Abstract
Background
Serving as N6-methyladenosine demethylases, the AlkB family is involved in the tumorigenesis of hepatocellular carcinoma (HCC). However, the molecular profiles and clinical values of the AlkB family in HCC are not well known.
Methods
Several bioinformatics tools and in vitro experiments were used to identify the immune-related profiles and prognostic values of AlkB family in HCC.
Results
In this study expression levels of ALKBH1/2/3/4/7 were all remarkably increased in HCC tissues when compared with normal tissues. Quantitative PCR (qPCR) and immunohistochemistry were used to validate the expression of AlkB family members in HCC tissues and normal liver tissues. In addition, high expression levels of ALKBH4 were negatively correlated with overall survival (OS) and disease-free survival (DFS) in patients with HCC. Increased ALKBH4 was also associated with pathological stage in HCC patients. The molecular profiles of AlkB family in HCC were mainly associated with peptidyl-serine modification, peptidyl-tyrosine modification, regulation of metal ion transport, etc. Furthermore, tumor-infiltrating immune cell analysis indicated that ALKBH1/2/3/4/5/6/7/8 and FTO were related to the infiltration of different immune cell, such as CD8+ T cells, macrophages, neutrophils, dendritic cells and CD4+ T cells. We also discovered that the methylation levels of ALKBH1/2/4/5/6/8 and FTO were remarkably reduced in HCC tissues.
Conclusions
Collectively, our findings may deepen the understanding of specific molecular profiles of the AlkB family in HCC pathology. In particular, ALKBH4 could serve as a promising prognostic candidate for treating HCC, and these results might potentiate the development of more reliable therapeutic strategies for patients with HCC.
Keywords: Hepatocellular carcinoma, AlkB family, Molecular profiles, Prognosis, Immune cell infiltration
Introduction
Hepatocellular carcinoma (HCC), accounting for 75%–85% of liver cancer cases, is one of the leading causes of cancer-associated deaths and it is frequently diagnosed worldwide, with approximately 841,000 new cases and 782,000 deaths yearly. The incidence of HCC has gradually increased in recent years (Feng et al., 2020; Huang et al., 2020). Currently, many scientific researchers have developed many comprehensive clinical therapeutic regimens for HCC, such as liver transplantation (LT), surgical resection, ablation treatment, arterial embolism, and systemic therapies (Akateh et al., 2019). However, to effectively improve the prognosis of patients with HCC, better treatments and novel biomarkers need to be further explored.
The AlkB family of Fe (II)- and α-ketoglutarate-dependent dioxygenases are demethylases, playing an important role in removing epigenetic inheritance information. They are closely related to the occurrence and development of many cancers. The AlkB family includes ALKBH1, ALKBH2, ALKBH3, ALKBH4, ALKBH5, ALKBH6, ALKBH7, ALKBH8 and FTO, and they are homologous enzymes (Cai et al., 2021; Wu et al., 2021). The AlkB family mediates many modification processes and stabilizes transcription and translation (Xu et al., 2020a). In addition, they also participate in DNA damage repair (Anindya, 2020), RNA and fatty acid metabolism and histone demethylation (Pilzys et al., 2019). It is worth noting that many studies have shown that the AlkB family is involved in the development of cancers, and the expression profiles of the AlkB family are different in various cancers (Geng et al., 2020). Accumulating evidence suggests that the AlkB family is a promising therapeutic biomarker for various cancers. Therefore, it is important to identify specific molecular profiles of the AlkB family.
Previous results have shown partial functions of the AlkB family in human cancers. However, the specific molecular profiles of AlkB family in HCC biology have not been well clarified, which is a major problem and worth attracting our attention. The AlkB family can be analyzed from multiple levels because of improvements in genetic testing and bioinformatics databases.
Materials & Methods
RNA extraction and quantitative PCR (qPCR)
Altogether 13 pairs of formalin-fixed, paraffin-embedded (FFPE) specimens of HCC and adjacent tissues were collected from Department of Pathology, Xiangya Hospital (Changsha, China). The ethics of our study has been approved by the Ethical Committee of Xiangya Hospital of Central South University. The ethical approval number is 202109083. Total RNA was extracted from FFPE tissue specimens using PureLink FFPE RNA Isolation Kit (K156002; Invitrogen, Waltham, MA, USA) according to the manufacturer’s protocol, followed by cDNA synthesis using a PrimeScript RT reagent kit (6210, Takara, China). The quantitative PCR (qPCR) was conducted with iTaq Universal SYBR green Supermix (172-5850; Bio-Rad, Hercules, CA, USA). β-actin was used as an internal control. The sequences of all primers used in this report are provided in Table 1. According to the previous reports (Buford et al., 2020; Li et al., 2020b; Pandelides et al., 2020; Zhou et al., 2020), the relative expression levels of AlkB family members were determined using the 2 −ΔΔCT method.
Table 1. The primers sequence of AlkB family members.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ALKBH1 | 5′-AGAAGCGACTAAACGGAGACC-3′ | 5′-GGGAAAGGTGTGTAATGATCTGC-3′ |
| ALKBH2 | 5′-GACTGGACAGACCTTCAAC-3′ | 5′-AGGAGACAGAGGCAATGG-3′ |
| ALKBH3 | 5′-AGATGTACTGGTTCCCTGGC-3′ | 5′-CCTCACGGAACACATGGTAG-3′ |
| ALKBH4 | 5′-GGTCAGCCTCAACCTCCTGT-3′ | 5′-TATCACGCTGTCCACCAAGG-3′ |
| ALKBH5 | 5′-GCTTCAGGGTATGGGAGTTG-3′ | 5′-TTCCAGGATCTGAGTGGATAGA-3′ |
| ALKBH6 | 5′-TGGACGGATTGGGTGCAAG-3′ | 5′-TCGAAGCAAATACTCCTCCTCT-3′ |
| ALKBH7 | 5′-CCATGAGATCCTTCGGGATGA-3′ | 5′-CGGCAGATCACGGAGATGC-3′ |
| ALKBH8 | 5′-TTAATGCCACCTAACAAGCCG-3′ | 5′-ATTGAGGGTAACATAGGCTCTCT-3′ |
| FTO | 5′-CCCTGTGAGCAGCAACATAAG-3′ | 5′-CAACCCGACCCAGTCTAAATC-3′ |
The protein levels of AlkB family in clinical samples
Base on the immunohistochemistry technology, the Human Protein Atlas (HPA) has been established to study the protein localization and expression in human cancer and normal tissues (Thul & Lindskog, 2018). Here, the HPA was applied to compare the protein expression of AlkB family members in HCC and normal tissues.
Functional and pathway enrichment analysis
The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes pathway (KEGG) enrichment analyses (Ju et al., 2019; Ju et al., 2020b) were analyzed to identify the biological functions and signaling pathways of AlkB associated neighboring genes. The GO and KEGG enrichment analyses were performed by WebGestalt (Liao et al., 2019).
The profiles of AlkB family analyzed with several databases
In this research, several reliable and multifunctional databases were utilized to analyze all aspects of expression profiles of AlkB family members in HCC (Table S1).
GEPIA2, an updated and enhanced web platform, include 198,619 isoforms and 84 cancer subtypes. GEPIA2 not only contains original functions and extends the analysis of transcript level (Tang et al., 2019). In this research, GEPIA2 was applied for analyzing differential RNA expression between HCC and normal tissues and exploring the correlation between the AlkB family and the stages of pathology. The p value was set as 0.05, and Student’s t-test was used to evaluate the expression and stage of HCC. Additionally, overall survival (OS) and disease-free survival (DFS) curves were also obtained from GEPIA2.
cBioPortal is an accessible and all-sided website that plays an important role in the visualization and analysis of genomics data in dimensions (Gao et al., 2013). Its data are derived from TCGA and contain more than 200 tumor genomes offering multiple analytical functions. In this study, we analyzed the mutational and coexpressed genes of the AlkB family using the Z score threshold of mRNA and protein ±2.0.
TIMER2.0, a comprehensive web resource, aims to analyze the infiltration of tumor immune cells (Li et al., 2020d). It collected and integrated the data of 10,897 tumors from 32 cancer types. In this research, we conducted a “gene module” to analyze the relationship between the expression levels of the AlkB family in HCC and tumor-infiltrating immune cells.
DiseaseMeth version 2.0 is a convenient and swift website that is mainly used for evaluating the level of aberrant DNA methylation of numerous tumors in 32,701 human samples (Xiong et al., 2017). In our studies, the “Analysis module” was utilized to evaluate the level of DNA methylation of the AlkB family in HCC patients.
Statistical analyses
The statistical tests were analyzed by using SPSS 12.0 software (IBM Analytics). GraphPad Prism 8 (GraphPad Software) was used to draw the bar graphs. The paired-samples t-test was used to analyze the data of qPCR. a p-value of less than 0.05 was considered to be statistically significant.
Results
Abnormal expression of AlkB family in HCC
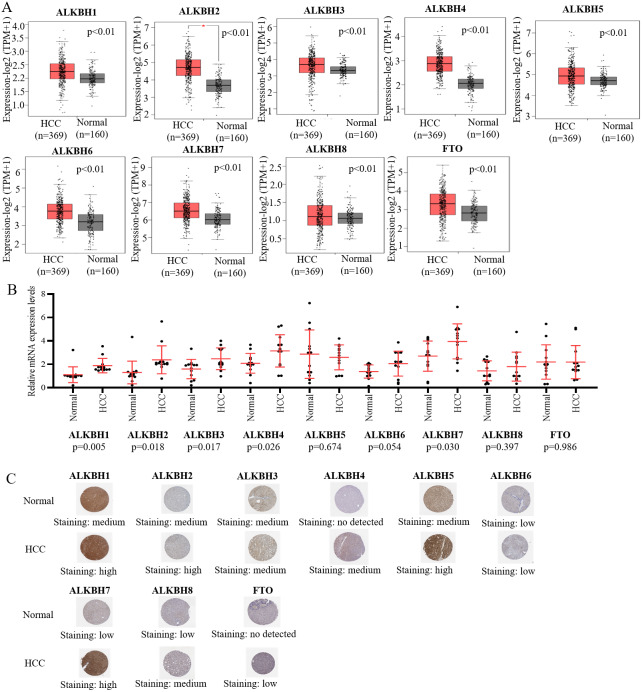
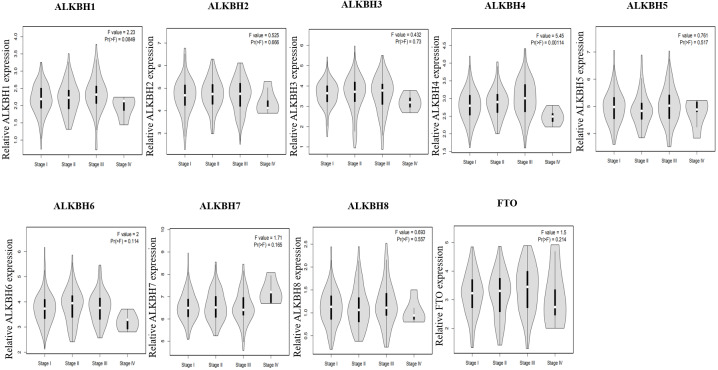
We utilized the GEPIA databases to retrieve the AlkB family, and the “Expression DIY module” was used to evaluate the expression level of the AlkB family between HCC and normal hepatic tissues. Fig. 1A shows that the transcriptional levels of ALKBH1/2/3/4/5/6/7/8 (p < 0.01) and FTO (p < 0.01) were all remarkably increased in HCC tissues when compared with normal tissues. We also evaluated the transcriptional levels of the Alkb family members in the HCC and paired adjacent normal tissues were. The result of qPCR showed that the mRNA expression levels of ALKBH1/2/3/4/7 are higher in tumor samples and lower in normal tissue samples and the others had no statistic difference (Fig. 1B). In addition, we used the HPA database to analyze the protein expression levels of Alkb family in HCC tissues and normal liver tissues. The results showed that ALKBH1/2/4/5/7/8 were medium or high expression in HCC tissues (Fig. 1C), which is consistent with their changes on the transcriptional levels. These findings are similar with other reports. For example, Ma et al. found that the expression level of ALKBH1 in HCC was strikingly elevated (Ma et al., 2019). The relationship between the expression level of the AlkB family and the stages of pathology was then evaluated. With the progression of tumors, ALKBH4 (p = 0.00114) was increasingly expressed in the first three stages (Fig. 2). Then, Xiantao Xueshu (https://www.xiantao.love/products) was used to further evaluate the relationship between ALKBH4 expression and HCC. The results revealed that ALKBH4 expression significantly increased with the increase in T stage (odds ratio = 1.629, p = 0.02). Increased ALKBH4 expression had the significance on pathological stage despite there was no statistical difference. The correlation between ALKBH4 and other characteristics in HCC patients, including N stage, M stage and histologic grade, needs to be further explored (Table 2). All in all, our results cast new light on the specific molecular profiles of the AlkB family in HCC.
Figure 1. The expression level of AlkB family in HCC tissues.
(A) The mRNA expression profiles of AlkB family members in HCC patients from GEPIA2 databases. (B) The mRNA expression level of AlkB family members in the clinical samples. (C) The HPA showed the immunohistochemical analysis of ALKB family in HCC tissue and normal tissue.
Figure 2. The relationship of AlkB family and pathology stages in HCC patients.
The GEPIA2 webtool was used to analyze the relevant between expression levels of AlkB family members and patients’ stages.
Table 2. Demographic characteristics of ALKBH4 expression in HCC.
| Characteristics | Total(N) | Odds Ratio (OR) | P value |
|---|---|---|---|
| T stage (T2&T3&T4 vs. T1) | 371 | 1.629 (1.082–2.460) | 0.020 |
| N stage (N1 vs. N0) | 258 | 2.773 (0.350–56.463) | 0.380 |
| M stage (M1 vs. M0) | 272 | 0.319 (0.016–2.525) | 0.325 |
| Pathologic stage (Stage II&Stage III&Stage IV vs. Stage I) | 350 | 1.513 (0.994–2.310) | 0.054 |
| Histologic grade (G2&G3&G4 vs. G1) | 369 | 1.578 (0.886–2.859) | 0.125 |
| Tumor status (With tumor vs. Tumor free) | 355 | 1.456 (0.955–2.224) | 0.081 |
| Age (>60 vs. <=60) | 373 | 1.125 (0.749–1.691) | 0.570 |
| Gender (Male vs. Female) | 374 | 1.187 (0.769–1.834) | 0.439 |
| Vascular invasion (No vs. Yes) | 318 | 0.960 (0.604–1.526) | 0.863 |
| Residual tumor (R1&R2 vs. R0) | 345 | 2.087 (0.791–6.119) | 0.151 |
| AFP (ng/ml) (>400 vs. <=400) | 280 | 1.548 (0.887–2.726) | 0.126 |
| Albumin(g/dl) (>=3.5 vs. <3.5) | 300 | 0.920 (0.536–1.578) | 0.760 |
The prognostic value of AlkB family in HCC patients
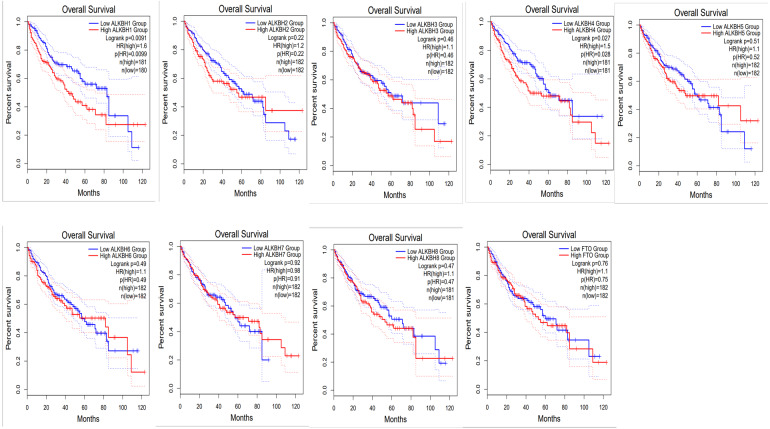
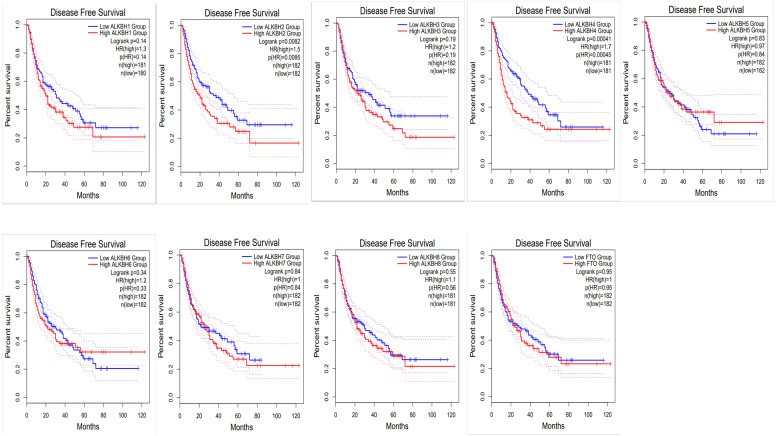
To identify the prognostic value of different expression levels of the AlkB family, we performed survival analysis and explored the interactive correlations. Figure 3 presents the OS curves. In this study, we found that remarkably increased ALKBH1 (p = 0.0099) and ALKBH4 (p = 0.028) expression were correlated with a bad prognosis (Fig. 3). Then, we also evaluated the DFS of patients with HCC. We observed that similarly, high expression levels of ALKBH2 (p = 0.0085) and ALKBH4 (p = 0.00041) were correlated with a poor DFS (Fig. 4). These data indicated the important roles of high expressed ALKBH4 on HCC prognosis.
Figure 3. The effect of AlkB family on overall survival of HCC patients.
GEPIA2 was used to evaluate the relationship of the AlkB family and OS in HCC patients.
Figure 4. The effect of AlkB family on disease free survival of HCC patients.
The relationship of the expression level of the AlkB family and DFS in HCC patients was analyzed by GEPIA2
Genetic alteration and enriched functional analysis of AlkB family in HCC patients
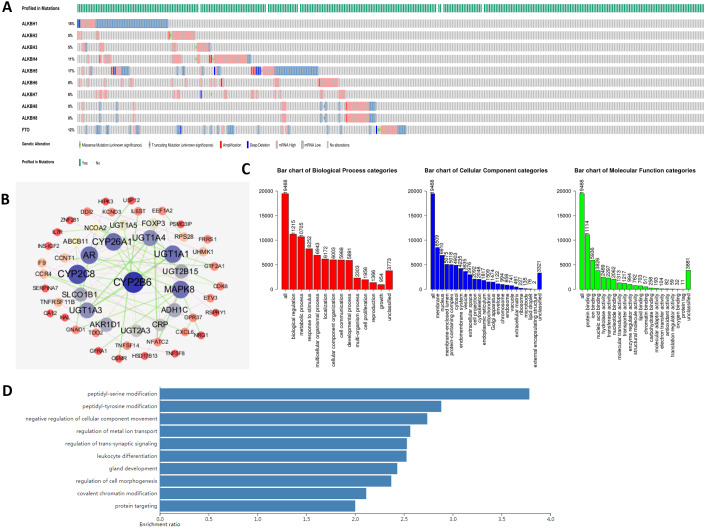
At the molecular level, an analysis of genetic alterations was conducted on the AlkB family in HCC based on the cBioPortal database. Our findings hinted that the genetic mutations occurred in ALKBH1/2/3/4/5/6/7/8 and FTO in HCC patients, with altered rates of 15, 5, 5, 11, 17, 8, 6, 8, and 12%, respectively (Fig. 5A). Moreover, we downloaded 19,909 genes related to HCC from the cBioPortal, and 155 genes were screened with p < 0.01 and —log ratio—> 0.85 (Table S2). Ultimately, using the protein-protein interaction analysis, we identified approximately 145 neighboring genes of the AlkB family. The hub genes mainly included Cytochrome P450 family 2 subfamily B member 6 (CYP2B6), CYP2C8, CYP26A1 and so on (Fig. 5B).
Figure 5. Genetic alterations and interaction analyses of the AlkB family in HCC patients.
(A) General mutations of the AlkB family in HCC. (B) The cBioPortal was used to identify the neighboring genes of AlkB family in HCC. (C) Bar chart of the biological process categories, cellular component categories and molecular function categories. (D) Bar chart of the KEGG enrichment results.
Then, we conducted gene ontology (GO) enrichment analysis of the interacting gene network of the AlkB family with WebGestalt. Figure 5C shows that in biological process (BP), biological regulation, metabolic process, response to stimulus, multicellular organismal process and localization were the top five most highly enriched categories. Meanwhile, at the cellular level, there were many enriched items, and the top 5 covered the membrane, nucleus, membrane-enclosed lumen, protein-containing complex and cytosol. Moreover, protein binding and ion binding were the richest categories in the molecular function (MF). In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis suggested that peptidyl-serine modification, peptidyl-tyrosine modification, negative regulation of cellular component movement, regulation of metal ion transport and regulation of transsynaptic signaling were the main signaling pathways in connection with the abnormal AlkB family members, which might play the potential roles in the occurrence and development of HCC (Fig. 5D).
Immune cell infiltration under the influence of AlkB family in HCC
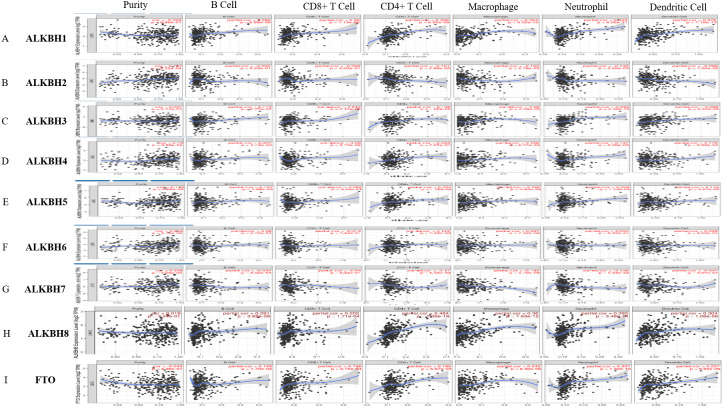
A reliable correlation analysis between tumor-infiltrating immune cells and the AlkB family was performed based on TIMER (Fig. 6). In detail, ALKBH1 was positively correlated with the infiltration of B cells (Cor =0.286, p = 6.89e−08), CD8+ cells (Cor = 0.26, p = 1.13e−06), CD4+ cells (Cor = 0.306, p = 6.82e−09), macrophages (Cor = 0.324, p = 9.02e−10), neutrophil (Cor = 0.843, p = 6.05e−11) and dendritic cells (Cor =0.376, p = 7.18e−13) (Fig. 6A). ALKBH2 had negative correlations with the infiltration of CD4+ cells (Cor = −0.151, p = 4.88e−03) and macrophages (Cor = −0.162, p = 2.76e−03) (Fig. 6B). ALKBH3 was positively correlated with the infiltration of CD8+ cells (Cor = 0.213, p = 7.14e−05), macrophages (Cor =0.22, p = 4.09e−05), neutrophils (Cor = 0.332, p = 2.46e−10) and dendritic cells (Cor = 0.288, p = 6.33e−08) (Fig. 6C). ALKBH4 had a positive relationship with infiltration of B cells (Cor = 0.207, p = 1.10e−04), CD4+ cells (Cor = 0.263, p = 7.21e−07), macrophages (Cor = 0.196, p = 2.77e−04), neutrophil (Cor = 0.147, p = 6.53e−03) and dendritic cells (Cor = 0.178, p = 9.99e−04) (Fig. 6D). ALKBH5 was positively associated with the infiltration of B cells (Cor =0.167, p = 1.88e−03), CD4+ cells (Cor = 0.205, p = 1.32e−04), macrophages (Cor = 0.192, p = 3.60e−04), neutrophil (Cor = 0.206, p = 1.14e−04) and dendritic cells (Cor = 0.116, p = 3.27e−02) (Fig. 6E). Figure 6F indicated positive correlations between ALKBH6 and the infiltration of CD4+ cells (Cor = 0.144, p = 7.28e−03). Conversely, negative correlations between ALKBH7 and CD4+ cells (Cor = −0.17, p = 1.56e−03), macrophages (Cor = −0.185, p = 5.79e−04) and neutrophil (Cor = −0.138, p = 1.03e−02) were showed in Fig. 6G. Surprisingly, ALKBH8 and FTO were positively correlated with all six types of immune cells analyzed (Figs. 6H–6I). We further utilized the Cox proportional hazard model to correct for confounders, including B cells, CD8+ T cells, CD4+ T cells, macrophages, neutrophils and the AlkB family. The results indicated that B cells, CD8+ T cells, macrophages and ALKBH4 were related to the prognosis of patients with HCC (Table S3).
Figure 6. The relationship of AlkB family and tumor-infiltrating immune cells.
(A-I) The effect of (A) ALKBH1, (B) ALKBH2, (C) ALKBH3, (D) ALKBH4, (E) ALKBH5, (F) ALKBH6, (G) ALKBH7, (H) ALKBH8 and (I) FTO on the abundance of immune cells in HCC patients.
The different methylation levels of AlkB family in HCC
There is a negative correlation between DNA methylation and gene expression levels (Gensous et al., 2020; Kronfol et al., 2020; Wang et al., 2020b; Zhang et al., 2020). In our results, the DNA methylation of ALKBH1/2/4/5 and FTO was decreased in HCC patients compared with healthy persons (p < 0.05) (Fig. S1). Therefore, we concluded that higher expression levels of ALKBH1/2/4/5 and FTO in HCC tissues might be due to downregulated DNA methylation levels.
Discussion
N6-methyladenosine is extensively involved in RNA modification (Zheng, Zhang & Sui, 2020), and it is closely related to the occurrence and development of various cancers (Li et al., 2020a; Xu et al., 2020b). Surprisingly, serving as demethylases, ALKBH5 and FTO facilitate the demethylation process of m6A modification (Toh et al., 2020). In addition, the AlkB family is also involved in DNA repair, transcription and translation, according to previous studies (Baldwin, Admiraal & O’Brien, 2020; Xu et al., 2020a). There are many findings indicated that m6A modification mediated by ALKBH5 and FTO participates in the development of HCC and plays a crucial role in suppressing proliferative and invasive abilities of HCC cells (Bian et al., 2021; Chen et al., 2020). The expression of ALKBH5 and FTO has been proved to be up-regulated in tumor tissues, which implies a poor prognosis of HCC patients (Li et al., 2019; Qu et al., 2021). Apart from that, m6A is evidently connected with HCC immune microenvironment. For example, FTO-mediated m6A demethylation gets involved in the response to anti-programmed cell death-1 (anti-PD-1)-based immunotherapy (Yang et al., 2019). Methyltransferase-like 3 (METTL3) and methyltransferase-like 114 (METTL114), the major m6A RNA methyltransferases, enhance the sensitivity of anti-PD-1 treatment (Wang et al., 2020a). Consequently, the AlkB family participates in the growth, prognosis and immune regulation of cancers from many aspects. However, the specific molecular profiles and detailed roles of the AlkB family in HCC are not well known.
First, we utilized GEPIA2 to explore the expression levels of the AlkB family, and we found that the AlkB family members were all expressed at a higher level compared with normal tissues. We evaluated expression levels in different pathological stages and found that ALKBH4 was increasingly expressed in the first three stages. This hinted that we might estimate the pathological stage of HCC by testing the expression level of ALKBH4. In addition, we observed that high expression of ALKBH4 was correlated with a poor prognosis in HCC. These results provide a novel insight into the vital roles of high expressed ALKBH4 in HCC.
Second, we genetically explored the correlation between the AlkB family and HCC. We found that the whole AlkB family had many genetic alterations in HCC patients, supporting again that the AlkB family might be involved in the tumorigenesis and progression of HCC.
Third, we performed GO enrichment analysis based on the WebGestalt database. We found that the functions of the AlkB family were mainly associated with peptidyl serine modification, peptidyl-tyrosine modification, negative regulation of cellular component movement, regulation of metal ion transport, regulation of transsynaptic signaling and leukocyte differentiation. As reported by previous studies, metal ion transport, transsynaptic signaling and leukocyte differentiation are related to hepatic tumor growth and progression (Hou et al., 2020; Ren et al., 2018). These results suggested that the AlkB family might participate in the progression of HCC by regulating these signaling pathways.
Then, previous studies showed that immune cell infiltration plays a crucial role in tumorigenesis, prognosis and therapies (Mukherjee et al., 2020; Xu et al., 2020c; Yan et al., 2020). Most T cells and macrophage-mediated phagocytosis are capable of killing tumor cells and preventing tumor growth (Feng et al., 2019). The results of our study showed that the expression levels of ALKBH1, ALKBH3, ALKBH5 and ALKBH8 were positively associated with macrophages, neutrophils, dendritic cells and CD4+ T cells, which suggested partial specific biological profiles of the AlkB family. Growing evidence has indicated that many cancer-associated genes serve as potential immune-associated prognostic biomarkers. The research results of Li et al. (2020c) demonstrated that ALKBH5 plays an important part in recruiting decreasingly of MDSCs and Tregs to restrict tumor growth. Ju et al. (2020a) have illustrated that erythroid 2 like 2 (NFE2L2) expression is positively associated with immune infiltration, which hints a more favorable prognosis in brain lower grade glioma.
Although we have made a comprehensive analysis of AlkB family from many aspects, there are still some issues that need to be addressed. First of all, we collected a small set of samples for the qPCR experiment. Secondly, our results exhibited a remarkable correlation between the expression of ALKBH4 and HCC. However, the specific mechanisms of their interaction remain to be explored. Finally, more in vitro and in vivo experiments should be conducted to confirm our results.
Conclusion
In conclusion, this article comprehensively explored the molecular profiles of AlkB family through multiple levels of analysis based on many bioinformatics databases. Furthermore, the ALKBH4 might show the tumor-promoting effect and negatively affect the prognosis of patients with HCC. These findings can deepen clinicians’ understanding of HCC and could potentiate the development of more accurate and reliable treatments for patients with HCC.
Supplemental Information
Acknowledgments
We thank Elsevier’s English Language Editing Service for assistance with the language editing.
Funding Statement
This study is supported by grants from the China Postdoctoral Science Foundation (2021T140754, 2020M672521), the National Natural Science Foundation of China (81803035), the Natural Science Foundation of Hunan Province (2020JJ5934, 2019JJ50932), and the Postdoctoral Science Foundation of Central South University (248485). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Bi Peng performed the experiments, prepared figures and/or tables, and approved the final draft.
Yuanliang Yan analyzed the data, prepared figures and/or tables, and approved the final draft.
Zhijie Xu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The ethics of our study has been approved by the Ethical Committee of Xiangya Hospital of Central South University and the ethical approval number is 202109083.
Data Availability
The following information was supplied regarding data availability:
The raw measurements for the qPCR are available in the Supplemental Files.
References
- Akateh et al. (2019).Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World Journal of Gastroenterology. 2019;25:3704–3721. doi: 10.3748/wjg.v25.i28.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anindya (2020).Anindya R. Single-stranded DNA damage: protecting the single-stranded DNA from chemical attack. DNA Repair. 2020;87:102804. doi: 10.1016/j.dnarep.2020.102804. [DOI] [PubMed] [Google Scholar]
- Baldwin, Admiraal & O’Brien (2020).Baldwin MR, Admiraal SJ, O’Brien PJ. Transient kinetic analysis of oxidative dealkylation by the direct reversal DNA repair enzyme AlkB. Journal of Biological Chemistry. 2020;295:7317–7326. doi: 10.1074/jbc.RA120.013517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian et al. (2021).Bian X, Shi D, Xing K, Zhou H, Lu L, Yu D, Wu W. AMD1 upregulates hepatocellular carcinoma cells stemness by FTO mediated mRNA demethylation. Clinical and Translational Medicine. 2021;11:e352. doi: 10.1002/ctm2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford et al. (2020).Buford TW, Sun Y, Roberts LM, Banerjee A, Peramsetty S, Knighton A, Verma A, Morgan D, Torres GE, Li Q, Carter CS. Angiotensin (1-7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. Geroscience. 2020;42:1307–1321. doi: 10.1007/s11357-020-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2021).Cai Y, Wu G, Peng B, Li J, Zeng S, Yan Y, Xu Z. Expression and molecular profiles of the AlkB family in ovarian serous carcinoma. Aging (Albany NY) 2021;13:9679–9692. doi: 10.18632/aging.202716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen Y, Zhao Y, Chen J, Peng C, Zhang Y, Tong R, Cheng Q, Yang B, Feng X, Lu Y, Xie H, Zhou L, Wu J, Zheng S. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Molecular Cancer. 2020;19:123. doi: 10.1186/s12943-020-01239-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2020).Feng D, Wang M, Hu J, Li S, Zhao S, Li H, Liu L. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases. Annals of Translational Medicine. 2020;8:553. doi: 10.21037/atm.2020.02.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2019).Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature Reviews Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2013).Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signaling. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng et al. (2020).Geng Y, Guan R, Hong W, Huang B, Liu P, Guo X, Hu S, Yu M, Hou B. Identification of m6A-related genes and m6A RNA methylation regulators in pancreatic cancer and their association with survival. Annals of Translational Medicine. 2020;8:387. doi: 10.21037/atm.2020.03.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensous et al. (2020).Gensous N, Garagnani P, Santoro A, Giuliani C, Ostan R, Fabbri C, Milazzo M, Gentilini D, di Blasio AM, Pietruszka B, Madej D, Bialecka-Debek A, Brzozowska A, Franceschi C, Bacalini MG. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: a pilot study from the NU-AGE project. Geroscience. 2020;42:687–701. doi: 10.1007/s11357-019-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou et al. (2020).Hou PP, Luo LJ, Chen HZ, Chen QT, Bian XL, Wu SF, Zhou JX, Zhao WX, Liu JM, Wang XM, Zhang ZY, Yao LM, Chen Q, Zhou D, Wu Q. Ectosomal PKM2 promotes HCC by inducing macrophage differentiation and remodeling the tumor microenvironment. Molecular Cell. 2020;78:1192–1206. doi: 10.1016/j.molcel.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2020).Huang X, Qin F, Meng Q, Dong M. Protein tyrosine phosphatase receptor type D (PTPRD)-mediated signaling pathways for the potential treatment of hepatocellular carcinoma: a narrative review. Annals of Translational Medicine. 2020;8:1192. doi: 10.21037/atm-20-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al. (2020a).Ju Q, Li X, Zhang H, Yan S, Li Y, Zhao Y. NFE2L2 is a potential prognostic biomarker and is correlated with immune infiltration in brain lower grade glioma: A pan-cancer analysis. Oxidative Medicine and Cellular Longevity. 2020a;2020:3580719. doi: 10.1155/2020/3580719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al. (2019).Ju Q, Zhao YJ, Dong Y, Cheng C, Zhang S, Yang Y, Li P, Ge D, Sun B. Identification of a miRNA-mRNA network associated with lymph node metastasis in colorectal cancer. Oncology Letters. 2019;18:1179–1188. doi: 10.3892/ol.2019.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju et al. (2020b).Ju Q, Zhao YJ, Ma S, Li XM, Zhang H, Zhang SQ, Yang YM, Yan SX. Genome-wide analysis of prognostic-related lncRNAs, miRNAs and mRNAs forming a competing endogenous RNA network in lung squamous cell carcinoma. Journal of Cancer Research and Clinical Oncology. 2020b;146:1711–1723. doi: 10.1007/s00432-020-03224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol et al. (2020).Kronfol MM, Jahr FM, Dozmorov MG, Phansalkar PS, Xie LY, Aberg KA, McRae M, Price ET, Slattum PW, Gerk PM, McClay JL. DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. Geroscience. 2020;42:819–832. doi: 10.1007/s11357-020-00181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020a).Li F, Wang H, Huang H, Zhang L, Wang D, Wan Y. m6A RNA methylation regulators participate in the malignant progression and have clinical prognostic value in lung adenocarcinoma. Frontiers in Genetics. 2020a;11:994. doi: 10.3389/fgene.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019).Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. American Journal of Translational Research. 2019;11:6084–6092. [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020b).Li M, Ni W, Zhang M, Liu S, Chen M, Hong X, Ma Y, Yu X, Wang W, Yang M, Hua F. MicroRNA-30/Cx43 axis contributes to podocyte injury by regulating ER stress in diabetic nephropathy. Annals of Translational Medicine. 2020b;8:1674. doi: 10.21037/atm-20-6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020c).Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, Gonzalez GM, Wang Y, Patel SP, Rana TM. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 2020c;117:20159–20170. doi: 10.1073/pnas.1918986117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020d).Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Research. 2020d;48:W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al. (2019).Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Research. 2019;47:W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2019).Ma CJ, Ding JH, Ye TT, Yuan BF, Feng YQ. AlkB homologue 1 demethylates N(3)-methylcytidine in mRNA of mammals. ACS Chemical Biology. 2019;14:1418–1425. doi: 10.1021/acschembio.8b01001. [DOI] [PubMed] [Google Scholar]
- Mukherjee et al. (2020).Mukherjee G, Bag S, Chakraborty P, Dey D, Roy S, Jain P, Roy P, Soong R, Majumder PP, Dutt S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLOS ONE. 2020;15:e0242058. doi: 10.1371/journal.pone.0242058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandelides et al. (2020).Pandelides Z, Thornton C, Faruque AS, Whitehead AP, Willett KL, Ashpole NM. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio) Geroscience. 2020;42:785–800. doi: 10.1007/s11357-020-00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilzys et al. (2019).Pilzys T, Marcinkowski M, Kukwa W, Garbicz D, Dylewska M, Ferenc K, Mieczkowski A, Kukwa A, Migacz E, Wolosz D, Mielecki D, Klungland A, Piwowarski J, Poznanski J, Grzesiuk E. ALKBH overexpression in head and neck cancer: potential target for novel anticancer therapy. Scientific Reports. 2019;9:13249. doi: 10.1038/s41598-019-49550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu et al. (2021).Qu S, Jin L, Huang H, Lin J, Gao W, Zeng Z. A positive-feedback loop between HBx and ALKBH5 promotes hepatocellular carcinogenesis. BMC Cancer. 2021;21:686. doi: 10.1186/s12885-021-08449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren et al. (2018).Ren T, Wang J, Zhang H, Yuan P, Zhu J, Wu Y, Huang Q, Guo X, Zhang J, Ji L, Li J, Zhang H, Yang H, Xing J. MCUR1-mediated mitochondrial calcium signaling facilitates cell survival of hepatocellular carcinoma via reactive oxygen species-dependent P53 degradation. Antioxid Redox Signal. 2018;28:1120–1136. doi: 10.1089/ars.2017.6990. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2019).Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Research. 2019;47:W556–W560. doi: 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul & Lindskog (2018).Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Protein Science. 2018;27:233–244. doi: 10.1002/pro.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh et al. (2020).Toh JDW, Crossley SWM, Bruemmer KJ, Ge EJ, He D, Iovan DA, Chang CJ. Distinct RNA N-demethylation pathways catalyzed by nonheme iron ALKBH5 and FTO enzymes enable regulation of formaldehyde release rates. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:25284–25292. doi: 10.1073/pnas.2007349117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020a).Wang L, Hui H, Agrawal K, Kang Y, Li N, Tang R, Yuan J, Rana TM. m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO Journal. 2020a;39:e104514. doi: 10.15252/embj.2020104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020b).Wang TX, Tan WL, Huang JC, Cui ZF, Liang RD, Li QC, Lu H. Identification of aberrantly methylated differentially expressed genes targeted by differentially expressed miRNA in osteosarcoma. Annals of Translational Medicine. 2020b;8:373. doi: 10.21037/atm.2020.02.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2021).Wu G, Yan Y, Cai Y, Peng B, Li J, Huang J, Xu Z, Zhou J. ALKBH1-8 and FTO: potential therapeutic targets and prognostic biomarkers in lung adenocarcinoma pathogenesis. Frontiers in Cell and Developmental Biology. 2021;9:633927. doi: 10.3389/fcell.2021.633927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong et al. (2017).Xiong Y, Wei Y, Gu Y, Zhang S, Lyu J, Zhang B, Chen C, Zhu J, Wang Y, Liu H, Zhang Y. DiseaseMeth version 2.0: a major expansion and update of the human disease methylation database. Nucleic Acids Research. 2017;45:D888–D895. doi: 10.1093/nar/gkw1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2020a).Xu B, Liu D, Wang Z, Tian R, Zuo Y. Multi-substrate selectivity based on key loops and non-homologous domains: new insight into ALKBH family. Cellular and Molecular Life Science. 2020a;78:129–141. doi: 10.1007/s00018-020-03594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2020b).Xu Z, Peng B, Cai Y, Wu G, Huang J, Gao M, Guo G, Zeng S, Gong Z, Yan Y. N6-methyladenosine RNA modification in cancer therapeutic resistance: current status and perspectives. Biochemical Pharmacology. 2020b;182:114258. doi: 10.1016/j.bcp.2020.114258. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2020c).Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Molecular Cancer. 2020c;19:160. doi: 10.1186/s12943-020-01278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2020).Yan Y, Zeng S, Gong Z, Xu Z. Clinical implication of cellular vaccine in glioma: current advances and future prospects. Journal of Experimental & Clinical Cancer Research. 2020;39:257. doi: 10.1186/s13046-020-01778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2019).Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C, He YY. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nature Communications. 2019;10:2782. doi: 10.1038/s41467-019-10669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2020).Zhang R, Li Y, Yu H, Liu L, Zhu C, Zuo S, Chen Z. An aberrant DNA methylation signature for predicting hepatocellular carcinoma. Annals of Translational Medicine. 2020;8:1667. doi: 10.21037/atm-20-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Zhang & Sui (2020).Zheng HX, Zhang XS, Sui N. Advances in the profiling of N(6)-methyladenosine (m(6)A) modifications. Biotechnology Advances. 2020;45:107656. doi: 10.1016/j.biotechadv.2020.107656. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2020).Zhou T, Yang M, Zhang G, Kang L, Yang L, Guan H. Long non-coding RNA nuclear paraspeckle assembly transcript 1 protects human lens epithelial cells against H2O2 stimuli through the nuclear factor kappa b/p65 and p38/mitogen-activated protein kinase axis. Annals of Translational Medicine. 2020;8:1653. doi: 10.21037/atm-20-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements for the qPCR are available in the Supplemental Files.