Abstract
Penicillium marneffei is a major cause of opportunistic infection in patients with AIDS in north and northeastern Thailand. A method for the quantitation of P. marneffei antigen in urine was developed by using fluorescein isothiocyanate-labelled purified rabbit hyperimmune immunoglobulin G in an enzyme-linked immunosorbent assay. This method was evaluated with 33 patients with culture-proven penicilliosis and 300 controls (52 healthy subjects, 248 hospitalized patients without penicilliosis) from the same area in which penicilliosis is endemic. Urinary antigen was found in all 33 (100%) patients with penicilliosis, with a median titer of 1:20,480. With undiluted samples, 67 (27%) of 248 hospital patients and 3 (6%) of 52 healthy controls were reactive. At a cutoff titer of 1:40, the urine antigen detection assay had a diagnostic sensitivity of 97% and specificity of 98% (positive predictive value, 84%; negative predictive value, 99.7%). This test offers a valuable and rapid method for the diagnosis of penicilliosis in patients with AIDS and could be a useful addition to conventional diagnostic methods in areas in which penicilliosis is endemic.
Penicilliosis, caused by the dimorphic fungus Penicillium marneffei—a facultative intracellular pathogen for humans—is a disseminated and progressive infection which is endemic to certain parts of southeast Asia and China (4, 6, 7, 21). The first natural infection in humans was described in 1973 in a man suffering from Hodgkin’s disease who had travelled to southeast Asia (5). Only 21 further cases were reported during the next 15 years (4, 9), most of which had no evidence of underlying impaired immunity. With the increasing prevalence of human immunodeficiency virus (HIV) infections in Thailand, Penicillium marneffei has emerged as a major opportunistic infection in AIDS patients (8, 19, 21). The common clinical manifestations of penicilliosis include fever, anemia, leukopenia, weight loss, diarrhea, hepatosplenomegaly, generalized lymphadenopathy, cough, and characteristic molluscum contagiosum-like lesions, predominantly on the face and trunk (4, 8, 19, 20, 21). The presentation may mimic tuberculosis, melioidosis, leishmaniasis, and other AIDS-related opportunistic infections, such as histoplasmosis and cryptococcosis (6, 8, 14, 21, 26). A presumptive diagnosis of penicilliosis can be made by finding characteristic yeast cells in smears of skin lesions, blood, bone marrow, or lymph nodes, but these may be confused easily with those of Histoplasma capsulatum and, occasionally, Cryptococcus neoformans or Leishmania sp. Definitive diagnosis relies upon the identification or isolation of P. marneffei in clinical specimens. However, conventional culture usually takes ≥3 days. Histological recognition of the pathogen (12, 15) is relatively straightforward, although the septa which distinguish P. marneffei from H. capsulatum may not always be seen. Identification of by means of an immunohistochemical approach (2) and exoantigen tests (7, 11, 17) is specific; however, these tests are also time-consuming and are not generally available. Rapid diagnosis is important, because disseminated P. marneffei infection has a high mortality, and effective antifungal treatments are available (7, 8, 18, 21).
A number of serological diagnostic tests have been developed for the detection of antibody to P. marneffei. These include an immunodiffusion test (12, 13, 23) and use of an indirect fluorescent antibody technique (25). We report the development and prospective evaluation of an enzyme-linked immunosorbent assay (ELISA) for the detection of P. marneffei antigen in urine, using a fluorescein isothiocyanate (FITC)-anti-FITC amplification system, in an area of northeastern Thailand in which penicilliosis is endemic.
MATERIALS AND METHODS
Patients.
The study was conducted between June 1995 and November 1997. Adult patients with suspected or confirmed penicilliosis admitted to Sappasitprasong Hospital, Ubon Ratchathani, northeastern Thailand, were included in the study. All patients were seen by one of the study team. Full clinical details were recorded on a standard form. Routine hematological and biochemical tests were performed, and HIV antibody was measured when indicated with the ELISA. As part of the routine diagnostic workup, blood (15 ml), urine, and where appropriate, lymph node biopsy or liver aspirate specimens were collected for bacterial and fungal culture. Smears were made from any suspicious skin lesions or aspirates, and were then fixed on a glass slide and stained with Gram and Wright’s stains. Throat and skin lesion swabs were also collected. All specimens were plated on Sabouraud’s medium (with added chloramphenicol) and cultured at 30°C in air for up to 3 weeks.
Urine samples were obtained on admission and, in cases of confirmed penicilliosis, at weekly intervals thereafter. All urine samples were stored at −30°C and thawed only at the time of testing.
Antigen preparation.
A stock culture of P. marneffei (a clinical isolate from a patient in Ubon Ratchathani) was maintained on horse blood agar (HBA) at 37°C for several days. Fission-form arthroconidia (yeast-like cells) of P. marneffei were grown in 50 ml of brain heart infusion broth (BHIB) at 37°C for 2 weeks while being rotated on a rotary shaker at 150 rpm. The culture was checked weekly for the degree of turbidity and microscopic morphology. After 2 weeks of incubation, the culture was centrifuged at 2,900 × g for 10 min. The cell pellet was washed, resuspended in 10% formal saline solution to the original volume, and then left overnight at room temperature in order to kill the organisms. After repeat centrifugation, the cell pellet was washed, resuspended in normal saline, and adjusted to approximately 2 × 108 cells/ml. After appropriate sterility checks, this was used as the immunogen for immunizing rabbits or as the control antigen for the ELISA.
Anti-P. marneffei IgG preparation.
Hyperimmune sera against P. marneffei were prepared by injecting three female New Zealand White rabbits subcutaneously in the dorsal area with an emulsion consisting of 1 ml of 2 × 108 cells/ml of killed-whole fission arthroconidia of P. marneffei and 2 ml of Freund’s incomplete adjuvant, at weekly intervals for 3 weeks. One week after the third injection, the rabbits were injected intravenously with 0.5 ml of 5 × 106 cells of killed-whole fission arthroconidia of P. marneffei per ml every 3 to 4 days for a further 2 weeks. Three days after the final injection, each rabbit was bled, and the serum was collected and pooled. This serum was tested for antibody against killed whole-fission arthroconidia of P. marneffei by using an immunodiffusion (ID) test similar to that described by Sekhon et al. (17). A single broad band was produced both before and after pooling the serum samples. The purified IgG fraction of the pooled immune serum was obtained by precipitation with 35% saturated ammonium sulfate and followed by fractionation by protein A–Sepharose CL-4B chromatography (Pharmacia, Uppsala, Sweden) by standard methods. The concentration of protein in the purified preparation was estimated by measuring the optical density at 280 nm.
Anti-P. marneffei IgG-FITC conjugate.
FITC-labeled antibody was prepared by the method of Samual and others (16). Briefly, FITC (5 mg/ml in dry absolute ethanol) was added at a molar ratio of 20:1 to a stirred preparation of purified anti-P. marneffei IgG, diluted in 0.1 M carbonate buffer, containing 0.5 M NaCl (pH 9.2) in the dark at room temperature. The reaction was allowed to continue for a further 60 min, and then the anti-P. marneffei IgG-FITC was separated from free FITC by gel filtration through a Sephadex G-25 M PD-10 column (Pharmacia) preblocked with 1% bovine serum albumin, and equilibrated with phosphate-buffered saline (PBS). Fractions containing conjugate were pooled and stored at 4°C in PBS (33% [vol/vol]) containing 0.02% merthiolate.
Urinary antigen ELISA.
Flat-bottom microtiter plates (Falcon 3912 Microtest III; Becton Dickinson, Oxnard, Calif.) were coated with capture antibody by adding 100 μl of purified rabbit anti-P. marneffei IgG (10 mg/ml) in PBS (pH 7.2) containing 0.02% (wt/vol) merthiolate to each well. The plates were incubated at 4°C for 48 h, and the wells were then washed three times with PBS containing 0.1% (vol/vol) Tween 20 (PBS-Tween).
Urine samples were heated in a boiling waterbath for 6 min, cooled, and centrifuged at 5,000 × g for 6 min to remove any precipitate. Urine (100 μl), either undiluted or diluted 1:10 and then serially diluted twofold in PBS-Tween, was added to each well. The plate was incubated at 37°C for 60 min and then washed three times in PBS-Tween. Anti-P. marneffei IgG-FITC (100 μl) diluted 1:2,000 in blocking buffer (PBS-Tween, 0.5 M sodium chloride, 3% bovine serum albumin) was then added to each well and the plate incubated as above. After further washing, 100 μl of the rabbit anti-FITC horseradish peroxidase conjugate (Dakopatts, Glostrup, Denmark), diluted 1:2,000 in blocking buffer, was added to each well followed by an incubation and washing step as described above. Finally, 100 μl of tetramethyl benzidine substrate was added, and this mixture was then incubated for 30 min at room temperature. The reaction was stopped by adding 100 μl of 2 M sulfuric acid. The results were read spectrophotometrically at 450 nm in a Titertek Multiscan MCC plate reader (Flow Laboratories, Ltd., Irvine, Ayrshire, Scotland). Blank wells were treated as described above but the test specimen was omitted. A positive reading was defined as a value greater than the mean plus 3 standard deviations of the optical density of the blank wells. One positive urine specimen from a patient with confirmed P. marneffei infection, a negative urine sample from one control, and a suspension of formalinized-killed whole cells of P. marneffei were used as internal controls throughout the period of study.
To evaluate the specificity of the method, merthiolate-killed BHIB cultures of various fungal species (Penicillium griseofulvum ATCC 48166, Penicillium chrysogenum ATCC 9480, Penicillium notatum ATCC 9478, Aspergillus terreus, Aspergillus fumigatus BCC 123, Aspergillus flavus BCC 235, Histoplasma capsulatum var. capsulatum, Candida albicans (2 strains), Candida kefyr, Cryptococcus neoformans var. neoformans, C. neoformans var. gattii, Sporothrix schenckii, and Trichosporon beigelii) were tested in the ELISA at various concentrations, together with six clinical strains of P. marneffei (3 hyphal and 4 yeast-like forms; one was used in both phases). Strains of Burkholderia pseudomallei (203a), Salmonella enteritidis and Staphylococcus aureus, common causes of systemic infection in Ubon Ratchathani, were also tested, in addition to a strain of Salmonella typhi. All samples were tested in duplicate, and each test was repeated three times.
Statistical analysis.
Data were analyzed by using SPSS for Windows, version 6.1 (SPSS, Inc., Chicago, Ill.) computer software. Continuous variables with a nonnormal distribution were transformed. The comparability between data from each group of patients was assessed by one-way analysis of variance and Tukey’s significant difference test or the Kruskal-Wallis one-way analysis of variance and Mann-Whitney U test, as appropriate. At each ELISA cutoff titer, the sensitivity (proportion of positives correctly identified by the test) and the specificity (proportion of negatives correctly identified by the test) were calculated. A receiver operating characteristic curve was then constructed by plotting sensitivity against (1 − specificity) at each value (1).
RESULTS
Preliminary evaluation.
Broth cultures of C. albicans, C. kefyr, C. neoformans var. neoformans, C. neoformans var. gattii, P. griseofulvum, P. chrysogenum, P. notatum, A. terreus, A. fumigatus, A. flavus, H. capsulatum var. capsulatum, and T. beigelii were nonreactive in the ELISA, even at cell concentrations of 107 cells/ml. All of the clinical strains of P. marneffei (both fission and hyphal forms) were reactive down to concentrations of 100 yeast cells/ml. Bacterial strains were all nonreactive, except for Staphylococcus aureus (108 CFU/liter), which was reactive only when undiluted.
Diagnostic sensitivity and specificity.
Urine samples from 33 HIV-seropositive adult Thai patients with culture-confirmed P. marneffei infection were collected on admission. Urine samples from 248 patients with other diagnoses were also tested. These included 34 urine samples from HIV-seropositive patients with fungal infections other than penicilliosis. Of these, 31 were from patients with culture-confirmed cryptococcosis, 1 was from a patient with culture-confirmed histoplasmosis, and 2 were from patients with culture-confirmed candidiasis. The remaining patient samples were from 168 patients with culture-confirmed melioidosis, 12 from patients with septicemia due to other bacterial species, 7 from patients with other bacterial infections, and 27 from culture-negative patients (who were being screened for melioidosis as part of another study). All patients in the control groups mentioned above were admitted to Sappasitprasong Hospital during the same period as the penicilliosis patients. They had demographic characteristics similar to those of the penicilliosis patients. Fifty-two urine samples from healthy volunteers living in Ubon Ratchathani also served as negative controls, and 3 were used for the calculation of the ELISA standard deviations.
The results of P. marneffei antigen detection in urine from patients with penicilliosis and the control groups are given in Table 1 and Fig. 1. The ELISA detected antigen in the urine samples of all 33 (100%) patients with penicilliosis, with a median titer of 1:20,480 (range, neat to 1:327,680). Of the 52 samples from healthy volunteers, 49 (94%) were negative for antigen, whereas 3 (6%) reacted in the assay when tested undiluted. A higher proportion, 67 (27%) of 248 urine samples, from inpatients with diagnoses other than penicilliosis were reactive in the assay, with a median titer of neat (range, neat to 1:5120) (Table 2). Of the 34 urine samples from patients with other fungal infections, 9 (26.5%) gave positive results (all had cryptococcosis). Of these, four (11.8%) were reactive in the assay only when tested undiluted, one (2.9%) was reactive at a titer of 1:10, 2 (5.9%) were reactive at a titer of 1:80, one was reactive at a titer of 1:160, and one was reactive at a titer of 1:5,120. False-positive results were also found in 39 (23.2%) of 168 urine samples from patients with melioidosis; 32 (19.0%) of these were reactive undiluted, 6 (3.6%) were reactive at a titer of 1:10, and 1 (0.6%) was reactive at a titer of 1:20. Ten (83.3%) of 12 urine samples from patients with other bacterial septicemias and 1 (14.3%) of 7 from patients with other bacterial infections gave positive results. In the group of patients with other septicemias, nine (75%) were reactive only when tested undiluted, and one (8.3%) was reactive at a titer of 1:5,120, whereas one (14.3%) of the patients with other bacterial infections was reactive at a titer of 1:160. False-positive results also occurred in 8 (29.6%) of 27 patients who were culture negative: 1 patient at a titer of 1:10, and the remainder in neat urine only.
TABLE 1.
Antigen detection test results for patients with P. marneffei infection and controls
| Patient group (no. of patients) | Median ELISA titer (range)a |
|---|---|
| Penicilliosis (33) | 20,480 (1–327,680) |
| Other fungal infection (34) | 0 (0–5,120) |
| Melioidosis (168) | 0 (0–20) |
| Other septicemia (12) | 1 (0–5,120) |
| Other bacterial infection (7) | 0 (0–160) |
| Culture negative (27) | 0 (0–10) |
| Normal healthy controls (52) | 0 (0–1) |
P < 0.001 for all comparisons with penicilliosis patients.
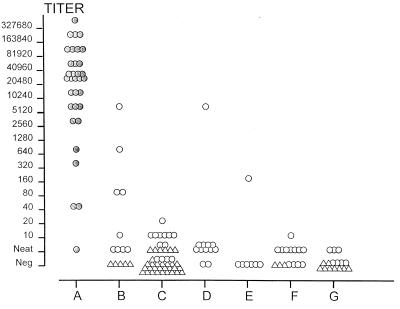
FIG. 1.
P. marneffei antigen titers in the urine collected on admission from 33 patients with penicilliosis and 300 control subjects and measured by ELISA. Group A (shaded circles) represents patients with culture-confirmed P. marneffei infection, group B had other fungal infections, group C had melioidosis, group D had other causes of bacterial septicemia, group E had other bacterial infections, group F had negative cultures, and group G were healthy controls. The triangles represent five patients, and the circles each represent one patient.
TABLE 2.
Sensitivity and specificity of P. marneffei urinary antigen ELISA at different cutoff values
| ELISA cutoff titer | % Sensitivity | % Specificity
|
||
|---|---|---|---|---|
| All (n = 300) | Other inpatients (n = 248) | Healthy controls (n = 52) | ||
| Neat | 100.0 | 76.7 | 73.0 | 94.2 |
| 10 | 97.0 | 95.0 | 94.0 | 100.0 |
| 20 | 97.0 | 97.7 | 97.2 | 100.0 |
| 40 | 97.0 | 98.0 | 97.6 | 100.0 |
| 80 | 90.9 | 98.0 | 97.6 | 100.0 |
| 160 | 90.9 | 98.7 | 98.4 | 100.0 |
| 320 | 90.9 | 99.0 | 98.8 | 100.0 |
| 640 | 87.9 | 99.0 | 98.8 | 100.0 |
| 1,280 | 84.9 | 99.3 | 99.2 | 100.0 |
| 2,560 | 84.9 | 99.3 | 99.2 | 100.0 |
| 5,120 | 78.8 | 99.3 | 99.2 | 100.0 |
| 10,240 | 69.7 | 100.0 | 100.0 | 100.0 |
| 20,480 | 60.6 | 100.0 | 100.0 | 100.0 |
| 40,960 | 33.3 | 100.0 | 100.0 | 100.0 |
| 81,920 | 24.2 | 100.0 | 100.0 | 100.0 |
| 163,840 | 12.1 | 100.0 | 100.0 | 100.0 |
| 327,680 | 3.0 | 100.0 | 100.0 | 100.0 |
The median ELISA titer for P. marneffei urinary antigen in the group of patients with penicilliosis (1:20,480; range, neat to 1:327,680) was significantly higher than those of the controls (Table 1), including the other fungal infections (median, negative; range, negative to 1:5,120; P < 0.0001), melioidosis (median, negative; range, negative to 1:20; P < 0.0001), other septicemia (median, negative; range, negative to 1:5,120 P < 0.0001), other bacterial infections (median, negative; range, negative to 1:160; P < 0.0001), those whose cultures were negative (median, negative; range, negative to 1:10; P < 0.0001), and normal healthy volunteers (median, negative; range, negative to neat; P < 0.0001).
Optimum cutoff titer.
The sensitivity and specificity of the ELISA in the diagnosis of penicilliosis are summarized in Table 2. Progressive dilution of urine samples reduced the sensitivity of the test, but the specificity increased to 100%. In order to achieve the best cutoff titer, a receiver-operating curve was used to assess the ability of the test to correctly identify patients with penicilliosis. This suggested that the best cutoff titer (i.e., the point that maximized the sum of the sensitivity and specificity) was at 1:40. At this cutoff titer, the ELISA was 97% sensitive and 98% specific (positive predictive value, 84.2%; negative predictive value, 99.7%). Repeated testing of a single sample with this initial titer gave values of 1:40 and 1:80. At a cutoff titer of 1:320, the specificity rose to 99%, but the sensitivity fell to 90% (positive predictive value, 91.3%; negative predictive value, 90.5%).
DISCUSSION
Penicilliosis is now recognized as one of the most important opportunistic infections in AIDS patients in Thailand. It is the fourth or fifth most common infection in this group of patients. Although in many cases, the diagnosis is readily made from microscopic examination of skin lesion smears, some patients do not have dermatological manifestations of the infection. In others, there may be confusion with histoplasmosis or disseminated cryptococcosis. Thus a rapid diagnostic or confirmatory test would be of clinical value and would direct appropriate antifungal therapy.
Yuen and colleagues (25) have developed an indirect immunofluorescence test to detect antibody in serum from patients infected with P. marneffei. All 78 healthy controls and 95 patients with fever from other causes had IgG titers of <1:40. Although there were only eight patients with P. marneffei infection in the series, all had IgG titers ≥160, but none had demonstrable IgM. Thus there was 100% sensitivity for this antibody test in this small series, although the study was performed in an area of low endemicity (Hong Kong) and included only two patients with other fungal infections (cryptococcosis). In an area of high endemicity, such as northern Thailand, background IgG seroprevalence could affect the specificity of the indirect fluorescent antibody IFA test.
Recently a specific 38-kilodalton antigen has been used to detect antibody to P. marneffei in HIV-seropositive patients in Thailand (3). Antibody was detected by immunoblotting in 30 (46%) of 65 patients with penicilliosis. In patients from an area in which P. marneffei is endemic, antibody was also found in 17 (25%) of 67 patients with cryptococcosis or candidiasis and in 45 (17%) of 262 asymptomatic HIV-positive individuals, but only 1 of 60 healthy controls. Thus, the specificity and usefulness of antibody tests for the diagnosis of penicilliosis in endemic areas remain uncertain. Other antigens from P. marneffei have been purified (10); one of these, a 61-kDa antigen, was recognized by sera from 18 of 21 (86%) P. marneffei-infected patients, but further evaluation of this in diagnostic tests is required.
Detection of P. marneffei antigen indicates active infection. However, it is known that there is some cross-reactivity between A. fumigatus and P. marneffei antigens, because antibody to A. fumigatus galactomannan reacts with P. marneffei in a latex agglutination test (22) and on immunohistochemical staining (2). A urinary antigen detection test developed for diagnosis of H. capsulatum var. capsulatum infection, using a rabbit IgG, has been reported to give positive results in 17 of 18 confirmed penicilliosis patients (24). Also, during the development of fluorescent-antibody tests for the tissue form of P. marneffei, Kaufman et al. (12) reported that the rabbit antiglobulin reacted with the yeast form of H. capsulatum but not with the hyphal form or the hyphal forms of A. flavus, A. fumigatus, and H. capsulatum or with Candida albicans, C. glabrata, or C. neoformans. Prior adsorption of the antiglobulin with yeast-form H. capsulatum could overcome this cross-reaction. These findings are consistent with those of Sekhon et al. (17), who reported no reactivity when rabbit antisera to mycelial elements of P. marneffei were tested with the exoantigens of eight monomorphic species of Penicillium or with hyphal antigens of Aspergillus spp. in the immunodiffusion test. Furthermore, Kaufman et al. (13) have developed an immunodiffusion test and a latex agglutination test to detect serum antigen. Both tests used rabbit antiserum raised against a culture filtrate of fission arthroconidia of P. marneffei which had the same antigens as those reported by Kaufman et al. (12). The tests were evaluated by using a small number of human sera. Serum antigen was detected by latex agglutination in 13 (76%) of 17 infected patients and by immunodiffusion in 10 (60%) patients. Antigen was not detected in serum samples from 15 normal Thai controls and six patients with cryptococcosis. P. marneffei antigen was also found in the urine from two infected patients, but not in six urine specimens containing Histoplasma antigen (13).
The polyclonal rabbit antiserum used in this study provides a highly sensitive and specific method for the diagnosis of penicilliosis in this area of endemicity. Of the 33 penicilliosis patients, 1 had detectable antigen only in neat urine (this patient had coinfection with C. neoformans) and a further 2 had titers of 1:40, while of the remaining 30 patients, 28 had titers of 1:2,560 or greater. Both of the patients with titers of 1:40 had very scanty skin lesions. One of these patients and the patient whose urine was positive only when undiluted had presented with disseminated cryptococcal infections. Because four of the six patients with false-positive antigen titers of >1:40 had cryptococcal infections, use of this cutoff titer alone for diagnosis would have lead to incorrect prescription of antifungal drugs in only two cases. Furthermore, these four patients were all HIV positive, and we cannot exclude the possibility that they were also infected with P. marneffei, although we were unable to culture it from suitable specimens. Of the other two patients with false-positive results, one was also HIV positive and had a Salmonella septicemia (titer, 1:5,120). This patient died shortly after blood cultures were collected and before other samples could be collected, so we cannot rule out coexisting penicillosis. The other patient (HIV test not done) was culture negative from blood and cerebrospinal fluid, but a coliform was grown from urine (titer, 1:160). This is not an area where leishmaniasis is prevalent, and so we cannot comment on test results for patients with this infection.
This antigen detection test should prove useful in diagnosis of P. marneffei infection and may be modifiable to a simple dot blot or latex agglutination form. The antigens recognized by the polyclonal antisera should now be characterized. The applicability of the test to serodiagnosis and the value of serial antigen detection in monitoring the response to treatment are currently being evaluated.
ACKNOWLEDGMENTS
We thank Wipada Chaowagul and Yupin Supputamongkol, Boongong Pimsa-ard, and Sayan Langla for assistance and Sornchai Looareesuwan (Faculty of Tropical Medicine, Mahidol University) and Siriwan Vanijanond (Department of Clinical Tropical Medicine) for support and encouragement.
This study was part of the Wellcome-Mahidol University, Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. London, United Kingdom: Chapman & Hall; 1995. pp. 396–439. [Google Scholar]
- 2.Arrese Estrada J, Stynen D, Van Cutsem J, Pierard-Franchimont C, Pierard G E. Immunohistochemical identification of Penicillium marneffei by monoclonal antibody. Int J Dermatol. 1992;31:410–412. doi: 10.1111/j.1365-4362.1992.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 3.Chongtrakool P, Chaiyaroj S C, Vithayasai V, Trawatcharegon S, Teanpaisan R, Kalnawakul S, Sirisinha S. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J Clin Microbiol. 1997;35:2220–2223. doi: 10.1128/jcm.35.9.2220-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Z L, Ribas J L, Gibson D W, Connor D H. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10:640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 5.DiSalvo A F, Fickling A M, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. 1973;59:259–263. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Duong T A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 7.Hilmarsdottir I, Meynard J L, Rogeaux O, Guermonprez G, Datry A, Katlama C, Brucker G, Coutellier A, Danis M, Gentilini M. Disseminated Penicillium marneffei infection associated with human immunodeficiency virus: a report of two cases and a review of 35 published cases. J Acquired Immune Defic Syndr. 1993;6:466–471. [PubMed] [Google Scholar]
- 8.Imwidthaya P. Update of penicilliosis marneffei in Thailand. Mycopathologia. 1994;127:135–137. doi: 10.1007/BF01102912. [DOI] [PubMed] [Google Scholar]
- 9.Jayanetra P, Nitiyanant P, Ajello L, Padhye A A, Lolekha S, Atichartakarn V, Vathesatogit P, Sathaphatayavongs B, Prajaktam R. Penicilliosis marneffei in Thailand: report of five human cases. Am J Trop Med Hyg. 1984;33:637–644. doi: 10.4269/ajtmh.1984.33.637. [DOI] [PubMed] [Google Scholar]
- 10.Jeavons L, Hamilton A J, Vanittanakom N, Ungpakorn R, Evans E G V, Sirisanthana T, Hay R J. Identification and purification of specific Penicillium marneffei antigens and their recognition by human immune sera. J Clin Microbiol. 1998;36:949–954. doi: 10.1128/jcm.36.4.949-954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman L, Standard P, Padhye A A. Exoantigen tests for the immunoidentification of fungal cultures. Mycopathologia. 1983;82:3–12. doi: 10.1007/BF00436938. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman L, Standard P G, Anderson S A, Jalbert M, Swisher B L. Development of specific fluorescent-antibody test for tissue form of Penicillium marneffei. J Clin Microbiol. 1995;33:2136–2138. doi: 10.1128/jcm.33.8.2136-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman L, Standard P G, Jalbert M, Kantipong P, Limpakarnjanarat K, Mastro T D. Diagnostic antigenemia tests for Penicilliosis marneffei. J Clin Microbiol. 1996;34:2503–2505. doi: 10.1128/jcm.34.10.2503-2505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kok I, Veenstra J, Rietra P J, Dirks-Go S, Blaauwgeers J L, Weigel H M. Disseminated Penicillium marneffei infection as an imported disease in HIV-1 infected patients: description of two cases and a review of the literature. Neth J Med. 1994;44:18–22. [PubMed] [Google Scholar]
- 15.Ma K F. Fine needle aspiration diagnosis of Penicillium marneffei infection. Acta Cytol. 1991;35:557–559. [PubMed] [Google Scholar]
- 16.Samual D, Patt R J, Abuknesha R A. A sensitive method of detecting proteins on dot and Western blots using monoclonal antibody to FITC. J Immunol Methods. 1988;107:217–224. doi: 10.1016/0022-1759(88)90221-9. [DOI] [PubMed] [Google Scholar]
- 17.Sekhon A S, Li J S K, Garg A K. Penicilliosis marneffei: serological and exoantigen studies. Mycopathologia. 1982;77:51–57. doi: 10.1007/BF00588658. [DOI] [PubMed] [Google Scholar]
- 18.Sirisanthana T, Supparatpinyo K, Perriens J, Nelson K E. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:1107–1110. doi: 10.1086/520280. [DOI] [PubMed] [Google Scholar]
- 19.Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1995;14:935–940. doi: 10.1097/00006454-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson K E, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–874. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 21.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem J, Meulemans L, Van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]
- 23.Viviani M A, Tortorano A M, Rizzardini G, Quirino L, Kaufman L, Padhye A A, Ajello L. Treatment and serological studies of an Italian case of Penicilliosis marneffei contracted in Thailand by a drug addict infected with the human immunodeficiency virus. Eur J Epidemiol. 1993;9:79–85. doi: 10.1007/BF00463094. [DOI] [PubMed] [Google Scholar]
- 24.Wheat J, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Nelson K, Bradsher R, Restrepo A. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 25.Yuen K Y, Wong S S, Tsang D N, Chau P Y. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]
- 26.Yuen W C, Chan Y F, Loke S L, Seto W H, Poon G P, Wong K K. Chronic lymphadenopathy caused by Penicillium marneffei: a condition mimicking tuberculous lymphadenopathy. Br J Surg. 1986;73:1007–1008. doi: 10.1002/bjs.1800731224. [DOI] [PubMed] [Google Scholar]