Abstract
The addition of trastuzumab to chemotherapy regimen is the standard of care for human epidermal growth factor receptor 2 (HER2)‐positive advanced gastric cancer; however, most patients eventually acquire trastuzumab resistance. Although some resistance mechanisms to trastuzumab‐based regimens have been proposed, further understanding is required for developing therapeutic strategies to overcome the resistance. In the present work, we attempted to determine the possible resistance mechanism to trastuzumab in a patient with HER2‐positive stage IV gastric adenocarcinoma. In this study, we first report the nucleotide change c.1899‐1G>A at the intron 15 acceptor splice site promoting exon 16 deletion of HER2 as the potential mechanism of trastuzumab resistance in HER2‐positive gastric adenocarcinoma.
Key Points
The combination of trastuzumab with chemotherapy is considered to be the standard therapy for HER2‐positive advanced gastric cancer (GC), but most of the patients eventually acquire trastuzumab resistance. The mechanisms of resistance to trastuzumab in GC are poorly characterized.
To the best of the authors' knowledge, this study is the first to implicate HER2 c.1899‐1G>A, which results in exon 16 skpping, as the acquired resistance mechanism to trastuzumab in HER2‐positive gastric adenocarcinoma.
This work provides insights into the potential molecular mechanism of trastuzumab resistance, which is crucial in developing effective therapeutic strategies for HER2‐positive GC patients refractory to trastuzumab.
Short abstract
This work attempted to determine the possible resistance mechanism to trastuzumab in a patient with HER2‐positive stage IV gastric adenocarcinoma.
Patient Story
In September 2018, a 30‐year‐old female was diagnosed with metastatic gastric cancer (stage IV). The histopathological diagnosis was poorly differentiated adenocarcinoma with HER2 overexpression (3+) (Fig. 1A, B). Her tumor marker levels in the plasma, including carcinoembryonic antigen (CEA; 64.22 μg/L) and cancer antigen (CA)19‐9 (5,789 U/mL), were elevated. The primary gastric biopsy and plasma samples underwent capture‐based next‐generation sequencing (NGS) using a panel consisting of 520 cancer‐related genes (OncoScreen Plus; Burning Rock Biotech, Guangzhou, China) for molecular analysis in September 2018. NGS consistently revealed HER2 amplification from both samples. NGS also showed microsatellite stability (MSS) and low tumor mutation burden (TMB; 2.4 mutations per Megabase [Mb]) in the tissue sample. Table 1 lists the mutations detected from the samples. Figure 1C illustrates the entire treatment history.
Figure 1.
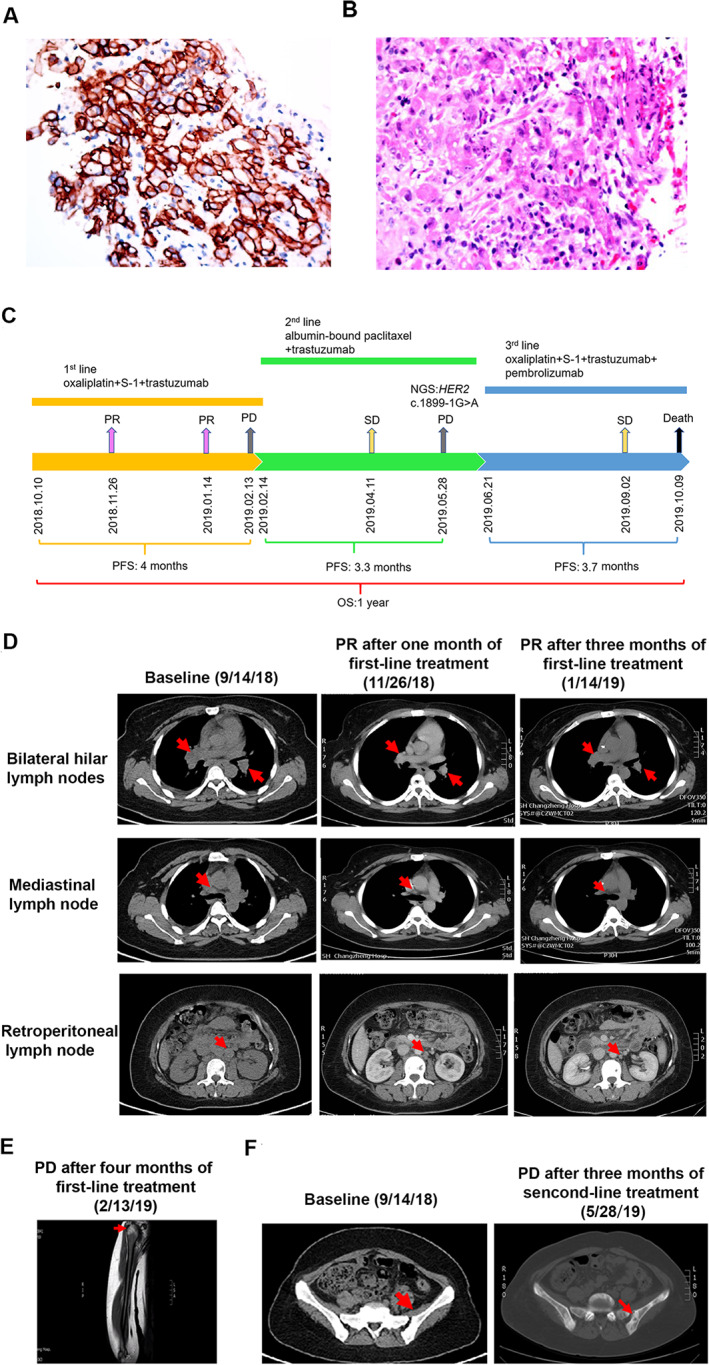
A summary of patient's treatment history. (A): Immunohistochemistry staining analyses showed the tumor cells were positive for HER2 expression (3+). (B): H&E staining showed a poorly differentiated adenocarcinoma. (C): The entire treatment procedure. (D): Chest and abdominal computed tomography (CT) scans at baseline and in November 2018 and January 2019 demonstrating PR in bilateral hilar lymph nodes, mediastinal lymph node, and retroperitoneal lymph node. (E): Magnetic resonance imaging scans in February 2019 showed the patient developed metastasis to right humerus after failure of first‐line treatment. (F): CT scans in May 2019 showed the patient developed metastasis to pelvis after failure of second‐line treatment. Abbreviations: HER2: human epidermal growth factor receptor 2; NGS, next‐generation sequencing; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Table 1.
Results from NGS‐based mutation analysis prior to trastuzumab treatment and after resistance to treatment with trastuzumab
| Gene/biomarker | At baseline prior to trastuzumab treatment | After trastuzumab resistance: plasma sample | ||||
|---|---|---|---|---|---|---|
| Primary tumor tissue | Plasma sample | |||||
| Variation | Abundance | Variation | Abundance | Variation | Abundance | |
| ARID1A | p.Glu1958fs | 5.63% | p.Glu1958fs | 17% | p.Glu1958fs | 28.09% |
| FGFR1 | ND | amplification | 3.86 CN | amplification | 4.4 CN | |
| TP53 | p.Arg267Trp | 9.58% | p.Arg267Trp | 22.26% | p.Arg267Trp | 31.95% |
| HER2 | amplification | 31.58 CN | amplification | 27.31 CN | amplification | 21.4 CN |
| HER2 | ND | ND | c.1899‐1G>A | 6.67% | ||
| TMB | 2.4 mutations/Mb | 2.4 mutations/Mb | 6.3 mutations/Mb | |||
| MSI status | MSS | NA | NA | NA | NA | |
Abbreviations: CN, copy number; fs, frameshift; Glu, glutamic acid; Mb, megabase; MSI, microsatellite instability; MSS, microsatellite stability; NA, not applicable/not tested; ND, not detected; NGS, next‐generation sequencing; Pro, proline; Ser, serine; TMB, tumor mutation burden; Trp, tryptophan.
The patient was initially treated on October 10, 2018, with trastuzumab combined with oxaliplatin S‐1. Chest and abdominal computed tomography (CT) scans, performed after two courses, showed partial response (PR; Fig. 1D). Her CEA level decreased to 9.31 μg/L, and CA19‐9 decreased to 674.9 U/mL. A review of her CT scans in January 2019 showed her disease remained as PR (Fig. 1D). After another month of treatment, she presented with right shoulder pain. Magnetic resonance imaging scans revealed the emergence of a new lesion in the proximal right humerus, evaluated as progressive disease (PD) with a progression‐free survival (PFS) of 4 months (Fig. 1E). At PD, her plasma CEA (171 μg/L) and CA19‐9 (3,192 U/mL) levels were markedly elevated.
Subsequently, her second‐line treatment was switched to albumin‐bound paclitaxel in combination with trastuzumab. Her best response was stable disease (SD); however, after five courses of treatment, she presented with pain on the pelvis and lower limbs. The abdominal CT examinations in May 2019 showed the newly developed pelvic metastasis with a PFS of 3.3 months (Fig. 1F). Her plasma CA19‐9 level increased to 8,850 U/mL. Plasma sample obtained was submitted for NGS. Compared with the NGS results prior to first‐line treatment, NGS revealed a decreased gene copy number of HER2 (21.4), the new mutation of c.1899‐1G>A in HER2, and higher TMB (6.3 mutations/Mb; Table 1). Cell‐based splicing reporter minigene assay [1] and sanger sequencing analysis confirmed that the splice site mutation c.1899‐1G>A in HER2 gene resulted in the loss of the entire exon 16 (Fig. 2). As third‐line treatment, she was administered a regimen of oxaliplatin, S‐1, trastuzumab plus intravenous pembrolizumab, based on the the detection of programmed cell death ligand‐1 (PD‐L1) expression level by combined positive score (CPS) of ≥10 in gastric biopsy prior to third‐line treatment. Treatment‐related grade 1 adverse events were reported by the patient. The patient achieved SD lasting for 3.7 months. She succumbed to her disease on October 9, 2019, with an overall survival of 12 months (Fig. 1C).
Figure 2.
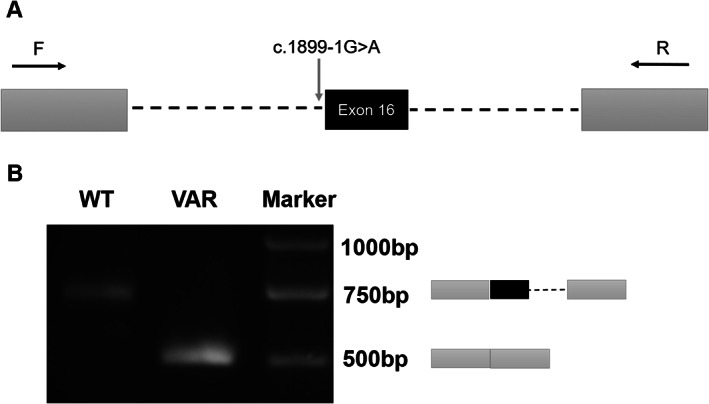
The HER2 c.1899‐1G>A variant resulted in aberrant splicing of exon 16. (A): Minigene assay was performed using human embryonic kidney 293T cells to investigate the impact of HER2 c.1899‐1G>A on the splicing of exon 16. HER2 exon 16 coding sequence are indicated by black boxes, 150 base‐pairs of 5’ and 3’ flanking intronic sequences are indicated by dash lines, two exons derived from SERPING1/CINH gene are indicated by dark gray boxes, and polymerase chain reaction (PCR) primers are indicated by black arrowhead. (B): Reverse transcription PCR (RT‐PCR) analysis of the spliced transcripts expressed from the wild‐type and mutant (c.1899‐1G>A) minigene constructs. RT‐PCR products were analyzed by agarose gel separation followed by sequencing of the different bands. The inclusion or the exclusion of HER2 exon 16 in each transcript is schematically indicated on the right. Abbreviations: F, forward primer; R, reverse primer; VAR, mutant construct containing the c.1899‐1G>A variant; WT, wild‐type minigene construct containing reference sequence.
Molecular Tumor Board
Genotyping Results and Interpretation of the Molecular Results
Capture‐based targeted sequencing results showed that the patient with HER2‐positive metastatic gastric adenocarcinoma harbored HER2 amplification with MSS and low TMB prior to first‐line treatment.
Although several HER2‐targeted therapies such as pertuzumab, lapatinib, trastuzumab emtansine, and trastuzumab deruxtecan (T‐DXd) have been approved for the treatment of patients with HER2‐positive breast cancer in either adjuvant or metastatic setting, these agents except for T‐DXd have failed to demonstrate significant survival benefits in patients with HER2‐positive advanced gastric or gastroesophageal junction (GEJ) cancer [2]. In recent years, an array of promising and novel anti‐HER2 therapeutic agents and their combinations for HER2‐positive gastric cancer (GC) have entered various stages of clinical development, such as tucatinib, margetuximab, and ZW45 [2].
Targeted therapeutic agents, such as trastuzumab and pembrolizumab, have been approved by the U.S. Food and Drug Administration (FDA) for use in gastric cancer. Trastuzamb is based on testing for HER2 positivity. Pembrolizumab is based on testing for microsatellite instability by polymerase chain reaction (PCR), mismatch repair deficiency by immunohistochemistry, PD‐L1 expression by CPS, or high TMB by NGS. TMB has emerged as a potential biomarker for predicting the tumor response to immune checkpoint inhibitors (ICIs). Elevated TMB increases the odds of generating immunogenic neoantigens, thus inducing a response to ICIs [3].
Trastuzumab, a humanized monoclonal antibody, specifically binds to HER2 and thus inhibits its homodimerization and phosphorylation, resulting in inhibition of the proliferation of HER2‐overexpressing tumor cells [4]. The combination of trastuzumab with chemotherapy is considered to be the standard therapy for HER2‐positive advanced GC, on the basis of the results from the Trastuzumab for Gastric Cancer trial indicating that HER2‐positive patients treated with trastuzumab plus chemotherapy have a longer overall survival (13.8 months vs. 11.1 months) [5]. In this study, the case benefited from trastuzumab combined with oxaliplatin S‐1 as first‐line treatment with a PFS of 4 months.
Although an objective response rate of 47% to 71% is observed when trastuzumab is used in combination with chemotherapy for first‐line treatment of HER2‐positive advanced GC, most of the patients eventually acquire trastuzumab resistance [2]. However, The molecular mechanisms of acquired resistance to trastuzumab in HER2‐positive GC remain elusive, which is due to the fact that NGS‐based molecular testing is still not a common practice for monitoring trastuzumab resistance in HER2‐positive GC. Compared with the NGS results prior to first‐line treatment, NGS on plasma smaple after progression on second‐line treatment revealed a decreased gene copy number of HER2 (21.4), a new mutation of c.1899‐1G>A in HER2 (Table 1), and a higher TMB. CPS of ≥10 was shown in gastric biopsy prior to third‐line treatment for testing PD‐L1 expression.
Several potential mechanisms of acquired resistance to trastuzumab‐based treatment in advanced HER2‐positive gastric or GEJ cancers have been identified, such as tumor heterogeneity in HER2 positivity and loss of HER2 positivity. The previous studies have demonstrated that 32%–69% of patients with HER2‐positive gastric cancer undergo HER2 loss in tumor rebiopsies at progression on trastuzumab‐based treatment [6, 7, 8]. Furthermore, approximately 6% of initially HER2‐negative patients with metastatic or recurrent gastric or GEJ cancers can turn out to have HER2‐positive metastatic lesions [9]. Tumor rebiopsies from metastatic lesions should be performed to ascertain HER2 status and heterogeneity in patients who progress on trastuzumab‐based treatment but is not likely possible in this case. The progression appears to be in bone only in the patient.
Functional and Clinical Significance of the Specific Mutation in the Particular Cancer
Our work revealed that HER2 c.1899‐1G>A resulted in the deletion of exon 16, which was consistent with a recent study [10]. Exon 16 of HER2 is 48 bases long, and its skipping causes an in‐frame loss of 16 amino acids in the juxta‐transmembrane region. The alternative mRNA splice variant of HER2 lacking exon 16 frequently occurs in breast cancers [10, 11, 12]. However, exon 16 deletion of HER2 (HER2D16) as a product of splice site mutation in HER2 gene is rare, with the frequency of 0.15% in GC [10].
With the exception of the nucleotide change (‐1G>A), other point mutations at the intron 15 acceptor splice site, such as ‐1G>C, have been previously reported as an oncogenic driver in lung cancer [10]. HER2D16 can drive lung tumorigenesis with an immunosuppressive tumor microenvironment in the genetically engineered mouse models [10]. HER2D16 resulting from c.1899‐1G>A has been implicated as a novel mechanism of osimertinib resistance in a patient with epidermal growth factor receptor (EGFR) L858R/T790M‐positive non‐small cell lung cancer (NSCLC). Furthermore, the combination of osimertinib with afatinib can reverse the osimertinib resistance driven by HER2D16 in EGFR T790M/L858R‐positive NSCLC cells [13]. Recently, a real‐world retrospective cohort study has demonstrated that HER2 exon 16 skipping contributes to the resistance mechanism to tyrosine kinase inhibitors in patients with EGFR‐mutated lung cancer and might be an oncogenic driver in breast, colorectal, gastric, and ovarian cancers [14]. Our and a previous study suggest that HER2D16 might potentially contribute to the mechanism of acquired resistance to trastuzumab and might serve as a novel therapeutical target in gastric cancer. HER2D16 as the potential mechanisms of resistance to trastuzumab‐based regimens is proposed in this work. However, we can not conclude that HER2 splice site mutation c.1899‐1G>A is the mediator of trastuzumab resistance in the context of lacking the validation from firm functional assays. Further in vitro and in vivo studies are needed to elucidate the oncogenic properties of point mutation c.1899‐1G>A in HER2‐positive gastric cancer and validate this splice site mutation as the mechanism of acquired resistance to trastuzumab in HER2‐positive gastric cancer.
Potential Strategies to Target the Pathway and Implications for Clinical Practice
Preclinical studies have revealed the effect of trastuzumab on inhibiting HER2D16 oncogenic activity, but yielded controversial results. Trastuzumab resistance has been demonstrated in vitro in HER2D16‐expressing breast cancer cell line [15], but several studies from the same group have demonstrated contradicting results, with one reporting that HER2D16‐transformed cells shows reduced sensitivity to trastuzumab and the other reporting that the cell proliferation induced by an increased HER2D16 expression in HER2‐positive GC patient‐derived xenografts is effectively inhibited by trastuzumab [11, 16, 17].
Our patient obtained clinical benefit from trastuzumab in combination with pembrolizumab and chemotherapy as the third‐line treatment with a PFS of 2 months, but she succumbed to her disease after another month treatment. Because the multidrug treatment with targeted agent, immune checkpoint inhibitor, and chemotherapy were administered simultaneously, we cannot delineate the lack of therapeutic effect of each agent. However, we speculate that the trastuzumab resistance mediated by the emergence of the HER2 c.1899‐1G>A contributed to the lack of response and short disease control with the multidrug regimen. T‐DXd is a novel HER2‐targeting antibody‐drug conjugate. Based on the findings of DESTINY‐Gastric01 trails [18], T‐DXd has been approved on January 15, 2021, by the FDA for the treatment of patients with locally advanced or metastatic HER2‐positive gastric or GEJ adenocarcinoma who have received a prior trastuzumab‐based regimen. Further studies are needed to illuminate the efficacy of T‐DXd and other anti‐HER2 agents in GC patients with acquired HER2D16.
Glossary of Genomic Terms and Nomenclature
Adenine (A): A nitrogenous purine base, which bonds with thymine (T) to form the A–T base pair in DNA and the A‐U base pair in RNA
Guanine (G): A nitrogenous purine base, which bonds with cytosine (C) to form the G‐C base pair
c.: a cDNA sequence
>: nucleotide substitutions
c.1899‐1G>A: the G to A substitution at the last nucleotide of intron 15 of the HER2 gene
Messenger RNA (mRNA): RNA that serves as a template for protein synthesis
Megabase (Mb): Unit of length for DNA fragments equal to 1 million nucleotides
Exon: DNA sequence portion of a gene that codes for the protein
Intron: DNA base sequence between exons
Author Contributions
Conception/design: Yuan‐Sheng Zang
Collection and/or assembly of data: Bao‐Dong Qin
Patient management: Xiao‐Dong Jiao, Ke Liu
Data analysis and interpretation: Xiao‐Dong Jiao, Bao‐Dong Qin, Bao‐Dong Qin, Haiwei Du, Jianxing Xiang
Manuscript writing: Xin‐Cheng Zhou, Yan Ling, Jun Liu, Xi He, Haiwei Du, Jianxing Xiang
Final approval of manuscript: Xiao‐Dong Jiao, Ke Liu, Ying Wu, Xin‐Cheng Zhou, Bao‐Dong Qin, Yan Ling, Jun Liu, Xi He, Haiwei Du, Jianxing Xiang, Yuan‐Sheng Zang
Disclosures
Jianxing Xiang: Burning Rock (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was funded by the Shanghai Municipal Science and Technology Committee (17511103400). This study was approved by the Ethics Committee of the Changzheng Hospital. Informed consent was obtained from the participant.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1.Gaildrat P, Killian A, Martins A et al. Use of splicing reporter minigene assay to evaluate the effect on splicing of unclassified genetic variants. Methods Mol Bio 2010;653:249–257. [DOI] [PubMed] [Google Scholar]
- 2.Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers 2020;12:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69–74. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Inomata M, Tojigamori M et al. Suppression of tumor growth in human gastric cancer with HER2 overexpression by an anti‐HER2 antibody in a murine model. Int J Oncol 2005;27:681–685. [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): A phase 3, open‐label, randomized controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 6.Pietrantonio F, Caporale M, Morano F et al. HER2 loss in HER2‐positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer 2016;139:2859–2864. [DOI] [PubMed] [Google Scholar]
- 7.Janjigian YY, Riches JC, Ku GY et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in HER2‐overexpressing esophagogastric (EG) tumors treated with trastuzumab. J Clin Oncol 2015;33(Suppl)63a. [Google Scholar]
- 8.Makiyama A, Sukawa Y, Kashiwada T et al. Randomized, phazse II study of trastuzumab beyond progression in patients with HER2‐positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T‐ACT Study). J Clin Oncol 2020;38:1919–1927. [DOI] [PubMed] [Google Scholar]
- 9.Park SR, Park YS, Ryu MH et al. Extra‐gain of HER2‐positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2‐negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer 2016;53:42–50. [DOI] [PubMed] [Google Scholar]
- 10.Smith HW, Yang L, Ling C et al. An ErbB2 splice variant lacking exon 16 drives lung carcinoma. Proc Natl Acad Sci USA 2020;117:20139–20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castagnoli L, Ghedini GC, Koschorke A et al. Pathobiological implications of the d16HER2 splice variant for stemness and aggressiveness of HER2‐positive breast cancer. Oncogene 2017;36:1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castiglioni F, Tagliabue E, Campiglio M et al. Role of exon‐16‐deleted HER2 in breast carcinomas. Endocr Relat Cancer 2006;13:221–232. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CC, Liao BC, Liao WY et al. Exon 16‐Skipping HER2 as a novel mechanism of osimertinib resistance in EGFR L858R/T790M‐positive non‐small cell lung cancer. J Thorac Oncol 2020;15:50–61. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Xu C, Ma Y et al. Clinical significance of ERBB2 exon 16 skipping: Analysis of a real‐world retrospective observational cohort study. ESMO Open 2020;5:e000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra D, Brumlik MJ, Okamgba SU et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther 2009;8:2152–2162. [DOI] [PubMed] [Google Scholar]
- 16.Castagnoli L, Iezzi M, Ghedini GC et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res 2014;74:6248–6259. [DOI] [PubMed] [Google Scholar]
- 17.Volpi CC, Pietrantonio F, Gloghini A et al. The landscape of d16HER2 splice variant expression across HER2‐positive cancers. Sci Rep 2019;9:3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shitara K, Bang YJ, Iwasa S et al. Trastuzumab deruxtecan in previously treated HER2‐positive gastric cancer. N Engl J Med 2020;382:2419–2430. [DOI] [PubMed] [Google Scholar]


