ABSTRACT
Background
The developing fetus requires adequate iron and produces its own hormones to regulate this process. Erythroferrone (ERFE) is a recently identified iron regulatory hormone, and normative data on ERFE concentrations and relations between iron status and other iron regulatory hormones at birth are needed.
Objectives
The objective of this study was to characterize cord ERFE concentrations at birth and assess interrelations between ERFE, iron regulatory hormones, and iron status biomarkers in 2 cohorts of newborns at higher risk of neonatal anemia.
Methods
Umbilical cord ERFE concentrations were measured in extant serum samples collected from neonates born to women carrying multiples (age: 21–43 y; n = 127) or teens (age: 14–19 y; n = 164). Relations between cord blood ERFE and other markers of iron status or erythropoiesis in cord blood were assessed by linear regression and mediation analysis.
Results
Cord ERFE was detectable in all newborns delivered between 30 and 42 weeks of gestation, and mean concentration at birth was 0.73 ng/mL (95% CI: 0.63, 0.85 ng/mL). Cord ERFE was on average 0.25 ng/mL lower in newborns of black as opposed to white ancestry (P = 0.04). Cord ERFE was significantly associated with transferrin receptor (β: 1.17, P < 0.001), ferritin (β: −0.27, P < 0.01), and hemoglobin (Hb) (β: 0.04, P < 0.05). However, cord hepcidin and the hepcidin:erythropoietin (EPO) ratio captured the most variance in newborn iron and hematologic status (>25% of variance explained).
Conclusions
Neonates born to teens and women carrying multiples were able to produce ERFE in response to neonatal cord iron status and erythropoietic demand. ERFE, however, did not capture significant variance in newborn iron or Hb concentrations. In these newborns, cord hepcidin and the hepcidin:EPO ratio explained the most variance in iron status indicators at birth.
Keywords: hepcidin, erythropoietin, newborn, hemoglobin, anemia, teens, multiple births
See corresponding editorial on page 2509.
Introduction
Anemia and inadequate iron accretion before birth are associated with adverse neonatal outcomes (1, 2). Iron provided to the developing fetus is derived from maternal iron stores and dietary iron absorption, and is dependent on efficient placental transfer of iron (3, 4). To date 3 hormones have been found to be involved in iron regulation and erythropoiesis: erythropoietin (EPO), erythroferrone (ERFE), and hepcidin. Both EPO and hepcidin are produced by the human fetus (5–8), but at present little information is available on ERFE status in newborns. Data are needed to characterize ERFE concentrations at birth and evaluate interactions between ERFE and other fetally produced hormones, and their association with newborn iron status biomarkers.
Erythropoiesis requires iron to support hemoglobin (Hb) production, therefore physiological regulatory mechanisms are necessary to ensure iron availability for this demand. EPO is a small glycoprotein responsible for regulating erythrocyte production (9) and is produced by the human fetus as early as 16 weeks of gestation (6) beginning in the liver and transitioning to the kidney by late gestation (10, 11). Fetal production of EPO is thought to remain relatively stable during normoxic conditions (12), but is upregulated in response to conditions leading to neonatal hypoxia (8, 13–16). EPO may also have nonerythropoietic roles because it has been linked to tissue protection (8, 17, 18) and immune regulation (19), and is potentially regulated by retinoic acid and thyroid hormone in an oxygen-dependent manner (20, 21). Hepcidin is the major systemic iron regulatory hormone (22) and is produced by the human fetus as early as the first trimester of pregnancy (5). Hepcidin production by the fetus is positively associated with inflammatory markers and elevated during infection (23–27). Studies in healthy newborns have found that umbilical cord blood hepcidin concentrations are strongly associated with neonatal iron status at birth (23, 25–32) and are more affected by intrauterine iron status at birth than maternal hepcidin when analyzed using a multiple birth model (29). It does not appear that maternal EPO or hepcidin cross the placenta (33–36) and therefore concentrations of these hormones in umbilical cord blood are assumed to be fetally produced.
In 2014, the hormone ERFE was identified and found to decrease hepcidin expression to increase the availability of circulating iron for erythropoiesis (37). Although a validated human ERFE assay was developed in 2017 (38), few normative data on umbilical cord ERFE concentrations in either preterm or term newborns have been published (39). Animal models have demonstrated that ERFE is produced specifically by the erythroblasts in response to elevated EPO (37). In humans ERFE is induced by elevated EPO in adults with clinical conditions (40, 41) or after recombinant human EPO administration (42). Increases in EPO have been associated with decreased hepcidin (42, 43), leading to increases in iron absorption and iron release from stores (44). Whether ERFE plays a mediating role between EPO and hepcidin, influences placental iron transport, or if ERFE crosses the placenta in humans all remain uncharacterized.
Owing to the lack of normative data on iron status indicators in cord blood, and the fact that relations between some of these indicators differ from those reported in older populations (27), normative neonatal data on iron biomarkers and regulatory hormones are needed. The objective of this article was to characterize umbilical cord ERFE concentrations in both term and preterm newborns in relation to other neonatal and maternal iron regulatory hormones and iron status indicators in a large cohort of newborns born to women at greater risk of gestational iron deficiency (ID). A secondary objective was to evaluate the ability of ERFE, hepcidin, EPO, and ratios between these hormones to explain variance in newborn iron status at birth.
Methods
Participants
ERFE was measured in umbilical cord blood serum obtained from 164 neonates born to pregnant teens (aged <19 y) receiving prenatal care in Rochester, NY between 2006 and 2012 and 127 neonates (twins, triplets, and quadruplets) born to adult women recruited from Strong Memorial Hospital and Highland Hospital in Rochester, NY from 2011–2014 (Supplemental Figure 1). All studies were approved by the institutional review boards of the University of Rochester and Cornell University and informed consent (or assent and parental consent in individuals aged <15 y) was obtained from parents of the newborns studied. Neonatal (27, 29, 35, 45, 46) and maternal (47–51) data have been published elsewhere.
Gestational age at birth was determined based on self-reported menstrual history and sonogram data or from the known date of in vitro fertilization when applicable. If menstrual history and sonogram data differed by >10 d, the earliest available ultrasound data were used to determine gestational age. Deliveries were classified as preterm (<37 weeks of gestation), early term (37–38 weeks of gestation), and term (≥38 weeks of gestation), with the earliest birth at 30 weeks of gestation. Low birth weight (LBW) was defined as birth weight <2500 g and small for gestational age (SGA) was defined as <10th percentile for growth rate using Fenton growth charts (52). Neonatal birth data were extracted from medical charts; maternal demographic information and prepregnancy BMI (ppBMI) were self-reported upon entry into the study.
Serum collection and biochemical analysis
Umbilical cord blood (∼15 mL) was collected at birth and nonfasted maternal blood (15 mL) was collected from women at mid-gestation (∼26 weeks of gestation) and delivery (∼39 weeks of gestation). Whole blood was sent to the University of Rochester core laboratory for assessment of Hb using a Cell-Dyn 4000 hematology analyzer (Abbott Diagnostics). Neonatal anemia was defined as cord Hb < 13.0 g/dL (53). Umbilical cord Hb cutoffs are not typically race-adjusted, but because the CDC recommends race-adjusting Hb concentrations by 0.4 g/dL in those <5 y of age (53) we also adjusted for race by decreasing the anemia cutoff for black newborns to Hb < 12.6 g/dL (53). Neonatal data were evaluated with and without this race-adjustment factor.
Serum ERFE was measured by ELISA (Intrinsic Lifesciences). Maternal and neonatal samples were run on the same plate and the interassay CV was 11.4%. Although the assay states the kit has a lower limit of detection (LOD) of 1.5 ng/mL, it provides quantitative measures of ERFE down to 0.001 ng/mL. Absolute values of this hormone were used for statistical analyses. Methods used to analyze the other iron status biomarkers have been published in detail (29, 35). In brief: serum ferritin (SF) (ELISA, RAMCO Laboratories), soluble transferrin receptor (sTfR) (ELISA, RAMCO Laboratories), serum iron (AAS, Perkin Elmer AAnalyst 800), and serum EPO, folate, and vitamin B-12 (all Siemens Immulite 2000) were measured in maternal and neonatal serum. In adults low SF reflects low body iron (i.e., ID) (54) and elevated sTfR reflects increased erythrocyte iron demand (i.e., functional ID) (55). It is unknown if these biomarkers provide the same information in a newborn that prioritizes iron for Hb production at the expense of other tissues (i.e., the brain) (56, 57). In the teen cohort serum hepcidin (ELISA, Intrinsic Lifesciences; LOD: 5 ng/mL), serum IL-6 (Millipore Magnetic Multiplex), and C-reactive protein (CRP) (Siemens Immulite 2000; LOD: 0.2 mg/L) were measured (35) with different assays than in the multiples cohort (hepcidin: ELISA, Bachem; LOD: 0.39 mg/L; serum IL-6 and CRP: ELISA, R&D Systems; CRP LOD: 0.077 mg/L) (48).
Statistical analysis
Nonnormally distributed values were transformed before statistical analysis. Neonatal characteristics and iron status indicators were compared between cohorts by ANOVA, chi-square test, or Wilcoxon's rank-sum test. Total body iron (mg/kg) was calculated with the following equation: −[log(sTfR/SF) − 2.8229] / 0.1207 (58). Erythrocyte iron (mg/kg) was calculated as previously described (56, 59) assuming the iron content of Hb is 3.47 g/kg (60) and a newborn blood volume of 80 mL/kg (61): neonatal Hb (g/L) × blood volume (L/kg) × birth weight (kg) × Hb iron content (mg/g). The storage iron pool (mg/kg) was calculated using the logarithmic relation between SF and body storage iron (62). The Hep:ERFE and Hep:EPO ratios were calculated to capture both iron status and erythropoietic drive. The ERFE:EPO ratio was expected to better capture erythropoietic drive and hypoxia. Indicators analyzed with different assays between cohorts (CRP, IL-6, and hepcidin) were standardized by dividing each measurement by its respective cohort's mean and dividing this number by the SD of the cohort. All analyses were performed using the multiples cohort maternal identification number as a random coefficient to control for lack of independence in the twins, triplets, and quadruplets. Pearson pairwise correlations were assessed between ERFE and iron status, hormones, and inflammatory markers. Determinants of ERFE were found through linear mixed models, where neonatal iron status indicators and maternal characteristics with P < 0.2 from bivariate correlation analysis were tested simultaneously and eliminated by backward selection until only statistically significant predictors remained. An R2 for linear mixed models was calculated following published methods (63). The intraclass correlation coefficient (ICC) for ERFE was calculated as the between-mother variance divided by the sum of the within-mother and between-mother variances as previously published (29). The aforementioned statistical analyses were performed using JMP 14.0 (SAS Institute Inc). To assess interrelations between ERFE, iron status indicators, Hb, and hormones, mediation models were developed using Stata 15 (StataCorp LLC). Finally, interaction terms were evaluated using Johnson-Neyman methods (64) to identify the cutoff at which the relation was no longer significant, using R (Rstudio, PBC). All analyses reported were evaluated in the full study cohort unless there was a significant interaction term with the study cohort, in which case separate analyses were presented for comparison purposes.
Results
Neonatal characteristics
Table 1 presents newborns’ characteristics. The majority of neonates born to women carrying multiples were born prematurely (63%) and delivered via cesarean delivery (72%), whereas the neonates born to teens tended to be delivered at term (90%) via vaginal delivery (88%). Among the premature multiples neonates, 89% were LBW (n = 77 of 87) and 21% were SGA (n = 14 of 66). In the whole population, 49% of newborns were born to black mothers and 19% of newborns were born to mothers who self-identified as Hispanic. Over 40% of newborns were delivered to mothers who entered pregnancy overweight or obese (based on self-reported ppBMI), and nearly half (49%) of the women gained more than the recommended amount of weight across gestation. Table 2 presents mean neonatal iron status indicators in each cohort. Overall, 18% of newborns were anemic at birth, and this value did not significantly change if the Hb cutoffs were adjusted for race (18% compared with 17%). The prevalence of anemia was significantly greater among neonates born to teens (P = 0.002). None of the newborns were folate deficient; however, 1 newborn in the teen cohort was deficient in vitamin B-12. Maternal iron status and further detailed information on neonatal iron homeostasis have been published (29, 35, 46, 48–50).
TABLE 1.
Characteristics of newborns born to women carrying multiple fetuses and teens carrying singletons1
| Variable | Whole population | Multiples cohort | Teen cohort |
|---|---|---|---|
| Neonatal characteristics | |||
| n | 291 | 127 | 164 |
| Gestational age, wk | 37.7 ± 3.1 | 34.9 ± 2.4 | 39.8 ± 1.34 |
| Early term (37.1–38 wk) | 50 | 32 | 104 |
| Preterm (32–37 wk) | 30 | 60 | 34 |
| Very preterm (<32 wk) | 3 | 8 | 04 |
| Birth weight, kg | 2.84 ± 0.68 | 2.29 ± 0.53 | 3.26 ± 0.434 |
| LBW (<2.5 kg) | 31 | 67 | 34 |
| VLBW (<1.5 kg) | 2 | 6 | 04 |
| SGA2 | 17 | 20 | 154 |
| LGA2 | 2 | 0 | 44 |
| APGAR (1 min) | 7.8 ± 1.5 | 7.7 ± 1.6 | 7.8 ± 1.4 |
| Male | 47 | 45 | 49 |
| Mode of delivery: cesarean delivery | 38 | 72 | 124 |
| Types of multiples | |||
| Twins | 71.7 | 71.7 | — |
| Triplets | 26.0 | 26.0 | — |
| Quadruplets | 2.4 | 2.4 | — |
| Maternal characteristics | |||
| n | 234 | 70 | 164 |
| Maternal age, y | 20.7 ± 6.1 | 29.9 ± 4.8 | 17.5 ± 1.14 |
| Race: Black/African American | 57 | 24 | 704 |
| Ethnicity: Hispanic | 21 | 7 | 274 |
| Maternal ppBMI, kg/m2 | 25.7 ± 6.3 | 27.6 ± 7.9 | 25.0 ± 5.64 |
| Underweight (<18.5) | 6 | 6 | 7 |
| Normal (18.5–24.9) | 51 | 44 | 52 |
| Overweight (25–29.9) | 21 | 21 | 19 |
| Obese (>29.9) | 22 | 29 | 21 |
| Gestational weight gain,3 kg | 17.7 ± 8.0 | 19.1 ± 8.3 | 17.1 ± 7.64 |
| Less than recommended | 19 | 33 | 134 |
| Recommended | 26 | 43 | 214 |
| More than recommended | 55 | 24 | 664 |
| Parity > 1 | 30 | 60 | 204 |
| Currently smoking | 5 | 6 | 4 |
Values are mean ± SDs or percentages unless otherwise indicated. LBW, low birth weight; LGA, large for gestational age; SGA, small for gestational age; VLBW, very low birth weight.
SGA was defined as <10th percentile for growth rate using the Fenton growth charts and LGA was defined as >10th percentile for growth rate using the Fenton growth charts.
Gestational weight gain categories were determined using the Institute of Medicine categories with adjustment for gestational age at delivery. For the teen cohort, recommended gestational weight gain was 12.7–18.1 kg for underweight women, 11.3–15.9 kg for normal-weight women, 6.8–11.3 kg for overweight women, and 5.0–9.1 kg (11–20 lb) for obese women. For the multiples cohort, recommended gestational weight gain was 22.7–28.1 kg for underweight women, 16.8–24.5 kg for normal-weight women, 14.1–22.7 kg for overweight women, and 11.3–19.1 kg for obese women.
Significant difference between cohorts, P < 0.05.
TABLE 2.
Iron status indicators in umbilical cord blood from newborns born to women carrying multiple fetuses and teens carrying singletons1
| Variable | n | Whole population | n | Multiples cohort | n | Teen cohort |
|---|---|---|---|---|---|---|
| Hb, g/dL | 224 | 14.8 ± 2.5 | 112 | 15.4 ± 2.18 | 112 | 14.2 ± 2.7 |
| Anemia | 40 | 18 | 11 | 108 | 29 | 26 |
| SF, μg/L | 288 | 111 (102, 121) | 126 | 104 (90.4, 119) | 162 | 117 (105, 132) |
| Erythrocyte iron,2 mg/kg | 224 | 40.9 ± 6.9 | 112 | 42.6 ± 5.68 | 112 | 39.2 ± 7.6 |
| Storage iron,3 mg/kg | 288 | 15.9 ± 7.5 | 126 | 15.3 ± 7.6 | 162 | 16.5 ± 7.3 |
| sTfR, mg/L | 288 | 6.8 (6.4, 7.1) | 126 | 6.0 (5.6, 6.4)8 | 162 | 7.5 (7.1, 8.0) |
| sTfR index4 | 288 | 3.4 (3.2, 3.5) | 126 | 3.0 (2.7, 3.3)8 | 162 | 3.7 (3.4, 3.9) |
| TBI,5 mg/kg | 223 | 53.6 ± 7.2 | 112 | 56.1 ± 6.28 | 111 | 51.0 ± 7.3 |
| Serum Fe, mg/L | 253 | 2.5 (2.3, 2.7) | 124 | 2.8 (2.6, 3.1)8 | 129 | 2.2 (2.0, 2.4) |
| IL-6,6 pg/mL | — | — | 123 | 3.6 (3.1, 4.2) | 145 | 9.0 (7.0, 11.6) |
| CRP,6 mg/L | — | — | 122 | 0.10 (0.09, 0.11) | 94 | 0.28 (0.23, 0.35) |
| Undetectable7 | — | — | 39 | 32 | 19 | 20 |
| Folate, nmol/L | 201 | 46.6 (43.7, 49.7) | 117 | 36.4 (34.3, 38.7) | 84 | 65.4 (59.9, 71.5) |
| <6.8 nmol/L | 0 | 0 | 0 | 0 | 0 | 0 |
| Vitamin B-12, pmol/L | 179 | 536 (495, 583) | 108 | 517 (464, 575) | 71 | 570 (501, 647) |
| <148 pmol/L | 1 | 1 | 0 | 0 | 1 | 1 |
Values are means ± SDs, geometric means (95% CIs) for transformed variables, or percentages. CRP, C-reactive protein; Fe, iron; Hb, hemoglobin; IL-6, interleukin-6; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron.
Erythrocyte iron (mg/kg) was calculated as neonatal hemoglobin (g/L) × estimated blood volume (L/kg) × birth weight (kg) × 3.4 (mg/g).
Storage iron (mg/kg) was calculated using the logarithmic relation between SF and body storage iron (62).
sTfR index was calculated as the following: sTfR/log10(SF).
TBI was calculated with the following equation: TBI (mg/kg) = −[log(sTfR/SF) − 2.8229] / 0.1207.
IL-6 and CRP were not assessed between cohorts because they were measured with different assays.
Undetectable was classified as below the limit of detection for CRP of this kit. In the teen cohort this was 0.2 mg/L and in the multiples cohort this was 0.077 mg/L.
Significant difference between cohorts, P < 0.05.
Neonatal ERFE and hormone concentrations
Table 3 presents mean ERFE concentrations, hormone concentrations, and ratios between these hormones. Ratios between these regulatory hormones were explored to capture neonatal adaptive responses in relation to iron status (hepcidin) or to alterations in erythropoietic drive and hypoxia (EPO and ERFE). Umbilical cord ERFE concentrations were measured in 85% of neonates born to women enrolled in the original study cohorts (29, 35, 46) and the CV of duplicate ERFE concentrations was <10% across the range of values measured. When data on variables from neonates with ERFE data were compared with neonates who did not have sufficient serum for ERFE analysis (n = 50), neonates with ERFE data had lower serum iron (P = 0.001) and higher birth weight (P = 0.03). There were no other significant differences between the 2 groups. At birth, the mean umbilical cord ERFE concentration was 0.73 ng/mL (95% CI: 0.63, 0.85 ng/mL), and neonatal ERFE needed to be log transformed to normalize the distribution. Umbilical cord ERFE was significantly lower in neonates who experienced intrauterine growth restriction (IUGR) (n = 18, P = 0.02). Umbilical cord ERFE did not significantly differ by cohort (P = 0.71), maternal ethnicity (P = 0.61), maternal parity (P = 0.39), maternal ppBMI (P = 0.40), sex of the neonate (P = 0.74), mode of delivery (P = 0.74), LBW classification (P = 0.98), or in those born prematurely (P = 0.66).
TABLE 3.
Iron regulatory hormone concentrations and hormone concentration ratios in newborns born to women carrying multiple fetuses and teens carrying singletons at birth1
| Variable | n | Whole population | n | Multiples cohort | n | Teen cohort |
|---|---|---|---|---|---|---|
| ERFE, ng/mL | 291 | 0.73 (0.63, 0.85) | 127 | 0.77 (0.62, 0.94) | 164 | 0.71 (0.57, 0.88) |
| EPO, mIU/mL | 273 | 24.15 (21.81, 26.73) | 124 | 17.4 (15.1, 20.0) | 150 | 31.7 (27.8, 36.1)3 |
| Hepcidin,2 ng/mL | — | — | 126 | 13.6 (11.4, 16.4) | 161 | 96.4 (84.8, 109.7) |
| Std hep | 287 | −0.008 ± 0.99 | 126 | 0.04 ± 0.4 | 161 | 0.08 ± 0.3 |
| Hep:EPO2 | — | — | 124 | 0.8 (0.6, 1.0) | 149 | 3.1 (2.6, 3.9) |
| Std hep:EPO | 272 | −0.59 ± 14.39 | 126 | −0.002 ± 0.08 | 149 | 0.007 ± 0.08 |
| Hep:ERFE2 | — | — | 126 | 18.0 (13.2, 24.5) | 161 | 136 (105, 177) |
| Std hep:ERFE | 287 | 0.003 ± 0.08 | 126 | 0.2 ± 2.4 | 161 | −1.2 ± 19.1 |
| ERFE:EPO | 273 | 0.03 (0.03, 0.04) | 124 | 0.04 (0.04, 0.05) | 150 | 0.02 (0.02, 0.03)3 |
Values are geometric means (95% CIs) unless otherwise indicated. EPO, erythropoietin; ERFE, erythroferrone; Hep, hepcidin.
Differences between cohorts were not assessed because Hep values were measured with different assays.
Significant difference between cohorts, P < 0.05.
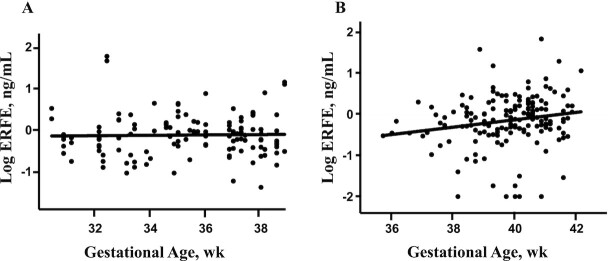
ERFE was detectable in all umbilical cord samples obtained from these cohorts, which ranged between 30 and 42 weeks of gestation at birth (Figure 1). All newborns in the multiples cohort were born between 30 and 38 weeks of gestation, and ERFE concentrations did not significantly vary as a function of gestational age at birth (β: 0.01, P = 0.72) (Figure 1A). However, newborns born to teens were generally born between 36 and 42 weeks of gestation and ERFE concentrations significantly increased as a function of gestational age at birth (β: 0.09, P = 0.02) (Figure 1B). After controlling for neonatal EPO or sTfR [2 indicators that were also significantly positively associated with gestational age at birth (35)], the relation between ERFE and gestational age was no longer significant (both P = 0.5).
FIGURE 1.
Effect of gestational age on cord ERFE differs between newborns born to women carrying multiple fetuses (A) and newborns born to teens carrying singletons (B). Bivariate correlations between neonatal ERFE and gestational age at birth. (A) The relation between ERFE and gestational age was β: 0.01, P = 0.72 in the multiples cohort. (B) The relation between ERFE and gestational age was β: 0.09, P = 0.02 in the teen cohort. ERFE, erythroferrone.
Neonatal ERFE associations with neonatal iron status indicators and hormones
Table 4 presents significant bivariate correlations between umbilical cord ERFE and umbilical cord iron status indicators and hormones. In the whole population, ERFE was significantly positively associated with Hb, sTfR, sTfR index, EPO, and erythrocyte iron and significantly inversely associated with SF and hepcidin. Although newborn ERFE was significantly positively associated with Hb, ERFE concentrations were not significantly lower in anemic neonates (0.61 ng/mL; 95% CI: 0.35, 0.78 ng/mL; n = 40) than in nonanemic neonates (0.76 ng/mL; 95% CI: 0.62, 0.92 ng/mL; n = 184) (P = 0.10). The strongest determinants of neonatal ERFE were neonatal EPO and sTfR, which together explained 21% of the variance in ERFE at birth (n = 274) (Table 5).
TABLE 4.
Umbilical cord erythroferrone is correlated with many iron status indicators and hormones in newborns born to women carrying multiple fetuses and teens carrying singletons1
| Whole population | Multiples cohort | Teen cohort | |
|---|---|---|---|
| Hemoglobin | 0.046 (224) | 0.097 (112) | 0.03 (112) |
| SF | −0.277 (288) | −0.468 (126) | −0.01 (162) |
| Erythrocyte iron2 | 2.617 (224) | 4.428 (112) | 1.11 (112) |
| Storage iron3 | −1.46 (288) | −4.487 (126) | −0.08 (162) |
| Transferrin receptor | 1.178 (288) | 0.907 (126) | 1.478 (162) |
| TBI4 | 0.01 (219) | 0.026 (111) | 0.00 (108) |
| sTfR index5 | 0.948 (288) | 0.798 (126) | 1.158 (162) |
| Serum iron | 0.12 (253) | 0.06 (124) | 0.23 (128) |
| Erythropoietin | 0.768 (269) | 0.728 (122) | 0.738 (147) |
| Hepcidin | −0.438 (283) | −0.488 (125) | −0.21 (158) |
Values are correlation coefficients (n) from regression models where multiples from 1 mother was controlled for by random effect. Models were also controlled for maternal race and cohort. SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron.
Erythrocyte iron (mg/kg) was calculated as neonatal hemoglobin (g/L) × estimated blood volume (L/kg) × birth weight (kg) × 3.4 (mg/g).
Storage iron (mg/kg) was calculated using the logarithmic relation between SF and body storage iron (62).
TBI was calculated with the following equation: TBI (mg/kg) = −[log(sTfR/SF) − 2.8229] / 0.1207.
sTfR index was calculated as the following: sTfR/log10(SF).
Indicates significant relationship with umbilical cord erythroferrone,P < 0.05.
Indicates significant relationship with umbilical cord erythroferrone,P <0.01.
Indicates significant relationship with umbilical cord erythroferrone,P < 0.001.
TABLE 5.
Neonatal determinants of erythroferrone in umbilical cord blood from newborns born to women carrying multiple fetuses and teens carrying singletons1
| Whole population (n = 274) | Multiples cohort (n = 110) | Teen cohort (n = 150) | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| EPO | 0.58 | <0.001 | 0.61 | <0.001 | 0.45 | 0.002 |
| sTfR | 0.66 | <0.001 | −0.60 | 0.03 | 1.17 | <0.001 |
| Hepcidin | −0.37 | 0.003 | ||||
| Erythrocyte iron | 0.02 | 0.01 | ||||
| Variance explained, % | 21 | 45 | 25 | |||
Values are correlation coefficients from regression models controlling for race and cohort and the maternal identification number was a random coefficient to account for multiples newborns being born from the same mother. EPO, erythropoietin; sTfR, soluble transferrin receptor.
In all neonates, the positive relation between ERFE and EPO was modified by neonatal Hb concentration (P-interaction = 0.02). In neonates with cord Hb < 17.7 g/dL, a significant relation between EPO and ERFE was evident, but in newborns with Hb > 17.7 g/dL (n = 17) no significant relation between EPO and ERFE was evident (Figure 2). Newborns with Hb > 17.7 g/dL had significantly higher EPO (P = 0.04), ERFE (P = 0.02), and sTfR (P = 0.01) and significantly lower hepcidin (P = 0.02) and SF (P = 0.004). Newborns with Hb > 17.7 g/dL were also on average 103 g smaller at birth (P = 0.03) and more were born SGA (P = 0.01), although no significant differences in the prevalence of preterm birth (P = 0.15) or IUGR (P = 0.35) were evident between groups.
FIGURE 2.
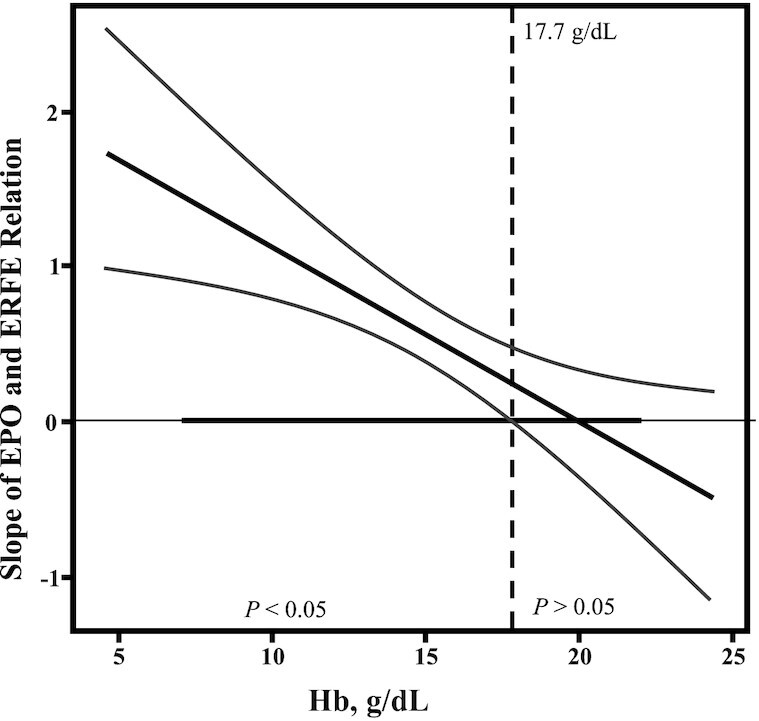
Relations between EPO and ERFE in cord blood were influenced by cord blood Hb concentrations. Johnson–Neyman analysis was undertaken to further assess the interaction between the independent variables EPO and Hb on the dependent variable ERFE concentration. The strength and significance of the relation between EPO and ERFE change as neonatal Hb concentration increases. The x axis indicates the range of Hb concentrations in umbilical cord blood. The y axis indicates the slope for the regression between EPO and ERFE. The thick solid black diagonal line demonstrates the slope of EPO and ERFE per Hb concentration, with the curved lines being the 95% CI. The thick solid black line on the x axis indicates the range of observed data. The dotted line indicates the Hb concentration (17.7 g/dL) for which neonates with Hb concentrations above this value no longer have a significant relation between EPO and ERFE, whereas neonates with Hb concentrations <17.7 g/dL have a significant relation between EPO and ERFE (P < 0.05). EPO, erythropoietin; ERFE, erythroferrone; Hb, hemoglobin.
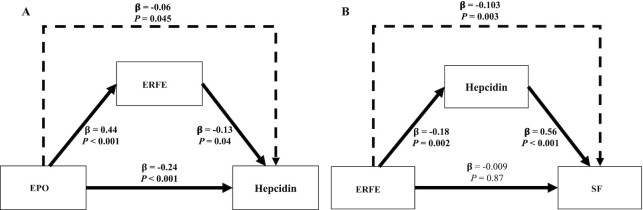
The utility of umbilical cord SF concentrations at birth is not fully understood. Although cord SF has been found to be positively associated with hepcidin, it is also often inversely associated with neonatal Hb concentrations (27). Further evaluation of the relation between ERFE and SF was undertaken using mediation analysis, and results suggest that the observed relation between ERFE and SF was indirect with hepcidin as the intermediary (P = 0.003) (Figure 3). Mediation analysis was also utilized to determine if ERFE played a mediating role in the observed EPO and hepcidin relation, given that this has been reported in animal models (37). Using this approach, the inverse correlation between EPO and hepcidin was found to only be minimally mediated by ERFE (β: −0.06, P = 0.045) (Figure 3).
FIGURE 3.
Direct and indirect relations between iron regulatory hormones and SF in umbilical cord blood in neonates born to women carrying multiple fetuses and teens carrying singletons. Mediation models were utilized to assess direct (consistent lines) and indirect (dashed line) associations between biomarkers in umbilical cord blood. (A) Direct relations (consistent line) between EPO as the independent variable and ERFE or hepcidin as the dependent variable as well as the direct relation between ERFE as the independent variable and hepcidin as the dependent variable; also, the indirect relation between EPO and hepcidin through ERFE (dashed line). (B) Direct relations (consistent line) between ERFE as the independent variable and SF or hepcidin as the dependent variable as well as the direct relation between hepcidin as the independent variable and SF as the dependent variable; also, the indirect relation between ERFE and SF through hepcidin (dashed line). Bolded coefficients and P values indicate significant effects (P < 0.05). EPO, erythropoietin; ERFE, erythroferrone; SF, serum ferritin.
Ratios between regulatory hormones may provide greater predictive ability in identifying newborns at increased risk of ID or anemia. In the whole population, SF was significantly positively associated with the hep:EPO (β: 0.27, P < 0.001, n = 273) and hep:ERFE (β: 0.17, P < 0.001, n = 288) ratios. sTfR was significantly inversely related to the hep:EPO ratio (β: −1.61, P < 0.001, n = 272) and the hep:ERFE ratio (β: −1.94, P < 0.001, n = 287), as was the sTfR/log(SF) index (hep:EPO: β: −1.71, P < 0.001, n = 272; hep:ERFE: β: −1.90, P < 0.001, n = 287). Inverse relations between Hb and the hep:EPO ratio (β: −0.04, P = 0.02, n = 212) and hep:ERFE ratio (β: −0.08, P < 0.001, n = 222) were also observed. Although there were significant inverse associations between Hb and the hep:EPO and hep:ERFE ratios, these ratios did not significantly differ between anemic and nonanemic newborns (P = 0.81 and P = 0.09, respectively). The ERFE:EPO ratio, however, was significantly higher in anemic than in nonanemic newborns (P = 0.03, n = 213). Serum iron was not significantly associated with any hormone ratio (all P > 0.1).
Significant differences in determinants of ERFE were evident between cohorts. A significant positive relation between ERFE and Hb was found among multiples newborns, and in these newborns ERFE was significantly inversely associated with hepcidin. However, in the newborns born to teens, ERFE was not significantly associated with either Hb or hepcidin. Significant differences in the mode of delivery were evident between cohorts, with a higher prevalence of vaginal delivery in the neonates born to teens. Because hepcidin is known to be significantly associated with the duration of labor (35), these associations were re-examined after controlling for the duration of labor, mode of delivery, and neonatal inflammation (IL-6); however, this did not alter the significance of the relation. Significant differences in the relation between Hb and the hep:ERFE ratio or the hep:EPO ratio were also evident between cohorts, with significant associations between Hb and these 2 ratios only being evident among the multiple birth newborns (P < 0.01).
Racial differences in neonatal ERFE
Neonatal ERFE was significantly lower in black than in white newborns (0.59 ng/mL; 95% CI: 0.45, 0.78 ng/mL compared with 0.89 ng/mL; 95% CI: 0.69, 1.16 ng/mL; P = 0.04) (Figure 4), and this difference remained significant after controlling for neonatal Hb (P = 0.004) or maternal Hb (P = 0.003). The ratio of ERFE to EPO was also significantly lower in black neonates (P = 0.02). The relation between Hb and ERFE significantly differed as a function of race, whereby a positive association between Hb and ERFE was found in white newborns (β: 0.05, P = 0.006, n = 116) but no significant association between these indicators was observed in black newborns (β: 0.04, P = 0.10, n = 108). Likewise, Hb was only significantly associated with EPO in white newborns (β: 0.03, P = 0.03, n = 110 compared with β: 0.01, P = 0.68, n = 103). These differences did not appear to be driven by differences in birth weight (P = 0.2), gestational age at birth (P = 0.81), maternal ppBMI (P = 0.6), maternal BMI at delivery (P = 0.7), or maternal weight gain (P = 0.6).
FIGURE 4.
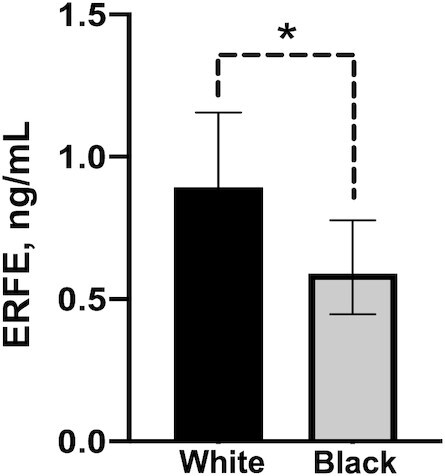
Cord ERFE in neonates of black or white women carrying multiples and black or white teens carrying singletons. Values are geometric means (95% CIs), n = 148 (black) or n = 141 (white). *P < 0.05. ERFE, erythroferrone.
Neonatal ERFE associations with maternal iron status indicators and hormones
In the whole population, neonatal ERFE was significantly positively associated with maternal ERFE concentrations at mid-gestation (β: 0.43, P < 0.001, n = 199) and at delivery (β: 0.39, P < 0.001, n = 237). Neonatal ERFE was, on average, 26% higher than maternal ERFE concentrations at mid-gestation and 19% higher than maternal ERFE concentrations at delivery. Neonatal ERFE was only significantly positively associated with maternal ERFE:EPO (β: 0.41, P < 0.001, n = 231). In addition, maternal ERFE (mid-gestation or delivery) was not significantly associated with neonatal iron status or hormone concentrations other than hep:ERFE and ERFE:EPO (both P < 0.001). Neonatal Hb and SF were not associated with any maternal hormone ratios. Neonatal hepcidin concentrations were significantly positively associated with maternal hep:EPO at delivery (β: 0.10, P = 0.003, n = 229) and neonatal EPO was associated with maternal hep:ERFE (β: −0.23, P < 0.001, n = 231). Other relations between maternal and neonatal iron status indicators have been published previously (29, 35).
ERFE in multiple birth newborns
Potential differences in ERFE concentrations between the twin, triplet, and quadruplet siblings were explored. The mean difference in ERFE between siblings was 1.12 ng/mL and mean differences were significantly greater between the triplet/quadruplet siblings than between twins (P = 0.03). Mean differences in ERFE also increased as chorionicity increased (P = 0.01) (mono: 0.31 ± 0.55 ng/mL, n = 12; di: 0.92 ± 0.33 ng/mL, n = 33; tri: 2.76 ± 0.61 ng/mL, n = 10). To further evaluate the impact of a shared uterine environment on neonatal ERFE concentrations, ICC values were calculated to assess differences in ERFE concentrations between siblings compared with differences observed between unrelated newborns. Values close to 0.5 suggest equal intra- and interuterine variance. The ICC value for ERFE was 0.58 (95% CI: 0.35, 0.64), which was similar to those previously reported for IL-6 and sTfR (29). Controlling for differences in amnionicity and chorionicity did not significantly change the calculated ICC value.
The ability of iron regulatory hormones, and ratios between these hormones, to explain variability in newborn iron status indicators was explored (Table 6). Cord ERFE explained 31% of the variance in cord hepcidin and 45% of the variance in cord EPO. However, ERFE was unable to explain a large portion of the variance in Hb, sTfR, SF, storage iron, or erythrocyte iron. Neonatal hepcidin was able to explain the most variance in cord Hb, erythrocyte iron, and the ratio of Hb to SF. Neonatal hepcidin explained more variance in neonatal Hb and iron status than did maternal hepcidin. In addition, the ratio of hepcidin to EPO was able to explain the most variance in sTfR, SF, sTfR index, and storage iron.
TABLE 6.
Umbilical cord hep:EPO and hep explain the most variance in iron status indicators in newborns born to women carrying multiple fetuses1
| Cord ERFE | Cord EPO | Cord hep | Maternal hep | Cord hep:ERFE | Cord hep:EPO | Cord ERFE:EPO | |
|---|---|---|---|---|---|---|---|
| Cord Hb | 3.7 | 7.7 | 25.9 | 0.6 | 18.3 | 22.2 | 6.2 |
| Cord SF | 14.8 | 29.4 | 41.8 | 0.2 | 36.6 | 49.1 | 6.4 |
| Erythrocyte iron2 | 4.5 | 7.0 | 26.9 | 11.5 | 19.4 | 22.4 | 6.7 |
| Storage iron3 | 15.0 | 29.3 | 44.7 | 2.5 | 37.2 | 49.2 | 6.5 |
| sTfR | 25.4 | 40.1 | 44.0 | 2.5 | 47.4 | 52.8 | 4.8 |
| Hb/SF | 14.7 | 24.9 | 48.1 | 0.1 | 38.5 | 18.6 | 0.9 |
| sTfR index4 | 25.7 | 44.7 | 49.9 | 0.4 | 54.0 | 61.9 | 4.0 |
| TBI5 | 4.0 | 8.8 | 1.0 | 3.1 | 1.0 | 9.3 | 6.1 |
| Serum iron | 0.9 | 3.5 | 0.6 | 0.6 | 0.1 | 4.5 | 4.3 |
Values are percentages of intrauterine variance explained. Intrauterine variance estimates were calculated to determine the amount of variance of each iron status indicator between siblings. Two models were generated: model 1 controlled for neonatal birth weight and model 2 controlled for neonatal birth weight and the iron regulatory hormone of choice. The percentage of intrauterine variance explained by the iron regulatory hormone was calculated as variance estimates of (model 1 − model 2)/model 1 × 100. EPO, erythropoietin; ERFE, erythroferrone; Hb, hemoglobin; hep, hepcidin; SF, serum ferritin; sTfR, soluble transferrin receptor; TBI, total body iron.
Erythrocyte iron (mg/kg) was calculated as neonatal hemoglobin (g/L) × estimated blood volume (L/kg) × birth weight (kg) × 3.4 (mg/g).
Storage iron (mg/kg) was calculated using the logarithmic relation between SF and body storage iron (62).
sTfR index was calculated as sTfR/log10(SF).
TBI was calculated with the following equation: TBI (mg/kg) = −[log(sTfR/SF) − 2.8229] / 0.1207.
Discussion
To our knowledge, the current study is the first to characterize neonatal ERFE at birth in 2 populations of newborns of which 18% were anemic at birth. ERFE was detected in all umbilical cord blood samples obtained in newborns born at 30–42 weeks of gestation, indicating the developing fetus produces this hormone as early as 30 weeks of gestation. Neonatal ERFE concentrations were higher than maternal ERFE concentrations and were significantly positively related to maternal ERFE. Although these newborns appeared to independently regulate ERFE production in relation to their individual iron status, this newly identified hormone did not capture more variability in neonatal iron or hematologic status than previously identified neonatal regulatory hormones or iron biomarkers. Of all indicators measured, neonatal hepcidin and the ratio of hepcidin to EPO were most strongly associated with neonatal iron and Hb concentrations.
The mean neonatal umbilical cord serum ERFE concentration in these newborns was 0.73 ng/mL (95% CI: 0.63, 0.85 ng/mL). The mean concentration observed in these neonates was 26% higher than maternal ERFE concentrations at mid-gestation and was only 19% higher than maternal values obtained at delivery. Currently, only 1 publication has reported ERFE concentrations in cord blood within a small cohort (n = 36) of newborns, where healthy term newborn ERFE concentrations were similar to those reported in these cohorts (1.0 ± 0.8 ng/mL, n = 10), and higher values were reported in newborns born to obese or diabetic mothers (2.8 ± 4.1 ng/mL, n = 13) (39). Furthermore, the mean neonatal ERFE concentration was >50% higher than published values reported in nonpregnant women (65) or healthy males (42, 65) using the same assay. The higher ERFE concentration in neonates may be a consequence of adaptation to the hypoxic intrauterine environment.
ERFE is an erythropoietic stress hormone that is produced by erythroblasts in response to stimulation by EPO (37). During fetal development, dynamic changes in erythropoiesis occur as the site of erythroblast production changes (66). The stage of development also affects the Hb isoform produced. Fetal hemoglobin (HbF) has an increased affinity for oxygen (67) but it is gradually replaced by adult hemoglobin (HbA) shortly after birth. The human fetus begins to produce both hepcidin (5) and EPO (6) relatively early in gestation, but similar data on ERFE are not available. In this cohort of newborns, ERFE was detected in samples obtained in newborns that were born across 30–42 weeks of gestation. Additional data are needed to identify the earliest stage at which the human fetus begins to produce this regulatory hormone, but significant associations found between ERFE and iron status biomarkers at birth suggest this hormone supports fetal erythropoietic iron demand in utero.
Under hypoxic conditions, EPO production is increased and binds to EPO-receptors on erythroblasts initiating the Janus kinase–signal transducers and activators of transcription (JAK-STAT) signaling cascade to increase ERFE expression (9, 37). Thus, serum ERFE concentrations are a product of circulating EPO and the number of erythroblasts. In these newborns the relation between EPO and ERFE was influenced by neonatal Hb concentrations but only among newborns with Hb < 17.7 g/dL. A significant relation was not evident among newborns with cord Hb concentrations >17.7 g/dL. These newborns were disproportionately SGA with significantly lower SF and higher concentrations of both EPO and ERFE. We speculate that neonates with Hb concentrations >17.7 g/dL may have experienced hypoxia because this would explain higher EPO and ERFE stimulating Hb production, and the increased iron utilization for erythropoiesis could lead to a reduction in iron stores.
ERFE is thought to downregulate hepcidin by sequestering bone morphogenetic protein 2/6, thus limiting the production of hepcidin (68). In these neonates, as expected, ERFE expression was increased in response to EPO, and both EPO and ERFE were inversely associated with hepcidin. However, we did not find that ERFE was the main driver of decreased hepcidin concentrations upon EPO stimulation. This finding suggests other hepcidin signaling pathways, such as those that respond to low serum iron, may have a larger regulatory role in this cohort of newborns.
Ratios between iron regulatory hormones may provide increased ability in explaining variability in neonatal iron status over individual hormone measures because ratios may address coordinated regulatory responses to both hypoxia and ID. For example, low hep:ERFE and hep:EPO suggest the coexistence of increased erythropoietic activity and ID, whereas high ERFE:EPO indicates the response of the erythron to EPO. Using this approach, the hep:EPO ratio explained the most variability in neonatal sTfR, SF, and storage iron concentrations. Neonatal hepcidin and hep:EPO explained the most variance of neonatal Hb and erythrocyte iron, which reflect the largest iron pool for the newborn. This suggests that these hormones play a large role in regulating neonatal RBC production and thus Hb status. We recently reported similar findings in the mothers of these neonates, whereby ratios of hepcidin to EPO also best predicted maternal iron status (69). In these newborns, the hep:EPO ratio was inversely associated with Hb and erythrocyte iron and positively associated with SF and storage iron concentrations. This finding is consistent with the significant inverse relation between SF and Hb in these newborns previously published, demonstrating hypoxia driving iron utilization for Hb production (27).
During fetal development adequate Hb production is essential for tissue oxygenation (57), and the fetus prioritizes iron use for RBC production over tissue stores (56, 57). The mass of the erythrocyte iron pool (mg/kg) in these newborns was significantly higher than their storage iron pool (mg/kg). We predicted that a higher prevalence of anemia would be found among multiple birth neonates because these neonates tended to be born prematurely to women who exhibited an increased prevalence of ID and anemia compared with that observed among teen mothers (48). However, we observed that the neonates born to pregnant teens, nearly all of whom were born at term, had a greater prevalence of anemia at birth. This finding may be a consequence of differential nutrient partitioning in teen pregnancies whereby biologically immature gravida may prioritize nutrients in support of their own continued growth, as has been reported in animal models of adolescent pregnancy (70). Further study of maternal–fetal nutrient partitioning and normative data in teen pregnancies are needed to identify newborns at risk of anemia so interventions can be initiated.
The CDC has set a lower Hb cutoff to define anemia in black adults and children than in white individuals (53), owing to a shift in Hb distribution between these 2 groups (71, 72). However, these race-specific criteria are frequently not applied, particularly among pediatric populations. In this cohort, prevalence of neonatal anemia did not significantly differ as a function of race, yet neonatal ERFE concentrations were significantly lower in black neonates. This relation remained significant after controlling for either maternal or neonatal Hb concentrations. The ratio of ERFE to EPO was also significantly lower in black neonates, suggesting that in these neonates more EPO was needed per unit increase in ERFE concentration. There are few other normative data on cord iron status biomarkers in these 2 groups with which to compare these findings, but given the growing awareness of the impact of genetics on nutrient metabolism (73, 74), more data are needed to understand the mechanisms responsible for ancestry-driven differences in iron metabolism.
There are several limitations that affect interpretation of these data. We did not obtain any data on Hb isoform concentrations in these newborns. At the time of birth 55%–65% of Hb is HbF (75); however, it has been found that infants with severe anemia have elevated fetal Hb at birth because the switch to HbA is delayed (76). We are not aware of other data that have examined whether the HbF to HbA transition differs between black and white newborns.
In conclusion, the wide range of Hb concentrations evident in this group of healthy neonates provided opportunities to characterize determinants of ERFE concentrations in umbilical cord blood and examine how this newly identified hormone was regulated in response to changes in hepcidin, EPO, and iron status. ERFE was present in all newborns studied and was produced as early as 30 weeks of gestation. Concentrations of ERFE in umbilical cord serum were significantly inversely related to neonatal iron status indicators and significantly positively related to erythropoietic drive as expected. Umbilical cord ERFE and its associations with erythropoietic indicators significantly differed between black and white newborns and further data are needed to explore possible mechanisms responsible for these differences. Using all currently identified hormones in the EPO, ERFE, and hepcidin signaling pathway, low cord blood hep:EPO ratios were most strongly associated with decreased SF and elevated sTfR in newborns. These findings may provide an additional measure of iron status at birth and help identify newborns that may have experienced in utero ID.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KOO: designed the research; KMD and KOO: conducted the research, analyzed and interpreted the data, and wrote the manuscript; RG and EKP: were responsible for the clinical implementation of the studies; RG, EKP, EN, and TG: assisted with the design of the research, analysis and interpretation of the data, and the preparation of the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Gerber Foundation (to KOO), USDA grants 2005-35200-15218 (to KOO) and 2009-35200-05171 (to KOO), and US NIH National Institute of Diabetes and Digestive and Kidney Diseases grant T32-DK007158 (to KOO). The Gerber Foundation had no conflict of interest in the study and was not involved in the study design or interpretation of the data.
Author disclosures: EN and TG are stockholders in and consultants for Intrinsic LifeSciences, LLC. All other authors report no conflicts of interest.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CRP, C-reactive protein; EPO, erythropoietin; ERFE, erythroferrone; Hb, hemoglobin; HbA, adult hemoglobin; HbF, fetal hemoglobin; ICC, intraclass correlation coefficient; ID, iron deficiency; IUGR, intrauterine growth restriction; LBW, low birth weight; LOD, limit of detection; ppBMI, prepregnancy BMI; SF, serum ferritin; SGA, small for gestational age; sTfR, soluble transferrin receptor.
Contributor Information
Katherine M Delaney, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Ronnie Guillet, Division of Neonatology, Department of Pediatrics, University of Rochester School of Medicine, Rochester, NY, USA.
Eva K Pressman, Department of Obstetrics and Gynecology, University of Rochester School of Medicine, Rochester, NY, USA.
Tomas Ganz, Center for Iron Disorders, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Elizabeta Nemeth, Center for Iron Disorders, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Kimberly O O'Brien, Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA.
Data availability
Data described in the article, code book, and analytic code will not be made available because of the composition of the patient population and the confidential nature of the data collected.
References
- 1. Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(4_suppl4):S560–71. [DOI] [PubMed] [Google Scholar]
- 2. Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69:S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1):257S–64S. [DOI] [PubMed] [Google Scholar]
- 4. Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26(127):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans P, Cindrova-Davies T, Muttukrishna S, Burton GJ, Porter J, Jauniaux E. Hepcidin and iron species distribution inside the first-trimester human gestational sac. Mol Hum Reprod. 2011;17(4):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckardt KU. The ontogeny of the biological role and production of erythropoietin. J Perinat Med. 1995;23(1–2):19–30. [DOI] [PubMed] [Google Scholar]
- 7. Thomas RM, Canning CE, Cotes PM, Linch DC, Rodeck CH, Rossiter CE, Huehns ER. Erythropoietin and cord blood haemoglobin in the regulation of human fetal erythropoiesis. BJOG. 1983;90(9):795–800. [DOI] [PubMed] [Google Scholar]
- 8. Teramo KA, Klemetti MM, Widness JA. Robust increases in erythropoietin production by the hypoxic fetus is a response to protect the brain and other vital organs. Pediatr Res. 2018;84(6):807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jelkmann W. Physiology and pharmacology of erythropoietin. Transfus Med Hemother. 2013;40(5):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polin RF, Fox WW. Fetal and neonatal physiology. 2nd ed. Philadelphia, PA: WB Saunders; 1998. [Google Scholar]
- 11. Dame C, Fahnenstich H, Freitag P, Hofmann D, Abdul-Nour T, Bartmann P, Fandrey J. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood. 1998;92(9):3218–25. [PubMed] [Google Scholar]
- 12. Forestier F, Daffos F, Catherine N, Renard M, Andreux JP. Developmental hematopoiesis in normal human fetal blood. Blood. 1991;77(11):2360–3. [PubMed] [Google Scholar]
- 13. Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fetal Neonatal Med. 2002;11(5):329–32. [DOI] [PubMed] [Google Scholar]
- 14. Jazayeri A, Tsibris JC, Spellacy WN. Fetal erythropoietin levels in growth-restricted and appropriately grown neonates with and without abnormal fetal heart rate tracings: a comparison with cord blood gases and Apgar scores. J Perinatol. 1999;19(4):255–9. [DOI] [PubMed] [Google Scholar]
- 15. Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95(2):105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teramo KA, Hiilesmaa VK, Schwartz R, Clemons GK, Widness JA. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med. 2004;32(3):240–7. [DOI] [PubMed] [Google Scholar]
- 17. Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264(5):405–32. [DOI] [PubMed] [Google Scholar]
- 18. Juul SE. Nonerythropoietic roles of erythropoietin in the fetus and neonate. Clin Perinatol. 2000;27(3):527–41. [DOI] [PubMed] [Google Scholar]
- 19. Peng B, Kong G, Yang C, Ming Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 2020;11(2):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fandrey J, Pagel H, Frede S, Wolff M, Jelkmann W. Thyroid hormones enhance hypoxia-induced erythropoietin production in vitro. Exp Hematol. 1994;22(3):272–7. [PubMed] [Google Scholar]
- 21. Kambe T, Tada-Kambe J, Kuge Y, Yamaguchi-Iwai Y, Nagao M, Sasaki R. Retinoic acid stimulates erythropoietin gene transcription in embryonal carcinoma cells through the direct repeat of a steroid/thyroid hormone receptor response element half-site in the hypoxia-response enhancer. Blood. 2000;96(9):3265–71. [PubMed] [Google Scholar]
- 22. Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. [DOI] [PubMed] [Google Scholar]
- 23. Lorenz L, Herbst J, Engel C, Peter A, Abele H, Poets CF, Westerman M, Franz AR. Gestational age-specific reference ranges of hepcidin in cord blood. Neonatology. 2014;106(2):133–9. [DOI] [PubMed] [Google Scholar]
- 24. Brickley EB, Spottiswoode N, Kabyemela E, Morrison R, Kurtis JD, Wood AM, Drakesmith H, Fried M, Duffy PE. Cord blood hepcidin: cross-sectional correlates and associations with anemia, malaria, and mortality in a Tanzanian birth cohort study. Am J Trop Med Hyg. 2016;95(4):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher AL, Sangkhae V, Presicce P, Chougnet CA, Jobe AH, Kallapur SG, Tabbah S, Buhimschi CS, Buhimschi IA, Ganz Tet al. Fetal and amniotic fluid iron homeostasis in healthy and complicated murine, macaque, and human pregnancy. JCI Insight. 2020;5(4):e135321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ichinomiya K, Maruyama K, Inoue T, Koizumi A, Inoue F, Fukuda K, Yamazaki Y, Arakawa H. Perinatal factors affecting serum hepcidin levels in low-birth-weight infants. Neonatology. 2017;112(2):180–6. [DOI] [PubMed] [Google Scholar]
- 27. Delaney KM, Guillet R, Fleming RE, Ru Y, Pressman EK, Vermeylen F, Nemeth E, O'Brien KO. Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples. J Nutr. 2019;149(3):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, Sankilampi U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–52. [DOI] [PubMed] [Google Scholar]
- 29. Ru Y, Pressman EK, Guillet R, Katzman PJ, Vermeylen F, O'Brien KO. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J Nutr. 2018;148(11):1716–22. [DOI] [PubMed] [Google Scholar]
- 30. Basu S, Kumar N, Srivastava R, Kumar A. Maternal and cord blood hepcidin concentrations in severe iron deficiency anemia. Pediatr Neonatol. 2016;57(5):413–19. [DOI] [PubMed] [Google Scholar]
- 31. Korlesky C, Kling PJ, Pham DQD, Ovasapyan AA, Leyns CEG, Weber MB, Coe CL. Cord blood erythropoietin and hepcidin reflect lower newborn iron stores due to maternal obesity during pregnancy. Am J Perinatol. 2019;36(5):511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chełchowska M, Maciejewski TM, Mazur J, Gajewska J, Zasimovich A, Ołtarzewski M, Ambroszkiewicz J. Active tobacco smoke exposure in utero and concentrations of hepcidin and selected iron parameters in newborns. Int J Environ Res Public Health. 2019;16(11):1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malek A, Sager R, Eckardt KU, Bauer C, Schneider H. Lack of transport of erythropoietin across the human placenta as studied by an in vitro perfusion system. Pflugers Arch. 1994;427(1–2):157–61. [DOI] [PubMed] [Google Scholar]
- 34. Schneider H, Malek A. Lack of permeability of the human placenta for erythropoietin. J Perinat Med. 1995;23(1–2):71–6. [DOI] [PubMed] [Google Scholar]
- 35. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, Pressman E, O'Brien KO. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016;79(1):42–8. [DOI] [PubMed] [Google Scholar]
- 36. Garcia-Valdes L, Campoy C, Hayes H, Florido J, Rusanova I, Miranda MT, McArdle HJ. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int J Obes. 2015;39(4):571–8. [DOI] [PubMed] [Google Scholar]
- 37. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ganz T, Jung G, Naeim A, Ginzburg Y, Pakbaz Z, Walter PB, Kautz L, Nemeth E. Immunoassay for human serum erythroferrone. Blood. 2017;130(10):1243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bahr TM, Ward DM, Jia X, Ohls RK, German KR, Christensen RD. Is the erythropoietin-erythroferrone-hepcidin axis intact in human neonates?. Blood Cells Mol Dis. 2021;88:102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanudel MR, Rappaport M, Chua K, Gabayan V, Qiao B, Jung G, Salusky IB, Ganz T, Nemeth E. Levels of the erythropoietin-responsive hormone erythroferrone in mice and humans with chronic kidney disease. Haematologica. 2018;103(4):e141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hall R, Peeling P, Nemeth E, Bergland D, McCluskey WTP, Stellingwerff T. Single versus split dose of iron optimizes hemoglobin mass gains at 2106 m altitude. Med Sci Sports Exerc. 2019;51(4):751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robach P, Gammella E, Recalcati S, Girelli D, Castagna A, Roustit M, Lundby C, Lundby AK, Bouzat P, Verges Set al. Induction of erythroferrone in healthy humans by micro-dose recombinant erythropoietin or high-altitude exposure. Haematologica. 2020;106(2):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mirciov CSG, Wilkins SJ, Hung GCC, Helman SL, Anderson GJ, Frazer DM. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica. 2018;103(10):1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinl GK, Gandelman JS, Katzman PJ, Ru Y, Guillet R, Pressman E, Cooper EM, O'Brien KO. Umbilical cord coiling in high-risk pregnancies: associations with determinants of adverse birth outcomes and iron status. Pediatr Dev Pathol. 2018;21(6):537–47. [DOI] [PubMed] [Google Scholar]
- 46. Ru Y, Pressman EK, Guillet R, Katzman PJ, Bacak SJ, O'Brien KO. Predictors of anemia and iron status at birth in neonates born to women carrying multiple fetuses. Pediatr Res. 2018;84(2):199–204. [DOI] [PubMed] [Google Scholar]
- 47. Steinl GK, Whisner CM, Pressman EK, Cooper EM, Groth SW, O'Brien KO. Patterns and correlates of self-reported physical activity in a cohort of racially diverse pregnant adolescents. J Pediatr Adolesc Gynecol. 2019;32(1):51–6. [DOI] [PubMed] [Google Scholar]
- 48. Ru Y, Pressman EK, Cooper EM, Guillet R, Katzman PJ, Kent TR, Bacak SJ, O'Brien KO. Iron deficiency and anemia are prevalent in women with multiple gestations. Am J Clin Nutr. 2016;104(4):1052–60. [DOI] [PubMed] [Google Scholar]
- 49. Lee S, Young BE, Cooper EM, Pressman E, Queenan RA, Olson CM, Guillet R, O'Brien KO. Nutrient inadequacy is prevalent in pregnant adolescents, and prenatal supplement use may not fully compensate for dietary deficiencies. Child Obes Nutr. 2014;6(3):152–9. [Google Scholar]
- 50. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Pressman E, O'Brien KO. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr. 2014;144(10):1524–32. [DOI] [PubMed] [Google Scholar]
- 51. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O'Brien KO. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod Sci. 2016;23(5):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. CDC . Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29. [PubMed] [Google Scholar]
- 54. Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290(22):1213–16. [DOI] [PubMed] [Google Scholar]
- 55. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta. 2003;329(1–2):9–22. [DOI] [PubMed] [Google Scholar]
- 56. Georgieff MK, Landon MB, Mills MM, Hedlund BE, Faassen AE, Schmidt RL, Ophoven JJ, Widness JA. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117(3):455–61. [DOI] [PubMed] [Google Scholar]
- 57. Zamora TG, Guiang SF 3rd, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. 2016;79(6):922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–63. [DOI] [PubMed] [Google Scholar]
- 59. Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121(1):109–14. [DOI] [PubMed] [Google Scholar]
- 60. Fomon SJ, Ziegler EE, Rogers RR, Nelson SE, Edwards BB, Guy DG, Erve JC, Janghorbani M. Iron absorption from infant foods. Pediatr Res. 1989;26(3):250–4. [DOI] [PubMed] [Google Scholar]
- 61. Mollison PL, Veall N, Cutbush M. Red cell and plasma volume in newborn infants. Arch Dis Child. 1950;25(123):242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saarinen UM, Siimes MA. Iron absorption from breast milk, cow's milk, and iron-supplemented formula: an opportunistic use of changes in total body iron determined by hemoglobin, ferritin, and body weight in 132 infants. Pediatr Res. 1979;13(3):143–7. [DOI] [PubMed] [Google Scholar]
- 63. Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–42. [Google Scholar]
- 64. Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: inferential and graphical techniques. Multivariate Behav Res. 2005;40(3):373–400. [DOI] [PubMed] [Google Scholar]
- 65. Appleby S, Chew-Harris J, Troughton RW, Richards AM, Pemberton CJ. Analytical and biological assessment of circulating human erythroferrone. Clin Biochem. 2020;79:41–7. [DOI] [PubMed] [Google Scholar]
- 66. Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE. Nathan and Oski's hematology of infancy and childhood. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 67. Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med. 2013;3(1):a011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang C-Y, Xu Y, Traeger L, Dogan DY, Xiao X, Steinbicker AU, Babitt JL. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood. 2020;135(6):453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Delaney KM, Guillet R, Pressman EK, Ganz T, Nemeth E, O'Brien KO. Serum erythroferrone during pregnancy is related to erythropoietin but does not predict the risk of anemia. J Nutr. 2021; May 12 (Epub ahead of print; doi: 10.1093/jn/nxab093). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wallace J, Bourke D, Da Silva P, Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction. 2001;122(3):347–57. [DOI] [PubMed] [Google Scholar]
- 71. Perry GS, Byers T, Yip R, Margen S. Iron nutrition does not account for the hemoglobin differences between blacks and whites. J Nutr. 1992;122(7):1417–24. [DOI] [PubMed] [Google Scholar]
- 72. Williams DM. Racial differences of hemoglobin concentration: measurements of iron, copper, and zinc. Am J Clin Nutr. 1981;34(9):1694–700. [DOI] [PubMed] [Google Scholar]
- 73. Stover PJ, Caudill MA. Genetic and epigenetic contributions to human nutrition and health: managing genome–diet interactions. J Am Diet Assoc. 2008;108(9):1480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. James WPT, Johnson RJ, Speakman JR, Wallace DC, Frühbeck G, Iversen PO, Stover PJ. Nutrition and its role in human evolution. J Intern Med. 2019;285(5):533–49. [DOI] [PubMed] [Google Scholar]
- 75. Wranne L. Studies on erythro-kinetics in infancy. VII. Quantitative estimation of the haemoglobin catabolism by carbon monoxide technique in young infants. Acta Paediatr. 1967;56(4):381–90. [DOI] [PubMed] [Google Scholar]
- 76. Bard H, Fouron J-C, Gagnon JG, Gagnon C. Hypoxemia and increased fetal hemoglobin synthesis. J Pediatr. 1994;124(6):941–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available because of the composition of the patient population and the confidential nature of the data collected.