Abstract
Extended-spectrum β-lactamases producing Escherichia coli (ESBL-EC) lend resistance to most β-lactam antibiotics. Because of limited treatment options, ESBL-EC infections are generally more difficult to treat, leading to higher hospital costs, reduced rates of microbiological and clinical responses, and a threat to the patient’s life. This study aimed to determine the antibiotic resistance pattern of ESBL-EC isolated from patients with urinary tract infection in Morocco. This retrospective laboratory-based study was conducted at Cheikh Khalifa International University Hospital, Casablanca, from January 2016 to June 2019. A total of 670 urine samples were collected from urinary tract infection patients and processed by standard microbiological methods. In vitro susceptibility testing to different antibiotics of all identified isolates of Escherichia coli (E. coli) was performed following Kirby–Bauer’s disc diffusion method on Mueller–Hinton Agar according to the EUCAST standards. The reviewing of ESBL-EC was confirmed by the appearance of a characteristically shaped zone referred to as a “champagne cork” using the Combined Disk Test. Among a total of 438 E. coli isolated from nonrepetitive urine samples, two hundred fifty-nine (59%) were ESBL-EC, of which 200 (77%) were isolated from adult patients (over the age of 50) and the majority were female. All ESBL-EC isolates were resistant to third-generation cephalosporin and quinolones and sensitive to carbapenem and fosfomycin. Knowledge of antimicrobial resistance patterns in ESBL-EC, the major pathogen associated with urinary tract infection, is indispensable as a guide in choosing empirical antimicrobial treatment.
Keywords: antibiotic resistance, ESBL, Escherichia coli, urinary tract infection, multidrug resistant
Introduction
Escherichia coli as an Enterobacteriaceae member is the most common causative bacteria associated with urinary tract infections up to 80% (Farzana et al., 2013; Shakya et al., 2017). β-Lactam antibiotics are the safest and most frequently prescribed antimicrobial agents for urinary tract infection (UTI) (Magale et al., 2016). They are a class of broad-spectrum antibiotics that contain a beta-lactam ring in their molecular structure (Ahmed et al., 2015; Rajabnia et al., 2019). Several studies prove the increased resistance of E. coli in UTI to beta-lactam antibiotics (Glasner et al., 2013; Tacconelli et al., 2018). Another worry is the “emergence of extended spectrum Beta-Lactamase (ESBL) producing bacteria” that hydrolyze beta-lactam ring, inactivate the antibiotic, and reduce consequently the number of treatment options (Ruano-de-pablo & Losada-pinedo, 2016; Rajabnia et al., 2019).
The first bacteria expressing acquired ESBL were identified in 1983 in Germany, and since 2000, ESBL-producing Escherichia coli (ESBL-EC) have spread throughout the world in both community and hospital settings (Kruglova & Stasheva, 2010; Rossignol et al., 2017; Rajabnia et al., 2019). Worryingly, the incidence of ESBL-EC continues to increase worldwide (Khanfar et al., 2009; Shakya et al., 2017). This situation leads to longer hospital stays, a dramatic increase in the consumption of antibiotics and consequently higher hospital expenses, and a trend toward higher mortality (Degnan et al., 2015; Cheng et al., 2016; Fernando et al., 2017). The beta-lactam antibiotics, in particular carbapenems, have been widely regarded as the treatment of choice for ESBL-EC infections (Nepal et al., 2017). However, the global emergence of beta-lactam antibiotic resistance strains of enterobacteriacae threatens the efficacy of this family of antibiotics (Stapleton et al., 2017; van Driel et al., 2019). Furthermore, frequent coresistance to sulfonamides and fluoroquinolones limits the availability of other therapeutic track (Cheng et al., 2016; Senard et al., 2020).
In Morocco, the ESBL-EC and its antibiotic susceptibility patterns have been very little studied. This kind of study would facilitate the understanding by clinicians of the therapeutic failure of the conventional antibiotic therapies used in serious infections caused by ESBL-EC and would guide them to adopt an appropriate empirical antibiotic therapy. In this perspective, we conducted a laboratory-based study in order to identify the ESBL-EC isolated from a patient with urinary tract infection and its phenotypic profiles of antibiotic susceptibility. The evolution of the ESBL-EC and some risk factors were also explored.
Methods
Study Design and Data Collection
This is a retrospective laboratory-based study of all isolates of ESBL-EC isolated from urine samples at the National Reference Laboratory (LNR) of Cheikh Khalifa International University Hospital (HCK) from January 2016 to June 2019. Patient data were retrieved from the bacteriology service information system of the LNR.
Patients with clinically suspected UTI were included in the study. We defined a significant monomicrobic bacteriuria as the culture of a single bacterial species from the urine sample at a concentration of >105 CFU/ml (urine bag). Patients with multimicrobial urine samples or isolates collected >72 h after hospitalization were excluded. We also excluded patients who were on antibiotics, patients who had hospital admission within the last 3 months, or patients on urinary catheters because we wanted to exclude recurrences due to partially treated urinary tract infections and healthcare-associated infections.
A total of 670 urine samples were included in this study; only the first isolate of E. coli from each patient was considered to avoid overestimation of resistance rates from different urine specimens. Each isolate was classified as being of community or hospital origin. Community samples included those originating from outpatient rooms of the hospital or of external general practitioners in the surrounding area. Hospital samples were provided both from the emergency department and hospital departments.
The collected samples according to current European recommendations were labeled properly and were immediately delivered to the LNR for further processing. When immediate delivery was not possible, the specimens were refrigerated at 4°C to 6°C (Cornaglia et al., 2012).
Laboratory Examinations of Samples
All urine samples were cultured on routine culture media by semiquantitative method as described in the World Health Organization (WHO) manual (Vandepitte et al., 2003). In short, 1 μl of urine (by using a calibrated wire loop) was inoculated on CLED (Oxoid Company, Britain) and blood agar plate (BioMèrieux, Lyon, France) by streaking and incubated aerobically for 24 h at 37°C. Isolates were isolated and identified depending on their morphology in Gram’s staining, cultural characteristics, and biochemical properties, as per the European Manual of Clinical Microbiology (Cornaglia et al., 2012).
Antimicrobial Susceptibility Testing
In vitro susceptibility testing to different antibiotics of all identified isolates of E. coli was performed following Kirby–Bauer’s disc diffusion method on Mueller–Hinton Agar (Hi-Media, India) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria version 6.0 (EUCAST, 2016). Antibiotics tested in our study include the following: amoxicillin (AMX; 10 μg), amoxicillin clavulanate (AMC; 20/10 μg), cefoxitin (FOX; 30 μg), piperacillin/tazobactam (TZP; 30/6 μg), ticarcillin (TIC; 85 μg), fosfomycin (FOS; 200 μg), gentamycin (GEN; 10 μg), tobramycin (TOB; 10 μg), amikacin (AN; 30 μg), norfloxacin (NX; 10 μg), cefalexin (CN; 30 μg), ceftriaxone (CTR; 30 μg), ceftazidime (CAZ; 30 μg), cefixime (CFM; 10 μg), cefotaxime (CTX; 30 μg), imipenem (IPM; 10 μg), ertapenem (ETP; 10 μg), co-trimoxazole (SXT; 1.25/23.75 μg) (Hi-Media, India), and colistin (CT; 10 μg) (Oxoid™, UK). The clinical interpretation (S/I/R) of results took into consideration diameters or breakpoints recommended by the EUCAST (2016). An isolate was considered as multidrug-resistant if it was resistant to ≥3 groups of antibiotics.
Screening and Confirmation of ESBL Producers
ESBL production was determined by the double-disk diffusion method, following the criteria established by the CA-SFM/EUCAST (EUCAST, 2016; Dubreuil et al., 2018). The presence of ESBL was concluded when the inhibition zone around cefotaxime (30 μg), ceftazidime (30 μg), cefepime (10 μg), or aztreonam (30 μg) antibiotic disks was enhanced on the side of the clavulanate-containing disk, resulting in a characteristically shaped zone referred to as a “champagne cork” (Garrec et al., 2011; van Driel et al., 2019; Senard et al., 2020). This method was carried out on Mueller Hinton Agar (Hi-Media, India), which was inoculated with the test strain and then incubated in ambient air for 16–18 h of incubation at 35 ± 2°C.
Quality Control
Each batch of media and reagents underwent sterility and performance testing. During antibiotic susceptibility test, quality control was performed using the control strains of E. coli ATCC 25922 (Hansen et al., 2014; Nellums et al., 2018).
Statistical Analysis
Statistical data analysis was performed using the chi-square test for testing relationships between categorical variables. Data were seized and analyzed by using IBM SPSS Statistics for Macintosh, Version 23.0 (IBM Corp, Armonk, NY, USA) and presented in percentage base distribution. Descriptive statistical analysis (including means and percentages to characterize data) was performed using Microsoft Excel, Version 16.16.21 (Redmond, WA, USA, Microsoft Corp.). All P values were two-sided, and P < 0.05 was considered statistically significant.
Results
During the study period, 670 urine samples meeting the inclusion criteria were collected from urinary tract infections from patients in different departments of the hospital (HCK). A total of 438 (65%) E. coli isolates were isolated from nonrepetitive urinary tract infections (UTIs) of which 259 (59%) were ESBL-EC. The prevalence study of ESBL-EC isolates from different clinical specimens was in favor of predominance in urine up to 74% (n=259) compared to other clinical samples (Figure 1A). Monitoring of these urinary tract infections due to ESBL-EC showed an upward trend, expressed more in women than in men, with an increase between 2016 and the end of 2019 from 2% to 44% in women and 8% to 34% in men, thus reaching a total increase from 4% to 40% (Figure 1B). In other words, out of 259 samples of ESBL-EC, 149 (58%) samples were of female patients and 110 (42%) were of male patients (Table 1). Approximately one-third of the patients had at least one comorbid disease.
Figure 1.
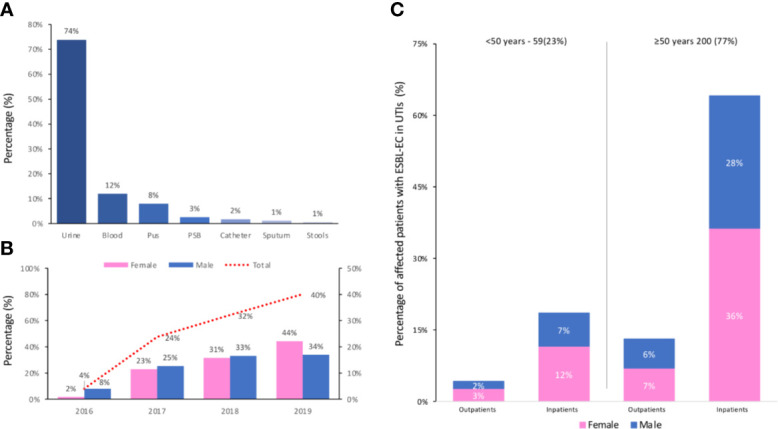
(A) Prevalence of ESBL-EC from different clinical specimens; Protected specimen brush (PSB). (B) Evolution of urinary tract infections due to ESBL-EC (Male, Female and Total) per year (from 2016 to 2019). (C) Age distribution of the affected patients with ESBL-EC (Male/Female) in the outpatient and inpatient groups.
Table 1.
Analysis of some risk factors in urinary E. coli isolates from 2016 to 2019.
| Total | Non-ESBL E. coli | ESBL producing E. coli | P* | |
|---|---|---|---|---|
| N = 438 | N = 179 | N = 259 | ||
| Sex | ||||
| Male | 162 (37%) | 52 (29%) | 110 (42%) | .004 |
| Female | 276 (63%) | 127 (71%) | 149 (58%) | |
| Age | ||||
| <50 | 123 (28%) | 64 (35%) | 59 (22%) | .003 |
| 0–10 | 32 (6%) | 18 (10%) | 14 (6%) | |
| 11–20 | 7(2%) | 7 (4%) | 0 (0%) | |
| 21–30 | 17(4%) | 17 (9%) | 0 (0%) | |
| 31–40 | 34 (8%) | 13 (7%) | 21 (8%) | |
| 41–50 | 33 (8%) | 9 (5%) | 24 (9%) | |
| >50 | 315 (72%) | 115 (65%) | 200 (77%) | |
| Origin | ||||
| Inpatients | 356 (81%) | 142 (79%) | 214 (83%) | .385 |
| Outpatients | 82 (19%) | 37 (21%) | 45 (17%) |
P*: “p value” obtained by the chi-square test provides to determine the existence of link between ESBL-EC and various risk factors.
The results of univariate analysis showed that patients’ gender and age (patients over the age of 50) were significantly associated with UTI due to ESBL-producing isolates (Table 1). Female gender was associated with increased risk of being infected by ESBL-EC compared to male (p = 0.004). In addition, patients over the age of 50 were found to have a higher risk of being infected by ESBL-EC compared to adults (p = 0.001).
Furthermore, the calculated p-value (p = 0.385) as displayed in Table 1 shows that the risk of contracting ESBL-EC is similar between the two groups studied (inpatients/outpatients). Among the affected patients with ESBL-EC, the age groups over 50 years of age (outpatients: 17%; inpatients: 83%) were the most numerous. In this age group, the female-to-male ratio was 1.25 (53% female) in the outpatient group, while in the inpatient group, this value was 1.38 (57% female). The age distribution of the affected patients with ESBL-EC (male/female) in the outpatient and inpatient groups is presented in Figure 1C.
The mean prevalence of UTI due to BLSE-EC in hospitalized patients during the study period (2016 to early 2019) was 66 (66%), and no significant difference per year was observed (data not shown).
The resistances observed in ESBL-EC sample are detailed in Table 2.
Table 2.
Resistances among ESBL-producing E. coli isolates.
| Antibiotic | Resistance | Susceptibility | Intermediate |
|---|---|---|---|
| Beta-lactam family | |||
| Amoxicillin (AMX) | 252 (100%) | 1 (0%) | 0 |
| Amoxicillin/clavulanic acid (AMC) | 171 (67%) | 83 (33%) | 0 |
| Piperacillin/tazobactam (TZP) | 97 (40%) | 115 (47%) | 32 (13%) |
| Ticarcillin (TIC) | 176 (98%) | 4 (2%) | 0 |
| Cefoxitin (FOX) | 25 (10%) | 212 (88%) | 3 (2%) |
| Ceftazidime (CAZ) | 225 (90%) | 18 (7%) | 8 (3%) |
| Cefotaxime (CTX) | 208 (91%) | 8 (3%) | 13 (6%) |
| Cefixime (CFM) | 231 (96%) | 7 (3%) | 2 (1%) |
| Imipenem (IPM) | 7 (3%) | 218 (96%) | 2 (1%) |
| Ertapenem (ETP) | 2 (1%) | 199 (98%) | 2 (1%) |
| Others antibiotic family | |||
| Gentamicin (GM) | 102 (41%) | 140 (56%) | 7 (3%) |
| Tobramycin (TM) | 183 (73%) | 57 (23%) | 10 (4%) |
| Amikacin (AN) | 46 (19%) | 162 (66%) | 37 (15%) |
| Norfloxacin (NX) | 231 (93%) | 14 (6%) | 4 (1%) |
| Trimethoprim/sulfamethoxazole (SXT) | 184 (80%) | 45 (20%) | 0 |
| Fosfomycin (FOS) | 5 (2%) | 235 (98%) | 0 |
| Colistin (CT/CL) | 1 (0%) | 223 (100%) | 0 |
Analysis of the β-lactam family shows that all strains of E. coli producing ESBLs are resistant to amoxicillin (AMX), 98% of strains are resistant to ticarcillin (TIC), and from 90% to 96% of strains are resistant to third-generation cephalosporins. Penicillin protected by beta-lactamase inhibitors such as piperacillin/tazobactam (TZP) and amoxicillin/clavulanic acid is no longer effective against ESBL-EC with resistance ranging from 40% to 67%. However, the analysis shows that the carbapenems are very effective against ESBL-EC with a sensitivity ranging from 96% for imipenem to 98% for ertapenem.
Table 2 shows also that ESBL-EC resistance affects other families of antibiotics with 93% resistance to quinolones (NX), 80% to sulfonamides (SXT), and 73% to tobramycin (TM). At the opposite, all ESBL-EC strains are sensitive to fosfomycin.
Moreover, all ESBL-EC were resistant either to at least two antibiotics belonging to two different families or to the protected penicillins (Table 3). This same table also shows that in the category of multidrug resistant, 47% of ESBL-EC are resistant to penicillin, protected penicillin, third-generation cephalosporins, and quinolones. It also shows that the total amount of multidrug-resistant isolates among ESBL-CE was 1% with resistance to cephalosporins (second and third generation), amoxicillin, combinations of antibiotics penicillin/beta-lactamase inhibitor, norfloxacin, tobramycin, gentamicin, and sulfamethazole/trimethoprim.
Table 3.
Multidrug resistant profile of ESBL-EC isolates.
| Resistance profile | No. of isolates with resistance profile [N (%)] | Resistance category |
|---|---|---|
| AMC, AMX | 150 (58%) | Drug resistant |
| CAZ, NX | 167 (65%) | Drug resistant |
| AMC, NX, AMX | 140 (54%) | Drug resistant |
| AMC, CAZ, NX, AMX | 121 (47%) | Multidrug resistant |
| AMC, CAZ, TM, NX, AMX | 101 (39%) | Multidrug resistant |
| AMC, CAZ, TM, GM, NX, AMX | 58 (23%) | Multidrug resistant |
| AMC, CAZ, TM, GM, SXT, NX, AMX | 48 (19%) | Multidrug resistant |
| AMC, FOX, CAZ, TM, GM, SXT, NX, AMX | 19 (7%) | Multidrug resistant |
| AMC, FOX, CAZ, TM, GM, AN, SXT, NX, AMX | 3 (1%) | Multidrug resistant |
AMX, amoxicillin; AMC, amoxicillin/clavulanic acid; FOX, cefoxitin; NX, norfloxacin; CAZ, ceftazidime; GM, gentamicin; TM, tobramycin; AN, amikacin; SXT, trimethoprim/sulfamethoxazole.
Discussion
The emergence and rapid dissemination of multidrug-resistant Enterobacteriaceae worries the whole world and, in particular, ESBL-producing enterobacteriaceae. Since the 2000s, ESBL-EC have been considered as serious pathogens both in nosocomial and community infections around the world, and their virulence varies by region (Shakya et al., 2017). Knowledge of the local epidemiology and its evolution over time is a key step in choosing the effective first-line antibiotic therapy adapted to each region. However, in our country, we are aware of the heavy burden we share due to the multitude of factors integrated in the characteristics of practice of medicine, policy, and the health system (Pokharel et al., 2019). Moreover, ESBL-EC pathogens are more common in females and in hospital settings (Figure 1C) and are for more than half of nosocomial origin, which is often very complicated to treat (Iqbal, 2019). ESBL-EC pose a major threat in the management of uropathogens and threaten the future activity of last-line molecules (Sbiti et al., 2017). In our study, more than two-thirds of the isolates (63%) were ESBL-EC. A high prevalence of ESBL-EC strains have been raised quite consistently from previous studies carried out in Morocco (Lahlou Amine et al., 2009; Romli et al., 2011; El Bouamri et al., 2014a; El bouamri et al., 2014b; Sbiti et al., 2017) and outside (Gravey et al., 2017; Critchley et al., 2019; Koguchi et al., 2020; Tan et al., 2020; van Hout et al., 2020).
In other words, 409 (61%) Enterobacteriaceae isolated from urine culture were ESBL-producing. Out of 438 isolates of E. coli, 259 (59%) are ESBL-EC and 179 (41%) are not ESBL-EC. A few other studies mentioned the prevalence of ESBL-EC isolates 37.11% (Rajabnia et al., 2019), 46.87% (Kulkarni et al., 2016), and 82.6% (Singh et al., 2016) respectively. These variations in the rate of ESBL-EC among UTI cases might be attributable to the local antibiotic prescribing policy, abuse of broad-spectrum antibiotics especially penicillin and third-generation cephalosporins, geographical difference, and hospitalization (Nepal et al., 2017; Parajuli et al., 2017). Furthermore, we did not observe a significant evolution in the rate of isolated ESBL-EC. Their incidence rate in urinary tract infection was 75%. However, an increase in the number of ESBL-EC is well described in different regions of the world (Fernando et al., 2017; Rajabnia et al., 2019; Rohde et al., 2020).
As seen in previous studies, sex, age, and hospitalization are proposed as risk factors (Lahlou Amine et al., 2009; Ahmed et al., 2015; Flammang et al., 2017). In the present study, among the positive E. coli cases, the prevalence rate of UTIs was found more in females, 276 (63%), than males, 172 (37%). As for positive ESBL-EC cases, the prevalence rate was 149 (58%) in females and 110 (42%) in males. Females are more frequently affected due to the anatomy of their genitals. In other words, the short length of the urethra and its closeness to the anus favor the proliferation of ESBL-EC (Tille, 2018). The patient’s sex is a risk factor of UTI (p= 0.004), which should be taken into account. In addition, the highest ESBL isolates were found in the age group over 50 years, including the prevalence of ESBL-EC organisms, which was above 77% (Table 1). The risk of ESBL-EC infection was significantly higher in the age group over 50 years than lower age groups (p < 0.003). Many studies confirm that advanced age (usually over 65 years) is a UTI risk factor for ESBL-EC (Heytens et al., 2017; Sbiti et al., 2017; van Driel et al., 2019). The reason for this may be due to the immunological status of these patients being more vulnerable to infections. Those under 50 years, including children, are not spared from this risk (Ruano-de-pablo & Losada-pinedo, 2016; Heytens et al., 2017). Likewise, our results on the prevalence of ESBL-EC in both the hospital (83%) and community (17%) settings are probably underestimated and drive us to suggest the need of systemic surveillance. The healthy carriers of ESBL-EC may contribute to the emergence of these bacteria in communities, which better explains the origin of the easy spread of resistances in a country, and the need of an appropriate national healthcare program.
According to the result of the antibiogram, the highest antibiotic resistance among ESBL-EC was observed with amoxicillin (100%), ticarcillin (98%), third-generation cephalosporin [cefixime (96%), cefotaxime (91%), and ceftazidime (90%)], and norfloxacin (93%). This study showed that ESBL-EC were resistant to third-generation cephalosporin and quinolones and sensitive to aminoglycoside and carbapenem. The highest antibiotic sensitivity was observed with colistin (100%), fosfomycin (98%), carbapenems [ertapenem (98%), imipenem (96%)], and cefoxitin (88%). Our findings are similar to some Moroccan studies (Lahlou Amine et al., 2009; El Bouamri et al., 2014a; El bouamri et al., 2014b; Sbiti et al., 2017) and others (Kulkarni et al., 2016; Ruano-de-pablo & Losada-pinedo, 2016; Tan et al., 2020) (Table 2).
We noted that many penicillins (combined with beta lactamase inhibitor) such as amoxicillin/clavulanic acid (67% resistance) or piperacillin/tazobactam (40%) used in practice as alternative antimicrobials were shown to be less effective against ESBL-EC. According to the standard guidelines, to use an antibiotic as an empirical antibiotic, it must have been tested >90% sensitive on the causative E. coli of that community (Bonnet et al., 2017). According to that criterion, only fosfomycin can be used as oral empirical antibiotics in our community. However, fosfomycin is currently unavailable in Morocco (Sbiti et al., 2017).
Moreover, several studies have concluded that the clinical picture of UTI cannot exactly predict whether it is ESBL UTI or not (Priyadharshana et al., 2019). The high resistance of ESBL-EC to many families of antibiotics reduced considerably the therapeutic options and maintains a continuous increase in the prescription of carbapenems (Lahlou Amine et al., 2009). Ertapenem, imipenem, doripenem, and meropenem are still the first choice of treatment for serious infections with ESBL-EC. It has been reported that >98% of the ESBL-EC, K. pneumonia, and P. mirabilis are still susceptible to these drugs (Fernando et al., 2017). But with the emergence of the carbapenem-resistant Enterobacteriaceae, some older drugs were found effective against ESBL-EC infections. Colistin and some aminoglycosides are considered effective in the treatment of carbapenem-resistant organisms (Fernando et al., 2017; Sbiti et al., 2017; Gajdács et al., 2019).
Senard et al. (2020), in a multicenter retrospective cohort study with propensity score analysis, showed that clinically and in terms of microbiological success, the efficacy of treatment with cefoxitin and carbapenems was similar in febrile UTIs due to ESBL-EC. However, cefoxitin is another antimicrobial drug missing from the Moroccan market.
Conclusions
Today, the use of prophylactic antibiotics in UTIs due to ESBL-EC is no longer recommended because it favors emergence and spread of antibiotic-resistant strains (World Health Organization, 2018). In addition, inappropriate use of antibiotics has been shown to play a major role in the emergence of multidrug-resistant (MDR) bacteria (World Health Organization, 2018). This is the reason why clinicians should ensure the prescription and use of appropriate antibiotics during the recommended times and at controlled doses in order to prevent emergence of MDR such as ESBL-EC (Table 3) (Barguigua et al., 2019).
Some limitations need to be considered when interpreting the results of our study. First, the study was retrospective in nature and was primarily based on data from urinary isolates infected with E. coli previously collected at Cheikh Khalifa Hospital only, and with a maximum of one isolate per patient. Second, in spite of the lack of the data on ciprofloxacin resistance due to accessibility issues for computer databases, we believe that our results follow the same trend reported by previous Moroccan studies (Lahlou Amine et al., 2009; Romli et al., 2011; El Bouamri et al., 2014a; Sbiti et al., 2017). Third, the molecular evaluation of antibiotic resistance in isolates has not been studied, though it is possible that isolates carry resistance genes but do not express them phenotypically. And therefore, they can transmit this resistance to other bacteria. Finally, all of our isolates tested were ESBL-EC, results that are not necessarily applicable to other Enterobacteriaceae. Nevertheless, our results are very interesting and clinically relevant as E. coli is the most commonly encountered ESBL from community-acquired infections. More in-depth surveys with larger sampling and collaborations with other hospitals would generate more attractive ideas.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
MK conceived and designed the study. RM, RB, and IS performed the experiments and analysis. FL, ID, and YA contributed with data and analysis. MK wrote the manuscript, with contributions and comments from all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the material and financial contribution of Mohammed VI University of Health Sciences (UM6SS) and the contributions made by Mr. Hamza Benhamid and Mrs. Salma Kettani Halabi from the National Institute for Statistics and Applied Economics (INSEA) during the statistical review of the manuscript.
References
- Ahmed I., Sajed M., Sultan A., Murtaza I., Yousaf S., Maqsood B., et al. (2015). Original Article: The Erratic Antibiotic Susceptibility Patterns of Bacterial Pathogens Causing. EXCLI J. 14, 916–925. 10.17179/excli2015-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barguigua A., Rguibi Idrissi H., Nayme K., Timinouni M. (2019). Virulence and Antibiotic Resistance Patterns in E. Coli, Morocco. EcoHealth 16 (3), 570–575. 10.1007/s10393-019-01404-8 [DOI] [PubMed] [Google Scholar]
- Bonnet R., Bru J.-P., Caron F., Cattoen C., Dubreuil L., Jarlier V., et al. (2017). Recommandations 2017 Du Comité De L’antibiogramme De La Société Française De Microbiologie. Société Française Microbiologie 1 (0), 125. [Google Scholar]
- Cheng M. F., Chen W. L., Huang I. F., Chen J. R., Chiou Y. H., Chen Y. S., et al. (2016). Urinary Tract Infection in Infants Caused by Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli: Comparison Between Urban and Rural Hospitals. Pediatr. Nephrol. 31 (8), 1305–1312. 10.1007/s00467-016-3338-0 [DOI] [PubMed] [Google Scholar]
- Critchley I. A., Cotroneo N., Pucci M. J., Mendes R. (2019). The Burden of Antimicrobial Resistance Among Urinary Tract Isolates of Escherichia Coli in the United States in 2017. PloS One 14 (12), 1–11. 10.1371/journal.pone.0220265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornaglia G., Courco R., Herrmann J.-L., Kahlmeter G. (2012). European Manual of Clinical Microbiology. [Google Scholar]
- Degnan L. A., Milstone A. M., Diener-West M., Lee C. K. K. (2015). Extended-Spectrum Beta-Lactamase Bacteria From Urine Isolates in Children. J. Pediatr. Pharmacol. Ther. 20 (5), 373–377. 10.5863/1551-6776-20.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil L., Goldstein F., Jarlier V., Morel C., Philippon A., Rouveix B., et al. (2018). Comité De L’antibiogramme De La Société Française De Microbiologie; CA-SFM. Société Française Microbiologie 86 (mm), 133–142. 10.1016/S0924-8579(03)00021-9 [DOI] [Google Scholar]
- El Bouamri M. C., Arsalane L., Kamouni Y., Berraha M., Zouhair S. (2014. a). Évolution Récente Du Profil Epidémiologique Des Entérobactéries Uropathogènes Productrices De β-Lactamases a Spectre Elargi a Marrakech, Maroc. Progres En Urologie 24 (7), 451–455. 10.1016/j.purol.2013.11.010 [DOI] [PubMed] [Google Scholar]
- El bouamri M. C., Arsalane L., Kamouni Y., Yahyaoui H., Bennouar N., Berraha M., et al. (2014. b). Profil Actuel De Résistance Aux Antibiotiques Des Souches D’Escherichia Coli Uropathogènes Et Conséquences Thérapeutiques. Progres En Urologie 24 (16), 1058–1062. 10.1016/j.purol.2014.09.035 [DOI] [PubMed] [Google Scholar]
- EUCAST . (2016). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf.
- Farzana R., Shamsuzzaman S. M., Mamun K. Z., Shears P. (2013). Antimicrobial Susceptibility Pattern of Extended Spectrum β-Lactamase Producing Gram-Negative Bacteria Isolated From Wound and Urine in a Tertiary Care Hospital, Dhaka City, Bangladesh. Southeast Asian J. Trop. Med. Public Health 44 (1), 96–103. [PubMed] [Google Scholar]
- Fernando M. M. P. S. C., Luke W. A. N. V., Miththinda J. K. N. D., Wickramasinghe R. D. S. S., Sebastiampillai B. S., Gunathilake M. P. M. L., et al. (2017). Extended Spectrum Beta Lactamase Producing Organisms Causing Urinary Tract Infections in Sri Lanka and Their Antibiotic Susceptibility Pattern -A Hospital Based Cross Sectional Study. BMC Infect. Dis. 17 (1), 1–6. 10.1186/s12879-017-2250-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammang A., Morello R., Vergnaud M., Brouard J., Eckart P. (2017). Étude Du Profil Des Résistances Bactériennes Dans Les Pyélonéphrites De L’enfant En 2014. Arch. Pediatrie 24 (3), 215–224. 10.1016/j.arcped.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Gajdács M., Ábrók M., Lázár A., Burián K. (2019). Comparative Epidemiology and Resistance Trends of Common Urinary Pathogens in a Tertiary-Care Hospital: A 10-Year Surveillance Study. Medicina (Lithuania) 55 (7), 356. 10.3390/medicina55070356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrec H., Drieux-Rouzet L., Golmard J. L., Jarlier V., Robert J. (2011). Comparison of Nine Phenotypic Methods for Detection of Extended-Spectrum β-Lactamase Production by Enterobacteriaceae. J. Clin. Microbiol. 49 (3), 1048–1057. 10.1128/JCM.02130-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner C., Albiger B., Buist G., Tambić Andrašević A., Canton R., Carmeli Y., et al. (2013). Carbapenemase-Producing Enterobacteriaceae in Europe: A Survey Among National Experts From 39 Countries, February 2013. Eurosurveillance 18 (28), 1–7. 10.2807/1560-7917.ES2013.18.28.20525 [DOI] [PubMed] [Google Scholar]
- Gravey F., Loggia G., de la Blanchardière A., Cattoir V. (2017). Bacterial Epidemiology and Antimicrobial Resistance Profiles of Urinary Specimens of the Elderly. Medecine Maladies Infectieuses 47 (4), 271–278. 10.1016/j.medmal.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Hansen F., Olsen S. S., Heltberg O., Justesen U. S., Fuglsang-Damgaard D., Knudsen J. D., et al. (2014). Characterization of Third-Generation Cephalosporin-Resistant Escherichia Coli From Bloodstream Infections in Denmark. Microb Drug Res. 20 (4), 316–324. 10.1089/mdr.2013.0157 [DOI] [PubMed] [Google Scholar]
- Heytens S., Boelens J., Claeys G., DeSutter A., Christiaens T. (2017). Uropathogen Distribution and Antimicrobial Susceptibility in Uncomplicated Cystitis in Belgium, a High Antibiotics Prescribing Country: 20-Year Surveillance. Eur. J. Clin. Microbiol. Infect. Dis. 36 (1), 105–113. 10.1007/s10096-016-2776-8 [DOI] [PubMed] [Google Scholar]
- Iqbal R. (2019). Determination of Epidemiology and Antimicrobial Susceptibility of Extended Spectrum Beta Lactamase Producing Uropathogens. J. Pakistan Med. Assoc. 69 (5), 690–694. [PubMed] [Google Scholar]
- Khanfar H. S., Bindayna K. M., Senok A. C., Botta G. A. (2009). Extended Spectrum Beta-Lactamases (ESBL) in Escherichia Coli and Klebsiella Pneumoniae: Trends in the Hospital and Community Settings. J. Infect. Dev. Ctries. 3 (4), 295–299. 10.3855/jidc.127 [DOI] [PubMed] [Google Scholar]
- Koguchi D., Murakami Y., Ikeda M., Dobashi M., Ishii J. (2020). Cefaclor as a First-Line Treatment for Acute Uncomplicated Cystitis: A Retrospective Single-Center Study. BMC Urol. 20 (1), 38. 10.1186/s12894-020-00605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglova E. N., Stasheva M. A. (2010). Research of the Indirect Method of Determination of the Woven Cloths Air Permeability. Izvestiya Vysshikh Uchebnykh Zavedenii Seriya Teknologiya Tekstil’noi Promyshlennosti 8 (7), 95–97. [Google Scholar]
- Kulkarni D. M., Bardapurkar S. A., Nilekar S. L., More S. R. (2016). Prevalence of Extended Spectrum β-Lactamase (ESBL) Producing E. Coli and Klebsiella Species in Urinary Isolates. J. Dent. Med. Sci. 15 6, 26–29. 10.9790/0853-1506052629 [DOI] [Google Scholar]
- Lahlou Amine I., Chegri M., L’Kassmi H. (2009). Épidémiologie Et Résistance Aux Antibiotiques Des Entérobactéries Isolées D’infections Urinaires a L’hôpital Militaire Moulay-Ismail De Meknès. Antibiotiques 11 (2), 90–96. 10.1016/j.antib.2008.10.004 [DOI] [Google Scholar]
- Magale H. I., Kassim I. A., Odera S. A., Omolo M. J., Jaoko W. G., Jolly P. E. (2016). Antibiotic Susceptibility of Organisms Causing Urinary Tract Infection in Patients Presenting at Kenyatta National Hospital, Nairobi. East Afr. Med. J. 92 (7), 333–337. [PMC free article] [PubMed] [Google Scholar]
- Nellums L. B., Thompson H., Holmes A., Castro-Sánchez E., Otter J. A., Norredam M., et al. (2018). Antimicrobial Resistance Among Migrants in Europe: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 18 (7), 796–811. 10.1016/S1473-3099(18)30219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal K., Pant N. D., Neupane B., Belbase A., Baidhya R., Shrestha R. K., et al. (2017). Extended Spectrum Beta-Lactamase and Metallo Beta-Lactamase Production Among Escherichia Coli and Klebsiella Pneumoniae Isolated From Different Clinical Samples in a Tertiary Care Hospital in Kathmandu, Nepal. Ann. Clin. Microbiol. Antimicrobials 16 (1), 1–7. 10.1186/s12941-017-0236-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli N. P., Maharjan P., Parajuli H., Joshi G., Paudel D., Sayami S., et al. (2017). High Rates of Multidrug Resistance Among Uropathogenic Escherichia Coli in Children and Analyses of ESBL Producers From Nepal. Antimicrobial Resistance Infect. Control 6 (1), 1–7. 10.1186/s13756-016-0168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokharel S., Raut S., Adhikari B. (2019). Tackling Antimicrobial Resistance in Low-Income and Middle-Income Countries. BMJ Global Health 4 (6), 4–6. 10.1136/bmjgh-2019-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadharshana U., Piyasiri L. B., Wijesinghe C. (2019). Prevalence, Antibiotic Sensitivity Pattern and Genetic Analysis of Extended Spectrum Beta Lactamase Producing Escherichia Coli and Klebsiella Spp Among Patients With Community Acquired Urinary Tract Infection in Galle District, Sri Lanka. Ceylon Med. J. 64 (4), 140. 10.4038/cmj.v64i4.8990 [DOI] [PubMed] [Google Scholar]
- Rajabnia M., Forghani M. S., Hasani S., Bahadoram M., Mohammadi M., Barahman M. (2019). Prevalence and Antibiotic Resistance Pattern of Extended Spectrum Beta Lactamase Producing Escherichia Coli Isolated From Urinary Tract Infection. J. Renal Injury Prev. 8 (2), 78–81. 10.15171/jrip.2019.15 [DOI] [Google Scholar]
- Rohde A. M., Zweigner J., Wiese-Posselt M., Schwab F., Behnke M., Kola A., et al. (2020). Prevalence of Third-Generation Cephalosporin-Resistant Enterobacterales Colonization on Hospital Admission and ESBL Genotype-Specific Risk Factors: A Cross-Sectional Study in Six German University Hospitals. J. Antimicrob. Chemother. 75 (6), 1631–1638. 10.1093/jac/dkaa052 [DOI] [PubMed] [Google Scholar]
- Romli A., Derfoufi O., Omar C., Hajjam Z., Zouhdi M. (2011). Enterobacteria ESBL Urinary Infections: Epidemiology and Resistance. Maroc Médical. 33 (1), 12–16. [Google Scholar]
- Rossignol L., Vaux S., Maugat S., Blake A., Barlier R., Heym B., et al. (2017). Incidence of Urinary Tract Infections and Antibiotic Resistance in the Outpatient Setting: A Cross-Sectional Study. Infection 45 (1), 33–40. 10.1007/s15010-016-0910-2 [DOI] [PubMed] [Google Scholar]
- Ruano-de-pablo L., Losada-pinedo B. (2016). Community-Onset Extended-Spectrum B -Lactamase Producing Escherichia Coli in Urinary Tract Infections in Children From 2015 to 2016. Medicine 96 (50), 2015–2017. 10.1097/MD.0000000000008571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbiti M., Lahmadi K., Louzi L. (2017). Profil Epidémiologique Des Entérobactéries Uropathogènes Productrices De Bêta-Lactamases a Spectre Elargi. Pan Afr. Med. J. 28, 1–8. 10.11604/pamj.2017.28.29.11402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senard O., Lafaurie M., Lesprit P., Nguyen Y., Lescure X., Therby A., et al. (2020). Efficacy of Cefoxitin Versus Carbapenem in Febrile Male Urinary Tract Infections Caused by Extended Spectrum Beta-Lactamase–Producing Escherichia Coli: A Multicenter Retrospective Cohort Study With Propensity Score Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 39 (1), 121–129. 10.1007/s10096-019-03701-0 [DOI] [PubMed] [Google Scholar]
- Shakya P., Shrestha D., Maharjan E., Sharma V. K., Paudyal R. (2017). ESBL Production Among E. Coli and Klebsiella Spp. Causing Urinary Tract Infection: A Hospital Based Study. Open Microbiol. J. 11 (1), 23–30. 10.2174/1874285801711010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Pattnaik D., Neogi D. K., Jena J., Mallick B. (2016). Prevalence of ESBL in Escherichia Coli Isolates Among ICU Patients in a Tertiary Care Hospital. J. Clin. Diagn. Res. 10 (9), DC19–DC22. 10.7860/JCDR/2016/21260.8544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton P. J., Lundon D. J., McWade R., Scanlon N., Hannan M. M., O’Kelly F., et al. (2017). Antibiotic Resistance Patterns of Escherichia Coli Urinary Isolates and Comparison With Antibiotic Consumption Data Over 10 Years 2005–2014. Irish J. Med. Sci. 186 (3), 733–741. 10.1007/s11845-016-1538-z [DOI] [PubMed] [Google Scholar]
- Tacconelli E., Sifakis F., Harbarth S., Schrijver R., van Mourik M., Voss A., et al. (2018). Surveillance for Control of Antimicrobial Resistance. Lancet Infect. Dis. 18 (3), e99–e106. 10.1016/S1473-3099(17)30485-1 [DOI] [PubMed] [Google Scholar]
- Tan K., Nguyen J., Nguyen K., Huse H. K., Nieberg P. H., Wong-Beringer A. (2020). Prevalence of the Carbapenem-Heteroresistant Phenotype Among ESBL-Producing Escherichia Coli and Klebsiella Pneumoniae Clinical Isolates. J. Antimicrob. Chemother., 75 (6), 1506–1512. 10.1093/jac/dkaa048 [DOI] [PubMed] [Google Scholar]
- Tille P. (2018). Bailey & Scott’s Diagnostic Microbiology. 14th Edition (United States: Mosby Elsevier; ). [Google Scholar]
- Vandepitte J., Engbaek K., Rohner P., Piot P., Heuck C. C., Organization W. H (2003). Basic Laboratory Procedures in Clinical Bacteriology. 2nd Ed. 2nd ed (Switzerland: World Health Organization; ). Available at: https://apps.who.int/iris/handle/10665/42696. [Google Scholar]
- van Driel A. A., Notermans D. W., Meima A., Mulder M., Donker G. A., Stobberingh E. E., et al. (2019). Antibiotic Resistance of Escherichia Coli Isolated From Uncomplicated UTI in General Practice Patients Over a 10-Year Period. Eur. J. Clin. Microbiol. Infect. Dis. 38 (11), 2151–2158. 10.1007/s10096-019-03655-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hout D., Verschuuren T. D., Bruijning-Verhagen P. C. J., Bosch T., Schürch A. C., Willems R. J. L., et al. (2020). Extended-Spectrum Beta-Lactamase (ESBL) Producing and Non-ESBL-Producing Escherichia Coli Isolates Causing Bacteremia in the Netherlands, (2014 – 2016) Differ in Clonal Distribution, Antimicrobial Resistance Gene and Virulence Gene Content. PloS One 15 (1), 1–14. 10.1371/journal.pone.0227604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018). WHO Report on Surveillance of Antibiotic Consumption. 2016-2018 Early Implementation (20 Avenue Appia, 1211 Geneva 27, Switzerland: World Health Organization; ). Available at: https://apps.who.int/iris/bitstream/handle/10665/277359/9789241514880-eng.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


