. Ann Oncol 2019;30:1298–1303. [DOI] [PubMed] [Google Scholar]
- 28.Fernández‐Martos C, Pericay C, Aparicio J et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging‐defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 study. J Clin Oncol 2010;28:859–865. [DOI] [PubMed] [Google Scholar]
- 29.van der Valk MJM, Marijnen CAM, Van Etten B et al. Compliance and tolerability of short‐course radiotherapy followed by preoperative chemotherapy and surgery for high‐risk rectal cancer – Results of the international randomized RAPIDO‐trial. Radiother Oncol 2020;147:75–83. [DOI] [PubMed] [Google Scholar]
- 30.Conroy T, Lamfichekh N, Etienne PL et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol 2020;38(suppl):4007a [Google Scholar]
- 31.Hospers G, Bahadoer R, Dijkstra E et al. Short‐course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol 2020;38(suppl):4006a. [Google Scholar]
- 32.Fokas E, Allgäuer M, Polat B et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO‐12. J Clin Oncol, 2019;37:3212–3222. [DOI] [PubMed] [Google Scholar]
- 33.Garcia‐Aguilar J, Patil S, Kim J et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol 2020;38(suppl):4008a. [Google Scholar]
- 34.Garcia‐Aguilar J, Chow OS, Smith DD et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marco MR, Zhou L, Patil S et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: Final results of a multicenter phase II trial. Dis Colon Rectum 2018;61:1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rödel C, Trojan J, Bechstein WO et al. Neoadjuvant short‐ or long‐term radio(chemo)therapy for rectal cancer: How and who should be treated? Dig Dis 2012;30(suppl 2):102–108. [DOI] [PubMed] [Google Scholar]
- 37.Van Dijk TH, Tamas K, Beukema JC et al. Evaluation of short‐course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762–1769. [DOI] [PubMed] [Google Scholar]
- 38.Markovina S, Youssef F, Roy A et al. Improved metastasis‐ and disease‐free survival with preoperative sequential short‐course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long‐course chemoradiotherapy: Results of a matched pair analysis. Int J Radiat Onco Biol Phys 2017;99:417–426. [DOI] [PubMed] [Google Scholar]
- 39.Kane C, Glynne‐Jones R. Should we favour the use of 5 × 5 preoperative radiation in rectal cancer. Cancer Treat Rev 2019;81:101908. [DOI] [PubMed] [Google Scholar]
- 40.Bujko K, Nowacki MP, Nasierowska‐Guttmejer A et al. Long‐term results of a randomized trial comparing preoperative short‐course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–1223. [DOI] [PubMed] [Google Scholar]
- 41.Ngan SY, Burmeister B, Fisher RJ et al. Randomized trial of short‐course radiotherapy versus long‐course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans‐Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 42.Latkauskas T, Pauzas H, Kairevice L et al. Preoperative conventional chemoradiotherapy versus short‐course radiotherapy with delayed surgery for rectal cancer: Results of a randomized controlled trial. BMC Cancer 2016;16:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou ZR, Liu SX, Zhang TS et al. Short‐course preoperative radiotherapy with immediate surgery versus long‐course chemoradiation with delayed surgery in the treatment of rectal cancer: A systematic review and meta‐analysis. Surg Oncol 2014;23:211–221. [DOI] [PubMed] [Google Scholar]
- 44.Ma B, Gao P, Song Y et al. Short‐course radiotherapy in neoadjuvant treatment for rectal cancer: A systematic review and meta‐analysis. Clin Colorectal Cancer 2018;17:320–330.e5. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Zheng B, Lu X et al. Preoperative short‐course radiotherapy and long‐course radiochemotherapy for locally advanced rectal cancer: Meta‐analysis with trial sequential analysis of long‐term survival data. PloS One 2018;13:e0200142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francois Y, Nemoz CJ, Baulieux J et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter‐sparing surgery for rectal cancer: The Lyon R90‐01 randomized trial. J Clin Oncol 1999;17:2396. [DOI] [PubMed] [Google Scholar]
- 47.Lefevre JH, Mineur L, Kotti S et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: A multicenter, randomized, controlled trial (GRECCAR‐6). J Clin Oncol 2016;34:3773–3780. [DOI] [PubMed] [Google Scholar]
- 48.de Campos‐Lobato LF, Geisler DP, da Luz Moreira A et al. Neoadjuvant therapy for rectal cancer: The impact of longer interval between chemoradiation and surgery. J Gastrointest Surg 2011;15:444–450. [DOI] [PubMed] [Google Scholar]
- 49.Wolthuis AM, Penninckx F, Haustermans K et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol 2012; 19:2833–2841. [DOI] [PubMed] [Google Scholar]
- 50.Sun Z, Adam MA, Kim J et al. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Am Coll Surg 2016;222:367–374. [DOI] [PubMed] [Google Scholar]
- 51.Mei SW, Liu Z, Wei FZ et al. Impact of interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer patients. World J Gastroenterol 2020;26:4624–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao W.TNT Versus Conventional CRT to Increase the Sphincter Preservation Rate for Distal LARC (TESS). 2019. Available at https://www.clinicaltrials.gov/ct2/show/NCT03840239. Accessed May 23, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Supplemental Table 1 MEDLINE search strategy


