Abstract
Listeria monocytogenes strains possess short repetitive extragenic palindromic (REP) elements and enterobacterial repetitive intergenic consensus (ERIC) sequences. We used repetitive element sequence-based PCR (rep-PCR) to evaluate the potential of REP and ERIC elements for typing L. monocytogenes strains isolated from humans, animals, and foods. On the basis of rep-PCR fingerprints, L. monocytogenes strains were divided into four major clusters matching origin of isolation. rep-PCR fingerprints of human and animal isolates were different from those of food isolates. Computer evaluation of rep-PCR fingerprints allowed discrimination among the tested serotypes 1/2a, 1/2b, 1/2c, 3b, and 4b within each major cluster. The index of discrimination calculated for 52 epidemiologically unrelated isolates of L. monocytogenes was 0.98 for REP- and ERIC-PCR. Our results suggest that rep-PCR can provide an alternative method for L. monocytogenes typing.
Listeria monocytogenes is an important food-borne pathogen, and various kinds of food have been implicated as sources of animal and human listeriosis (12). Since there are great differences in pathogenic potential among strains of L. monocytogenes (18), useful information can be obtained from typing.
Serotyping and phage typing have traditionally been used for the differentiation and characterization of L. monocytogenes isolates (2, 31). Since the vast majority of clinical isolates belong to serotype 1/2a, 1/2b, or 4b, serotyping has limited value as an epidemiological tool (12, 32). Phage typing has been useful in studies of some listeriosis outbreaks but also has limited value, as not all strains are typeable (12, 29). Improved discrimination between L. monocytogenes isolates has been attained by the development of molecular typing methods such as restriction enzyme analysis (3, 9, 36), pulsed-field gel electrophoresis (7, 8, 27), multilocus enzyme electrophoresis (MEE) (6, 17), esterase typing (14, 15), ribotyping (3), and random amplification of polymorphic DNA (RAPD) (4, 5, 11, 24).
Repetitive element sequence-based PCR (rep-PCR) is a recently described method which generates DNA fingerprints that allow discrimination between bacterial strains (34). The term rep-PCR refers to the general methodology involving the use of oligonucleotide primers based on short repetitive sequence elements that are dispersed throughout the prokaryotic kingdom. The palindromic units, or repetitive extragenic palindromes (REP), constitute the best-characterized family of repetitive bacterial sequences (16).
REP sequences are 35 to 40 bp long and include an inverted repeat. A second family of repetitive elements, called intergenic repeat units or enterobacterial repetitive intergenic consensus (ERIC) sequences, are larger elements of 124 to 127 bp and contain a highly conserved central inverted repeat (19). REP and ERIC sequences were used as primer binding sites to amplify the genomes of a variety of bacteria by PCR (10, 22, 23, 28, 30, 33, 37). Our previous study showed that REP and ERIC sequences are also present in the genus Listeria and that they are useful for species and strain discrimination (21).
In this work, REP- and ERIC-PCR were used to generate DNA fingerprints of L. monocytogenes strains isolated from humans, animals, and foods. The objective of this investigation was to evaluate the potential of rep-PCR for typing L. monocytogenes isolates of various origins.
MATERIALS AND METHODS
Isolates.
Sixty-four L. monocytogenes isolates were examined by rep-PCR. Among them, 52 strains were of unrelated origin (Table 1). Geographical origin, time of isolation, and maintenance of L. monocytogenes isolates prior to the beginning of this research are shown in Table 2. Bacteria were maintained on brain heart infusion agar (Oxoid Ltd., London, United Kingdom) at 4°C after the beginning of this research. All isolates were biochemically confirmed to be L. monocytogenes and serotyped at the Belgian National Reference Center for Listeriosis (Institute of Hygiene and Epidemiology, Brussels) (32).
TABLE 1.
Characteristics of L. monocytogenes strains isolated from humans, animals, and foods and REP and ERIC types
| Designation
|
Originb | Sero-type | REP type
|
ERIC type
|
|||
|---|---|---|---|---|---|---|---|
| This study | Sourcea | VCc | CAd | VC | CA | ||
| 1 | MF1 | Child 1, CSF | 1/2b | 1 | A4 | 1 | A1 |
| 2 | MF2 | Child 1, navel | 1/2b | 1 | A4 | 1 | A1 |
| 3 | MF3 | Child 1, auditory passage | 1/2b | 1 | A4 | 1 | A1 |
| 4 | MF4 | Child 1, eyes | 1/2b | 1 | A4 | 1 | A1 |
| 5 | MF5 | Child 1, skin | 1/2b | 1 | A3 | 1 | A1 |
| 6 | MF6 | Child 1, blood | 1/2b | 1 | A3 | 1 | A1 |
| 7 | MF7 | Mother 1, cervix | 1/2b | 1 | A3 | 1 | A1 |
| 8 | MF8 | Mother 1, lochia | 1/2b | 1 | A3 | 1 | A1 |
| 9 | MF9 | Human 2, CSF | 4b | 2 | A1 | 2 | A2 |
| 10 | MF10 | Human 3, CSF | 4b | 2 | A2 | 2 | A2 |
| 11 | MF11 | Human 4, auditory passage | 4b | 2 | A1 | 2 | A2 |
| 12 | MF12 | Human 4, blood | 4b | 2 | A1 | 2 | A2 |
| 13 | MF13 | Human 5, / | 1/2c | 3 | A5 | 3 | A4 |
| 14 | MF14 | Human 5, / | 1/2c | 3 | A5 | 3 | A4 |
| 15 | MF15 | Child 6, auditory passage | 4b | 2 | A1 | 2 | A2 |
| 16 | MF16 | Mother 6, cervix | 4b | 2 | A1 | 2 | A2 |
| 17 | MF17 | Child 6, blood | 4b | 2 | A1 | 2 | A2 |
| 18 | MF18 | Human 7, / | 4b | 2 | A1 | 2 | A2 |
| 19 | MF19 | Human 8, CSF | 4b | 2 | A1 | 2 | A2 |
| 20 | MF20 | Human 9, blood | 1/2b | 4 | B3 | 4 | B5 |
| 21 | MF21 | Human 10, CSF | 1/2b | 5 | B2 | 5 | B2 |
| 22 | MF22 | Human 11, / | 1/2b | 5 | B2 | 6 | B6 |
| 23 | MF24 | Human 12, / | 4b | 6 | B4 | 5 | B4 |
| 24 | MF25 | Human 13, / | 4b | 6 | B4 | 5 | B4 |
| 25 | MF26 | Human 14, / | 1/2a | 7 | B6 | 7 | B8 |
| 26 | MF27 | Human 15, blood | 4b | 8 | B1 | 8 | B1 |
| 27 | MF28 | Human 15, auditory passage | 4b | 8 | B1 | 8 | B1 |
| 28 | B574 | Sheep 1, brain | 4b | 6 | B4 | 5 | B3 |
| 29 | B282 | Sheep 2, brain | 4b | 6 | B4 | 5 | B3 |
| 30 | B139 | Sheep 3, brain | 4b | 6 | B4 | 5 | B3 |
| 31 | B429 | Sheep 4, brain | 1/2a | 9 | B5 | 7 | B7 |
| 32 | B254 | Sheep 5, brain | 1/2a | 9 | B5 | 7 | B7 |
| 33 | VF321/17 | Sheep 6, brain | 4b | 6 | B4 | 5 | B3 |
| 34 | IPH61 | A, Dairy Queen salad | 4b | 10 | C10 | 9 | C14 |
| 35 | IPH68 | A, chicken salad | 1/2c | 11 | C1 | 10 | C7 |
| 36 | IPH77 | B, vienna salad | 1/2a | 12 | C8 | 10 | C3 |
| 37 | IPH129 | C, turkey salad | 1/2b | 13 | C2 | 11 | C9 |
| 38 | IPH132 | D, French salad | 1/2a | 14 | C9 | 10 | C1 |
| 39 | IPH139 | E, frozen kajmak | 1/2a | 11 | C3 | 10 | C6 |
| 40 | IPH143 | F, cheese with vegetables | 1/2b | 15 | C15 | 12 | C15 |
| 41 | IPH144 | F, Smoked cheese | 1/2b | 15 | C13 | 12 | C15 |
| 42 | IPH145 | B, Vienna salad | 1/2b | 15 | C13 | 9 | C11 |
| 43 | IPH151 | D, French salad | 1/2a | 16 | C4 | 10 | C5 |
| 44 | IPH152 | D, squid salad | 1/2a | 16 | C4 | 10 | C5 |
| 45 | IPH153 | G, paste | 4b | 15 | C11 | 9 | C12 |
| 46 | IPH156 | H, Russian salad | 3b | 15 | C12 | 9 | C11 |
| 47 | IPH160 | H, Russian salad | 1/2a | 17 | C6 | 9 | C11 |
| 48 | IPH161 | H, liptaver | 1/2b | 18 | C14 | 13 | C4 |
| 49 | IPH165 | B, Alps salad | 1/2c | 16 | C5 | 10 | C8 |
| 50 | IPH173 | B, egg grease | 3b | 18 | C14 | 9 | C13 |
| 51 | IPH134 | I, rough seitan | 1/2a | 12 | C7 | 10 | C2 |
| 52 | IPH123 | G, Tatarian beefsteak | 1/2b | 18 | C14 | 12 | C10 |
| 53 | IPH141 | J, Tatarian beefsteak | 1/2b | 19 | D4 | 9 | D3 |
| 54 | IPH146 | K, Tatarian beefsteak | 4b | 19 | D6 | 9 | D3 |
| 55 | IPH147 | L, Tatarian beefsteak | 1/2b | 19 | D4 | 14 | D1 |
| 56 | IPH149 | M, Tatarian beefsteak | 1/2b | 19 | D5 | 14 | D2 |
| 57 | IPH150 | D, Tatarian beefsteak | 1/2a | 20 | D2 | 15 | D5 |
| 58 | IPH166 | N, Tatarian beefsteak | 1/2a | 21 | D3 | 15 | D5 |
| 59 | VF2648 | O, steamed chicken | 1/2a | 20 | D1 | 15 | D4 |
| 60 | VF2746/11 | O, steamed chicken | 1/2a | 20 | D1 | 15 | D4 |
| 61 | VF2856/15 | O, steamed chicken | 1/2a | 20 | D1 | 15 | D4 |
| 62 | VF2855/17 | O, steamed chicken | 1/2a | 20 | D1 | 15 | D4 |
| 63 | VF2856/18 | O, steamed chicken | 1/2a | 20 | D1 | 15 | D4 |
| 64 | VFM10 | O, steamed chicken | 1/2b | 22 | D4 | 16 | A3 |
MF, Medical Faculty, Institute of Microbiology, University of Ljubljana, Ljubljana, Slovenia; B, Central Veterinary Research Institute, Sofia, Bulgaria; VF, Veterinary Faculty, University of Ljubljana; IPH, Institute of Public Health of the Republic of Slovenia, Department of Sanitary Microbiology, Ljubljana, Slovenia.
Human subjects were labelled 1 to 15; sheep were labelled 1 to 6; and food manufacturers were labelled A to O. For human subjects, those in whom isolates of related origin were found are given the same number (e.g., mother 1 and child 1 produced related isolates). Kajmak is salted cream fermented for a short time; Alps salad contains mayonnaise, sliced sausages, and mixed vegetables; liptaver is fresh cheese with red paprika, onion, and salt; seitan is a vegetarian substitute for meat. A slash mark indicates that no data about exact origin were available. CSF, cerebrospinal fluid.
VC, determined by visual comparison of banding patterns.
CA, determined by computer analysis on the basis of the Jaccard coefficient (SJ ≥ 80%).
TABLE 2.
Geographical origin, time of isolation, and maintenance of L. monocytogenes isolates
| Geographical region of origin of isolatea | Isolate designation | Date of isolation | Maintenance of isolates before the present research was begun |
|---|---|---|---|
| Ljubljana | 1–27 | 1980–1985 | Bacterial strain storage medium (Sanofi Diagnostics, Pasteur, Marnes-la-Coquette, France) at 4°C without subculturing |
| Smoljan (Rodopi mountain) | 28 | 1972 | Lyophilized |
| 30 | 1983 | ||
| 31, 32 | 1984 | ||
| Sofia (Kostenez) | 29 | 1972 | Lyophilized |
| Ljubljana | 33 | 1985 | Nutrient agar at 4°C subcultured every 4 mo |
| Ljubljana | 34–58 | 1992–1993 | Microbank system (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada) subcultured every 6 mo |
| Ptuj | 59–64 | 1993 | Nutrient agar at 4°C subcultured every 4 mo |
The regions of Ljublana and Ptuj are in central and northeastern Slovenia, respectively.
Preparation of genomic DNA.
DNA was extracted from bacterial cells grown overnight at 37°C in brain heart infusion broth (Oxoid) by the method of Flamm et al. (13) as follows. Bacterial cells from 2 ml of culture were pelleted by centrifugation at 13,000 × g for 2 min, washed in 1 ml of 1× SSC (150 mM NaCl plus 15 mM sodium citrate [pH 7.0]), suspended in 100 μl of lysozyme solution (10 mM sodium phosphate [pH 7.0], 20% sucrose, 4 mg of lysozyme [Boehringer, Mannheim, Germany] per ml), and incubated for 45 min at 37°C. To these suspensions, 200 μl of TE buffer (20 mM Na2-EDTA, 50 mM Tris-HCl [pH 8.0]), 100 μl of Sarkosyl solution (5% Sarkosyl [Boehringer] in TE buffer), and 100 μl of proteinase K solution (25 mg/ml [Boehringer] in TE buffer) were added and incubated at 37°C for 1 h. Cell lysates were extracted once with phenol and twice with chloroform. Precipitation of nucleic acids was done with sodium acetate (final concentration, 0.3 M) and 2 volumes of ethanol (100%). The DNA was dissolved in sterile water, and the concentration of the DNA was determined spectrophotometrically at 260 nm.
rep primers and rep-PCR conditions.
For REP-PCR, the (18-mer) primers REP 1R-I (5′-IIIICGICGICATCIGGC-3′) and REP 2-I (5′-ICGICTTATCIGGCCTAC-3′) and for ERIC-PCR, the (22-mer) primers ERIC 1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC 2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) (34) were used. The REP 1R-I and REP 2-I primers contain the nucleotide inosine (I) at ambiguous positions in the REP consensus (34). Inosine can form Watson-Crick base pairs with A, T, G, or C. PCRs were carried out as described by Versalovic et al. (34) with 25 ng of template DNA per reaction for REP-PCR and 35 ng of template DNA for ERIC-PCR. Amplification reactions were performed in 25 μl of a solution containing 25 pmol of each of the two opposing primers (Isogen, Amsterdam, The Netherlands), 200 μM each deoxynucleoside triphosphate (Pharmacia, Uppsala, Sweden), 2.5 mM MgCl2 (Perkin-Elmer, Norwalk, Conn.), 50 mM KCl–10 mM Tris-HCl (pH 8.3), and 0.35 U of Goldstar DNA polymerase (Eurogentec S. A., Seraing, Belgium). Amplifications were performed with a DNA thermocycler (Cetus model 9600 [Perkin-Elmer]) with the following temperature profiles: for REP-PCR, 1 cycle at 95°C for 3 min; 30 cycles at 90°C for 30 s, at 40°C for 1 min, at 72°C for 1 min; and 1 cycle at 72°C for 8 min; for ERIC-PCR, 1 cycle at 95°C for 5 min; 30 cycles at 90°C for 30 s, at 50°C for 30 s, at 52°C for 1 min, at 72°C for 1 min; and 1 cycle at 72°C for 8 min.
Analysis of rep-PCR products.
rep-PCR products (12 μl) were separated by electrophoresis on a 1.5% agarose gel (SeaKem LE agarose; FMC Bioproducts, Rockland, Maine) in 1× TBE buffer (2 mM EDTA, 0.1 M Tris-HCl, 0.1 M boric acid [pH 8]) at a constant voltage of 4 V/cm. After being stained with ethidium bromide, the gel was photographed under UV transillumination with Polaroid 665 film. The DNA molecular weight marker X from Boehringer was used as a size standard.
First, DNA fingerprints of the isolates were compared for similarity by visual inspection of the band patterns. Two fingerprints were considered different if the presence or absence of at least two bands differed in one of the patterns. Variations in band intensity were not considered to be differences. Bands that were too faint to be interpreted when reproduced were not considered.
Subsequently, gel images were scanned (ScanJet4p; Hewlett-Packard Co., Palo Alto, Calif.), digitized, and stored as TIFF files. These files were converted (track resolution, 450 pixels [px]), normalized (normalization settings: resolution, 350 px;smoothing, 3; background subtraction: rolling disk, intensity 6), and analyzed (band settings: band search filters: minimal profiling, 0.27%; minimal area, 0.25%; band comparison settings: position tolerance, 0.85%; increase, 0%; clustering bands, unweighted pair group method using arithmetic averages (UPGMA), and Jaccard coefficients were compared with GelCompar software (version 3.1; Applied Maths, Kortrijk, Belgium) (1). DNA bands detected by computer were carefully verified by visual examination to correct unsatisfactory detection. The normalization program allowed alignment of gels by associating internal reference bands. The similarities between DNA fingerprints were calculated with the band-matching Jaccard coefficient (SJ) (28). The proportion of bands common to two strains, A and B, is defined as SJ = nAB/(nA + nB − nAB), where nAB is the number of bands common to A and B, and nA and nB are the total numbers of bands for A and B, respectively. This Jaccard coefficient ranges from 0 to 1.0, where 1.0 represents 100% identity (presence and position) for all bands in the two fingerprints being compared. A band-matching tolerance of 0.8% was chosen. DNA fingerprints from 298 to 6,100 bp were compared. Cluster analysis of similarity matrices was performed by UPGMA.
RESULTS
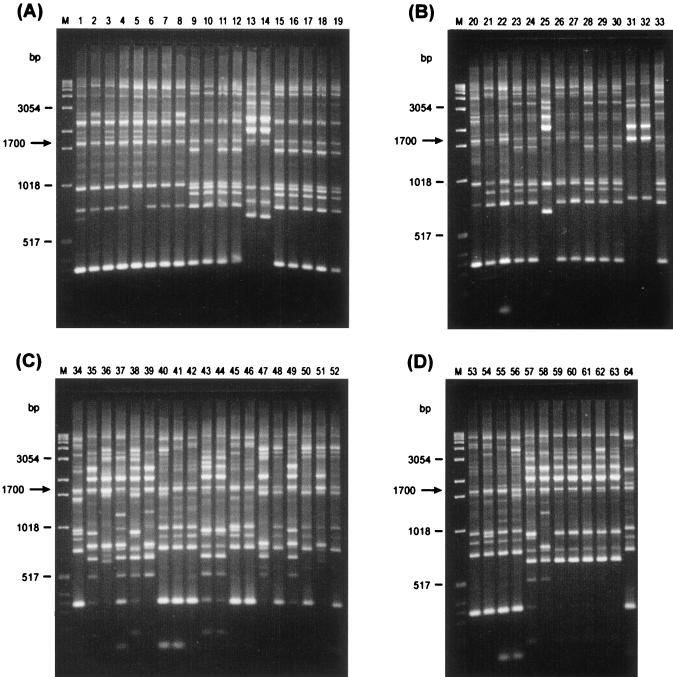
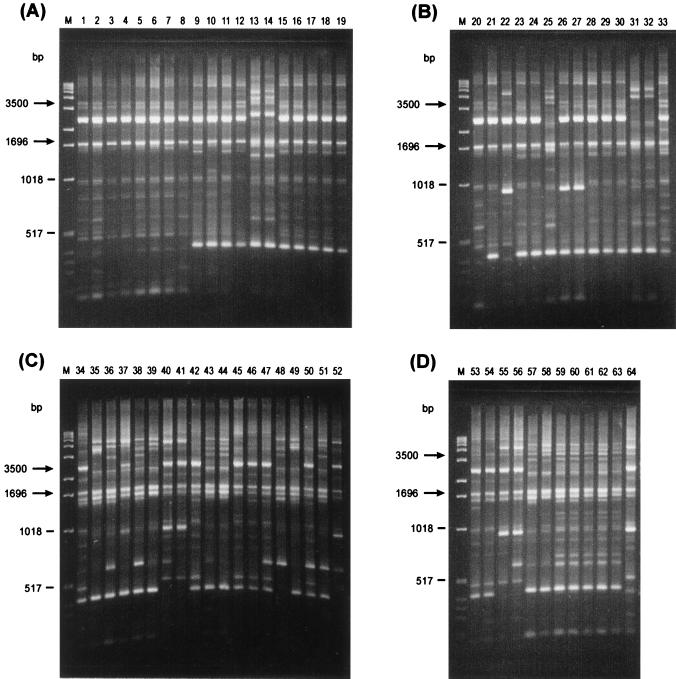
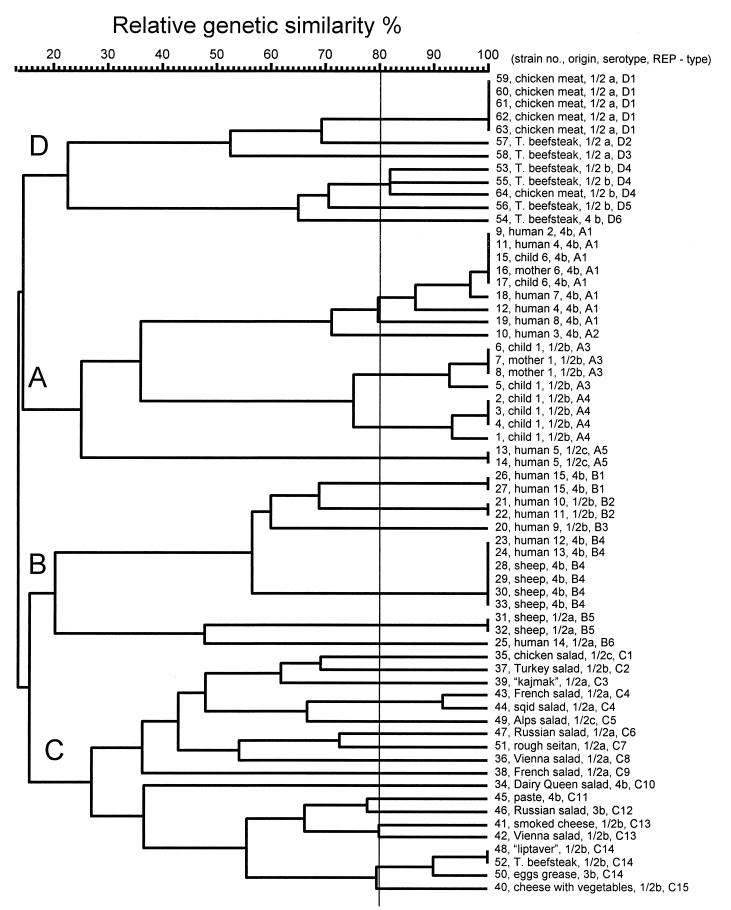
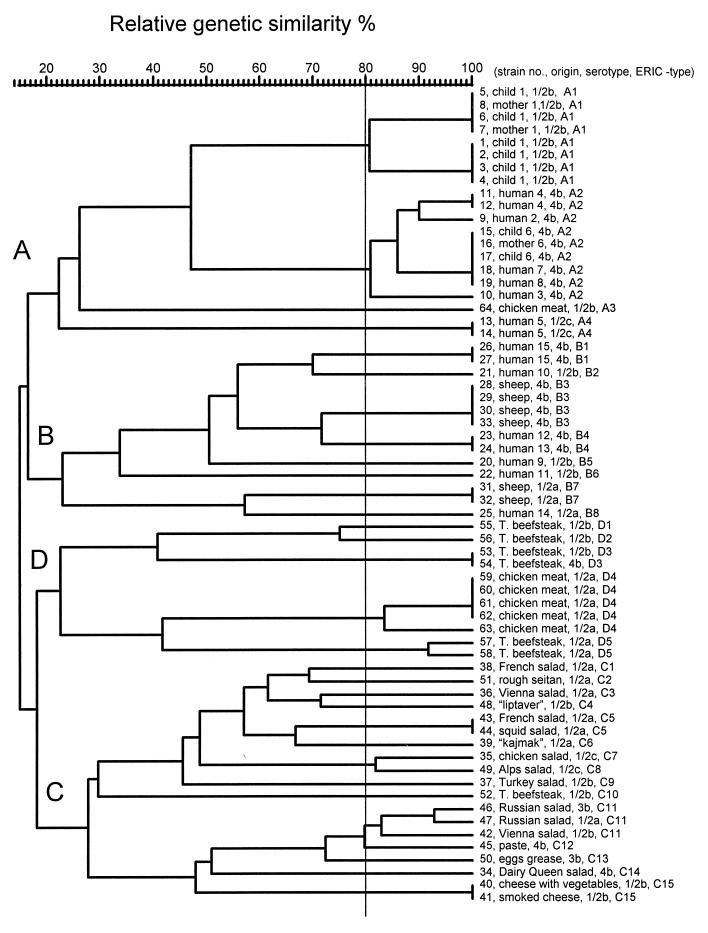
rep-PCR of genomic DNA from L. monocytogenes isolates generated multiple DNA fragments in sizes ranging between 298 and 6,100 bp (Fig. 1 and 2). One common band of about 1,700 bp, for REP-PCR (Fig. 1), and two common bands, of about 1,696 and 3,500 bp, for ERIC-PCR (Fig. 2), were present in all L. monocytogenes strains tested. Visual comparison of banding patterns revealed 22 distinct REP profiles and 16 distinct ERIC profiles (Fig. 1 and 2 and Table 1) for the 52 unrelated strains tested. Related strains (isolated from a mother and a newborn or different isolates from the same patient) produced identical REP and ERIC profiles. REP and ERIC profiles of L. monocytogenes strains isolated from foods were clearly distinct from REP and ERIC profiles of human and animal L. monocytogenes isolates (Fig. 1 and 2). In contrast, four of six L. monocytogenes animal isolates (no. 28 to 30 and 33) had the same DNA banding pattern as that of two human isolates (no. 23 and 24). All those isolates (no. 23, 24, 28 to 30, and 33), which are REP type 6 and ERIC type 5, belong to serotype 4b. It is, however, worth noting that not all ERIC type 5 strains are serotype 4b strains, as the human isolate no. 21 is ERIC type 5, serotype 1/2b (Table 1). Subsequently, DNA fingerprints were analyzed by using a computer program for comparative analysis of DNA electrophoresis patterns. After normalization and alignment of the different DNA profiles, the relative genetic similarity among L. monocytogenes isolates was calculated and visualized by cluster analysis. The estimated relationships among isolates on the basis of REP and ERIC fingerprints are indicated on the dendrograms in Fig. 3 and 4,, respectively. The dendrograms clearly show that the strains examined are divided into four distinct groups designated A, B, C, and D. Strains were assigned to group A, B, C, or D regardless of which type of PCR (REP or ERIC) was used. The low degree of relative genetic similarity between these four groups is less than 20%. Group A consists of human isolates, group B consists of human and animal isolates, and groups C and D consist of food isolates. Within each of the four groups A, B, C, and D, a further differentiation of rep profiles was established at 80% relative genetic similarity. This second level of clustering enabled the same or greater differentiation among strains than did serotyping, with the exception of REP type C14 and ERIC types C11 and D3 (no. 53 and 54) (Table 1). This computer evaluation (at SJ = 80%) suggested the existence of 33 different REP patterns and 35 different ERIC patterns (Fig. 3 and 4). Computers work with a certain resolution to discriminate bands from each other, and this can differ from visual interpretation by eye. The index of discrimination (DI) of Hunter and Gaston (20) calculated for 52 epidemiologically unrelated isolates was 0.98 for REP- and ERIC-PCR. As expected, we found a lower DI (0.72) for serotyping. By visual analysis, the DIs calculated for 52 epidemiologically unrelated isolates were 0.96 for REP-PCR and 0.94 for ERIC-PCR. The lower DI obtained by visual analysis may be compensated for by better REProducibility, and this is a very important but often neglected criterion for the assessment of a typing method.
FIG. 1.
REP-PCR fingerprints of human (lanes A1 to A19 and B20 to B27), animal (lanes B28 to B33), and food (lanes C34 to C52 and D53 to D64) isolates of L. monocytogenes. Lanes M contain molecular size markers.
FIG. 2.
ERIC-PCR fingerprints of human (lanes A1 to A19 and B20 to B27), animal (lanes B28 to B33), and food (lanes C34 to C52 and D53 to D64) isolates of L. monocytogenes. Lanes M contain molecular size markers.
FIG. 3.
Dendrogram representing genetic relationships between L. monocytogenes isolates based on REP-PCR fingerprints.
FIG. 4.
Dendrogram representing genetic relationships between L. monocytogenes isolates based on ERIC-PCR fingerprints.
REP- and ERIC-PCR provided a similar degree of discrimination within the tested serotypes (Table 3). At a relative genetic similarity of 80%, there was complete discrimination between serotypes 1/2a, 1/2b, and 4b. Strains 50 and 46, both serotype 3b, could be discriminated from the other serotypes at a relative genetic similarity of 90%. In general, fewer L. monocytogenes rep types were found among human and animal isolates than among food isolates. As for visual comparison of banding patterns, rep types found among human and animal isolates are different from those found among food isolates, regardless of the serotype. Serotype 4b is present in human, animal, and food isolates, but human and animal serotype 4b isolates clearly belong to other rep types than food isolates. The different rep types present among food isolates show a relative genetic similarity lower than 20% to rep types found among human and animal isolates, with the exception of one strain (no. 64) isolated from chicken, which is more closely related to human L. monocytogenes isolates than to the other food isolates (Fig. 3 and 4). REP- and ERIC-PCR for strain 64 were repeated with freshly prepared DNA in order to confirm the results.
TABLE 3.
Ability of rep-PCR to discriminate between L. monocytogenes isolates
| Serotype | No. of unrelated isolates | No. of REP typesa | No. of ERIC typesa |
|---|---|---|---|
| 1/2a | 17 | 11 | 10 |
| 1/2b | 14 | 10 | 13 |
| 1/2c | 3 | 3 | 3 |
| 3b | 2 | 2 | 2 |
| 4b | 16 | 7 | 7 |
REP and ERIC types were based on determination by computer analysis with the Jaccard coefficient (SJ ≥80%).
DISCUSSION
In this study, rep-PCR was used as a tool to characterize L. monocytogenes strains isolated from humans, animals, and foods. Both methods, REP- and ERIC-PCR, showed great possibilities for the typing of L. monocytogenes isolates. L. monocytogenes isolates which are closely linked epidemiologically as well as some unrelated strains (animal isolates 28 to 30 and 33 and human isolates 23 and 24) showed very similar REP- and ERIC-PCR fingerprints by visual inspection. Computer-aided comparison of electrophoresis patterns confirmed this visual impression (Table 1). REP and ERIC types correlated well with serotypes and generally provided greater differentiation within and between human and animal isolates.
Among food isolates of L. monocytogenes, a great diversity of fingerprints was observed. REP and ERIC profiles for food isolates were clearly different from the profiles obtained for human and animal isolates of L. monocytogenes.
The 64 L. monocytogenes isolates were divided into four groups, A, B, C, and D, separated at a relative genetic similarity of less than 20%. This first level of clustering was based on the origin of the L. monocytogenes strains—human, animal, or food. The second level of clustering (at SJ = 80%) allowed at least the same level of differentiation between strains as serotyping. The results of our clustering of L. monocytogenes strains are different from the division of L. monocytogenes strains by restriction fragment length polymorphism (33) or MEE (17). Vines et al. (35) examined 29 strains of L. monocytogenes and divided them into one group containing serovars 1/2a, 3a, and 1/2c and another group comprising serovars 1/2b, 3b, and 4b. These groups did not correlate with human or environmental origin. Harvey and Gilmour (17) divided 141 L. monocytogenes strains into ET group I, containing serovars 1/2b, 4a, 4b, 4c, 4d, and 4e, and ET group II, containing serovars 1/2a, 1/2c, and 3a. The DI of Hunter and Gaston (20), being 0.98 for REP-PCR or for ERIC-PCR, is in the same range as the one determined by RAPD with the combination of results obtained with two or three primers (5).
The division of the 64 strains of L. monocytogenes by rep-PCR into two groups (A and B) containing human and animal isolates and another two groups (C and D) containing food isolates showed that no similarity between strains isolated from humans or animals and strains isolated from foods is present (Fig. 3 and 4). The exception, strain 64, isolated from chicken, showed by ERIC-PCR higher similarity with human isolates than with other food isolates (SJ = 26%). This observation could lead to the hypothesis that only a minor portion of the L. monocytogenes strains present in food products are infectious for humans and animals. McLauchlin (25) and Notermans et al. (26) reached a similar conclusion that not all L. monocytogenes bacteria present in food cause disease because of the interstrain differences in their virulence properties. Because only 64 strains were included in this study, these observations should be validated by the examination of a larger set of strains.
The potential of rep-PCR as an efficient and sensitive molecular typing tool for L. monocytogenes should be further evaluated by the examination of L. monocytogenes isolates associated with food-borne epidemics. rep-PCR may serve as a rapid screening method to classify a large number of isolates into clusters. Results of this study add further evidence to the idea that rep-PCR may be broadly applicable for fingerprinting bacteria which possess repetitive elements such as REP or ERIC sequences.
ACKNOWLEDGMENTS
We are grateful to A. Genicot (Belgian National Center for Listeriosis) for serotyping and to M. De Loose and J. De Riek (Plant Breeding Station, Merelbeke, Belgium) for assistance with the computer-aided evaluation of rep-PCR fingerprints.
B. Jeršek was supported by a fellowship from the Ministry of Science and Technology, Slovenia, during her stay at the Centre of Agricultural Research, Department for Animal Product Quality (DVK).
REFERENCES
- 1.Anonymous. GelCompar, comparative analysis of electrophoresis patterns. Kortrijk, Belgium: Applied Maths; 1994. [Google Scholar]
- 2.Audurier A, Martin C. Phage typing of Listeria monocytogenes. Int J Food Microbiol. 1989;8:251–257. doi: 10.1016/0168-1605(89)90022-6. [DOI] [PubMed] [Google Scholar]
- 3.Baloga A O, Harlander S K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991;57:2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black S F, Gray D J, Fenlon D R, Kroll R G. Rapid RAPD analysis for distinguishing Listeria species and Listeria monocytogenes serotypes using a capillary air thermal cycler. Lett Appl Microbiol. 1995;20:188–190. doi: 10.1111/j.1472-765x.1995.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 5.Boerlin P, Bannerman E, Ischer F, Rocourt J, Bille J. Typing of Listeria monocytogenes: a comparison of polymorphic DNA with 5 other methods. Res Microbiol. 1995;146:35–49. doi: 10.1016/0923-2508(96)80269-5. [DOI] [PubMed] [Google Scholar]
- 6.Boerlin P, Rocourt J, Piffaretti J C. Taxonomy of genus Listeria by using multilocus enzyme electrophoresis. Int J Syst Bacteriol. 1991;41:59–64. doi: 10.1099/00207713-41-1-59. [DOI] [PubMed] [Google Scholar]
- 7.Brosch R, Chen J, Luchansky J B. Pulsed-field fingerprinting of Listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar. Appl Environ Microbiol. 1994;60:2584–2592. doi: 10.1128/aem.60.7.2584-2592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchrieser C, Brosch R, Catimel B, Rocourt J. Pulsed-field gel electrophoresis applied for comparing Listeria monocytogenes strains involved in outbreaks. Can J Microbiol. 1993;39:395–401. doi: 10.1139/m93-058. [DOI] [PubMed] [Google Scholar]
- 9.Carriere C, Allerdet-Servent A, Bourg G, Audurier A, Ramuz M. DNA polymorphism in strains of Listeria monocytogenes. J Clin Microbiol. 1991;29:1351–1355. doi: 10.1128/jcm.29.7.1351-1355.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farber J M, Addison C J. RAPD typing for distinguishing species and strains in the genus Listeria. J Appl Bacteriol. 1994;77:242–250. doi: 10.1111/j.1365-2672.1994.tb03070.x. [DOI] [PubMed] [Google Scholar]
- 12.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamm R K, Hinricks D J, Thomashow M F. Introduction of pAMβ1 into L. monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect Immun. 1984;44:157–161. doi: 10.1128/iai.44.1.157-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilot P, Andre P. Characterization of five esterases from Listeria monocytogenes and use of their electrophoretic polymorphism for strain typing. Appl Environ Microbiol. 1995;61:1661–1665. doi: 10.1128/aem.61.4.1661-1665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilot P, Genicot A, Andre P. Serotyping and esterase typing for analysis of Listeria monocytogenes populations recovered from foodstuffs and from human patients with listeriosis in Belgium. J Clin Microbiol. 1996;33:1007–1010. doi: 10.1128/jcm.34.4.1007-1010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilson E J, Clement J M, Brulag D, Hofnung M. A family of dispersed repetitive extragenic palindromic DNA sequences in E. coli. EMBO J. 1984;3:1417–1421. doi: 10.1002/j.1460-2075.1984.tb01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey J, Gilmour A. Application of multilocus enzyme electrophoresis and restriction fragment length polymorphism analysis to the typing of Listeria monocytogenes strains isolated from raw milk, nondairy foods, and clinical and veterinary sources. Appl Environ Microbiol. 1994;60:1547–1553. doi: 10.1128/aem.60.5.1547-1553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hof H, Hefner P. Pathogenicity of Listeria monocytogenes in comparison to other Listeria species. Infection. 1988;16:141–144. doi: 10.1007/BF01639737. [DOI] [PubMed] [Google Scholar]
- 19.Hulton C S, Higgins C F, Sharp P M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other Enterobacteriaceae. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 20.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26:2456–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeršek B, Tcherneva E, Rijpens N, Herman L. Repetitive element sequence based PCR for species and strain discrimination in the genus Listeria. Lett Appl Microbiol. 1996;23:55–60. doi: 10.1111/j.1472-765x.1996.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 22.Judd A K, Schneider M, Sadowsky M J, de Bruijn F J. Use of repetitive sequences and the polymerase chain reaction technique to classify genetically related Bradyrhizobium japonicum serocluster 123 strains. Appl Environ Microbiol. 1993;59:1702–1708. doi: 10.1128/aem.59.6.1702-1708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louws F J, Fulbright D W, Stephens C T, de Bruijn F J. Specific genomic fingerprints of phytophatogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazurier S I, Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992;143:499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- 25.McLauchlin J. The pathogenicity of Listeria monocytogenes: a public health perspective. Rev Med Microbiol. 1997;8:1–14. [Google Scholar]
- 26.Notermans S, Dufrenne J, Teunis P, Chackraborty T. Studies on the risk assessment of Listeria monocytogenes. J Food Protect. 1998;61:244–248. doi: 10.4315/0362-028x-61.2.244. [DOI] [PubMed] [Google Scholar]
- 27.Proctor M E, Brosch R, Mellen J W, Garrett L A, Kaspar C W, Luchansky J B. Use of pulsed-field gel electrophoresis to link sporadic cases of invasive listeriosis with recalled chocolate milk. Appl Environ Microbiol. 1995;61:3177–3179. doi: 10.1128/aem.61.8.3177-3179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera I G, Chowdhury M A R, Huq A, Jacobs D, Martins M T, Colwell R R. Enterobacterial repetitive intergenic consensus sequences and the PCR to generate fingerprints of genomic DNAs from Vibrio cholera O1, O139, and non-O1 strains. Appl Environ Microbiol. 1995;61:2898–2904. doi: 10.1128/aem.61.8.2898-2904.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocourt J, Audurier A, Courtieu A L, Durst J, Ortel S, Schrettenbrunner A, Taylor A G. A multi-centre study on the phage typing of Listeria monocytogenes. Zentbl Bakteriol Hyg A. 1985;259:489–497. doi: 10.1016/s0176-6724(85)80081-x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Barradas M C, Hamill R J, Houston E D, Georghiou P R, Clarridge J E, Regnery R L, Koehler J E. Genomic fingerprinting of Bartonella species by repetitive element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeliger H P R, Hoehne K. Serotyping of Listeria monocytogenes and related species. In: Bergan T, Norris J, editors. Methods in microbiology. New York, N.Y: Academic Press; 1979. pp. 31–48. [Google Scholar]
- 32.Seeliger H P R, Jones D. Genus Listeria. In: Sneath P H A, Mair N S, Shrape M E, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1235–1245. [Google Scholar]
- 33.Sharples G J, Lloyd R G. A novel repeated DNA sequence located in the intergenic regions of bacterial chromosomes. Nucleic Acids Res. 1990;18:6503–6508. doi: 10.1093/nar/18.22.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in Eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vines A, Reevs M W, Hunter S, Swaminathan B. Restriction fragment length polymorphism in four virulence-associated genes of Listeria monocytogenes. Res Microbiol. 1992;143:281–294. doi: 10.1016/0923-2508(92)90020-o. [DOI] [PubMed] [Google Scholar]
- 36.Wesley I V, Ashton F. Restriction enzyme analysis of Listeria monocytogenes strains associated with foodborne epidemics. Appl Environ Microbiol. 1991;57:969–975. doi: 10.1128/aem.57.4.969-975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods C R, Versalovic J, Koeuth T, Lupski J R. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J Clin Microbiol. 1993;31:1927–1931. doi: 10.1128/jcm.31.7.1927-1931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]