ABSTRACT
Background
Iron is critical for fetal development. Neonates of obese women may be at risk for poor iron status at birth as a result of maternal inflammation-driven overexpression of hepcidin.
Objectives
The objective of this study was to determine differences in placental transfer of oral iron (57Fe) and expression of placental transferrin receptor 1 (TFR1) and ferroportin (FPN) mRNA and protein and their association with maternal and neonatal iron-related parameters, including maternal hepcidin, among women with and without prepregnancy (PP) obesity.
Methods
57Fe ingested during the third trimester of pregnancy was recovered in venous umbilical cord blood among 20 PP obese [BMI (in kg/m2): 30.5–43.9] and 22 nonobese (BMI: 18.5–29.0) women aged 17–39 y. Placental TFR1 and FPN mRNA and protein expression were quantified via qPCR and Western blot. Maternal and neonatal markers of iron status and regulation, as well as inflammation, were measured. Descriptive and inferential statistical tests (e.g., Student t test, Pearson correlation) were used for data analysis.
Results
There was no difference in cord blood enrichment of 57Fe or placental mRNA or protein expression of TFR1 or FPN among the women with and without PP obesity. Maternal hepcidin was not correlated with cord blood enrichment of 57Fe or placental FPN mRNA or protein expression. Maternal log ferritin (corrected for inflammation) was inversely correlated with log percent enrichment of 57Fe in cord blood (partial r = –0.50; P < 0.01, controlled for marital status) and protein expression of TFR1 (r = –0.43; P = 0.01).
Conclusions
Placental iron trafficking did not differ among women with and without PP obesity. Findings reinforce the importance of maternal iron stores in regulating placental iron trafficking.
Keywords: iron transport, iron status, pregnancy, placenta, obesity, stable isotope
Introduction
Iron deficiency (ID) is a major cause of anemia among infants worldwide (1, 2). Reduced fetal iron bioavailability has profound consequences, many of which are irreversible, for the developing fetal brain given iron's role in brain structural development and function (3–8). Specifically, a reduction in iron flow to the fetus causes both short- and long-term effects on neurocognitive development even after restoring iron stores early in life (9). Thus, disruptions to the flow of iron in the gestational period may have profound and lasting health effects.
The fetus is dependent on obtaining iron from maternal circulation via the placenta. Although not definitive, placental iron transport is thought to be regulated by maternal, placental, and fetal signals (10, 11). Two proteins responsible for placental iron trafficking are transferrin receptor 1 (TFR1) and ferroportin (FPN) (12, 13). TFR1, expressed on the apical side of the placental syncytiotrophoblast, is primarily responsible for mediating the uptake of transferrin-bound iron from the maternal circulation into the placenta. FPN, expressed on the basolateral membrane of the syncytiotrophoblast, is primarily responsible for iron export from the placenta to fetal circulation (10, 14). Recently, we showed in animals that placental TFR1 is increased and FPN expression is decreased in the context of severe maternal ID [third-trimester hemoglobin (Hb): 8.50 ± 0.70 g/dL] to preserve placental iron and function of the placental electron transport chain (11). This “selfish” action by the placenta demonstrates a hierarchy of needs, by prioritizing the health of the placenta over the fetus. This suggests that when maternal iron bioavailability is decreased, less iron is transferred across the placenta to the fetus.
Hepcidin controls systemic iron metabolism through its regulation of the FPN iron exporter and promotes FPN degradation, thereby reducing iron efflux from intestinal enterocytes (i.e., diet), reticuloendothelial macrophages, and hepatocytes. Hepcidin expression is suppressed by iron deficiency and erythropoiesis and increased by inflammation. In pregnancy, a woman's ability to access iron from her stores and diet is increased (15). This compensatory mechanism helps to fulfill increasing maternal iron needs, placental iron uptake, and iron flux to the fetus as the pregnancy progresses (16). It is believed this mechanism is largely supported by suppression of maternal hepcidin. If inflammation is the dominating signal, hepcidin can become inappropriately increased, resulting in reduced iron export into the circulation from diet and stores (17).
Few studies have objectively examined factors that impact iron transfer across the human placenta in vivo. During the third trimester of pregnancy, O'Brien et al. (18) administered 10 mg of an oral iron (57Fe) and 0.6 mg of an intravenous iron (58Fe) stable iron isotope to 41 healthy pregnant Peruvian women with a BMI (in kg/m2) ranging from 21.7 to 31.4. They found that the transfer of 57Fe to the fetus was inversely regulated in response to maternal iron status; in contrast, 58Fe was not related to maternal iron status. Another study from this group found that the expression of placental TFR mRNA was inversely associated with circulating maternal hepcidin concentrations in 18 healthy pregnant women [Hb 11.4 g/dL (range 10.1–12.9 g/dL); prepregnancy (PP) BMI 32.5 (range 23.3–46.5)] (19). Young et al. (20) also evaluated placental iron transfer of maternally ingested dietary heme and nonheme iron, labeled with stable iron isotopes (57Fe and 58Fe) during the third trimester of pregnancy in relation to maternal and neonatal iron status and hepcidin among 20 women (Hb 11.1 ± 1.20 g/dL; PP BMI 24.7 ± 7.00). They found elevated maternal hepcidin and total body iron (TBI) were significantly associated with decreased placental iron transport to the fetus. Finally, Best et al. (21) looked at placental FPN and found midgestation hepcidin was inversely associated with placental TFR1 protein, and maternal serum soluble transferrin receptor 1 (sTFR1) at midgestation was associated with FPN protein among 154 adolescent mothers with a PP BMI of 24.9 ± 5.70 and Hb of 11.5 ± 1.30 g/dL at delivery. Considering the impact of inflammation on hepcidin regulation, it is important to explore how conditions associated with increased maternal inflammation impact placental iron trafficking.
There is evidence that neonates born to obese mothers have a lower iron endowment at birth compared with neonates born to nonobese mothers, suggesting maternal–placental–fetal iron trafficking may be impaired (22–24). Obesity is a significant public health problem among pregnant women in the United States, with recent statistics indicating over a third of women enter pregnancy obese (BMI ≥30) (25). In a recent analysis, our team found no difference in iron utilization in the third trimester of pregnancy in women with and without PP obesity. However, women with PP obesity had a higher frequency of anemia despite higher TBI, even when corrected for inflammation, suggesting some degree of iron sequestration (26). This finding indicates that in pregnancies complicated by obesity, iron transfer to the fetus may be compromised given maternal iron is less bioavailable. To our knowledge, only 1 study has examined the effect of maternal obesity on placental TFR1 protein expression. Garcia-Valdes et al.(22) found that maternal PP BMI was not associated with placental TFR1 expression. However, we are not aware of any published human studies objectively examining the effects of maternal BMI on iron transfer across the placenta.
The focus of this study was to examine the impact of maternal PP obesity on transfer of iron across the placenta (primary outcome). Our secondary objective was to examine differences in placental TFR1 and FPN mRNA and protein expression by maternal PP BMI group (obese compared with nonobese) and associations with placental iron transfer and maternal and neonatal iron-related and clinical characteristics (secondary outcomes). We hypothesized that PP obesity would be characterized by lower transfer of iron across the placenta.
Participants and Methods
Fifty-two women seeking prenatal care at the University of Illinois at Chicago (UIC) Center for Women's Health were recruited in their third trimester of pregnancy (29–33 wk of gestation) between 2014 and 2017 to study the effect of maternal PP obesity on maternal and neonatal iron status and regulation. Forty-two women [20 PP obese (PP BMI 30.5–43.9) and 22 PP nonobese (PP BMI 18.5–28.9)] were successfully followed to labor and delivery. Eligibility for the study included singleton pregnancy, 17–45 y old (9 women were ≤19 y of age), and PP BMI ≥18.5. Women were excluded for any health conditions or medications that might have impacted study outcomes. Maternal outcome data have been published (26). All women provided written informed consent. Study procedures and materials were approved by the UIC Institutional Review Board (#2015–0353).
Stable isotope preparation
The stable isotope 57Fe was purchased at >95% enrichment (Trace Sciences International). The isotope was prepared as a sterile, pyrogen-free, ferrous sulfate solution following methods previously published (18, 20, 27–29). The isotopic composition of the ferrous sulfate tracer solution was verified via inductively coupled plasma mass spectrometry and tested for sterility (Baylor College of Medicine). The target dosage of 57Fe was 8.40 mg and was ingested along with 2.00 mL of raspberry syrup (Humco) containing 0.39% ascorbic acid to enhance palatability (18–20, 27–29).
Antenatal research visits
Methods pertaining to the antenatal period have been described in detail elsewhere (26). Briefly, at 32–34 wk of gestation, a baseline visit was scheduled and women ingested an iron isotope solution. Prior to ingesting the 57Fe, women were fasted for 90 min and refrained from all vitamin, mineral, and dietary supplements for 48 h. Weight and height were measured using a calibrated digital scale and stadiometer, and antecubital venous blood was obtained. Women returned 2 wk later (34–36 wk of gestation) to undergo venipuncture and to complete clinical and survey assessments.
Upon admission for labor and delivery, a maternal venous blood sample was obtained. Immediately following delivery of the placenta, umbilical venous cord blood was collected at the bedside. Maternal and cord blood samples were used immediately or processed for serum following standardized procedures and stored at –80°C or sent to a local commercial laboratory for analysis. The placenta was transported and processed within 30 min. Forty-one placentas were collected; 1 was inadvertently discarded by clinical staff following cord blood collection. Placental dimensions (i.e., weight, length, thickness, width) were determined after removing the cord and membranes. The placentas were divided into quadrants and a core sampled systematically from each quadrant, avoiding fibrotic, calcified, or infarcted areas. The maternal and fetal membranes were removed from each core and divided into smaller sections, and a small section from each of the 4 cores was placed into a cryovial to provide a relatively representative sample of the placenta. Several aliquots were immediately frozen for protein analysis. Placenta samples were stored at –80°C until analyzed.
Following the participant's discharge, mode of delivery, gestational age at delivery, infant weight, and infant sex were abstracted from the electronic medical record.
Isolation of 57Fe from cord blood samples
Whole blood was digested with 2 mL nitric acid (Ultrex JT Baker) using a hot plate (19, 27). Then, 2 mL ultrapure 6M hydrochloric acid (Ultrex, JT Baker) was used to reconstitute the evaporated digested samples. Anion exchange chromatography was used to extract Fe from the digested blood samples. The eluate was dried on a hot plate. Next, 40 μL 3% nitric acid was used to reconstitute the dried eluate. Extracted Fe samples (8 μL), silica gel (6 μL) (Sigma-Aldrich), and 0.23 mol/L phosphoric acid (2 μL) were loaded onto degassed ultrapure zone-refined rhenium filaments (H. Cross). Iron isotope ratios were obtained via magnetic sector thermal ionization mass spectrometry as previously described (19, 29).
Calculation of Fe transfer to fetus
The oral Fe tracer administered to the pregnant woman is 1) incorporated into the maternal RBCs or used for maternal stores, 2) incorporated into placental proteins/enzymes, or 3) transported across the placenta to be incorporated into fetal RBCs or used for fetal Fe stores. To assess iron transfer to the fetus, we examined the Δ% excess of 57Fe in cord blood, which reflects the degree to which the natural abundance ratio (57Fe/56Fe) was increased as a result of RBC incorporation of the stable Fe tracer, as previously described (18, 20). We also examined the amount of Fe tracer in the neonate as a fraction of the tracer dose absorbed by the mother based on the amount recovered in maternal and neonatal RBCs, also previously described (20).
Laboratory procedures
Maternal and cord Hb was measured from whole blood with the Hemocue point-of-care monitor (Abbott). Hb <11.0 g/dL was indicative of maternal anemia for nonblack women and adjusted downward by 0.80 g/dL to 10.2 g/dL for black women per Institute of Medicine guidelines (16). Anemia among neonates was defined as cord blood Hb <13.0 g/dL (30, 31).
Serum iron, transferrin saturation (TSAT) and ferritin were measured at a local commercial laboratory (Quest Diagnostics). sTFR1 was measured from serum in duplicate using an immunoassay kit (R&D Systems). Our laboratory's intraassay CV for this assay was 5.30%. sTFR1 >28.1 nmol/L was also indicative of ID (32). TBI was calculated using sTFR1 and serum ferritin measurements following the equation devised by Cook et al. (33).
Serum hepcidin was measured using a competitive immunoassay (Intrinsic LifeSciences). The lower level of detection for this assay was 5.00 ng/mL (34). Values below the lower level of detection were assigned a value of 2.50 ng/mL.
Serum erythropoietin (EPO) was measured in duplicate using an immunoassay kit (R&D Systems). Our laboratory's intra-assay CV for this assay was 2.60%. Serum IL-6 was measured in duplicate using an immunoassay kit (R&D Systems). Our laboratory's intra-assay CV for this assay was 5.70%. Serum high-sensitivity C-reactive protein (hs-CRP) was measured by a local commercial laboratory (Quest Diagnostics). High-sensitivity assays can measure concentrations as low as 0.18 mg/L. hs-CRP assays are based on nephelometric analysis of antigen–antibody complexes using monoclonal antibodies with sufficient sensitivity to detect low concentrations of CRP.
Maternal iron biomarker adjustment for maternal inflammation
Maternal serum ferritin, sTFR1, and TBI were adjusted for individuals with hs-CRP >5.00 mg/L using the correction factor methods developed for nonpregnant women of reproductive age by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project (35–37).
Because the volume of umbilical venous cord blood varied from cord to cord, this limited our ability to conduct the planned assays for all 42 dyads. We were limited to the following for cord analyses: cord Hb, n = 40; cord hepcidin, n = 37; cord ferritin, EPO, TSAT, iron, and sTFR1, n = 36; and cord IL-6, n = 25.
qPCR for TFR1 and FPN mRNA
Samples were harvested following delivery and submerged into RNAlater stabilizing reagent (Ambion). Samples were thawed on ice, excess RNAlater was blotted off, and RNA was isolated using TRIzol reagent according to the manufacturer's instructions (Invitrogen). RNA was quantified by absorbance at 260 nm, and 500 ng mRNA was converted to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative PCR was performed with SsoAdvanced SYBR Green Supermix (Bio-Rad), using primers GCM1 (forward: 5′-CTGACAAGGCTTTTTTCTTCACA-3′; reverse: 5′-CCAGACGGGACAGGTTT-3′), FPN (forward: 5′-TTACCAGAAAACCCCAGCTCTAG-3′; reverse: 5′-AGTCTTTCACACCCATTAGATGAG-3′), and TFR1 (forward: 5′-AGTTGAACAAAGTGGCACGAG-3′; reverse: 5′-GCAGTTGGCTGTTGTACCTC-3′). Samples were run in duplicate on a CFXconnect instrument (Bio-Rad). TFR1 and FPN mRNA was measured in placentas from 21 PP obese and 20 PP nonobese women.
Western blot for placenta proteins
Tissues were lysed by homogenization in RIPA buffer (Santa Cruz Biotechnology, sc-24,948). Tissue lysates were cleared at 17,000 × g for 15 min at 4ºC. Protein was quantified using a BCA Assay (ThermoFisher Pierce, 23,225). FPN samples were prepared in Laemmli sample buffer without reducing agent and not boiled. All other proteins samples were prepared in Laemmli buffer with DTT and incubated at 100°C for 5 min. Samples were resolved on Bio-Rad 4–20% TGX gels, electroblotted on nitrocellulose (Trans-Bot Turbo System; Bio-Rad), and imaged with a ChemiDoc XRS+ system (Bio-Rad). The antibodies were human monoclonal antibodies 38G6 (Western), 38C8 (immunofluorescence), Amgen for FPN and monoclonal antibody H68.4, and ThermoFisher Scientific for TFR1. Membranes were stripped using 0.2N NaOH for 10 min at room temperature and reprobed for loading controls. Quantification was performed using Image Lab Software, version 5.2.1 (Bio-Rad). Proteins were measured in n = 18 obese and n = 19 nonobese placentas due to limited protein availability.
Other measures
Gestational weight gain was determined as the average weekly rate of weight gain between the first recorded weight in pregnancy in the medical record and weight at the 34–36 wk of gestation research visit divided by the number of weeks between the measures (38).
Dietary intake data were collected at each research visit (32–24 and 34–36 gestational weeks) via multiple-pass 24-h diet recall using Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota) (38, 39). The nutrient data from the 2 recalls were averaged, and data pertaining to total (food plus supplements) and food-based dietary iron are reported.
Participants also completed surveys related to their medical and reproductive history, sociodemographics (e.g., age, race/ethnicity, education), and lifestyle factors (e.g., smoking).
Statistical analysis
Data were analyzed with SAS v9.4 (SAS Institute). Each variable was tested for normality using model residuals. Corrected maternal serum ferritin, maternal TSAT, cord serum EPO, cord serum IL-6, and cord Δ% excess 57Fe were transformed using a logarithmic transformation. For these variables, a back-transformed geometric least squares mean and 95% CI are presented in lieu of the log-transformed value. To examine differences in characteristics of the pregnant women by prepregnancy BMI category (PP obese compared with nonobese), Student t test or the Wilcoxon 2-sample test for nonparametric data was used for continuous variables, and differences in categorical variables were assessed with χ2 or Fisher exact test. Characteristics found to differ between the nonobese and obese women were examined for confounding effects on the iron/hematologic outcomes, placental isotope transfer variables, and placental mRNA and protein FPN and TFR1 expression. Specifically, we first examined if the maternal characteristics were associated with the predictor variable and outcome in simple generalized linear models. Second, we estimated the measure of association before and after adjusting for the potential confounding variable. A change in the estimated measure of association of 10% or more was deemed evidence of confounding and included as a confounder in subsequent analysis as appropriate. Generalized linear models were used to test for differences in maternal and neonatal cord iron–related parameters, enrichment of 57Fe in cord blood, and placenta mRNA and protein TFR1 and FPN expression by prepregnancy BMI group with the confounding variable included if appropriate. Pearson product moment and Pearson partial correlation coefficients (r) were calculated to examine the direction and strength of the relation among maternal PP BMI, maternal iron-related indices, placental transfer of 57Fe and placental iron transporter protein, and mRNA expression and placental weight. Pearson product moment and Pearson partial correlation coefficients (r) were also used to examine the direction and strength of the relations among neonatal cord iron–related indices, neonatal characteristics, placental transfer of 57Fe, and placental iron transporter mRNA and protein expression. A separate linear model with PP BMI on the x-axis and observed (nontransformed) Δ% enrichment 57Fe in cord blood on the y-axis was conducted and a corresponding unadjusted scatterplot visualized to examine individual estimates (Figure 1). Boxplots were also produced to provide a visual depiction of the placenta protein expression of TFR1 and FPN by maternal PP BMI group (Figure 2). Last, given the evidence suggesting that dysregulation of maternal–fetal iron trafficking may be restricted to women with severe obesity (40, 41), we explored differences in placental transfer of 57Fe, placental iron trafficking mRNA/proteins, and neonatal cord iron–related indices in women with PP BMI ≥35.0 compared with women who were not severely obese using Student t test or the Wilcoxon rank-sum test. Statistical significance was set at P < 0.05.
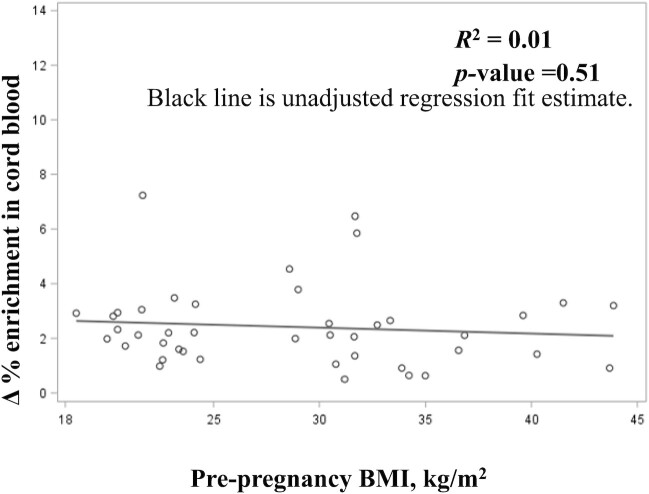
FIGURE 1.
Enrichment of oral iron (57Fe) in cord blood by maternal prepregnancy BMI. Scatterplot of unadjusted linear model details prepregnancy BMI on the x-axis and observed (nontransformed) Δ% enrichment of 57Fe in cord blood on the y-axis, n = 42.
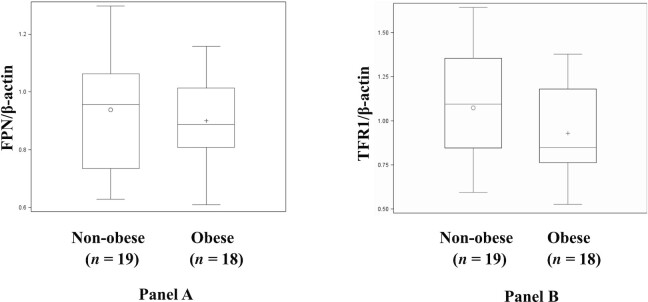
FIGURE 2.
(A) Western blotting to determine protein expression of ferroportin (FPN) normalized to β-actin from placentas from obese and nonobese pregnancies. (B) Western blotting to determine protein expression of placental transferrin receptor 1 (TFR1) normalized to β-actin from placentas from obese and nonobese pregnancies. Box plots show the mean, median, and distribution of TFR1 or FPN protein expression by maternal BMI group (n = 18, obese; n = 19, nonobese). P value for box plots is from the generalized linear model (proc GLM).
Results
Forty-two women were included in the analysis. Four women were overweight PP. We conducted a sensitivity analysis removing these women; results remained unchanged. Moreover, 5 women who were nonobese PP were obese at the time of delivery. We repeated the analyses based on maternal BMI at delivery (obese, n = 25; nonobese, n = 17). However, we found no differences in this approach to categorizing the women and present the data based on maternal PP BMI herein.
Study participant characteristics are shown in Table 1. The mean BMI at delivery for the women was 39.5 in the obese group and 28.1 in the nonobese group (P < 0.01). The rate of gestational weight gain between the groups was not significantly different even after adjusting for PP BMI (data not shown). A larger proportion of black women were obese compared with nonobese, and a larger portion of women reported being single in the obese compared with nonobese group. Half of the women in the nonobese group had a college education compared with only 15% in the obese group. Dietary iron intake between groups was not significantly different. Three women in the obese group developed preeclampsia in the third trimester. There were no significant differences between the groups regarding birth or infant outcomes, including rate of C-section, gestational age at delivery, infant birthweight, or placenta weight.
TABLE 1.
Characteristics of pregnant women by prepregnancy BMI category (n = 42)1
| Variable | Obese (n = 20) | Nonobese (n = 22) | P value |
|---|---|---|---|
| Maternal age, y | 25.0 ± 6.6 | 27.0 ± 6.5 | 0.31 |
| Black or African American, % (n) | 65 (13) | 27 (6) | 0.02 |
| Hispanic or Latina, % (n) | 25 (5) | 32 (7) | 0.87 |
| Relationship status = single, % (n) | 65 (13) | 14 (3) | 0.001 |
| Employed full-time, % (n) | 60 (12) | 68 (15) | 0.58 |
| College graduate (≥4 y), % (n) | 15 (3) | 50 (11) | 0.02 |
| Receiving WIC benefits, % (n) | 65 (13) | 45 (10) | 0.20 |
| Receiving SNAP benefits, % (n) | 30 (6) | 32 (7) | 0.90 |
| Delivery BMI, kg/m2 | 39.5 ± 5.5 | 28.1 ± 4.1 | <0.0001 |
| Rate of gestational weight gain/wk, kg | 0.41 ± 0.32 | 0.44 ± 0.19 | 0.76 |
| Dietary and supplemental iron intake, mg (IQR) | 35.0 (19.8) | 49.6 (33.4) | 0.09 |
| Gravida | 2.5 ± 1.7 | 2.2 ± 1.3 | 0.56 |
| Gestational age at delivery, wk | 38.8 ± 1.5 | 38.9 ± 1.3 | 0.80 |
| C-section, % (n) | 40 (8) | 18 (4) | 0.17 |
| Infant birthweight, g | 3251 ± 550 | 3256 ± 445 | 0.97 |
| Infant sex = male, % (n) | 50 (10) | 50 (11) | 1.0 |
| Placenta weight, g | 473 ± 92.9 | 440 ± 89.2 | 0.25 |
Data are presented as means ± SDs unless otherwise indicated. Wilcoxon rank-sum and t tests were used to compare continuous variables, and χ2 and Fisher exact tests were used to compare categorical variables between groups. Statistical significance was set at P < 0.05. SNAP, Supplemental Nutrition Assistance Program; WIC, Women, Infants, and Children.
Serum maternal and cord iron status and inflammatory indicators by maternal PP BMI category are shown in Table 2. The only variable among the women that was significantly different was sTFR1. Women in the obese group had significantly lower sTFR1 compared with the nonobese women. (sTFR1 corrected using BRINDA was only 1% lower than the uncorrected value, and thus the original value was used for the group comparison; data not shown.) There were no differences among the neonatal parameters by maternal BMI group.
TABLE 2.
Maternal third-trimester and neonatal cord iron status, inflammatory, and iron regulatory indicators by prepregnancy BMI category1
| Variable | Obese women (n = 20) | Nonobese women (n = 22) | Neonates birthed by obese women (n = 20) | Neonates birthed by nonobese women (n = 22) |
|---|---|---|---|---|
| Hemoglobin, g/dL | 10.7 (10.0–11.3) | 11.2 (10.6–11.7) | 13.6 (12.6–14.7) | 14.6 (13.6–15.7) |
| Anemia, % (n)2 | 40.0 (8.00) | 32.0 (7.00) | 35.0 (7.00) | 13.0 (3.00) |
| Ferritin, ng/mL3 | 14.5 (10.7–20.0) | 9.33 (7.08–12.6)4,5 | 129 (79.1–179) | 170 (120–219) |
| TBI, mg/kg3 | 5.63 (4.22–7.04) | 4.02 (2.68–5.35) | 11.1 (9.3–12.9) | 12.1 (10.4–13.9) |
| Iron, μg/dL | 57.1 (48.2–66.0) | 63.1 (54.7–71.6) | 137 (117–158) | 146 (126–167) |
| sTFR1, nmol/L3 | 25.7 (21.0–30.3) | 32.2 (27.8–36.7)5 | 44.1 (38.4–49.8) | 39.2 (33.5–44.9) |
| TSAT, % | 12.3 (10.0–14.8)4 | 11.5 (9.55–13.8)4 | 57.2 (48.8–65.7) | 62.7 (54.2–71.1) |
| Hepcidin, ng/mL | 22.7 (19.0–26.4) | 20.6 (17.1–24.1) | 131 (83.5–179) | 108 (61.4–155) |
| hs-CRP, mg/L | 8.30 (5.97–10.6) | 5.20 (3.02–7.38) | — | — |
| IL-6, pg/mL | 1.98 (1.41–2.55) | 2.11 (1.56–2.66) | 6.61 (4.79–9.33)4 | 5.75 (3.98–8.32)4 |
| EPO, mIU/mL | 32.5 (24.3–40.6) | 33.9 (26.1–41.7) | 24.5 (16.9–36.3)4 | 34.7 (2.39–51.3)4 |
Data are presented as standard least squares means (95% CIs) from generalized linear models (proc GLM) unless otherwise indicated. The maternal groups and neonatal groups were compared separately using generalized linear models for continuous variables and χ2 and Fisher exact tests for categorical variables. Maternal hepcidin was controlled for educational attainment, maternal TBI was controlled for marital status, maternal CRP was controlled for educational attainment, and cord iron and cord TSAT were controlled for WIC participation. Hemoglobin was measured from whole blood, and the other markers were measured from serum. EPO, erythropoietin; hs-CRP, high-sensitivity C-reactive protein; sTFR1, soluble transferrin receptor; TBI, total body iron; TSAT, transferrin saturation; WIC, Women, Infants, and Children.
Maternal anemia was defined as <10.2 g/dL for black women and <11.0 g/dL for nonblack women (16). Cord anemia was defined as hemoglobin <13.0 g/dL (30, 31).
Maternal serum ferritin was corrected for inflammation following the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia methods; corrected value was used to calculate TBI (36).
Data are presented as geometric least squares means (95% CIs).
Denotes P < 0.05.
There was no statistically significant difference in enrichment of 57Fe in cord blood of neonates born to women with or without PP obesity using the 2 methods for calculating Fe transfer to the fetus (Table 3, Δ% enrichment in cord blood was adjusted for marital status). Figure 1 shows the results of an unadjusted linear model examining individual nontransformed enrichment of 57Fe in cord blood by maternal PP BMI; the model was not significant.
TABLE 3.
Enrichment of 57Fe in cord blood following maternal ingestion during the third trimester of pregnancy among women who were obese and nonobese prepregnancy1
| Variable | Obese women (n = 20) | Nonobese women (n = 22) | P value |
|---|---|---|---|
| Enrichment in cord blood, Δ%2,3 | 3.16 (2.63–3.80) | 3.09 (2.63–3.72) | 0.90 |
| Maternal ingested Fe tracer present in neonate at birth, %4 | 6.13 (4.57–7.68) | 5.57 (4.05–7.09) | 0.61 |
Data are presented as standard least squares means (95% CIs) from generalized linear models (proc GLM). Δ% enrichment in cord blood was controlled for marital status. Statistical significance was set at P < 0.05. Analysis was run from whole cord blood samples. Fe, iron.
Percent excess was adjusted for the natural abundance of isotope administered (0.02,317 for 57/56Fe).
Data are presented as geometric least squares means (95% CIs).
Quantity of tracer dose in neonate divided by average tracer dose administered to mother.
Table 4 and Figure 2 report the placental TFR1 and FPN gene and protein expression results by PP BMI group. Placental TFR1 and FPN mRNA and protein expression were not significantly different among the groups.
TABLE 4.
Placental iron transporter gene and protein expression among women who were obese and nonobese prepregnancy1
| Variable | Obese women (n = 18) | Nonobese women (n = 19) | P value |
|---|---|---|---|
| Transferrin receptor mRNA, –ΔCt (GCM1-TFR1) | 1.85 (1.38–2.32) | 1.37 (0.91–1.83) | 0.15 |
| Transferrin receptor protein/β-actin | 0.93 (0.79–1.07) | 1.07 (0.94–1.21) | 0.15 |
| Ferroportin mRNA, –ΔCt (GCM1-FPN) | 1.02 (0.61–1.44) | 0.67 (0.26–1.07) | 0.22 |
| Ferroportin protein/β-actin | 0.90 (0.82–0.98) | 0.94 (0.86–1.02) | 0.52 |
Data are presented as least squares means (95% CIs) from generalized linear models (proc GLM). Statistical significance was set at P < 0.05. FPN, ferroportin; GCM1, glial cells missing transcription factor 1; TFR1, transferrin receptor 1.
Pearson correlation coefficients examined associations between percent enrichment of 57Fe in cord blood, placental TFR1 and FPN mRNA and protein expression, and maternal and cord iron–related inflammatory parameters. Maternal-corrected log serum ferritin was significantly inversely correlated with percent enrichment of 57Fe in cord blood controlling for marital status (partial r = –0.43; P = 0.005). Maternal-corrected log serum ferritin and corrected log TBI were significantly inversely correlated with placental TFR1 protein expression (log serum ferritin: r = –0.43; P = 0.01; TBI = r = –0.44, P < 0.01). Maternal sTFR1 was significantly positively correlated with placental FPN protein expression (r = 0.34, P = 0.04). No other maternal, placental, or cord blood parameters were significantly correlated with percent enrichment of 57Fe in cord blood or the placental genes or proteins.
Last, we explored differences in placental transfer of 57Fe, placental iron trafficking proteins, and neonatal cord iron–related indices among women with PP BMI ≥35.0 (n = 6) compared with <35.0 (n = 35) and found no difference in these parameters among the 2 groups of women (data not shown).
Discussion
To our knowledge, this is the first study to demonstrate that there was no significant difference among women who were obese and nonobese PP in enrichment of nonheme Fe in cord blood following maternal ingestion of 57Fe in the third trimester of pregnancy. We also did not find any significant correlations between maternal PP BMI and percent enrichment of 57Fe in cord blood. However, we did find that maternal iron stores were significantly inversely associated with percent enrichment of 57Fe in the cord blood at delivery. This is consistent with what others have found, and it emphasizes that maternal iron stores influence iron transfer to the fetus and are a likely mechanism protecting the placenta and fetus from iron excess (11, 18, 20).
We also observed that TFR1 and FPN mRNA and protein expression were not different between the groups. This is partly consistent with reporting from Garcia-Valdes et al. (22), who did not find a significant correlation between either TFR1 mRNA or protein concentrations in the placenta and maternal PP BMI. In addition, Yang et al. (42) reported similar placental TFR1 protein expression among women with and without gestational diabetes (GDM), a condition that often accompanies obesity in pregnancy. However, placental FPN protein expression was significantly higher among GDM compared with non-GDM women. Like Garcia-Valdes et al. (22), we also found that placental TFR1 protein expression was associated with maternal ferritin in a reciprocal fashion. Best et al. (21) also previously reported that maternal iron stores predict placental TFR1 expression. Together, these findings reinforce the importance of maternal iron stores in regulating placental iron trafficking. It is important to acknowledge that our sample was more racially and ethnically diverse, with almost half self-identifying as black and a third self-identifying as Hispanic, whereas Garcia-Valdes et al. (22) and Yang et al. (42) enrolled women who were primarily Caucasian (96.2–97.8%) or Asian (100%). Little is known regarding racial-ethnic variation of the placental transcriptome or proteome as it relates to iron trafficking. Exploring the role of genetic variation in driving functional differences of the human placenta as it relates to nutrient transfer is of importance and should be explored further.
We did not observe significant differences in cord Hb or cord iron–related parameters among neonates born to PP obese and nonobese women. Similarly, Garcia-Valdes et al. (22) found no difference in the iron status of neonates, based on cord blood parameters, birthed by obese or nonobese women. However, both Cao et al. (41) and Phillips et al. (24) found that neonates born to obese mothers had higher cord Hb concentrations (15.5 ± 2.40 compared with 14.0 ± 2.80 g/dL and 16.6 ± 2.00 compared with 15.9 ± 2.00 g/dL, respectively; P < 0.03), despite lower cord ferritin (145 ± 95.5 compared with 147 ± 94.7 ng/mL and 138 ± 7.50 compared with 168 ± 9.50 ng/mL, respectively; P < 0.01). Moreover, they showed that iron was partitioned to Hb compared with tissue stores, which could have significant repercussions for neurodevelopment.
In our subanalysis of women with severe obesity (n = 6), there were no differences in cord 57Fe, placental gene or protein expression of TFR1 or FPN1, or cord hematologic and iron status markers. However, this should be interpreted with caution given the small sample size. There is evidence that maternal PP BMI ≥35.0 is associated with elevated maternal hepcidin and poorer maternal and cord iron status (41, 43). Studies focused specifically on examining placental iron trafficking and neonatal iron status among women with extreme obesity are warranted.
Limitations and strengths
Our conclusions are limited by several features of the current study. First, we did not power the study to detect differences between the groups on placental transfer of the isotope or on placenta transport proteins. Our sample size is small, which may have impacted our ability to detect significant differences between groups. Second, cord blood and placentas were not available for all the biomarkers of interest, often because volume was limited, or the sample integrity was compromised, resulting in missing data for the maternal, neonatal, and placental iron-related markers. We also used an indirect measure of neonatal iron status (i.e., cord blood), which could have influenced our results. Although an exclusion criterion for this study was bacterial or viral infections, a participant may have developed an infection unbeknownst to the participant and the research team, impacting results. Delaney et al. (44) recently reported that maternal RBC iron incorporation of an orally ingested iron tracer, a variable used in the calculation of placental 57Fe transfer, may be underestimated when iron utilization by the placenta is overlooked, potentially biasing our results. Last, we did not correct for multiple comparison, but pairwise comparisons were planned for a priori.
Nonetheless, this study has multiple strengths that should be highlighted. We longitudinally followed the ingestion of a maternal stable iron isotope in the third trimester of pregnancy to incorporation in cord blood at delivery. We also comprehensively assessed iron and inflammatory markers in mothers and indirectly in neonates via cord blood. Although this design does not allow for determination of the causal direction of any of the associations, this design provides valuable information on the maternal–neonatal dyad in relation to iron homeostasis. It is also important to note that our sample was racially and ethnically diverse compared with other adult obese cohorts in the published literature, with almost half identifying as black and a third identifying as Hispanic.
Conclusions
To conclude, transfer of the orally administered 57Fe from mother to fetus in the third trimester was not significantly related to by PP BMI. Future research should examine Fe transfer earlier in pregnancy or in larger cohorts of racially and ethnically diverse women, including women with class 2 and class 3 PP obesity (PP BMI ≥35.0) to determine the impact of severe obesity on Fe transfer to the fetus. Iron deficiency can have lifelong lasting effects on neurodevelopment of the offspring, affecting emotional and cognitive ability of school-age children (6). Addressing and understanding Fe regulation in high-risk populations is of paramount importance to ensuring the health of future generations.
Acknowledgments
We thank Drs. Steven Abrams and Zhensheng Chen for assistance preparing the isotope solution, Amy Haara and the clinic and hospital staff for facilitating recruitment and data collection efforts, and Alyshia Hamm and Nicollette Kessee for their technical assistance. This work would not have been possible without the commitment and dedication of the research participants.
The authors’ responsibilities were as follows—MDK and LT-H: designed research and wrote the paper; MDK, LT-H, KO, EN, VS, VD, RR, LW, GE, KC, NOH, BL, LP, AM, and BH: conducted research; LT-H: performed statistical analysis; CEF, GE, KO, EN, and VS: provided valuable knowledge and scientific consultation throughout the study; and all authors read and approved the final manuscript.
Notes
Supported by the Robert Wood Johnson Foundation Nurse Faculty Scholars Program (#72117); National Institute on Minority Health and Health Disparities (U54 MD012523; Subaward #088917) and National Heart, Lung, and Blood Institute (R34 HL155481-01); University of Illinois College of Nursing Dean's Award; Department of Medicine, University of Illinois; and National Center for Advancing Translational Sciences, NIH ( UL1TR002003). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author disclosures: EN is a shareholder of and scientific adviser for Intrinsic LifeSciences and Silarus Therapeutics and is a consultant for Ionis Pharmaceuticals, Protagonist Therapeutics, and Vifor Pharma. All other authors report no conflicts of interest.
Abbreviations used: BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; EPO, erythropoietin; Fe, iron; FPN, ferroportin; GDM, gestational diabetes; Hb, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; ID, iron deficiency; PP, prepregnancy; sTFR1, serum soluble transferrin receptor 1; TBI, total body iron; TFR1, transferrin receptor 1; TSAT, transferrin saturation; UIC, University of Illinois at Chicago; WIC, Women, Infants, and Children.
Contributor Information
Lisa Tussing-Humphreys, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Bazil LaBomascus, Northwestern Feinberg School of Medicine, Chicago, IL, USA.
Kimberly O'Brien, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Elizabeta Nemeth, Center for Iron Disorders, Department of Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Veena Sangkhae, Center for Iron Disorders, Department of Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Alana D Steffen, Department of Biobehavioral Nursing Science, University of Illinois at Chicago, Chicago, IL, USA.
Karla Castellanos, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Victoria DeMartelly, Department of Obstetrics and Gynecology, University of Chicago, Chicago, IL, USA.
Rungnapa Ruchob, Mahidol University, Bangkok, Thailand.
Lauren Welke, Abbvie, Chicago, IL, USA.
Nefertiti OjiNjideka Hemphill, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Lacey Pezley, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Andrew McLeod, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago, IL, USA.
Bruni Hirsch, Saint Anthony Hospital, Chicago, IL, USA.
Gloria Elam, Department of Obstetrics and Gynecology, University of Illinois at Chicago, Chicago, IL, USA.
Carol Estwing Ferrans, Department of Biobehavioral Nursing Science, University of Illinois at Chicago, Chicago, IL, USA.
Mary Dawn Koenig, Department of Human Development Nursing Sciences, University of Illinois at Chicago, Chicago, IL, USA.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request in deidentified form.
References
- 1.Fomon SJ, Nelson SE, Ziegler EE. Retention of iron by infants. Annu Rev Nutr. 2000;20:273–90. [DOI] [PubMed] [Google Scholar]
- 2.Kretchmer N, Beard JL, Canison S. The role of nutrition in the development of normal cognition. Am J Clin Nutr. 1996;63:997S–1001S. [DOI] [PubMed] [Google Scholar]
- 3.Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;71:173–96. [DOI] [PubMed] [Google Scholar]
- 4.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. [DOI] [PubMed] [Google Scholar]
- 5.DeUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. [DOI] [PubMed] [Google Scholar]
- 8.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341:1–4. [DOI] [PubMed] [Google Scholar]
- 9.Kaar JL, Crume T, Brinton JT, Bischoff KJ, McDuffie R, Dabelea D. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J Pediatr. 2014;165:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao C, Fleming MD. The placenta: the forgotten essential organ of iron transport. Nutr Rev. 2016;74:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelić M, Ganz T, Nemeth E. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2020;130:625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–9. [DOI] [PubMed] [Google Scholar]
- 13.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 14.Heaton SJ, Eady JJ, Parker ML, Gotts KL, Dainty JR, Fairweather-Tait SJ, McArdle HJ, Srai KS, Elliott RM. The use of BeWo cells as an in vitro model for placental iron transport. Am J Physiol Cell Physiol. 2008;295:C1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S–64S. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine (U.S.). Committee on the Prevention Detection and Management of Iron Deficiency Anemia Amoung U.S. Children and Women of Childbearing Age, Institute of Medicine (U.S.) Food and Nutrition Board, Earl R, Woteki CE. Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among U.S. children and women of childbearing age. Washington (DC): National Academies Press; 1993. [PubMed] [Google Scholar]
- 17.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman AXL, Braunschweig C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity. 2010;18:1449–56. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. 2003;77:924–30. [DOI] [PubMed] [Google Scholar]
- 19.Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O'Brien KO. Utilization of iron from an animal-based iron source is greater than that of ferrous sulfate in pregnant and nonpregnant women. J Nutr. 2010;140:2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young MF, Griffin I, Pressman E, Mcintyre AW, Cooper E, Mcnanley T, Harris ZL, Westerman M, O'Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012;142:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best CM, Pressman EK, Cao C, Cooper E, Guillet R, Yost OL, Galati J, Kent TR, O'Brien KO. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J. 2016;30:3541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;39:571–8. [DOI] [PubMed] [Google Scholar]
- 23.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link?. J Perinatol. 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, Coe CL, Kling PJ. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–5. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig MD, Klikuszowian E, O'Brien KO, Pauls H, Steffen A, DeMartelly V, Ruchob R, Welke L, Hemphill N, LaBomascus Bet al. Prepregnancy obesity is not associated with iron utilization during the third trimester. J Nutr. 2020;150:1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, O'Brien KO. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89:533–8. [DOI] [PubMed] [Google Scholar]
- 28.Kastenmayer P, Davidsson L, Galanz P, Cherouvrier F, Hercberg S, Hurrell RF. A double stable isotope technique for measuring iron absorption in infants. Br J Nutr. 1994;71:411–24. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien KO, Zavaleta N, Caulfield LE, Yang D-X, Abrams SA. Influence of prenatal iron and zinc supplements on supplemental iron absorption, red blood cell iron incorporation, and iron status in pregnant Peruvian women. Am J Clin Nutr. 1999;69:509–15. [DOI] [PubMed] [Google Scholar]
- 30.Diagne I, Archambeaud MP, Diallo D, d'Oiron R, Yvart J, Tchernia G. Paramètres érythrocytaires et réserves en fer dans le sang du cordon. Arch Pediatr. 1995;2:208–14. [DOI] [PubMed] [Google Scholar]
- 31.Paterakis GS, Lykopoulou L, Papassotiriou J, Stamulakatou A, Kattamis C, Loukopoulos D. Flow-cytometric analysis of reticulocytes in normal cord blood. Acta Haematol. 1993;90:182–5. [DOI] [PubMed] [Google Scholar]
- 32.Baillie FJ, Morrison AE, Fergus I. Soluble transferrin receptor: a discriminating assay for iron deficiency. Clin Lab Haematol. 2003;25:353–7. [DOI] [PubMed] [Google Scholar]
- 33.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 34.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–7. [DOI] [PubMed] [Google Scholar]
- 35.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJet al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mei Z, Namaste SM, Serdula M, Suchdev PS, Rohner F, Flores-Ayala R, Addo OY, Raiten DJ. Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:383S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJet al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:372S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang A, Ji Z, Zhao W, Hu H, Yang Q, Chen D. Rate of gestational weight gain and preterm birth in relation to prepregnancy body mass indices and trimester: a follow-up study in China. Reprod Health. 2016;13:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harnack L, Stevens M, Van Heel N, Schakel S, Dwyer JT, Himes J. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls. J Food Compos Anal. 2008;21;S78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn AC, Begum S, White SL, Dalrymple K, Gill C, Alwan NA, Kiely M, Latunde-Dada G, Bell R, Briley ALet al. Relationships between maternal obesity and maternal and neonatal iron status. Nutrients. 2018;10;1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23:613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang A, Zhao J, Lu M, Gu Y, Zhu Y, Chen D, Fu J. Expression of hepcidin and ferroportin in the placenta, and ferritin and transferrin receptor 1 levels in maternal and umbilical cord blood in pregnant women with and without gestational diabetes. Int J Environ Res Public Health. 2016;13:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, Kaciroti N, Zhang Z, Lozoff B. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr. 2016;70:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delaney KM, Guillet R, Pressman EK, Caulfield LE, Zavaleta N, Abrams SA, O'Brien KO. Iron absorption during pregnancy is underestimated when iron utilization by the placenta and fetus is ignored. Am J Clin Nutr. 2020;112:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request in deidentified form.