Main text
In an article previously published in Molecular Therapy, a high incidence of cancer formation was reported in mice transduced with adeno-associated viral (AAV) vector.1 Tumors from hepatectomized mice transduced with a self-complementary AAV (scAAV) vector containing a strong enhancer/promoter (CMV/beta-actin) but lacking a transgene or polyadenylation signal (“CBA-null,” Figure 1A) were more frequently associated with vector DNA than tumors in the control group that had been transduced with a conventional scAAV vector expressing a reporter gene. Most tumors caused by CBA-null vector insertions in the host chromosome were associated with common proto-oncogenes or tumor suppressors. These studies were performed in the tumor-prone mouse strain C3H/HeJ and in mice with severe combined immunodeficiency (SCID). Facilitated by the specific design of the CBA-null vector, proto-oncogene activation in liver tumors was linked to readthrough transcription or transcriptional enhancer effects on the target gene caused by rAAV integration.
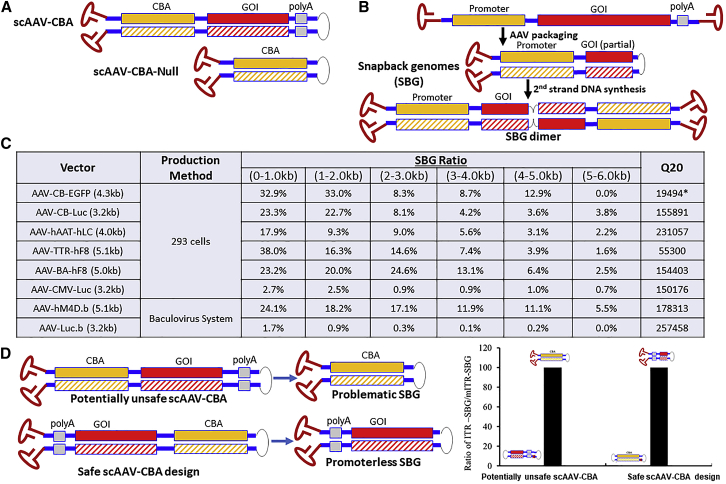
Figure 1.
Subgenomic AAV vector molecules with increased risk for cancer formation by “readthrough” gene activation.
(A) Illustration of known vector designs that induce genotoxicity as described previously in Molecular Therapy (CBA, CMV enhancer/chicken beta actin promoter).1 (B) Illustration of SBG formed during rAAV production and of the dimer that is formed upon 2nd stranded DNA conversion. (C) Summary of the amount of SBG molecules in rAAV preparations. The SBG ratio indicates the number of molecules with SBG configuration divided by the number of all sequenced rAAV genomes in the same size range (based on Pacbio Single Molecules analysis of pooled vector DNA). For example, SBG in size range 4,000–5,000 means the complementary region is 2,000–2,500 nucleotides. Q20: long reads with = Q20 (99%) single-molecule accuracy generated using the circular consensus sequencing analysis method. SEQUEL sequencing was used, except for the one RSII sequencing library indicated with asterisks (∗), which had shorter reads. (D) Comparison of two scAAV vector designs. Placing the promoter in close proximity to the mutant ITR (which lacks the terminal resolution site) produces dimeric forms beneficial for transgene expression (with 2 copies of the enhancer in close proximity) and generates only “harmless” SBGs lacking a promoter. Vectors with either orientation were produced and sequenced. Problematic SBG contents was determined and graphed.
To our surprise, we found that viral particles containing such “promoter-only” or “snapback” vector genome (SBG), among other unconventional genome configurations, are inadvertently generated during viral replication and are detected in all vector preparations that we have analyzed to date. SBG configurations identical to the engineered scAAV-null vector in McCarty’s study are generated during the replication of rAAVs with conventional designs, which we hypothesize result from linkage of fragments derived from plus and minus vector DNA genomes (Figure 1B). Ultimately, packaging of these derivative molecules into capsids via the AAV-ITR results in accumulation of virions carrying the promoter only, along with other subpopulations containing genome deletions or incompletely packaged genomes. As illustrated in Figure 1C, SBG rAAV genome configurations carrying only the promoters can be found to varying degrees in multiple vector preparations using different production systems. Such vector particles share similar physical and biochemical properties as those containing the correct, non-rearranged expression cassette. Thus, one may not be able to easily remove these compromised genomic particles from therapeutic vectors by downstream processing during manufacturing.
Not unexpectedly, the total abundance of such SBGs depends on the specific design of each vector. In rAAV with a CB promoter, there appears to be a hotspot in the promoter itself for forming snapback molecules, resulting in partial elimination of the promoter element. The proportion of snapback molecules containing the CB promoter is ∼40% of sub-genomic particles with genomes smaller than 1 kb (Figure 1C). In other vectors, the abundance of SBG molecules differs, suggesting a need to better characterize these by-products before patient use. For example, in large expression cassettes (such as coagulation factor VIII for the treatment of hemophilia A), a small promoter is necessary in order not to exceed the AAV packaging capacity. Since not all SBGs present in vector preparations will have similar effects upon integration, the SBG alone does not necessarily cause genotoxicity. However, in a vector preparation where most of these snapback molecules contain the entire promoter but lack a poly(A) sequence, the risk is elevated for high readthrough after chromosome integration, as is the potential for genotoxicity. Ironically, use of tissue-specific promoters would restrict potentially “harmful” events to designated target tissues or cell types. In our hands, the level of generation of such unwanted snapback molecules in each vector preparation varies depending on the actual nucleotide composition of the promoter along with the strength and size of the promoter.
We propose that the culprit in rAAV associated with the risk of cancer formation in published studies is a recombined genome that is inadvertently generated during the AAV production process. Because of the relatively small amount of SBGs, compared with that of other forms in the final vector preparations, and the perceived need for a tumor-prone target cell, cancer formation has not been observed thus far in current human clinical trials.2 Nonetheless, the potential risk that these molecular forms of “promoter-only” vectors may contribute to genotoxicity warrants particular attention since they universally exist in rAAVs. Further, use of strong promoters could contribute to additional molecular events with undesired effects in host cells. It has been reported that the vector dose, enhancer/promoter selection, and the timing of gene delivery are all critical factors associated with incidence of hepatocellular carcinoma (HCC).3,4 With increased doses, the potential for such contaminants further increases. In our opinion, to eliminate these potentially dangerous defective genomes, design and technology development of AAV vectors, including analytics of vector preparations, will need to be substantially overhauled and viewed in an entirely new light going forward. Figure 1D shows an example for how this concept can be applied to generate a safer vector design. When simply switching the orientation of the expression cassette in the scAAV genome (where the terminal resolution site in one ITR is mutated), the resulting configuration leads to formation of dimers with two enhancer copies in close proximity (which could benefit transgene expression). More importantly regarding safety, any potential SBG generated from this configuration will not carry a functional promoter because AAV packaging initiates from the functional ITR at the 3′ end. Therefore, correct orientation eliminates promoter-only molecules. We argue that avoidance of potentially deleterious SBGs should be included in clinical vector design alongside already existing criteria such as removal of CpG elements, alternative open reading frames, and inadvertent splice sites.
Whether or not AAV vectors carry a cancer risk has been a highly disputed topic in the field of human gene therapy. Wild-type AAV (wtAAV) has largely been deemed nonpathogenic.5 However, monitoring of wtAAV DNA in tumor and non-tumor liver tissues of 1,461 patients led to the detection of clonal wtAAV insertions in approximately 30 cases of non-cirrhotic liver.6 Additional reports have implied insertional mutagenesis caused by wtAAV.6, 7, 8, 9 Recombinant AAV vectors (rAAV) carry only 145 nt wtAAV sequences, namely, the inverted terminal repeats (ITRs). Thus far, rAAVs have been tested in 223 clinical trials, and two of these vectors have achieved FDA approval for treatment of Leber’s congenital amaurosis type 2 and Spinal Muscular Atrophy type 1, with others likely to follow. However, in many cases rAAV is delivered at extremely high doses to cell types (e.g., striatum, slow twitch muscle, Muller cells, etc.) that wtAAV may never encounter. In addition, rAAVs are typically engineered for high-level transgene expression without latency, while wtAAV evolved to maintain a stable latent infection by cellular repression of p5 promoter via YY1 protein.10 As a consequence, the true safety profiles of rAAVs used for gene therapy have yet to be established. It is not clear how the above-mentioned differences from their parental ancestors may impact rAAV-host interactions. And yet, some murine studies have shown oncogenic events caused by rAAV transduction similar to those found in Dr. McCarty’s study.1 In one study, AAV vectors were shown to induce HCC in neonatal mice with mucopolysaccharidosis type VII and eventually even in normal mice.11,12 Other studies also revealed integration events that may cause cancer.3,13,14 It was shown that AAV integration into the RNA imprinted and accumulated in nucleus (Rian) locus, and the resulting overexpression of proximal microRNAs and retrotransposon-like 1 (Rtl1) were associated with HCC.3,4 In contrast, many other studies failed to observe the development of tumors in mouse models. In a recent canine study, expansion of transduced hepatocytes was observed in dogs that had received rAAVs expressing coagulation factor VIII, although no malignancy was observed up to a 10-year follow-up period.15 In our opinion, it would be beneficial if the vector preparations used in these studies were analyzed for the presence of promoter-only SBGs.
Contributor Information
R. Jude Samulski, Email: rjs@med.unc.edu.
Weidong Xiao, Email: xiaow@iu.edu.
References
- 1.Rosas L.E., Grieves J.L., Zaraspe K., La Perle K.M., Fu H., McCarty D.M. Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol. Ther. 2012;20:2098–2110. doi: 10.1038/mt.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George L.A., Ragni M.V., Rasko J.E.J., Raffini L.J., Samelson-Jones B.J., Ozelo M., Hazbon M., Runowski A.R., Wellman J.A., Wachtel K. Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B. Mol. Ther. 2020;28:2073–2082. doi: 10.1016/j.ymthe.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandler R.J., LaFave M.C., Varshney G.K., Trivedi N.S., Carrillo-Carrasco N., Senac J.S., Wu W., Hoffmann V., Elkahloun A.G., Burgess S.M., Venditti C.P. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Invest. 2015;125:870–880. doi: 10.1172/JCI79213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler R.J., Sands M.S., Venditti C.P. Recombinant Adeno-Associated Viral Integration and Genotoxicity: Insights from Animal Models. Hum. Gene Ther. 2017;28:314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D., Li B., Xu T., Hu R., Tan D., Song X., Jia P., Zhao Z. VISDB: a manually curated database of viral integration sites in the human genome. Nucleic Acids Res. 2020;48(D1):D633–D641. doi: 10.1093/nar/gkz867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Bella T., Imbeaud S., Peneau C., Mami I., Datta S., Bayard Q., Caruso S., Hirsch T.Z., Calderaro J., Morcrette G. Adeno-associated virus in the liver: natural history and consequences in tumour development. Gut. 2020;69:737–747. doi: 10.1136/gutjnl-2019-318281. [DOI] [PubMed] [Google Scholar]
- 7.Nault J.C., Datta S., Imbeaud S., Franconi A., Mallet M., Couchy G., Letouzé E., Pilati C., Verret B., Blanc J.F. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat. Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 8.Berns K.I., Byrne B.J., Flotte T.R., Gao G., Hauswirth W.W., Herzog R.W., Muzyczka N., VandenDriessche T., Xiao X., Zolotukhin S., Srivastava A. Adeno-Associated Virus Type 2 and Hepatocellular Carcinoma? Hum. Gene Ther. 2015;26:779–781. doi: 10.1089/hum.2015.29014.kib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan G.J., Dane A.P., Hallwirth C.V., Smyth C.M., Wilkie E.E., Amaya A.K., Zhu E., Khandekar N., Ginn S.L., Liao S.H.Y. Identification of liver-specific enhancer-promoter activity in the 3′ untranslated region of the wild-type AAV2 genome. Nat. Genet. 2017;49:1267–1273. doi: 10.1038/ng.3893. [DOI] [PubMed] [Google Scholar]
- 10.Houbaviy H.B., Usheva A., Shenk T., Burley S.K. Cocrystal structure of YY1 bound to the adeno-associated virus P5 initiator. Proc. Natl. Acad. Sci. USA. 1996;93:13577–13582. doi: 10.1073/pnas.93.24.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donsante A., Miller D.G., Li Y., Vogler C., Brunt E.M., Russell D.W., Sands M.S. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 12.Donsante A., Vogler C., Muzyczka N., Crawford J.M., Barker J., Flotte T., Campbell-Thompson M., Daly T., Sands M.S. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 2001;8:1343–1346. doi: 10.1038/sj.gt.3301541. [DOI] [PubMed] [Google Scholar]
- 13.Wang P.R., Xu M., Toffanin S., Li Y., Llovet J.M., Russell D.W. Induction of hepatocellular carcinoma by in vivo gene targeting. Proc. Natl. Acad. Sci. USA. 2012;109:11264–11269. doi: 10.1073/pnas.1117032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong L., Malani N., Li M., Brady T., Xie J., Bell P., Li S., Jones H., Wilson J.M., Flotte T.R. Recombinant adeno-associated virus integration sites in murine liver after ornithine transcarbamylase gene correction. Hum. Gene Ther. 2013;24:520–525. doi: 10.1089/hum.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen G.N., Everett J.K., Kafle S., Roche A.M., Raymond H.E., Leiby J., Wood C., Assenmacher C.A., Merricks E.P., Long C.T. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021;39:47–55. doi: 10.1038/s41587-020-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]