Abstract
While the developed nations are discussing giving a third dose of the COVID-19 vaccine to immunocompromised individuals, there are still challenges that are of global concern, especially in developing countries. The Delta variant which is predominantly responsible for the disease burden has now been reported in over 148 countries. The catastrophe caused in the Indian subcontinent has highlighted some associations, most notable being the unprecedented rise in the cases of mucormycosis in COVID-19 patients referred to as CAM (COVID-19 associated mucormycosis). This life-threatening opportunistic fungal infection which was historically associated with immunosuppression has reached a new peak as its incidence has increased many folds with the advent of COVID-19. Here we present one of the very first Case reports on how to post COVID immunosuppression state, uncontrolled blood sugar levels in the background of diabetic ketoacidosis led to the development of pulmonary mucormycosis with superimposed pulmonary tuberculosis and later Sino-nasal mucormycosis eventually leading to life-threatening massive hemoptysis, causing mortality of a post-COVID-19 infected middle-aged diabetic Asian male patient who presented twenty days after COVID-19 infection. However, our patient did not have risk factors such as severe COVID-19 infection requiring hospitalization, use of steroids or other immunomodulatory drugs like remdesivir or tocilizumab. Our case report aims to bring forth this post COVID pulmonary mucormycosis with pulmonary tuberculosis association as well as highlight the fact that tuberculosis is still a major public health burden that should not be forgotten in the fight to combat the pandemic.
Keywords: Post COVID-19, Pulmonary mucormycosis, Pulmonary tuberculosis, Massive, Haemoptysis, Bronchospopy, Co-infection
1. Introduction
Amid the global rampage caused by the Delta variant, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has set a new record. The cumulative number has crossed over 206 million cases with more than 4.4 million deaths [1]. A noteworthy finding is an unprecedented rise in the number of cases of the morbid fungal disease mucormycosis, which is now increasingly being associated with COVID-19. Mucormycosis has a global incidence varying from 0.005 to 1.7 per million population. In India, however, the prevalence is estimated to be 140 per million, which is 80 times higher than the developed countries [2]. Data indicates, since the advent of COVID-19 until the beginning of April 2021, India has contributed to almost 71% of global cases of mucormycosis in patients with COVID-19 [3]. Another significant finding amidst the pandemic is the rise of the worldwide burden of tuberculosis, which remains the leading cause of death amongst infectious diseases [4]. Both the conditions are airborne, have considerable overlap in their symptoms, have similar social determinants, target the lungs, and impair host immune responses [5]. We hereby, present a first Case report of pulmonary and rhino-sinusoidal mucormycosis and pulmonary tuberculosis in a post-COVID-19 infected patient. It is crucial to learn from the disease trends in the South-East Asian and European regions during the past few months and be vigilant as the cases begin to rise predominantly in the Western Pacific region and region of Americas.
2. Case report
A 48 years old Asian male diagnosed with type 2 diabetes mellitus two years back presented to our emergency with complaints of left-sided chest pain, radiating to back and dry cough for 25 days along with a low-grade fever of 99.8 °F without any diurnal variation, relieved on medications for 20 days. There was no complaint of shortness of breath, orthopnea, abdominal discomfort, headache, or contact with a tuberculosis patient; however, he had a history of insignificant weight loss and loss of appetite for two months. He was on injectable human insulin (30/70) as 12 units subcutaneously before breakfast and eight units before dinner, and his blood dextrose level was uncontrolled since he reported positive for COVID-19 20 days back. However, he did not need hospitalization, steroids, or oxygen therapy and was managed conservatively in a COVID care center. The characteristic clinical, laboratory, and treatment profile of the patient have been tabulated in Table 1. On presentation, he had a blood pressure of 144/90 mmHg in the right radial artery, a random blood glucose level of 478 mg/dL with positive urinary ketones, a pulse rate of 89/min regular, and oxygen saturation of oxygen 98% under room air. General physical examination was unremarkable, and on chest auscultation, bilateral coarse crepitations were heard more over left interscapular and inframammary areas. An electrocardiograph was done, which showed normal sinus rhythm, and a portable bedside chest radiograph showed bilateral hilar prominence with a possibility of consolidation or a mass lesion (left > right), as shown in Fig. 1. A nasal and oropharyngeal swab for SARS-CoV-2 was taken, which was reported negative. The patient's arterial blood gas showed metabolic acidosis with a pH of 7.32, bicarbonate as 16.8 mEq/L, and normal partial pressure of oxygen and carbon dioxide. On presentation, a provisional diagnosis of newly diagnosed hypertensive with type 2 diabetes mellitus complicated by diabetic ketoacidosis and a lower respiratory tract infection with a query of perihilar mass was made. The patient's blood samples for routine blood parameters were withdrawn along with blood and urine cultures before starting intravenous antibiotics. On admission, blood parameters revealed a hemoglobin of 10.1g/dL, TLC of 13,400 cells/mm3, DLC of 91% neutrophils, 8% lymphocytes, platelets of 4.1 lakhs/mm3, ALT of 50 U/L, AST of 41U/L, LDH of 352 U/L, D-dimer of 497 ng/ml, INR of 1.14, IL-6 of 34 (<7pg/ml) and highly sensitive CRP of 212 mg/L. The patient had a normal renal function test, serum electrolytes, calcium, phosphate, total proteins, and serum albumin levels. As a result, treatment was started with empirical antibiotics as a piperacillin-tazobactam combination with levofloxacin along with strict blood glucose control with an insulin infusion and intravenous fluids. Subsequently, a Contrast-Enhanced Computed Tomography Angiography (CECT- Angio) of the chest was performed, which depicted findings consistent with Pulmonary Mucormycosis and bilateral ground-glass opacities in lower lobes without any evidence of pulmonary artery thrombosis, as shown in Fig. 2, and a normal transthoracic 2D-echo study. On the second day, as the patient recovered from diabetic ketoacidosis, injection liposomal amphotericin B (5–10mg/kg/day) at a dose of 300mg intravenously daily was added to the treatment with the aim of a total dose of 4 g along with strict monitoring of kidney function and serum electrolytes. Later, as the patient continued to have a low-grade fever and dry cough, a bronchoscope-guided bronchial biopsy of the left lower lobe mass was performed. Serial sections of processed tissue demonstrated large areas of necrosis and inflammatory granulation tissue along with aseptate branched fungal hyphae visualized as consistent with mucormycosis. However, an Acid-Fast Bacilli (AFB) stain was negative. As the patient continued to experience low-grade fever, injection vancomycin was added to the treatment because of an elevated serum procalcitonin level, i.e., 1.8 (>2ng/ml for high risk of infection), although his urine microscopy, blood and urine cultures were sterile after 48 hours of incubation. Peripheral smear for malaria antigen, serologies for HIV, hepatitis A, B, C, D, E, dengue, rickettsia, scrub typhus, Leptospira, beta 2 glucans, aspergillosis, galactomannan, and repeated urine routine microscopy, blood and urine cultures reported negative. The patient's blood glucose was taken care of by human (plain) intravenous insulin injections and intravenous fluids, and potassium supplementations. On the seventh day, he developed cough with expectoration, hence sputum for AFB, CBNAAT (Cartridge Based Nucleic Acid Amplification), gram stain, culture sensitivity, and fungal cultures were sent for analysis. On the ninth day, his sputum for AFB was reported positive. CBNAAT showed no rifampicin resistance; hence weight-based anti-tubercular therapy (ATT) (isoniazid 300mg, rifampicin 450 mg, pyrazinamide 1200mg and ethambutol 800mg) along with tablet pyridoxine 20mg were added further to the treatment. The patient started experiencing vomiting and abdominal pain on the eleventh day with deranged liver function tests (AST -127U/L, ALT-98, Total Bilirubin −1.7), hepatic modified ATT (injection streptomycin 0.75g daily, tablet ethambutol 800mg, and tablet levofloxacin 750 mg alternate day) was initiated. However, his abdominal ultrasonography was normal. On the thirteenth day, tab Posaconazole 300mg was also added to treatment. On the sixteenth day, he experienced right nasal congestion with blackish crusting of the nasal septum, a nasal swab for 20% KOH mount was sent, which showed aseptate branched fungal hyphae consisting of mucormycosis, as shown in Fig. 3. A CECT of paranasal sinuses and orbit was done, which showed findings of Sino-nasal mucormycosis, as shown in Fig. 2. Hence, a FESS (Functional Endoscopic Sinus Surgery) guided biopsy and local debridement were done, as shown in Fig- 3. However, on the twentieth day, the patient developed massive hemoptysis and went into hypovolemic shock. Hence, Ryle's tube was inserted, two packed cell volumes of blood were transfused along with inotropes, and the patient was planned for an emergency therapeutic bronchoscopy, however as the patient's GCS (Glasgow Coma Scale) deteriorated patient was electively intubated. Unfortunately, the patient succumbed to his illness on the very next day. Written consent was taken from the patient's relative to reproduce the clinical data.
Table 1.
Timeline showing characterstic clinico- laboratory and treatment profile of the patient during hospital stay.
| Day 1 | Day 5 | Day 25 | Day 26/27 | Day 32–34 | Day 36 | Day 41 | Day 45 | |
|---|---|---|---|---|---|---|---|---|
| Clincal Profile | Type 2 diabetes mellitus on injectable insulin therapy Chest pain (dull aching) Dry cough | Low grade fever, chest pain, dry cough | Presented to hospital With DKA | Perihilar mass evaluated | Developed productive cough | Developed vomiting, abdominal pain, deranged LFT | Right nasal congestion and crusting of nasal septum | Massive hemoptysis, hypovolemic shock |
| Investigations | Tested COVID + | COVID - status CXR s/o B/L LRTI perihilar lung mass | CECT chest, angiography, Suspected pulmonary mucormycosis, Confirmed after bronchoscopic biopsy | Sputum for AFB + CBNAAT for Rifampicin resistance negative | Daignosed with ATT induced hepatotoxicity | KOH mount and MRI parental sinuses confirmed sino-nasal mucormycosis | Ryle's tube inserted, 2 PCV transfused, Inotropes started, Elective intubation in view of declining GCS | |
| Treatment | Admitted to hospital, piperacillin/tazobactam, levofloxacin, insulin | Started on Amphotericin B, antibiotics continued | Started on ATT | Started on Heaptic modified ATT | FESS guided biopsy and local debridement performed | Patient died on Day 46 |
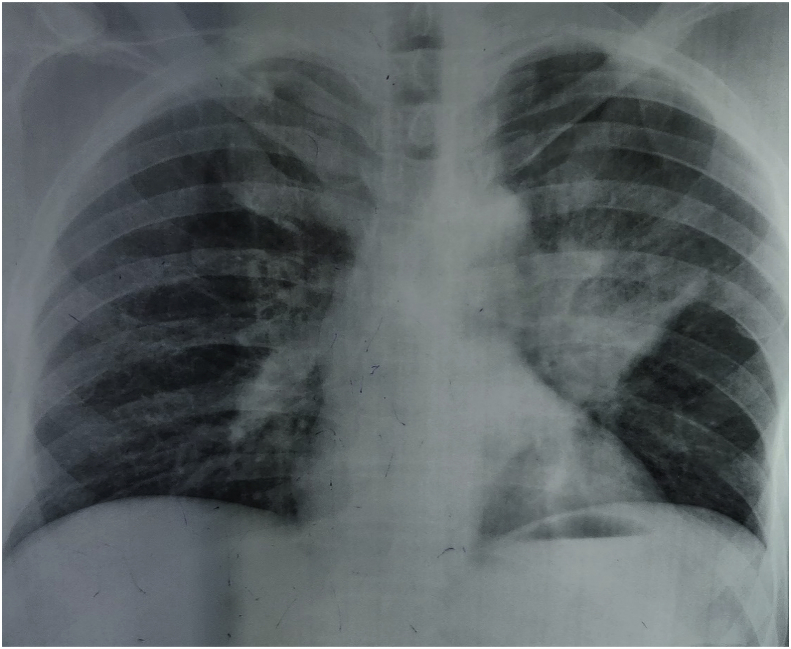
Fig. 1.
Chest radiograph: Showing bilateral middle zone (left > right) perihilar opacities with possibility of consolidation or left perihilar mass along with prominent bronchovascular marking.
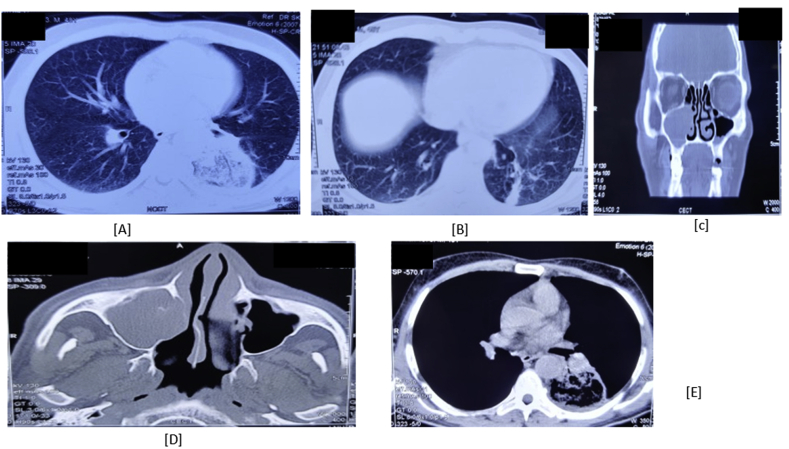
Fig. 2.
[A], [B] and [E] CECT Chest showing a well-defined area in the superior and anteromedial segments of left lower lobe comprising of peripheral consolidation, central ground glass opacities and areas of cavitation suggestive of pulmonary mucormycosis along with bilateral ground glass opacities in peripheral and basal segments of bilateral lower lobes. [C] and [D]: CECT paranasal sinuses with orbit shows soft tissue thickening of right and left maxillary sinuses, and destruction of right maxillary sinus medial wall along with blocked right osteomeatal complex without orbital involvement suggestive of Sino-Nasal mucormycosis.
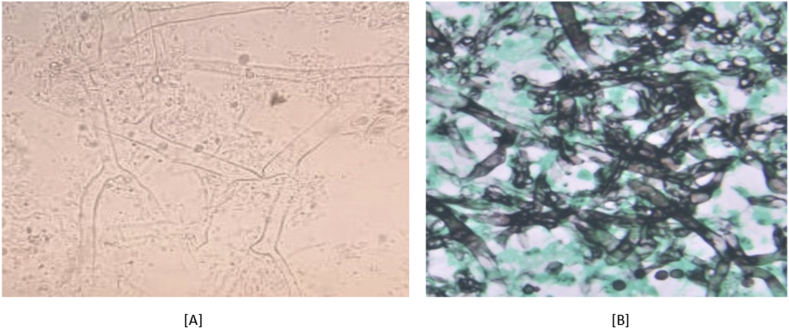
Fig. 3.
[A]: Fungal KOH showing aseptate broad fungal hyphae consistent with mucormycosis. [B] Showing magnified view of silver methenamine stain in which 1–6 fungal hyphae seen.
3. Discussion
Mucormycosis is a rare but angio-invasive disease caused by a group of fungi called Mucoromycetes. This ubiquitous mold is found anywhere from the soil, plant surfaces, decaying vegetables to fallen leaves, compost, and animal manure [6]. Individuals who have decreased immunity are specifically at high risk of being affected by this life-threatening opportunistic infection. The susceptible groups are post-COVID recovered, immunosuppressed, those on long-term corticosteroids, chemotherapy, and iron chelation therapy. Individuals with uncontrolled hyperglycemia irrespective of the diagnosis of diabetes mellitus, unchecked iron overload states, transplant, malignancy, burns, neutropenia, monocytopenia, tuberculosis, HIV, and chronic kidney disease are highly vulnerable [7]. Our patient had risk factors like a history of COVID-19 infection 20 days before presenting to our hospital, uncontrolled blood sugar levels, and diabetic ketoacidosis. Although our Case had been COVID-19 positive 20 days before, he didn't require hospitalization, steroids, or oxygen therapy. COVID-19 associated mucormycosis (CAM) refers to individuals infected with mucormycosis while on treatment or after recovering from COVID-19 [8]. The most common form of this condition is rhino-orbital-cerebral mucormycosis (ROCM), which includes Sino-nasal (involvement of nose and paranasal sinuses), limited rhino-orbital disease, and rhino-orbital-cerebral disease (extension to CNS) [9]. ROCM is followed by cutaneous, pulmonary, gastrointestinal, and disseminated disease [7,10]. Pulmonary mucormycosis accounts for 10% of the cases [8]. COVID-19 can worsen or even precipitate diabetes mellitus by causing glycemic abnormalities that can persist even up to two months after recovery [11]. The mechanisms altering the glucose metabolic control in these patients include infection followed by proliferation in the islet cells causing direct pancreatic injury and the storm of cytokines (predominantly IL-6) released, causing insulin resistance. Superimposed treatment with agents such as corticosteroids and remdesivir further contributes to hyperglycemia [8]. Severe COVID-19 and diabetic ketoacidosis are implicated in causing elevated ferritin and serum iron levels causing free radical damage [12,13]. Acidosis has also been involved in impairing phagocyte function [8]. Although our case never had a severe COVID-19 infection and was not prescribed drugs like corticosteroids or remdesivir, his blood glucose levels started to worsen after he got infected with SARS-COV2 twenty days back, as he presented with uncontrolled hyperglycemia and diabetic ketoacidosis. Post COVID-19 immunosuppression has also been mentioned in the literature, which again is a risk factor in the pathogenesis of mucormycosis [7]. Hence, these interactions explain pulmonary mucormycosis, which further spread to cause sino-nasal involvement, as evident by the CECT chest and CECT paranasal sinuses (demonstrating orbital involvement). A bronchoscopic biopsy later confirmed the diagnosis.
Interestingly, various studies have explored the association between COVID-19 and tuberculosis; most of them have attributed the findings to either post TB development of COVID or a co-infection [14,15]. A Case report highlights the development of tuberculosis post recovery from COVID-19 in a young patient without any risk factors [16]. Moreover, in a case report by “Gautam S and colleagues,” tubercular empyema thoracic presented acutely with COVID-19 co-infection in a diabetic Asian male patient [17]. Superimposed to pulmonary mucormycosis, our patient also developed pulmonary tuberculosis as detected on sputum for AFB and without rifampicin resistance in CBNAAT. As a result, anti-tubercular therapy (ATT) was started. However, our patient later developed complications like hepatitis, as evident from deranged LFTs after ATT initiation; hence hepatic modification of doses was done. Intensified case-finding initiatives to detect tuberculosis in post-COVID patients taken by the Government of Kerala is a step forward in the fight against eradicating tuberculosis [18]. Our goal is to highlight the need for post-COVID diabetic patients to be screened timely for detection of tuberculosis and invasive fungal infections such as mucormycosis and diagnosed with a high index of suspicion at the earliest.
4. Conclusion
With our very first case report on pulmonary mucormycosis along with pulmonary tuberculosis in a post COVID-19 infected patient, we would like to urge clinicians to focus more on these uncontrolled diabetic post COVID-19 infected patients, even without classical risk factors like severe COVID-19 infection requiring hospitalization, steroid intake or intake of any other immunomodulator drugs like remdesivir or tocilizumab, who are high risk of deveoping mucormycosis and tuberculosis. Hence, strict monitoring of blood sugar in diabetic patients is of utmost improtance especially in COVID-19 infected and post covid-19 patients to combat this yet another growing epidemic of mucormycosis especially in Indian subcontinent.
Funding
No organized funding source was used in study conduction.
Declaration of competing interest
The all authors declare that they have no conflict of interest.
Acknowledgements
None.
References
- 1.WHO, Weekly operational update on COVID-19 - 23 August 2021, Issue No. 69; Emergency Situational Updates, Accessed August 24, 2021.
- 2.WHO, Emergencies/home/coronavirus disease(COVID-19)/mucormycosis, Accessed August 24, 2021.
- 3.John T.M., Jacob C.N., Kontoyiannis D.P. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel) 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alagna R., Besozzi G., Codecasa L.R. Celebrating world tuberculosis day at the time of COVID-19. Eur. Respir. J. 2020;55(4) doi: 10.1183/13993003.00650-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tassi Mousquer Gabriel, Peres Alessandra, Fiegenbaum Marilu. Pathology of TB/COVID-19 Co-Infection: the phantom menace. Tuberculosis. 2021 Jan;126 doi: 10.1016/j.tube.2020.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley Hagen, American Society of Microbiology COVID-19-Associated mucormycosis: triple threat of the pandemic. July 15, 2021. https://asm.org/Articles/2021/July/COVID-19-Associated-Mucormycosis-Triple-Threat-of
- 7.National Centre for Disease Control . June, 2021. Directorate general of health services, government of India; CD alert; pp. 2230179–2232021.https://www.ncdc.gov.in/WriteReadData/l892s/22911839231625743853.pdf O/O IDSP-NCDC. [Google Scholar]
- 8.Pal R., Singh B., Bhadada S.K. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses. 2021 doi: 10.1111/myc.13338. 10.1111/myc.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson K.L., Wang M., Canalis R.F., Abemayor E. Rhinocerebral mucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107(7):855–862. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montefusco L., Ben Nasr M., D'Addio F. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim A.S., Spellberg B., Edwards J., Jr. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr. Opin. Infect. Dis. 2008;21(6):620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perricone C, Bartoloni E, Bursi R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. 2020;68(4):213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tadolini M., Codecasa L.R., García-García J.M. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur. Respir. J. July 9, 2020;56(1) doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta N., Ish P., Gupta A., Malhotra N. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Eur. Respir. J. Nov 2020;56(5) doi: 10.1183/13993003.03408-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahid A., Iqbal N., Moeen S., Irfan M. Post COVID-19 tuberculosis: an emerging threat of pandemic. Monaldi Arch. Chest Dis. 2021 doi: 10.4081/monaldi.2021.1749. 10.4081/monaldi.2021.1749,31],3,2021. [DOI] [PubMed] [Google Scholar]
- 17.Gautam S., Pandit S., Bhadoria Tubercular empyema thoracic: an acute presentation with COVID-19 co-infection. J. Clin. Diagn. Res. 2021;15(6):6–9. Article in English | EMBASE | ID: covidwho-1302770. [Google Scholar]
- 18.Government of Kerala, Health and Family Welfare Department . June 14, 2021. COVID-19 outbreak control and prevention state cell, intensified case finding for TB among post COVID patients. No 34/31/F2/2020/Health. Accessed August 24, 2021. [Google Scholar]