Abstract
Background
Chemotherapy‐induced toxicities lead to therapy dose reduction or delay, affecting patient outcomes. This systematic review and meta‐analysis evaluated the impact of relative dose intensity (RDI) on survival in adult patients with solid tumor cancer on nonadjuvant‐based chemotherapy regimens.
Methods
PubMed, Embase, and Web of Science databases were searched for peer‐reviewed English journal articles or congress abstracts evaluating association between RDI and survival; observational studies, case series of ≥20 patients, and clinical trials published between 2013 and 2020 were eligible. Meta‐analyses were conducted to quantify the association between RDI levels and overall survival (OS) among studies reporting a hazard ratio (HR) for OS by similar tumor types, regimens, and RDI. Forest plots represented summary HR and 95% confidence interval (CI); Cochran's Q and I2 tests evaluated study heterogeneity.
Results
Overall, 919 articles were reviewed and 22 included; seven were eligible for meta‐analysis. Significantly shorter OS at RDI <80% versus ≥80% and <85% versus ≥85% was observed upon meta‐analysis of four carboplatin‐based studies for breast, non‐small cell lung, or ovarian cancer (HR 1.17; 95% CI: 1.07–1.27) and three FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based studies for colorectal or pancreatic cancer (HR 1.39; 95% CI: 1.03–1.89). Grade 3 or higher hematologic toxicities were higher for carboplatin‐based regimens (thrombocytopenia: 14%–22%; anemia: 15%–19%; neutropenia: 24%–58%) than FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based regimens (thrombocytopenia: 1%–4%; anemia: 5%–19%; neutropenia: 19%–47%).
Conclusion
The results suggested longer OS with RDI ≥80% or ≥85% for both regimens, indicating that management of toxicities across treatment modalities may contribute to maintenance of higher RDI and benefit survival for patients with advanced solid tumors.
Implications for Practice
Chemotherapy‐induced toxicities lead to dose reduction and/or treatment delay, thus affecting patient outcomes. Results of this systematic review and meta‐analysis, evaluating the impact of relative dose intensity (RDI) on survival of patients with solid tumors on nonadjuvant‐based chemotherapy regimens, demonstrate a longer overall survival with RDI levels of at least 80% for patients with solid tumors on carboplatin‐based and FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based chemotherapy regimens, suggesting a protective effect of maintaining RDI ≥80% or ≥ ‐85%. Although grade 3 or higher hematologic toxicities occurred more in carboplatin‐based studies, managing toxicities across treatment regimens may contribute to maintenance of higher RDI and ultimately benefit overall survival.
Keywords: Carboplatin‐based regimens, FOLFOX/FOLFIRI‐based regimens, Meta‐analysis, Overall survival, Progression‐free survival, Relative dose intensity
Short abstract
Chemotherapy‐induced toxicities are a challenge to optimal treatments and dosing regimens for cancer patients. This review evaluates the effect of relative dose intensity, including dose delays and reductions, on survival in patients with solid tumors receiving nonadjuvant‐based chemotherapy.
Introduction
Chemotherapy‐induced toxicities are a persistent challenge to optimal treatments and dosing regimens for patients with cancer [1, 2]. Although most chemotherapy regimens are dosed based on patient‐specific parameters (body surface area or body weight), these regimens are often complicated by the occurrence of severe to life‐threatening conditions, including febrile neutropenia, anemia, and thrombocytopenia, thus warranting a reduction in dose or delay in planned treatment [2, 3]. However, clinical evidence suggests that improved outcomes are achieved with standard chemotherapy regimens in a dose‐dependent manner; patients receiving higher dose intensities experience better overall survival (OS), progression‐free survival (PFS), and disease‐free survival than patients receiving lower dose intensities than planned [1, 4, 5].
Relative dose intensity (RDI), the ratio of the delivered dose intensity (dose per unit body surface area per unit time [mg/m2 per week]) to the standard or planned dose intensity for a chemotherapy regimen, is a summary measure commonly used to describe dose delays and/or reductions occurring with a chemotherapy regimen [1, 4]. A decrease in RDI below 85% (or below 80% in some studies) has been considered to be a clinically significant reduction from standard or planned therapy, and maintaining RDI has been associated with improved survival in advanced ovarian and breast cancer in both randomized clinical trials and retrospective observational studies [4, 5, 6, 7, 8].
Clinical practice surveys have shown that a substantial proportion of patients are treated at relatively low dose intensities, representing a potential reason for treatment failure in patients with curable malignancies [9]. A systematic review of the impact of RDI on survival in patients with metastatic lung, breast, or ovarian cancer receiving chemotherapy between January 2000 and April 2013 [1] concluded that maintaining an RDI of ≥85% had a favorable impact on survival. No reviews have since been published regarding the effects of RDI on a broader population of patients with cancer receiving chemotherapy. Thus, the purpose of this systematic review and meta‐analysis was to evaluate the impact of RDI, including dose delays and reductions, on survival in patients with solid tumor cancer receiving nonadjuvant‐based chemotherapy in publications from 2013 through 2020.
Materials and Methods
Study Search
The review was conducted per Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [10]. A systematic literature search was performed in PubMed, Embase, and Web of Science databases to identify peer‐reviewed English journal articles or congress abstracts, and ClinicalTrials.gov to identify clinical trials with results reporting the impact of RDI or therapy dose delays and/or dose reductions on survival in adult patients with cancer treated with nonadjuvant‐based chemotherapy. No other gray literature was considered. Search strings included terms for neoplasms, RDI, and survival (supplemental online Table 1). The search strategy was adapted to meet the search specifications of each included database and was designed to capture outcomes of interest and stratification variables in the scope of the literature review. These were translated into indexed Medical Subject Headings (MeSH) and plain language text‐word terms using the National Library of Medicine MeSH thesaurus. Boolean operators were used to combine the final list of search terms into a comprehensive search strategy adding limit filters of language and date to ensure only the most relevant studies were included in the final search yield. Studies published prior to 2013 were filtered in the initial review of search results based on the Havrilesky et al. 2015 review article having an upper date limit of 2013 [1].
Study Selection
Study selections were documented through DistillerSR, a specialized software program designed for tracking and managing literature reviews, resulting in a fully auditable and transparent review process. Study eligibility criteria were defined a priori by study population, interventions, and outcomes (Table 1). Studies reporting a measure of RDI or treatment dose delays and/or reductions and evaluating overall and/or progression‐free survival were included. Eligible publications included prospective or retrospective observational studies, case series of ≥20 patients, and clinical trials. Studies reporting RDI and the cumulative incidence of grade 3 or higher hematologic toxicities during treatment were also evaluated. Articles identified in the literature searches were uploaded into DistillerSR and de‐duplicated by title and author. Studies were then screened by title and abstract against the inclusion and exclusion criteria; 10% of the abstracts were evaluated by an independent reviewer for quality control (QC). Articles designated as relevant were evaluated at full‐text level by two reviewers independently to determine agreement on inclusion (100% QC). The screening results were recorded, maintained, and assessed using DistillerSR.
Table 1.
Inclusion and exclusion criteria for narrative review and meta‐analysis
| Inclusion criteria | Exclusion criteria |
|---|---|
|
Adult patients with cancer with solid tumors, regardless of tumor location or stage. ► Limited to breast, ovarian, and pancreatic cancer and NSCLC and CRC. a |
Studies of patients without cancer, pediatric patients, or patients with blood tumors (leukemia, lymphoma, myeloma). |
|
Studies evaluating nonadjuvant chemotherapy regimens providing a measure of RDI. ► Limited to carboplatin‐based or FOLFOX‐ or FOLFIRI‐based regimens. a |
Studies without evaluation of chemotherapy regimens or only including adjuvant therapies, alternative, homeopathic, naturopathic, or traditional Chinese regimens. |
|
Studies including comparison of different levels of RDI ► Limited to RDI comparisons of < 85% vs. ≥85% or < 80% vs. ≥80% (including one study of < 79% vs. ≥79%). a |
Studies without any RDI level comparisons. |
|
Studies evaluating survival measures, such as overall, disease‐free, or progression‐free survival. ► Limited to overall survival. a |
Studies not evaluating any survival measures. |
| Studies published in English from 2013 to 2019. Prospective or retrospective observational cohort studies, clinical trials, case‐control studies, and case series with n ≥ 20 were eligible. | Studies not published in English, studies published prior to 2013, nonhuman studies (laboratory, preclinical, or animal studies), case series with <20 patients, opinion pieces, reviews, and case reports. |
Bold text indicates an additional criterion imposed for inclusion of articles for the meta‐analysis after a review of characteristics of studies identified through initial literature review. Non‐bold text indicates original study eligibility criteria from the systematic literature review.
Abbreviations: CRC, colorectal cancer; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; FOLFOX, folinic acid (leucovorin), fluorouracil (5‐FU), and oxaliplatin; NSCLC, non‐small cell lung cancer; RDI, relative dose intensity.
Data Extraction and Synthesis
Data were extracted in DistillerSR for all studies deemed relevant to the current analysis in the full‐text review stage. The abstraction form was reviewed before any data extraction to ensure that all appropriate fields were captured, and an initial small sample of articles was extracted to determine that the form was able to capture the appropriate information from the articles. Specific items extracted included study characteristics (study design, duration, follow‐up time, inclusion and exclusion criteria, and patient demographics), disease characteristics (tumor type, tumor stage, and previous therapies or surgeries), treatment characteristics (treatment regimen, dosage, number of cycles, concomitant treatment, RDI, and dose delays and/or reductions), and prevalence of dose‐limiting toxicities (neutropenia, thrombocytopenia, leukopenia, fatigue, etc.).
Risk of Bias and Study Quality Assessment
Studies were evaluated for risk of bias and study quality using several methods based on study design. Case‐control and cohort studies were assessed using the Newcastle‐Ottawa Scale [11], and clinical trials were assessed using the Cochrane Risk of Bias Tool [12]. The Newcastle‐Ottawa Scale score ranges from 0 to 9, with lower scores indicating higher risk of bias and higher scores indicating lower risk of bias. The Cochrane Risk of Bias Tool scores studies as having a low risk of bias, some concerns for risk of bias, or high risk of bias using five bias domains. The approach to address the potential for differences in baseline prognostic factors such as tumor stage and Eastern Cooperative Oncology Group performance status between RDI levels was evaluated for each study. During the systematic literature review, we abstracted patient factors, including sex, race, time from diagnosis, dosing regimen, comorbidities, earlier therapies, and treatment line number. Our primary concern for observational studies was confounding of the RDI‐survival association by disease stage. The risk of bias tool for cohort studies addressed whether tumor stage was used as an adjustment factor in the analyses, and studies restricting inclusion to a particular subpopulation (e.g., all patients with stage II–III colon cancer) were noted.
Statistical Analysis
Fixed‐effect meta‐analyses were conducted based on the results of the systematic review. Studies eligible for meta‐analysis reported a hazard ratio (HR) for OS and were categorized by tumor type, chemotherapy regimen, and evaluated RDI threshold(s). Categories that contained at least three studies were included for meta‐analysis. Studies that used an RDI threshold of 80% and those that used a threshold of 85% were not separated into distinct meta‐analyses because these thresholds were considered sufficiently similar. Specific thresholds used in each study are reported throughout results tables and figures. The primary objectives of the meta‐analysis were to determine the summary strength of association between lower and higher RDI levels (<80% vs. ≥80% or < 85% vs. ≥85%) of carboplatin‐based chemotherapy regimens and OS in studies of ovarian or breast cancer or non‐small cell lung cancer (NSCLC), and the summary strength of association between lower and higher RDI levels (<80% vs. ≥80% or < 85% vs. ≥85%) of FOLFOX‐ or FOLFIRI‐based chemotherapy regimens and OS in studies of colorectal cancer (CRC) or pancreatic cancer. Fixed‐effect meta‐analyses of HRs were conducted using Comprehensive Meta‐Analysis software (version 3.0; Biostat, Englewood, NJ). The effect of chemotherapy RDI on survival was summarized, weighting all included studies by the inverse of their variance [13]. Forest plots were used to represent HRs and 95% confidence intervals (CIs); values of p < .05 were considered significant.
Potential sources of heterogeneity in the impact of RDI on survival in each analysis were evaluated using the Cochran's Q and I2 statistical tests. The I2 statistics indicate the percentage of systematic variability across studies (range: 0%–100%), with larger values depicting greater heterogeneity [14].
Results
Search Results
Figure 1 shows a PRISMA flow diagram detailing the flow of study inclusion and exclusion at each level, with reasons for exclusion at the full‐text level. We identified 1,442 English language articles from the three databases (Fig. 1). After removing duplicate articles, 919 studies were screened at the level of title and abstract; 789 studies were assessed at the full‐text level. Overall, 623 studies were excluded, primarily because they were published prior to 2013 (n = 351) or they only evaluated adjuvant or neoadjuvant therapies (n = 113). In total, 166 studies were eligible for inclusion, among which 137 did not report any comparison between the RDI levels and seven belonged to the same trial or cohort. Finally, 22 studies were included in the abstraction database; seven were eligible for meta‐analysis. Most of the studies used the Hryniuk calculation method [15] to assess the ratio of the actual or delivered dose intensity to the planned or standard dose intensity. The method used to calculate RDI was not cited by all of the included studies.
Figure 1.
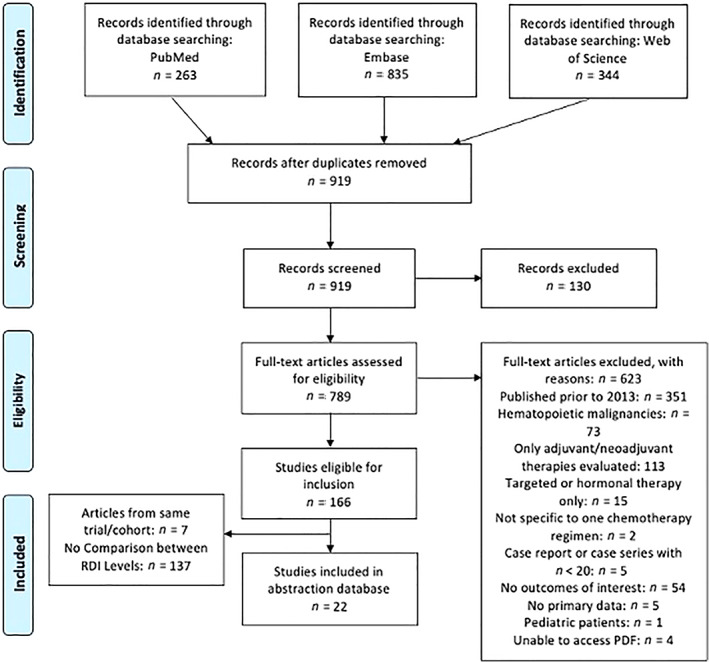
PRISMA diagram for study inclusion. Source: Moher et al. [10]. Abbreviation: RDI, relative dose intensity.
Clinical Characteristics of Selected Studies
Of the 22 included studies, 6 (27.3%) evaluated pancreatic cancer, 4 (18.2%) ovarian cancer, 3 (13.6%) CRC, 3 (13.6%) gastric cancer, 2 (9.1%) breast cancer, 1 (4.5%) esophageal cancer, 1 (4.5%) prostate cancer, 1 (4.5%) NSCLC, and 1 (4.5%) lung cancer. Interventions included platinum‐based regimens (9 [40.9%]), FOLFIRI‐, FOLFOX‐, or FOLFIRINOX‐based regimens (5 [22.7%]), taxane‐based regimens (8 [36.4%]), and irinotecan monotherapy (1 [4.5%]). Most of the studies were observational (21 [95.5%]), with only one clinical trial (4.5%); 11 (50%) of the studies were conducted in Japan, followed by the U.S. (3 [13.6%]), Spain (2 [9.1%]), Australia (2 [9.1%]), China (1 [4.5%]), South Korea (1 [4.5%]), Denmark (1 [4.5%]), and 1 (4.5%) in multiple countries.
Outcomes of the Systematic Literature Review
The studies included in the systematic literature review (n = 22) [5, 16, 17, 18, 19, 20, 36] were grouped by chemotherapy regimen. Most studies reported outcomes of OS or PFS. Eight studies reported median OS [16, 17, 18, 19, 20, 21] and PFS [16, 17, 18, 19, 22, 23] but not HR. RDI cutoffs ranged from 0% (vs. 100%) for 5‐fluorouracil bolus [22] to 80%, 85%, or 90% in several studies describing FOLFIRI‐, FOLFOX‐, or FOLFIRINOX‐based regimens [28, 29, 30, 31], including one with RDI cutoff of 77% [17] and one with RDI 79% [29]. The differences in OS between RDI categories were up to approximately 14 months [16, 17, 18, 19, 29, 32, 33]. For convenience in the following results summary, we considered a difference of at least 1 month to be clinically significant; however, the choice of this value is subjective and may vary by patient population and tumor type. Where survival time differences were statistically compared, we reported the level of significance in Figures 2 and 3. Of the 10 studies that reported median OS in high and low RDI categories, six reported at least 1 month longer OS with higher than with lower RDI [16, 22, 23, 29, 30, 32], three did not show any significant difference within 1 month [18, 19, 33], and one reported at least 1 month longer median OS with lower than with higher RDI [18] (Fig. 2). Three of the comparisons that reported more than 1 month longer median OS with higher RDI reported p < .05 for the difference, and all were for <80% versus ≥80% RDI (Fig. 2). Nine studies reported median PFS in high and low RDI categories, among which five reported at least 1 month longer PFS with higher than with lower RDI [16, 24, 26, 29, 32], three reported no significant differences within 1 month [18, 19, 24], and two reported at least 1 month longer median PFS with lower than with higher RDI [17, 22] (Fig. 3). Six comparisons reporting more than 1 month longer median PFS with higher RDI reported significance level of p < .05; one study of FOLFIRI in patients with CRC reported longer median PFS with RDI below 60% (p < .05; Fig. 3). Seven studies reported both OS and PFS [16, 17, 18, 19, 22, 29, 32]. In one study of patients with metastatic CRC on oxaliplatin and irinotecan‐based chemotherapy regimens, PFS increased slightly with higher RDI, whereas OS remained unchanged in the mFOLFOX6 regimen (modified FOLFOX, six cycles postoperative); both OS and PFS increased with higher RDI with the FOLFIRI regimen [29]. Conversely, in another study on patients with metastatic CRC, OS increased, whereas PFS decreased with higher RDI of ramucirumab plus modified FOLFIRI [22]. Higher RDI of nab‐paclitaxel improved OS and PFS in a study on patients with advanced/recurrent gastric cancer [16], whereas another study reported improved OS with lower RDI of gemcitabine plus nab‐paclitaxel in unresectable pancreatic cancer [17]. No difference in OS and PFS was observed with change in RDI in two studies in advanced gastric cancer [18] and metastatic pancreatic cancer [19].
Figure 2.
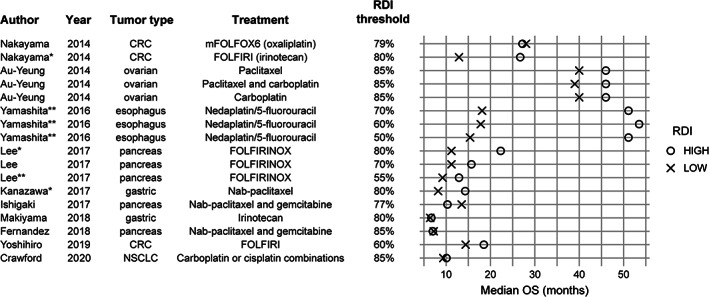
Median OS by RDI category. * Indicates p < .05 reported for the difference in median OS; ** indicates .05 ≤ p < .10. Unmarked comparisons reported p > .10 or did not report p values. Abbreviations: CRC, colorectal cancer; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; FOLIRINOX, folinic acid (leucovorin), fluorouracil (5‐FU), irinotecan, and oxaliplatin; mFOLFOX6, modified folinic acid (leucovorin), fluorouracil (5‐FU), and oxaliplatin (six cycles postoperative); NSCLC, non‐small cell lung cancer; OS, overall survival; RDI, relative dose intensity.
Figure 3.
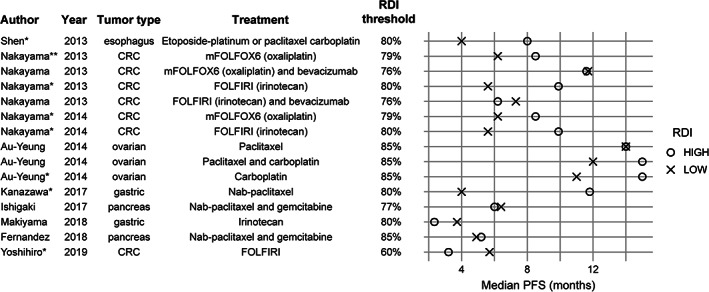
Median PFS by RDI category. * Indicates p < .05 reported for the difference in median PFS; ** indicates .05 ≤ p < .10. Unmarked comparisons reported p > .10 or did not report p values. Abbreviations: CRC, colorectal cancer; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; mFOLFOX6, modified folinic acid (leucovorin), fluorouracil (5‐FU), and oxaliplatin (six cycles postoperative); PFS, progression‐free survival; RDI, relative dose intensity.
Taxane (paclitaxel, nab‐paclitaxel, docetaxel) and gemcitabine‐based regimens were reported in eight studies of patients with breast, pancreatic, prostate, or gastric cancer (supplemental online Table 2). RDI cutoffs ranged from 63.6% [30] to 90% [27]. Kushnir et al. reported significantly improved OS of patients with metastatic castrate‐sensitive prostate cancer per 10% increase in RDI of docetaxel [31]. Low RDI levels were associated with decreased OS and PFS in three studies [16, 32], whereas no significant associations between RDI and OS and/or PFS were identified in four studies [17, 19, 27, 30].
Meta‐Analysis Results
Overall, seven studies were included in two separate meta‐analyses (Table 2). The narrative summary identified two major categories for meta‐analyses, all evaluating RDI levels <80% versus ≥80% or < 85% versus ≥85%: (a) carboplatin‐based regimens for breast or ovarian cancer or NSCLC and (b) FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based regimens for CRC or pancreatic cancer. Five estimates from four studies were included in the meta‐analysis for OS of carboplatin‐based regimens for ovarian, NSCLC, or breast cancer [5, 28, 29, 33] (Fig. 4). The summary HR was 1.17 (95% CI: 1.07–1.27), demonstrating a significant increased risk of mortality for RDI levels <80% versus ≥80% or < 85% versus ≥85%. No significant heterogeneity was present in this analysis, with HRs ranging from 1.14 (95% CI: 0.71–1.81) for paclitaxel and carboplatin in ovarian cancer in a study of Japanese patients [33] to 1.22 (95% CI: 0.77–1.94) for the same combination and tumor type in a study of American patients [5]. Four estimates from three studies were included in the meta‐analysis for OS of FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based regimens for CRC or pancreatic cancer [24, 25, 26] (Fig. 4). The summary HR was 1.39 (95% CI: 1.03–1.89), demonstrating a significant increased risk of mortality at RDI levels <80% versus ≥80% or < 85% versus ≥85%. Results were somewhat heterogeneous (I2 = 57%; p = .07), with HRs ranging from 0.90 (0.41–2.21) for oxaliplatin RDI <79% in the mFOLFOX6 regimen [25] to 2.72 (1.22–6.04) for irinotecan RDI <80% in the FOLFIRI regimen [25].
Table 2.
Studies included in the meta‐analyses, 2013–2019 (overall survival)
| Author, year | Tumor type | Chemotherapy | Dosage | n | RDI comparison |
|---|---|---|---|---|---|
| Studies evaluating carboplatin‐based regimens for ovarian or breast cancer or NSCLC | |||||
| Bun 2019 [33] | Ovarian | Paclitaxel/carboplatin | PPaclitaxel (80 mg/m2 on days 1, 8, and 15) and carboplatin (AUC 6 mg/mL per minute on day 1) in a 21‐day cycle | 244 | <80% vs. ≥80% |
| Denduluri 2018 [5] | Ovarian | Paclitaxel/carboplatin | Carboplatin/paclitaxel every 3 weeks: 5 AUC/175 mg/m2 | 170 | <85% vs. ≥85% |
| Au‐Yeung 2014 [28] | Ovarian | Paclitaxel/carboplatin | Carboplatin AUC 5–6, paclitaxel 175 mg/m2 | 537 | <85% vs. ≥85% |
| Crawford 2020 [29] | NSCLC | Platinum‐based therapies: carboplatin/paclitaxel, pemetrexed/carboplatin, carboplatin/bevacizumab/paclitaxel, pemetrexed/cisplatin, carboplatin/pemetrexed/bevacizumab, carboplatin/gemcitabine |
Carboplatin + paclitaxel 5 AUC/175 mg/m2; Carboplatin + pemetrexed 5 AUC/500 mg/m2; Carboplatin + bevacizumab + paclitaxel 5 AUC/15 mg/kg/175 mg/m2; Pemetrexed + cisplatin 500 mg/m2/75 mg/m2; Carboplatin + pemetrexed + bevacizumab 5 AUC/500 mg/m2/15 mg/kg; Carboplatin + gemcitabine 5 AUC/1,000 mg/m2 × 2 (days 1 and 8) |
3,866 | <85% vs. ≥85% |
| Denduluri 2018 [5] | Breast | The most common chemotherapy regimens were paclitaxel/bevacizumab q4w, albumin‐bound paclitaxel q3w, and weekly paclitaxel |
Paclitaxel/bevacizumab every 4 weeks 240 mg/m2/30 mg/kg, six cycles; Albumin‐bound paclitaxel every 3 weeks 300 mg/m2, six cycles; Paclitaxel weekly 80 mg/m2, 25 cycles; Docetaxel every 3 weeks 75 mg/m2, eight cycles; Carboplatin/paclitaxel every 3 weeks 6 AUC/175 mg/m2, eight cycles; Vinorelbine weekly 25 mg/m2, 25 cycles; Carboplatin/paclitaxel every 4 weeks 6 AUC/240 mg/m2, six cycles |
874 | <85% vs. ≥85% |
| Studies evaluating FOLFOX, FOLFIRI, or FOLFIRINOX regimens in colorectal or pancreatic cancer | |||||
| Nakayama 2014 [25] | FOLFIRI | Irinotecan (150 mg/m2) and folinic acid (200 mg/m2) followed by a bolus infusion of fluorouracil (400 mg/m2) and subsequent continuous infusion of fluorouracil (2,400 mg/m2), repeated every 2 weeks until disease progression | 36 | <80% vs. ≥80% | |
| Colorectal | mFOLFOX6 | Six cycles of mFOLFOX6, consisting of oxaliplatin (85 mg/m2) and folinic acid (200 mg/m2), followed by a bolus infusion of fluorouracil (400 mg/m2) and subsequent continuous infusion of fluorouracil (2,400 mg/m2), repeated every 2 weeks followed by maintenance therapy with oral S‐1. Reintroduction of mFOLFOX6 therapy was scheduled after four cycles of S‐1 or upon tumor progression | 30 | <79% vs. ≥79% | |
| Kobayashi 2019 [24] | Pancreas | FOLFIRINOX | Irinotecan: 180 mg/m2; oxaliplatin: 85 mg/m2; 5‐FU bolus: 400 mg/m2; 5‐FU continuous infusion: 2,400 mg/m2 | 359 |
Irinotecan: <75% vs. ≥75% Oxaliplatin: <70% vs. ≥75% 5‐FU bolus: 0% vs. >0% 5‐FU continuous infusion: <80% vs. ≥80% |
| Lee 2017 [26] | Pancreas | FOLFIRINOX | 85 mg/m2 for oxaliplatin; 180 mg/m2 for irinotecan; 400 mg/m2 for 5‐FU as a bolus and 2,400 mg/m2 for 5‐FU via continuous infusion every 2 weeks | 133 | <80% vs. ≥80% |
AUC, area under the curve; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; FOLFOX, folinic acid (leucovorin), fluorouracil (5‐FU), and oxaliplatin; FOLIRINOX, folinic acid (leucovorin), fluorouracil (5‐FU), irinotecan, and oxaliplatin; mFOLFOX6, modified FOLFOX (six cycles postoperative); FU, fluorouracil; NSCLC, non‐small cell lung cancer; q3w, every 3 weeks; q4w, every 4 weeks; RDI, relative dose intensity.
Figure 4.
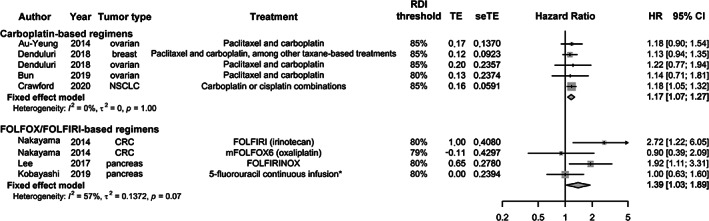
Association of RDI with OS in studies of FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based and carboplatin‐based regimens. *, Kobayashi et al. (2019) evaluated 5‐flurouracil infusion as part of an evaluation of constituents of FOLFIRINOX. Abbreviations: CI, confidence interval; CRC, colorectal cancer; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; FOLIRINOX, folinic acid (leucovorin), fluorouracil (5‐FU), irinotecan, and oxaliplatin; HR, hazard ratio; mFOLFOX6, modified folinic acid (leucovorin), fluorouracil (5‐FU), and oxaliplatin (six cycles postoperative); NSCLC, non‐small cell lung cancer; OS, overall survival; RDI, relative dose intensity; seTE, standard error of treatment effect; TE, treatment effect.
Risk of Bias Assessment
Risk of bias could not be evaluated in one study of pooled data from clinical trials because of insufficient information about the design of the original studies [25]. Among the 21 cohort studies, the score for the Newcastle‐Ottawa Scale assessments ranged from 3 to 8 with a median score of 6 out of a possible 9. The most frequent reasons for losing points on the risk of bias assessment included not describing the nonexposed cohort (typically because nonexposed cohorts were excluded from the study), having <1 year of follow‐up, and not controlling for tumor stage or other additional factors. However, this apparent deficit was mitigated by restricting cohorts to specific tumor stages in these studies (thus obviating the need for tumor stage adjustment in the analyses). Therefore, confounding by disease stage was addressed for all included studies. Another concern was that several studies did not report whether follow‐up time was sufficient to observe the survival outcomes of interest. This deficit may affect the precision of HR and survival time estimates in observational studies. Other factors evaluated in the risk of bias analysis related to ascertainment of exposure and outcomes and comparability of the exposure groups, which were well defined in nearly all studies. Overall, the risk of bias related to the outcomes of interest was generally low in the included observational studies. The Cochrane Risk of Bias assessment was conducted on the clinical trial included and had insufficient information reported in the trial to confirm a low risk of bias [21].
Safety Assessments
Grade 3 or higher hematologic toxicities were reported by 10 studies (two with age stratification) [16, 18, 20, 26, 27, 29, 30, 33] (Fig. 5). The cumulative incidence of grade 3 or higher hematologic toxicities was higher for carboplatin‐based regimens than FOLFOX‐ or FOLFIRI‐based regimens (thrombocytopenia: 14%–22% vs. 1%–4%; anemia: 15%–19% vs. 5%–19%; neutropenia: 24%–58% vs. 19%–47%).
Figure 5.
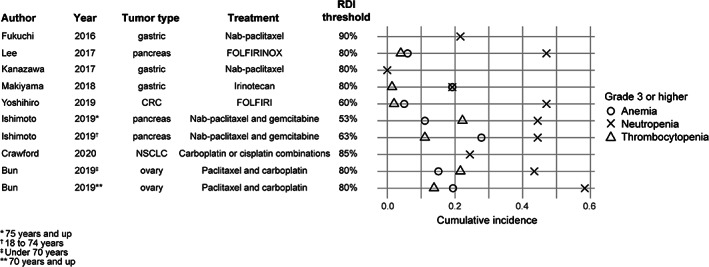
Cumulative incidence of grade 3 or higher hematologic toxicities in studies that reported RDI and survival associations. Abbreviations: CRC, colorectal cancer; FOLFIRI, folinic acid (leucovorin), fluorouracil (5‐FU), and irinotecan; FOLIRINOX, folinic acid (leucovorin), fluorouracil (5‐FU), irinotecan, and oxaliplatin; NSCLC, non‐small cell lung cancer; RDI, relative dose intensity.
Discussion
This systematic literature review and meta‐analysis identified 22 published scientific studies evaluating the impact of RDI on survival outcomes in patients with solid tumors from 2013 to 2020. The need to group studies by diverse tumor types, treatment regimens, and different RDI levels reduced the number within each classification and made meta‐analysis infeasible for most studies. However, in two categories of studies—four carboplatin‐based and three FOLFOX‐, FOLFIRI‐, or FOLFIRINOX‐based—meta‐analysis evaluation was feasible, and the association between low RDI and poor OS was consistent.
The results of the meta‐analysis showed a significant risk of increased mortality among patients with cancer treated with carboplatin‐based or FOLFOX‐ or FOLFIRI‐based regimens at RDI <80% or <85% compared with those treated at RDI ≥80% or ≥85%%. These results are consistent with those reported in the review by Havrilesky et al., in which patients with advanced or metastatic lung, breast, or ovarian cancer treated with chemotherapy had favorable survival with an RDI ≥85% [1].
The effect of RDI on survival may be confounded by other prognostic factors like age, body composition, comorbidities, and chemotherapy‐induced toxicities. Body composition was found to be a major determinant of chemotherapy tolerance and adherence [37] with greater visceral and intramuscular adiposity increasing the risk of delivering less than the planned dose of chemotherapy, thereby reducing the efficacy of chemotherapy. Feliciano et al. reported a 30% increase in risk of death with low RDI among patients with breast cancer and obesity [37]. Another study demonstrated a positive correlation between obesity and reduced dose intensity of carboplatin, which negatively impacted PFS in patients with advanced serous ovarian cancer [28]. Another prognostic factor, age, was not found to affect RDI similarly, with the same chemotherapy efficacy observed for elderly and nonelderly patients with cancer [5, 38]. However, elderly patients are at increased risk of chemotherapy‐induced toxicities like febrile neutropenia and asthenia, which often lead to lowering of RDI and reduced treatment efficacy [38].
The inclusion of various therapeutic constituents in the regimens studied may have introduced heterogeneity in the effects of RDI on survival. For example, of the five studies of carboplatin‐based regimens, two also included bevacizumab, which may have influenced survival. A study of the addition of bevacizumab to mFOLFOX6 provided evidence that the combination allows a lower RDI of mFOLFOX6 to achieve equivalent survival times compared with higher RDI without bevacizumab [22]. However, in the studies included in the meta‐analyses, there was no evidence that constituents were added depending on RDI threshold; therefore, the additional constituents are unlikely to be confounders of the summary RDI‐survival associations observed.
In the current study, grade 3 or higher neutropenia had the highest cumulative incidence among hematologic toxicities, whereas anemia and thrombocytopenia occurred at similar frequencies. Febrile neutropenia, a complication of significant neutropenia, is considered a medical emergency with a risk of mortality requiring immediate hospitalization that may result in delays and/or reductions to planned chemotherapy [39]. Primary prophylaxis with granulocyte‐colony stimulating factors may improve patient outcomes by reducing the depth and duration of neutropenia, thereby reducing the risk of febrile neutropenia and helping to maintain planned chemotherapy dose intensity [39].
Chemotherapy‐induced thrombocytopenia may also result in reduced RDI and treatment delays and/or reductions [40]. Studies have indicated the highest prevalence of thrombocytopenia in patients with solid tumors with CRC, NSCLC, and ovarian cancer [40, 41] and with platinum‐based and gemcitabine‐based chemotherapy regimens [42, 43]. Several therapies, including thrombopoietin receptor agonists romiplostim and eltrombopag, have been shown to be effective in correcting platelet counts [43, 44, 45, 46]. Suppression of hematopoiesis is a common adverse effect of chemotherapy, resulting in hematologic toxicities that eventually lead to delay or reduction of cancer therapy because of inadequate blood counts [47]. Thus, the results from the current study as well as earlier studies suggest that maintaining RDI while preventing neutropenia and managing other hematologic toxicities may ultimately benefit OS.
There are several limitations to this systematic review. Although each study addressed potential confounding by including only advanced or metastatic disease and/or by statistical adjustment for stage, additional disease severity, or comorbidity, some of the association between lower RDI and survival may still be attributed to confounding factors like worse performance status. Additionally, most of the studies included in this review were retrospective, observational studies based in clinical or institutional patient populations, and variations in clinical practice likely influenced which patients received high versus low RDI regimens. Generalizability may also be influenced by the geographic representation of the studies published. Many of the studies identified in the literature review and three of the nine studies included for meta‐analysis were from Japan, with studies from the U.S., Australia, and South Korea also contributing. A lack of published evidence from other regions limits generalizability, and publication bias may have resulted in fewer studies with findings of no RDI‐survival association being available in the literature. The clinical trial [21] was adjudicated to have “some concerns” per the Cochrane Risk of Bias scale. For example, the randomization method was not reported, and it was unclear whether patients were aware of their regimen assignment, which influenced the bias score.
The results of the systematic literature review were also limited by demographic and baseline characteristics; chemotherapy treatment patterns and management of toxicities may differ in other regions or patient populations. Because meta‐analyses were conducted only with studies or estimates reporting similar RDI levels per category for tumor types and chemotherapy regimens, the results for the meta‐analyses were only generalizable to those study populations. We identified several studies that reported median OS and/or PFS times stratified by RDI level; however, statistical comparisons were not always reported, and those that were reported were usually unadjusted for potential confounding factors. Although those studies were not included in the meta‐analyses, we presented their results graphically as supporting evidence of the RDI association with longer survival times. Furthermore, many publications did not describe the method of RDI calculation, whereas others did not define any specific RDI threshold but instead compared high and low dose regimens, which impacted the ability to evaluate the association between RDI and survival outcomes. A standardization of the RDI calculation and of its reporting is needed to determine the impact of maintaining planned dose intensity on the outcomes in patients with advanced solid tumors [1]. Moreover, predicting an RDI decrease before treatment initiation or during treatment is difficult, despite potential planned dose delays or reductions unrelated to chemotherapy‐induced toxicity.
Conclusion
This review and meta‐analysis summarized recent evidence and quantified the effect of RDI on survival outcomes. Although multiple factors contribute to reductions in RDI, hematologic toxicities are common and may be preventable. As cancer therapy evolves, ongoing research can continue to elucidate the associations between RDI, dose delays and reductions, and survival outcomes in patients with solid tumor cancer.
Author Contributions
Conception/design: Carrie M. Nielson, Lauren C. Bylsma, Jon P. Fryzek, Hossam A. Saad, Jeffrey Crawford
Collection and/or assembly of data: Lauren C. Bylsma
Data analysis and interpretation: Carrie M. Nielson, Lauren C. Bylsma, Jon P. Fryzek, Hossam A. Saad, Jeffrey Crawford
Manuscript writing: Carrie M. Nielson, Lauren C. Bylsma, Jon P. Fryzek, Hossam A. Saad, Jeffrey Crawford
Final approval of manuscript: Carrie M. Nielson, Lauren C. Bylsma, Jon P. Fryzek, Hossam A. Saad, Jeffrey Crawford
Disclosures
Carrie M. Nielson: Amgen (E, OI); Lauren C. Bylsma: EpidStrategies (E), Amgen (RF—institution); Jon P. Fryzek: EpidStrategies (E), Amgen (RF—institution); Hossam A. Saad: Amgen (E, OI); Jeffrey Crawford: Amgen, AstraZeneca, Coherus, Enzychem, G1 Therapeutics, GlaxoSmithKline, Merck, Pfizer, Spectrum (C/A), AstraZeneca, Genentech, Helsinn, Pfizer (RF), Beyond Spring, G1 Therapeutics, Merrimack, Mylan, Roche (other—Data Safety Monitoring Board member).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Acknowledgments
This study was supported by Amgen Inc. Medical writing support was provided by Debasri Mukherjee of Cactus Lifesciences (part of Cactus Communications Pvt Ltd.) funded by Amgen Inc.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1.Havrilesky LJ, Reiner M, Morrow PK et al. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol 2015;93:203–210. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw 2009;7:99–108. [DOI] [PubMed] [Google Scholar]
- 3.Huitema ADR, Spaander M, Mathôt RAA et al. Relationship between exposure and toxicity in high‐dose chemotherapy with cyclophosphamide, thiotepa and carboplatin. Ann Oncol 2002;13:374–384. [DOI] [PubMed] [Google Scholar]
- 4.Denduluri N, Patt DA, Wang Y et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw 2015;13:1383–1393. [DOI] [PubMed] [Google Scholar]
- 5.Denduluri N, Lyman GH, Wang Y et al. Chemotherapy dose intensity and overall survival among patients with advanced breast or ovarian cancer. Clin Breast Cancer 2018;18:380–386. [DOI] [PubMed] [Google Scholar]
- 6.Loibl S, Skacel T, Nekljudova V et al. Evaluating the impact of relative total dose intensity (RTDI) on patients’ short and long‐term outcome in taxane‐ and anthracycline‐based chemotherapy of metastatic breast cancer ‐ a pooled analysis. BMC Cancer 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RK, Poniewierski MS, Laskey RA et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi‐agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol 2013;129:74–80. [DOI] [PubMed] [Google Scholar]
- 8.Ben‐David Y, Rosen B, Franssen E et al. Meta‐analysis comparing cisplatin total dose intensity and survival. Gynecol Oncol 1995;59:93–101. [DOI] [PubMed] [Google Scholar]
- 9.Chu E, DeVita VT Jr.Principles of Cancer Management. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed.Philadelphia, PA: Lippincott Williams & Wilkins, 2005:295–306. [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O'Connell D et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa Hospital Web site. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 26, 2020. [Google Scholar]
- 12.RoB 2: A revised Cochrane risk‐of‐bias tool for randomized trials . Cochrane Methods Web site. Available at https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. Accessed July 26, 2020.
- 13.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hryniuk WM. The importance of dose intensity in the outcome of chemotherapy. Important Adv Oncol 1988:121–141. [PubMed] [Google Scholar]
- 16.Kanazawa Y, Fujita I, Kakinuma D et al. Initial experience with nab‐paclitaxel for patients with advanced gastric cancer: Safety and efficacy. Anticancer Res 2017;37:2715–2720. [DOI] [PubMed] [Google Scholar]
- 17.Ishigaki K, Ozaka M, Kataoka S et al. The relationship between antitumor effects and relative dose intensity of gemcitabine plus nab‐paclitaxel for unresectable pancreatic cancer. J Clin Oncol 2017;35(suppl 4):419a. [Google Scholar]
- 18.Makiyama A, Arimizu K, Hirano G et al. Irinotecan monotherapy as third‐line or later treatment in advanced gastric cancer. Gastric Cancer 2018;21:464–472. [DOI] [PubMed] [Google Scholar]
- 19.Fernández A, Salgado M, García A et al. Prognostic factors for survival with nab‐paclitaxel plus gemcitabine in metastatic pancreatic cancer in real‐life practice: The ANICE‐PaC study. BMC Cancer 2018;18:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshihiro T, Kusaba H, Makiyama A et al. Efficacy and safety of ramucirumab plus modified FOLFIRI for metastatic colorectal cancer. Int J Clin Oncol 2019;24:508–515. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita H, Takenaka R, Okuma K et al. Prognostic factors in patients after definitive chemoradiation using involved‐field radiotherapy for esophageal cancer in a phase II study. Thorac Cancer 2016;7:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama G, Hayashi N, Tanaka C et al. Addition of bevacizumab to compensate for the negative influence of attenuated relative dose intensity of cytotoxic agents on the outcome in patients with metastatic colorectal cancer. J Clin Oncol 2013;31(suppl 15):e14614a. [Google Scholar]
- 23.Shen Y, Fan M, Zhao W et al. Management of primary thoracic small cell esophageal carcinoma: Analysis of 20 consecutive cases. Int J Radiat Oncol Biol Phys 2013;87(suppl 2):S297. [Google Scholar]
- 24.Kobayashi S, Ueno M, Omae K et al. Influence of initial dose intensity on efficacy of FOLFIRINOX in patients with advanced pancreatic cancer. Oncotarget 2019;10:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama G, Tanaka C, Uehara K et al. The impact of dose/time modification in irinotecan‐ and oxaliplatin‐based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol 2014;73:847–855. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Kim JW, Ahn S et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer 2017;76:125–133. [DOI] [PubMed] [Google Scholar]
- 27.Fukuchi M, Mochiki E, Ishiguro T et al. Efficacy of nab‐paclitaxel as second‐line chemotherapy for unresectable or recurrent gastric cancer. Anticancer Res 2016;36:6699–6703. [DOI] [PubMed] [Google Scholar]
- 28.Au‐Yeung G, Webb PM, DeFazio A et al. Impact of obesity on chemotherapy dosing for women with advanced stage serous ovarian cancer in the Australian Ovarian Cancer Study (AOCS). Gynecol Oncol 2014;133:16–22. [DOI] [PubMed] [Google Scholar]
- 29.Crawford J, Denduluri N, Patt D et al. Relative dose intensity of first‐line chemotherapy and overall survival in patients with advanced non‐small‐cell lung cancer. Support Care Cancer 2020;28:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishimoto U, Kinoshita A, Hirose Y et al. The efficacy and safety of nab paclitaxel plus gemcitabine in elderly patients over 75 years with unresectable pancreatic cancer compared with younger patients. Cancer Chemother Pharmacol 2019;84:647–654. [DOI] [PubMed] [Google Scholar]
- 31.Kushnir I, Mallick R, Ong M et al. Docetaxel dose‐intensity effect on overall survival in patients with metastatic castrate‐sensitive prostate cancer. Cancer Chemother Pharmacol 2020;85:863–868. [DOI] [PubMed] [Google Scholar]
- 32.Saito K, Nakai Y, Isayama H et al. The efficacy and safety of gemcitabine + nab‐PTX for recurrence and refractory pancreatic cancer. J Clin Oncol 2017;35(suppl 4):485a.28029328 [Google Scholar]
- 33.Bun S, Yunokawa M, Ebata T et al. Feasibility of initial treatment in elderly patients with ovarian cancer in Japan: A retrospective study. Int J Clin Oncol 2019;24:1111–1118. [DOI] [PubMed] [Google Scholar]
- 34.Jordan S, Anuradha S, Donovan P, Webb P. Variations in primary chemotherapy and survival amongst australian women with epithelial ovarian cancer. Int J Gynecol Cancer 2014;24(9 Suppl. 4):230–230. [Google Scholar]
- 35.Molby A, Laursen B, Falkmer U, McCulloch T, Jensen N, Poulsen L. 54PUp front chemotherapy dosing and relative dose intensity in extensive stage small cell lung cancer. Ann Oncol 2017;28(suppl_2). [Google Scholar]
- 36.Pérez‐Fidalgo JA, Bermejo B, Chirivella I et al. Retrospective analysis of the use of G‐CSF and its impact on dose response for anthracycline plus taxane‐based schedules in early breast cancer. Clin Transl Oncol 2014;16:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feliciano EMC, Chen WY, Lee V et al. Body composition, adherence to anthracycline and taxane‐based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol 2020;6:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JW, Lee KW, Kim KP et al. Efficacy and safety of FOLFIRI regimen in elderly versus nonelderly patients with metastatic colorectal or gastric cancer. The Oncologist 2017;22:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madry R, Poplawska L, Haslbauer F et al. Results of a prospective dose intensity and neutropenia prophylaxis evaluation programme (DIEPP) in cancer patients at risk of febrile neutropenia due to myelosuppressive chemotherapy. Wien Klin Wochenschr 2016;128:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Aravind S, Ranganathan G et al. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: A descriptive study of a large outpatient oncology practice database, 2000‐2007. Clin Ther 2009;31 pt 2:2416–2432. [DOI] [PubMed] [Google Scholar]
- 41.Hitron A, Steinke D, Sutphin S et al. Incidence and risk factors of clinically significant chemotherapy‐induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract 2011;17:312–319. [DOI] [PubMed] [Google Scholar]
- 42.Ten Berg MJ, van den Bemt PMLA, Shantakumar S et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: Results from a retrospective hospital‐based cohort. Drug Saf 2011;34:1151–1160. [DOI] [PubMed] [Google Scholar]
- 43.Bussel JB, Cheng G, Saleh MN et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 2007;357:2237–2247. [DOI] [PubMed] [Google Scholar]
- 44.Kuter DJ, Bussel JB, Lyons RM et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double‐blind randomised controlled trial. Lancet 2008;371:395–403. [DOI] [PubMed] [Google Scholar]
- 45.Shaw J, Kilpatrick K, Eisen M et al. The incidence and clinical burden of immune thrombocytopenia in pediatric patients in the United States. Platelets 2020;31:307–314. [DOI] [PubMed] [Google Scholar]
- 46.Al‐Samkari H, Marshall AL, Goodarzi K et al. The use of romiplostim in treating chemotherapy‐induced thrombocytopenia in patients with solid tumors. Haematologica 2018;103:e169–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker PS, Griffiths EA, Alwan LM et al. NCCN guidelines insights: Hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw 2020;18:12–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.


