Abstract
Introduction
Approximately 50% of patients with non-metastatic prostate cancer are treated with radical prostatectomy (RP). While some men will be cured with surgery alone, a substantial proportion will experience cancer recurrence. Androgen-directed therapy (ADT) is an effective adjuvant therapy for patients treated with prostate radiation. Comparatively, the efficacy of ADT in surgical patients has not been well-studied.
Methods
A systematic search of MEDLINE, Embase, and the Cochrane Library from inception to July 2020 was performed. Randomized trials comparing ADT with RP vs. prostatectomy alone in patients with clinically localized prostate cancer were included. Neoadjuvant ADT and adjuvant ADT interventions were assessed separately. The primary outcomes were cancer recurrence-free survival (RFS) and overall survival (OS). Pathological outcomes following neoadjuvant ADT were also evaluated.
Results
Fifteen randomized trials met eligibility criteria; 11 evaluated neoadjuvant ADT (n=2322) and four evaluated adjuvant ADT (n=5205). Neoadjuvant ADT (three months of treatment) did not improve RFS (hazard ratio [HR] 0.90, 95% confidence interval [CI] 0.74–1.11) or OS (HR 1.22, 95% CI 0.62–2.41). Neoadjuvant ADT significantly decreased the risk of positive surgical margins (relative risk [RR] 0.48, 95% CI 0.41–0.56) and extraprostatic tumor extension (RR 0.75, 95% CI 0.64–0.89). Adjuvant ADT improved RFS (HR 0.65, 95% CI 0.45–0.93) but did not improve OS (HR 1.02, 95% CI 0.84–1.24).
Conclusions
Neoadjuvant ADT causes a pathological downstaging of prostate tumors but has not been found to delay cancer recurrence nor extend survival. Few studies have evaluated adjuvant ADT. Trials are needed to determine the benefits and harms of intermediate- or long-term adjuvant ADT for RP patients.
Introduction
Approximately 50% of patients with non-metastatic prostate cancer are treated by radical prostatectomy (RP).1 While some men will be cured with surgery alone, approximately 40% will experience cancer recurrence.2 The combination of conservative management of low-risk prostate cancer and aggressive surgical treatment of high-risk patients has resulted in a greater number of patients certain to experience cancer recurrence after surgery.3
Androgen-directed therapy (ADT) is a hormonal mediated class of treatments that encompasses orchiectomy, gonadotropin-releasing hormone (GnRH) agonists/antagonists, androgen receptor antagonists, non-steroidal estrogens, and androgen-related enzyme inhibitors. ADT prolongs survival for patients with metastatic prostate cancer.4 Approximately 90% of these men respond initially to ADT, however, treatment resistance eventually occurs due to the emergence of ADT-resistant tumor cells. While ADT is not curative for patients with metastases, randomized clinical trials have consistently shown that 6–18 months of ADT improves survival for patients receiving pelvic radiation for non-metastatic disease. As such, ADT has become the standard of care for high-risk men treated with primary radiation.5,6
Several observational studies suggest ADT may also benefit patients treated with surgery. In cohorts of RP patients, adjuvant ADT has been associated with improved cancer-specific survival, as well as reduced clinical and prostate-specific androgen (PSA) recurrence.7–10 Despite cohort studies showing a beneficial role for ADT when added to RP, clinical practice guidelines do not advocate adjuvant ADT following surgery (with the exception of those found to have lymph node metastases).
Thus, the objective of this study was to systematically review the literature for randomized controlled trials (RCTs) evaluating the use of ADT with RP for treatment of prostate cancer. Specifically, we examined whether ADT, administered before or immediately after RP improves survival and surgical outcomes when compared to RP alone in patients with clinically localized prostate cancer.
Methods
Protocol registration and eligibility
The protocol for this review was registered on PROSPERO (no. CRD42019120866) in March 2019. To guide our literature search strategy, we focused on RP patients (population), ADT (intervention), compared to placebo or standard care (comparison), for cancer-related events (outcome). RCTs evaluating neoadjuvant (started prior to RP) and adjuvant (started after RP and prior to PSA recurrence) ADT were included. ADTs included those aimed to decrease androgens (orchiectomy, GnRH agonists or antagonists, CYP17A1 inhibitors) or androgen receptor antagonists. We did not restrict inclusion to any specific dose, duration, or route of drug administration.
The primary outcome was cancer recurrence-free survival (RFS), including biochemical recurrence (i.e., PSA recurrence), local clinical recurrence, distant clinical metastasis, or receipt of salvage therapies, such as radiotherapy. Secondary outcomes included overall survival (OS), and for neoadjuvant studies, surgical margin status, pathological tumor stage, and lymph node metastases. Where reported, the harms of therapy (hot flashes, anemia, cognitive impairment, fatigue, gynecomastia, osteoporosis, obesity, cardiovascular disease, and diabetes), and patient-reported quality of life were captured and described.
Information sources
A comprehensive and systematic literature search of peer-reviewed, indexed databases MEDLINE, Embase, and the Cochrane Library was conducted to identify relevant studies. The search was last conducted on July 31, 2020.
Search
The search strategy was developed by an information specialist and a surgical urologic oncologist (Appendix; available at cuaj.ca). No language and date restrictions were imposed on full-text articles; conference abstracts were limited to 2015 onward. Relevant historical cohort or prospective studies were included in the search criteria and reviewed at the full-text stage but were removed in the final review. Other published works, including letters, editorials, and comments, were excluded. Literature and systematic review articles were also excluded; however, reference lists were sourced for additional studies.
Study selection
Titles and abstracts retrieved from the literature search were screened for inclusion by two independent reviewers (AN and AF). All duplicates were removed. The full-text articles of potentially relevant titles and abstracts were retrieved and screened for final eligibility by the same reviewers. Any disagreements were resolved by consensus, or by a third-party reviewer (RB). Studies meeting eligibility criteria were included in the systematic review and studies reporting one or more of the outcomes of interest were included in the meta-analyses. If multiple publications pertaining to the same trial were identified, the most recent publication and data were used. The study selection was documented and reported using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram.11
Data collection process
The data extraction form was developed and pilot-tested by two independent reviewers (AN and AF). Data extraction was performed in duplicate and compared. Disagreements were reconciled by consensus and consultation with a third party (RB), when necessary.
Data items
Data items included: publication traits (year of publication, journal, authorship list, country, funding sources); study population (eligibility criteria, number of patients, age, race, comorbidities); cancer characteristics (grade, stage, preoperative PSA); intervention (type of ADT, dose, route, duration, and timing of administration); comparator (standard of care vs. placebo); and outcomes of interest (PSA recurrence, local recurrence, metastases, death, receipt of pelvic radiation, and adverse events). For neoadjuvant ADT trials, surgical outcomes (margin status, lymph node involvement, pathological staging) were also collected. Cancer staging reported using the Whitmore-Jewett system was converted to the American Joint Committee on Cancer Tumor stage system as follows: stage B (0–2)=pT2, stage C (1–2)=pT3.
Risk of bias in individual studies
Two independent reviewers (AN and AF) evaluated the risk of bias for each included study. Guided by the Cochrane risk of bias tool,12 five domains were graded as “high-risk,” “moderate-risk,” or “low-risk” for bias: randomization process, deviation from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result. Any disagreements were resolved through consensus or by a third party (RB). Risk of bias for each domain, outcome, and included study were reported.
Summary measures
For dichotomous outcomes, summary measures were risk ratios with associated 95% confidence intervals (CIs). For continuous outcomes, summary measures were absolute mean difference with associated 95% CIs. For time-to-event measures, hazard ratios (HRs) were extracted from individual studies or calculated from the available data using previously described methods.13 Forest plots were used to present the outcomes of individual studies and the pooled estimate of effect across all studies with the corresponding 95% CIs. Quality-of-life outcomes were not pooled but described in the narrative analysis.
Synthesis of results
We used a random-effects model to perform all meta-analyses, as it provides a more conservative estimate than the fixed-effects model and makes more realistic assumptions regarding the existence of heterogeneity between studies. Pooling of data and data analysis was performed using RevMan 5.3 (Cochrane Collaboration, Oxford, U.K.). Neoadjuvant and adjuvant ADT were analyzed as separate interventions. Preplanned subgroup analyses were performed, stratifying patients by type of ADT (GnRH agonist/antagonist, androgen receptor antagonists, orchiectomy, or combined treatments) and cancer stage (clinical TNM staging for ADT administered prior to surgery; pathological TNM staging for ADT administered after surgery).
The heterogeneity of effect sizes (i.e., statistical heterogeneity) across included studies was examined using the I2 statistic, interpreted in categories of low (0–25%), moderate (25–50%), and substantial (50–100%) heterogeneity.
Risk of bias across studies
Funnel plots were used to assess publication bias. The quality of evidence was assessed using The Grading of Recommendations Assessment, Developing, and Evaluation (GRADE) approach.14
Results
Study selection
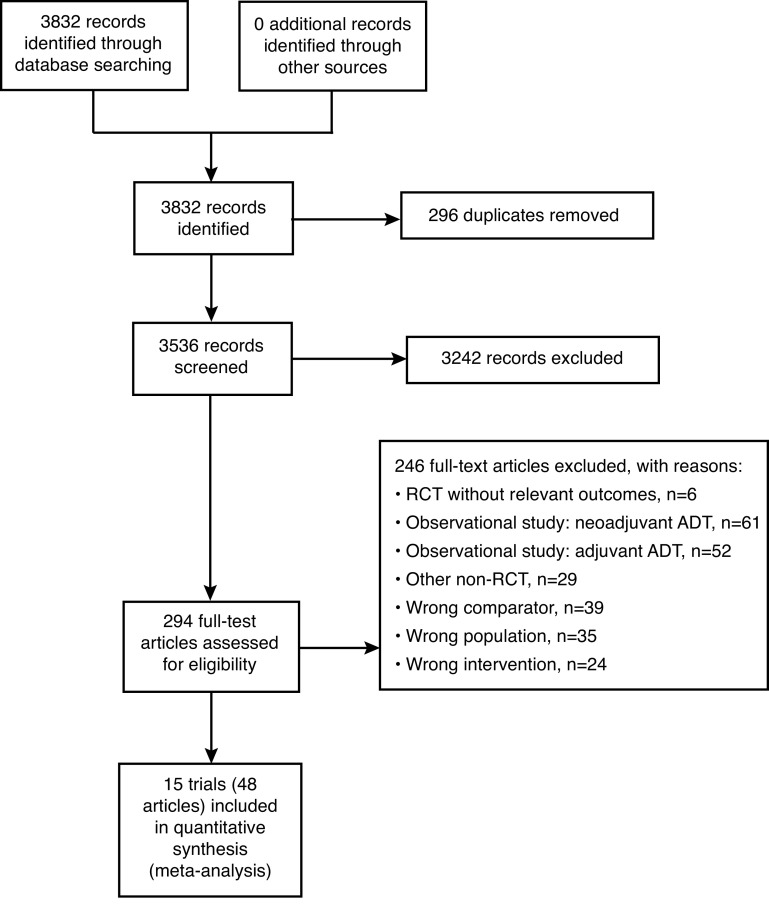
Our systematic literature search identified 3832 records. After removing 296 duplicates, 3536 titles and abstracts were screened, of which 3242 were deemed not relevant to the research question. Of the 294 full-text articles, 15 trials described in 48 articles met our eligibility criteria and were included in quantitative analysis (Fig. 1).
Fig. 1.
Selection flow diagram. ADT: androgen directed therapy; RCT: randomized controlled trial.
Study characteristics
A total of 11 RCTs evaluating neoadjuvant ADT and four RCTs evaluating adjuvant ADT published between 1980 and 2010 met our eligibility criteria.15–29 Seven trials originated in North America, seven in Europe, and one trial included sites worldwide. Study characteristics are described in Table 1.
Table 1.
Study characteristics
| Author (year) | Country (ies) | Patient population | Patients randomized (n) | Treatment | Comparator | Outcomes assessed |
|---|---|---|---|---|---|---|
| Neoadjuvant ADT | ||||||
|
| ||||||
| GnRH agonist | ||||||
| Aus (2002)15 | Sweden, Denmark | T1b-T3aNXM0 Age <75 >10-year life expectancy |
126 (initial report: 112) | Triptorelin 3.75mg IM monthly (+ cyproterone acetate 50mg BID 1 week before and 2 weeks after first injection for prophylaxis against flare) for 3 months, followed by RP (n=63) | RP (n=63) |
|
| Dalkin (1996)16 | USA | cT1c, T2a or T2b PSA >4.0 ng/ml >10-year projected survival |
61 (5 did not undergo surgery) | Goserelin 3.6ng SC monthly for 3 months, followed by RP with bilateral PLND (n=28) | RP with bilateral PLND (n=28) |
|
| Prezioso (2004)17 | Italy | Histopathologically proven prostatic carcinoma T1a-T2b >5-year life expectancy WHO performance status ≤2 |
183 randomized (16 not included in analysis) | Leuprolide acetate 3.75mg IM monthly for 3 months, followed by RP with PLND (n=81) | RP with PLND (n=86) |
|
| Androgen receptor antagonist | ||||||
| Gravina (2007)18 | Italy | cT2-T3a No previous hormonal, radio- or chemotherapy No previous investigational agents >10-year life expectancy |
119 | Bicalutamide 150 mg daily for 4 months, followed by RP (n=61) | RP (n=58) |
|
| Klotz (2003)19 | Canada | cT1-T2 Negative bone scan PSA <50 ng/ml PAP <twice normal (<1.8 u/L) |
213 (13 did not undergo surgery) | Cyproterone acetate 300mg daily for 3 months, followed by RP (n=104) | RP (n=96) |
|
| Scattoni (2006)20 | Italy | cT1c/T2a Gleason score ≤8 with HGPIN |
90 | Bicalutamide 150 mg daily for 3 months, followed by RP with bilateral PLND (n=45) | RP with bilateral PLND (n=45) |
|
| GnRH agonist + androgen receptor antagonist | ||||||
| Labrie (1997)21 | Canada | Histopathologically proven adenocarcinoma of prostate Localized PCa >10-year life expectancy |
161 | Depo-lupron 7.5 mg IM every 28 days + flutamide 250mg TID for 3 months, followed by RP (n=90) | RP (n=71) |
|
| Schulman (2000)22 | Netherlands, Belgium | T2–3 NxM0 PSA <100 ng/ml |
487 (21 not eligible, 64 not included in analysis) | Goserelin 3.6 mg SC monthly + flutamide 250 mg TID for 3 months, followed by RP (n=192) | RP (n=210) |
|
| Schulman (2000)22 | Netherlands, Belgium | T2–3 NxM0 PSA <100 ng/ml |
487 (21 not eligible, 64 not included in analysis) | Goserelin 3.6 mg SC monthly + flutamide 250 mg TID for 3 months, followed by RP (n=192) |
RP (n=210) |
|
| Selli (2002)23 | Italy | T2–T3, N0, M0 | 431 (24 did not undergo surgery, 14 not included in analysis) | Goserelin acetate 3.5 mg SC every 28 days + bicalutamide 50 mg daily for 3 months (short-term) or 6 months (long-term), followed by RP with bilateral PLND (Short-term ADT: n=143; long-term ADT: n=122) | RP with bilateral PLND (n=128) |
|
| Soloway (2002)24 | USA, Puerto Rico | cT2b Normal bone scan PSA <50 ng/ml Age <75 |
303 randomized (21 not included in analysis; 7 did not undergo surgery) | Leuprolide 7.5 mg IM monthly + flutamide 250 mg TID for 3 months, followed by RP (n=137) | RP (n=138) |
|
| Yee (2010)25 | USA | cT1–2 >10-year life expectancy Karnofsky performance status = 100 |
148 randomized (11 did not receive surgery, 1 not included in analysis) | Goserelin acetate 3.6 mg SC monthly + flutamide 250 mg TID for 3 months, followed by RP (n=72) | RP (n=64) |
|
| Overall: | 2322 patients randomized | |||||
| 11 RCTs | 2125 patients analyzed | |||||
|
| ||||||
| Adjuvant ADT | ||||||
|
| ||||||
| Androgen receptor antagonist | ||||||
| Iversen (2010) | North America Europe South Africa Australia Israel | Localized PCa: T1–2, N0/Nx) Locally advanced PCa: T3–4, or any N; or and T N+ |
4454 (64 also received RT) | RP followed by bicalutamide 150 mg for 2 years or until disease progression (n=2236) | RP followed by placebo (n=2218) |
|
| Wirth (2004)27 | Germany, Austria | pT3–4, N0 | 352 (43 not included in analysis) | RP with PLND followed by flutamide 250 mg TID (n=152) | RP with PLND followed by observation (n=157) |
|
| Byar (1980)28 | USA | Stage I: Incidentally diagnosed prostate cancer; no tumor palpable rectally Stage II: Palpable tumor localized to prostate gland No bony metastases | 299 (52 not included in analysis) | RP followed by diethylstilbestrol 5 mg (Stage 1: n=43; Stage 2: n=82) | RP followed by placebo (Stage 1: n=46; Stage 2: n= 76) |
|
| GnRH agonist or orchiectomy | ||||||
| Messing (2006)29 | USA | cT1b or T2 with lymph node metastases RP + PLND | 100 (2 not eligible) | RP with bilateral PLND followed by goserelin 3.6 mg SC monthly until local recurrence or orchiectomy (n=47) | RP with bilateral PLND followed by observation (n=51) |
|
| Overall: | 5205 patients randomized | |||||
| 4 RCTs | 5108 patients analyzed | |||||
ADT: androgen-directed therapy; BID: twice daily; GnRH: gonadotropin-releasing hormone; HGPIN: high-grade prostatic intraepithelial neoplasia; IM: intramuscular; LN: lymph node; OS: overall survival; PAP: prostatic acid phosphatase; PCa: prostate cancer; PLND: pelvic lymph node dissection; PSA: prostate-specific antigen; PSM: positive surgical margins; RCT: randomized controlled trial; RFS: recurrence-free survival; RP: radical prostatectomy; SC: subcutaneous; TID: three times daily; WHO: World Health Organization.
Neoadjuvant ADT
Eleven studies with a total of 2322 patients investigated the effect of neoadjuvant ADT before RP compared to surgical treatment alone (Table 1). From these studies, RFS, OS, surgical margin status, organ confinement of disease, and lymph node involvement were reported and evaluated.
RFS
RFS was reported in five trials involving 1277 patients (Table 1). PSA recurrence threshold varied between 0.1 ng/mL to 1.0 ng/mL, and some trials also included local recurrence, metastases, and death into a composite recurrence outcome (Supplementary Table 1; available at cuaj.ca). Median followup ranged from 4–8 years. Overall, the pooled estimate demonstrates a small but statistically insignificant reduction in postoperative recurrence with neoadjuvant ADT (pooled HR 0.90, 95% CI 0.74–1.11), with low heterogeneity between studies (Chi2=1.12, df=4 [p=0.89], I2=0%) (Fig. 2).
Fig. 2.

Forest plot of hazard ratios of recurrence-free survival for neoadjuvant androgen-directed therapy (ADT) with prostatectomy (RP) vs. RP alone. CI: confidence interval.
OS
Four studies involving 974 patients reported OS, from which two studies (339 patients) reported time to death. The pooled estimate from those two studies suggests there is no difference in OS between patients treated with three months of neoadjuvant ADT compared to surgery alone (pooled HR 1.22, 95% CI 0.62–2.41) (Supplementary Fig. 1; available at cuaj.ca). Additionally, neoadjuvant ADT did not reduce the risk of prostate cancer associated death (Supplementary Fig. 2; available at cuaj.ca).
Positive surgical margins
All eleven trials of neoadjuvant ADT examined surgical margins (Supplementary Fig. 3; available at cuaj.ca). The pooled estimate demonstrates neoadjuvant ADT for 3–6 months reduces the risk of positive surgical margins compared to no treatment (pooled relative risk [RR] 0.48, 95% CI 0.41–0.56). There was low statistical heterogeneity between trials (Chi2=14.3, df=11 [p=0.22], I2=23%). A similar risk reduction was also found when stratifying studies according to type of ADT (Supplementary Fig. 3; available at cuaj.ca).
Longer durations of neoadjuvant treatment further decreased the risk of positive margins. However, these rates were not significantly different between three and six months of treatment (25.9% with three months ADT vs. 18.7% with six months ADT, p=0.295).23
In studies that stratified patients by cancer stage, neoadjuvant ADT improved margins for both cT2 and cT3 prostate cancers.21–23 Positive margins ranged from 8–26% with neoadjuvant ADT compared to 34–47% without ADT in patients with cT2 disease, and ranged from 8–42% with neoadjuvant ADT compared to 33–76% without ADT in patients with cT3 disease.
Pathological extraprostatic extension (EPE)
Eight studies investigated pathological tumor stage, involving 1710 patients (Table 1). The administration of neoadjuvant ADT reduced the risk of EPE (pooled RR 0.75, 95% CI 0.64–0.89), however, there was substantial statistical heterogeneity in the findings (Chi2=24.4, df=8 [p=0.002], I2=67%) (Supplementary Fig. 4; available at cuaj.ca). When stratifying trials according to type of ADT, only combination ADT (GnRH and androgen receptor antagonist) demonstrated a statistically significant reduction in EPE, however, these findings were also heterogeneous (pooled RR 0.65, 96% CI 0.51–0.82; Chi2=14.4, df=4 [p=0.006], I2=72%).
Pathological lymph node metastases
Six trials involving 1395 patients investigated pathological lymph node metastases. Overall, the reduction in lymph node metastases with neoadjuvant ADT was not statistically significant (pooled RR 0.67, 95% CI 0.43–1.04) (Supplementary Fig. 5; available at cuaj.ca). The findings had low statistical heterogeneity (Chi2=5.62, df=5 [p=0.34], I2=11%). Similarly, there were no differences in distant metastases between patients receiving neoadjuvant ADT and those in the control group (Supplementary Fig. 6; available at cuaj.ca). Analysis of the three studies that used combination ADT demonstrated an overall reduction in lymph node metastases (pooled RR 0.61, 95% CI 0.42–0.90) (Supplementary Fig. 5; available at cuaj.ca).
Adverse events
Two studies described side effects from ADT. The most common adverse effects of GnRH agonists were flushing and increased perspiration,17 while three of 45 patients withdrew from bicalutamide due to gynecomastia, mastodynia, and gastrointestinal intolerance.20 One death from acute myocardial infarction was also reported in a patient receiving ADT.17
Adjuvant ADT
Four trials evaluated adjuvant ADT following RP (Table 1). These trials evaluated bicalutamide for two years, long-term flutamide, long-term diethylstilbestrol (DES), or long-term goserelin/orchiectomy.
RFS
Pooled survival data of 4906 patients from three trials demonstrated a significant benefit of adjuvant ADT on recurrence (pooled HR 0.65, 95% CI 0.45–0.93) but substantial heterogeneity was present (Chi2=20.0, df=3 [p=0.0002], I2=85%) (Fig. 3). The definition of recurrence varied between studies (Supplementary Table 1; available at cuaj.ca). Patients treated with DES were not included in this analysis, as recurrence was described in rate per 1000 patient months.
Fig. 3.
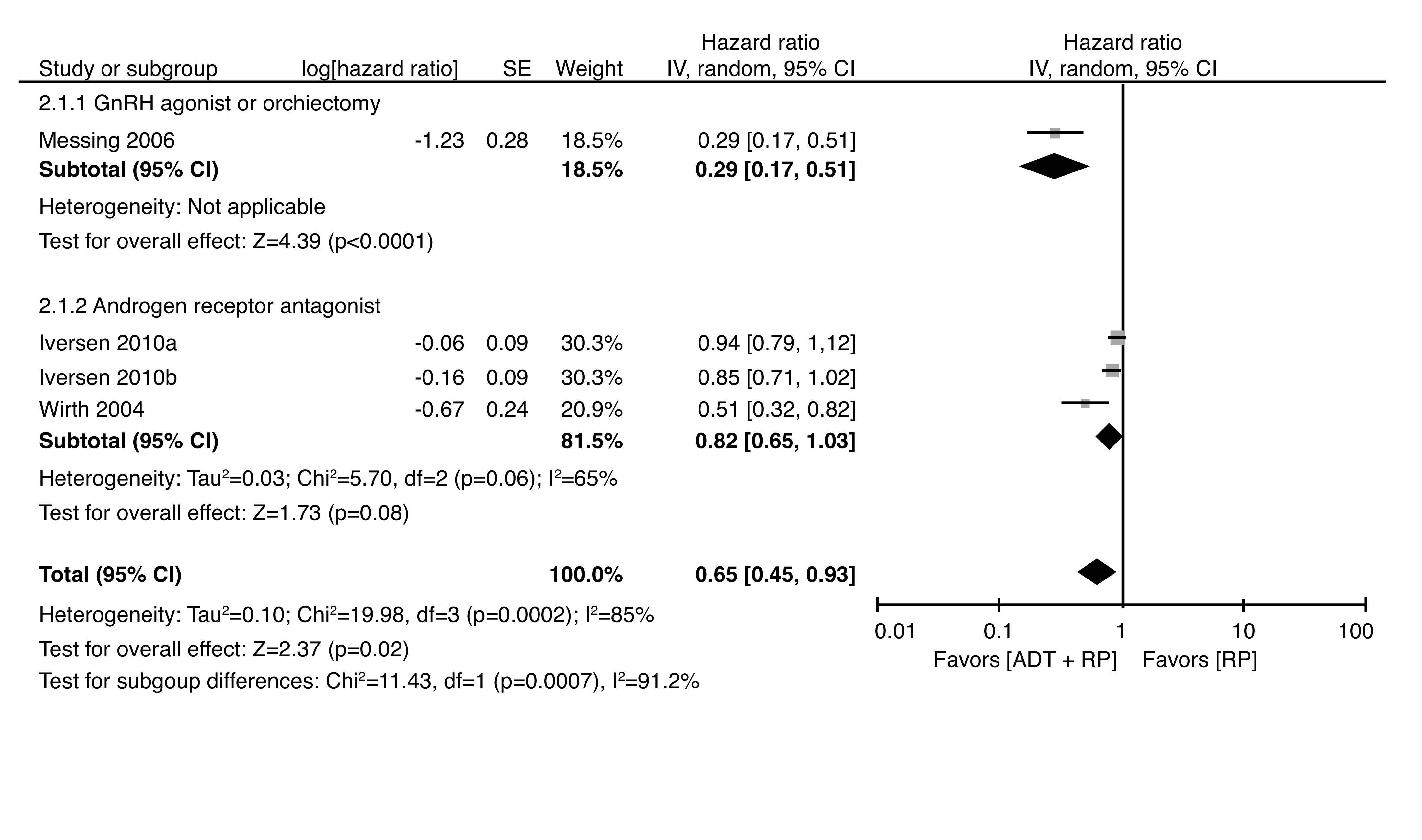
Forest plot of hazard ratios of recurrence-free survival for adjuvant androgen-directed therapy (ADT) with prostatectomy (RP) vs. RP alone. *Iversen 2010a corresponds to clinically localized prostate cancer; Iversen 2010b corresponds to locally advanced prostate cancer. CI: confidence interval.
Larger benefits of ADT were observed in patients with higher stages of disease. Among patients with pT3–T4, node-negative disease, adjuvant ADT delayed recurrence (HR 0.51, 95% CI 0.32–0.81).27 Similarly, marginal significance was achieved favoring ADT in patients with locally advanced disease (HR 0.85, 95% CI 0.71–1.01).26 In lymph node-positive patients, recurrence was also significantly reduced with adjuvant treatment (47% vs. 86%, HR 3.42, 95% CI 1.96–5.98, p<0.0001).29
OS
Data pooled from the four included trials (5205 patients) demonstrated no difference in survival between patients followed with observation and adjuvant hormone therapy (pooled HR 1.02, 95% CI 0.84–1.24). The results between studies were moderately heterogeneous (Chi2=9.75, df=5 [p=0.08], I2=49%) (Supplementary Fig. 7; available at cuaj.ca).
The only study that found an improvement in survival included patients with node-positive prostate cancer treated with orchiectomy or goserelin.29 At 11.9 years median followup, death occurred in 36% of patients treated with ADT compared to 55% of control patients. No improvement in survival was observed in trials that used DES or androgen receptor antagonists.26,27
Prostate cancer death
Three trials involving 4853 patients assessed prostate cancer death. The pooled estimate suggests there is a 40% reduction in death due to prostate cancer between patients treated with adjuvant ADT and control following RP, but this difference was not statistically significant (pooled RR 0.60, 95% CI 0.31–1.18, Chi2=13.45, df=4 [p=0.009], I2=70) (Supplementary Fig. 8; available at cuaj.ca).
Adverse events
The most common side effects of adjuvant bicalutamide was breast pain (73.7% treated vs. 7.6% placebo) and gynecomastia (68.8% treated vs. 8.3% placebo), resulting in the withdrawal of 29.3% and 10.0% of patients from the trial, respectively.26 Flutamide-related side effects included nausea, vomiting, and hepatotoxicity, and were the major reason for withdrawal from treatment.27 A significantly greater proportion of patients treated with goserelin experienced hot flashes (59% vs. 0%, p<0.001), gynecomastia (22% vs. 2%, p<0.01), gastrointestinal effects (26% vs. 6%, p<0.01), hematological effects (20% vs. 4%, p=0.02), and weight gain (17% vs. 2%, p=0.05). Increases in urinary frequency and non-specific genitourinary symptoms were also observed, however, all patients who experienced any adverse event were able to continue treatment.29 Death due to cardiovascular disease was slightly higher in patients treated with ADT (DES: 38.4% treated vs. 35.2% untreated; bicalutamide: 2.6% treated vs. 2.1% untreated; goserelin/orchiectomy: 4.3% treated vs. 2.0% untreated).26,28,29
Risk of bias within studies
Most of the included studies scored as “some concerns” on the overall risk of bias assessment (Supplementary Fig. 9; available at cuaj.ca). Randomization and allocation concealment were adequately described in six trials. Two studies were centrally randomized by telephone;25,29 one study used block randomization through an external party,18 and two described computer randomization.19,26 Labrie et al used a random permuted block randomization.21 Only two trials used a placebo to blind patients to their assigned intervention;26,28 all other studies used an observation arm as the control group. Deviations from the intended intervention were appropriately described in most studies; prevailing reasons for deviations included side effects of treatment or patients not undergoing surgery. Ten studies used appropriate intention-to-treat analysis. Only one study did not document reasons for dropout or withdrawal.28
Bias varied within the studies according to the outcome of interest. Surgical pathology is dependent on the interpretation of the pathologist; while four studies blinded pathologists to the intervention,15,20,21,24 neoadjuvant hormone therapy alters the architecture of the cells and can be difficult for pathologists to interpret without the knowledge of receipt of treatment.
RFS was determined based on a combination of PSA tests, clinical findings, and imaging findings, which rely on the interpretation of the physician. The Cochrane algorithm would classify this outcome as high-risk,12 however, all studies followed appropriate clinical practices; therefore, we considered these outcomes to be only of some concern. Measurements of OS were not affected by knowledge of treatment received. Outcomes were not measured differently between intervention and comparator groups. Few studies had an available study protocol or were registered, however, many trials had multiple reports that demonstrated consistent analysis between publications.
Risk of bias across studies
Publication bias was assessed using funnel plots (Supplementary Figs. 10, 11; available at cuaj.ca). For neoadjuvant ADT trials, small asymmetry was seen in the funnel plots for RFS and positive surgical margins. No asymmetry was observed in the funnel plots for OS, organ-confined disease, and lymph node involvement. For adjuvant ADT trials, slight asymmetry was found for all outcomes, suggesting small study effects favoring treatment with ADT. For OS, asymmetry was likely due to the different study populations, where highest benefit for OS with treatment occurred in patients with lymph node metastases.
Strength of evidence
The GRADE evidence profile is presented in Supplementary Table 2 (available at cuaj.ca). While the strength of evidence for most outcomes was low, the cumulative evidence for surgical margin status was high.
Discussion
Many patients will experience recurrence following RP.30 These patients have no preventative treatment options. Adjuvant pelvic radiation was previously considered to be an option for these patients; however, results of the ARTISTIC meta-analysis demonstrated adjuvant radiotherapy provided no additional benefit over early salvage radiotherapy (HR 0.95, 95% CI 0.75–1.21, p=0.70).31 Among potential alternatives, ADT may be considered. ADT is currently indicated for use in metastatic prostate cancer patients and as an adjunct to primary radiation for patients with non-metastatic prostate cancer, however, the utility of this treatment in surgical patients may not be well-understood.
Two systematic reviews and meta-analyses were published in 2009 and described ADT in patients undergoing RP.32,33 The collective evidence demonstrated a benefit of neoadjuvant ADT on surgical margin status, however, neither neoadjuvant nor adjuvant ADT improved RFS or OS. The findings from previous reviews were limited due to incomplete followup in a number of trials. The current review has captured additional trials and final followup results from previously described studies. More recently, Tosco et al conducted a wide-ranging systematic review focused on recent publications evaluating systemic treatments in combination with primary radiation or prostatectomy.34 In that review, only two trials of ADT with RP were identified, neither comparing ADT to observation or placebo.34 Thus, this systematic review and meta-analysis sought to comprehensively examine the literature and summarize the available evidence surrounding the use of ADT with RP.
Neoadjuvant ADT
The meta-analysis of neoadjuvant ADT trials demonstrated that short-term hormone therapy (3–6 months) prior to prostatectomy improves surgical margin status and may lead to pathological downstaging of tumors. Despite these pathological improvements, short-term ADT provided no recurrence or survival benefits.
Longer durations of neoadjuvant ADT have demonstrated greater improvements in pathological outcomes, with smaller tumor volumes and continued regression seen at 6–8 months of neoadjuvant treatment.23,35,36 It is possible that the absence of survival benefit is due to insufficient duration or intensity of ADT therapy, or that the studies were underpowered to show a difference in these outcomes.
There are at least two ongoing trials investigating neoadjuvant ADT in high-risk prostate cancer patients compared to prostatectomy alone; one studying the androgen receptor antagonist apalutamide, and the other studying combinations of ADT (Supplementary Table 3; available at cuaj.ca).37 Like the trials included in this review, neoadjuvant ADT durations being examined are between 3–6 months. One of the limitations of neoadjuvant studies is the necessary delay in surgical treatment required. For longer durations of treatment, an adjuvant or combined neoadjuvant/adjuvant approach may be preferred.
Adjuvant ADT
Only four clinical trials evaluated long-term survival outcomes of adjuvant ADT following RP. Although no improvements in OS were observed, there was delayed recurrence, particularly among patients with high risk of recurrence. Remarkably, the most recent results of these trials were published over a decade ago, and many contemporary methods of ADT have not been evaluated. Three of the four trials have limited relevance to contemporary clinical practice, as they examined treatment with either synthetic non-steroidal estrogen or androgen receptor antagonists at doses that are not currently recommended. The heterogeneity between the few studies examining adjuvant ADT limits our ability to make definitive conclusions regarding the utility of this treatment. In the absence of contemporary clinical trials, several large cohort studies have found that patients treated with immediate adjuvant ADT had delayed metastasis, and prolonged recurrence-free and cancer-specific survival, especially in higher-risk patients.7–9 Additionally, RCTs have shown 6–36 months of ADT prolongs survival of patients receiving primary radiation.38–40 Therefore, modern trials are needed to determine if ADT in the postoperative setting is beneficial.
Currently, there are few trials evaluating adjuvant therapy following RP publicly registered on clinicaltrial.gov (Supplementary Table 3; available at cuaj.ca). One, registered in Russia and China, has been successfully completed (NCT01753297). This study evaluates the effect of nine months of triptorelin immediately following prostatectomy in high-risk prostate cancer patients. Results from this study have yet to be published. Two other studies evaluating disease recurrence following longer durations of ADT (leuprolide acetate or apalutamide) are ongoing, with final reports expected in 2027.
Salvage ADT
A number of trials suggest some benefit of ADT as a salvage therapy. The ARTS trial investigated treatment with dutasteride in 294 patients with biochemical failure following RP or radiation therapy.41 Over the 24-month treatment period, compared to placebo, salvage dutasteride significantly delayed PSA doubling time (RR 0.34, 95% CI 0.23–0.50) and disease recurrence (RR 0.41, 95% CI 0.25–0.67), (defined as PSA doubling time ≤3 months, PSA>20 ng/mL for subjects who underwent radiotherapy, or PSA>10 ng/mL for subjects who underwent prostatectomy with ≥50% increase from baseline, clinical recurrence, metastatic disease, or additional prostate cancer rescue therapy).41
The timing of salvage treatment has also been shown to influence survival outcomes. One study evaluated the effects of hormone deprivation in 120 men with detectable PSA following prostatectomy.42 Patients were either treated with two years of finasteride or observed for one year and treated with finasteride for the subsequent year. Though not statistically significant, fewer recurrences were observed in the early treatment group (12% vs. 19%).42 In another study of men with PSA relapse after curative treatment or disease not suitable for treatment, those who immediately received ADT had better OS compared to those who received ADT after two years from randomization (HR 0.55, 95% CI 0.30–1.00).43 To our knowledge, there are currently no ongoing trials investigating the use of ADT as a salvage monotherapy following prostatectomy, however, trials using combinations of ADT with salvage radiation therapy and/or chemotherapy are ongoing.
Other systemic treatments
The perioperative use of non-ADT treatments has been investigated and is an emerging area of research. These therapies include chemotherapy, immunotherapy, and poly ADP ribose polymerase (PARP) inhibitors, and are being studied both with and without concomitant ADT.34 The use of chemotherapy alone in patients undergoing prostatectomy did not provide survival benefit, while initial results suggest combined chemotherapy and hormone therapy may improve RFS.44,45 Small trials of vaccine-based immunotherapies and checkpoint inhibitors in localized prostate cancer have shown these therapies result in histological tumor response, however, there are no available data on long-term outcomes at this time.46
Limitations
Study level
It is important to recognize that most of the studies included in this review were found to have some risk or high risk of bias. The findings presented may be influenced by lack of blinding, patient dropouts, and missing outcome data. Of note, the Early Prostate Cancer study, the largest trial included in this review, has a number of issues that contribute to the heterogeneity of our findings.26 A small proportion of patients who had been treated with radiation therapy were included in the comparison, and North American sites of the trial followed different protocols (primarily, excluding patients with lymph node involvement).26 Moreover, many of these trials evaluated older ADT agents, including anti-androgen monotherapy, which are currently not recommended for use in the clinical setting. Overall, eight of the eleven neoadjuvant ADT trials and only one of the four adjuvant ADT trials examined current methods of ADT (orchiectomy, GnRH agonist/antagonist).
Review level
Disease recurrence and OS were reported either as dichotomous outcomes at the final followup or as survival curves. Most of the survival curves presented did not provide the number at risk at interval time points; as such, it was not feasible to estimate the number of events at a common time point between studies. Thus, the relative risk of death and recurrence incorporates data from a range of time points spanning several years. While robust statistical measures were used to extract data from Kaplan-Meier curves, these methods still require estimations of the original curves and may lead to some imprecision in calculated HRs. For most studies investigating neoadjuvant ADT, time from randomization to surgery was not reported. Two studies indicated patients who did not receive ADT underwent surgery approximately six weeks after randomization, and patients receiving ADT underwent surgery approximately one week following treatment cessation.19,21 This may result in skewed survival outcomes favoring neoadjuvant ADT. Finally, patient populations and interventions varied widely between studies, particularly among the four adjuvant ADT trials. Different disease stages, forms of ADT, and length of treatment were considered in this review. Besides the limitation of the small number of trials, the heterogeneity between studies suggests a true estimate of the effect of adjuvant ADT cannot be confidently determined.
Conclusions
Based on the available literature, short-term neoadjuvant ADT causes a pathological downstaging of prostate tumors but does not reduce the risk of cancer recurrence or extend survival. The available studies evaluating adjuvant ADT are heterogeneous, however, suggest a potential role of ADT in delaying disease recurrence. Adverse events associated with perioperative ADT are generally tolerable by patients and may be reversible upon ADT cessation. Further trials are needed to evaluate the benefits and harms of ADT in surgical patients.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
See related commentary on page 280
Competing interests: Dr. Fergusson is a member of Data Safety Monitoring Board for Altimmune. Dr. Cagiannos has been an advisory board member for Abbvie, Bayer, and Janssen; and had received honoraria from Astellas, Bayer, and Ferring. Dr. Morash has been an advisory board member for Amgen, Astellas, Bayer, Ferring, Janssen, Sanofi, and TerSera. Dr. Breau participated in a bladder cancer advisory board for Ferring. No other authors report any competing personal or financial interests related to this work.
Funding: This study was supported by grant funding from the Ottawa Chapter of the TELUS Ride For Dad and the Nation Valley ARFD, the University of Ottawa Department of Surgery, and The Ottawa Hospital Academic Medical Organization.
This paper has been peer-reviewed.
To answer the multiple-choice questions associated with this article, go to: www.cuasection3credits.org/cuajaugust2021. This program is an Accredited Self-Assessment Program (Section 3) as defined by the Maintenance of Certification Program of The Royal College of Physicians & Surgeons of Canada, and approved by the Canadian Urological Association. Remember to visit MAINPORT (www.mainport.org/mainport/) to record your learning and outcomes. You may claim a maximum of 1 hour of credit.
References
- 1.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montroy J, Elzayat E, Morash C, et al. Long-term patient outcomes from the first year of a robotic surgery program using multi-surgeon implementation. Can Urol Assoc J. 2018;12:38–43. doi: 10.5489/cuaj.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witherspoon L, Lau JL, Breau RH, et al. Reducing overtreatment of prostate cancer by radical prostatectomy in Eastern Ontario: A population-based cohort study. CMAJ Open. 2018;6:E197–E201. doi: 10.9778/cmajo.20170149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 5.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: Risk stratification, shared decision-making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 6.Voog JC, Paulus R, Shipley WU, et al. Cardiovascular mortality following short-term androgen deprivation in clinically localized prostate cancer: An analysis of RTOG 94-08. Eur Urol. 2016;69:204–10. doi: 10.1016/j.eururo.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui SA, Boorjian SA, Blute ML, et al. Impact of adjuvant androgen deprivation therapy after radical prostatectomy on the survival of patients with pathological T3b prostate cancer. BJU Int. 2011;107:383–8. doi: 10.1111/j.1464-410X.2010.09565.x. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui SA, Boorjian SA, Inman B, et al. Timing of androgen deprivation therapy and its impact on survival after radical prostatectomy: A matched cohort study. J Urol. 2008;179:1830–7. doi: 10.1016/j.juro.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–7. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 10.Sato YT, Fukuhara H, Suzuki M, et al. Long-term results of radical prostatectomy with immediate adjuvant androgen deprivation therapy for pT3N0 prostate cancer. BMC Urol. 2014;14:13. doi: 10.1186/1471-2490-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schünemann H, Higgins J, Vist G, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins J, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019) Cochrane; 2019. [Accessed Jan. 8, 2021]. Available at: www.training.cochrane.org/handbook. [DOI] [Google Scholar]
- 15.Aus G, Abrahamsson PA, Ahlgren G, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: A 7-year followup of a randomized controlled trial. BJU Int. 2002;90:561–6. doi: 10.1046/j.1464-410X.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 16.Dalkin BL, Ahmann FR, Nagle R, et al. Randomized study of neoadjuvant testicular androgen ablation therapy before radical prostatectomy in men with clinically localized prostate cancer. J Urol. 1996;155:1357–60. doi: 10.1016/S0022-5347(01)66266-9. [DOI] [PubMed] [Google Scholar]
- 17.Prezioso D, Lotti T, Polito M, et al. Neoadjuvant hormone treatment with leuprolide acetate depot 3.75 mg and cyproterone acetate, before radical prostatectomy: A randomized study. Urol Int. 2004;72:189–95. doi: 10.1159/000077113. [DOI] [PubMed] [Google Scholar]
- 18.Gravina GL, Festuccia C, Galatioto GP, et al. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology. 2007;70:728–33. doi: 10.1016/j.urology.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Klotz LH, Goldenberg SL, Jewett MAS, et al. Long-term followup of a randomized trial of 0 vs. 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–4. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 20.Scattoni V, Montironi R, Mazzucchelli R, et al. Pathological changes of high-grade prostatic intraepithelial neoplasia and prostate cancer after monotherapy with bicalutamide 150 mg. BJU Int. 2006;98:54–8. doi: 10.1111/j.1464-410X.2006.06204.x. [DOI] [PubMed] [Google Scholar]
- 21.Labrie F, Cusan L, Gomez JL, et al. Neoadjuvant hormonal therapy: The Canadian experience. Urology. 1997;49:S56–64. doi: 10.1016/S0090-4295(97)00170-2. [DOI] [PubMed] [Google Scholar]
- 22.Schulman CC, Debruyne FMJ, Forster G, et al. 4-year followup results of a European, prospective, randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. Eur Urol. 2000;38:706–13. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 23.Selli C, Montironi R, Bono A, et al. Effects of complete androgen blockade for 12 and 24 weeks on the pathological stage and resection margin status of prostate cancer. J Clin Pathol. 2002;55:508–13. doi: 10.1136/jcp.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–6. doi: 10.1016/S0022-5347(05)65393-1. [DOI] [PubMed] [Google Scholar]
- 25.Yee DS, Lowrance WT, Eastham JA, et al. Long-term followup of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU Int. 2010;105:185–90. doi: 10.1111/j.1464-410X.2009.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iversen P, McLeod DG, See WA, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: Final results from the bicalutamide Early Prostate Cancer programme at a median followup of 9.7 years. BJU Int. 2010;105:1074–81. doi: 10.1111/j.1464-410X.2010.09319.x. [DOI] [PubMed] [Google Scholar]
- 27.Wirth MP, Weissbach L, Marx FJ, et al. Prospective randomized trial comparing flutamide as adjuvant treatment vs. observation after radical prostatectomy for locally advanced, lymph node-negative prostate cancer. Eur Urol. 2004;45:267–70. doi: 10.1016/j.eururo.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Byar D. VACURG studies of post-prostatectomy survival. Scand J Urol Nephrol Suppl. 1980;55:113–6. [PubMed] [Google Scholar]
- 29.Messing EM, Manola J, Yao J, et al. Immediate vs. deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 30.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 31.Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020 doi: 10.1016/S0140-6736(20)31952-8. S0140-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shelley MD, Kumar S, Coles B, et al. Adjuvant hormone therapy for localised and locally advanced prostate carcinoma: A systematic review and meta-analysis of randomised trials. Cancer Treat Rev. 2009;35:540–6. doi: 10.1016/j.ctrv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Shelley MD, Kumar S, Wilt T, et al. A systematic review and meta-analysis of randomized trials of neo-adjuvant hormone therapy for localized and locally advanced prostate carcinoma. Cancer Treat Rev. 2009;35:9–17. doi: 10.1016/j.ctrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Tosco L, Briganti A, D’Amico AV, et al. Systematic review of systemic therapies and therapeutic combinations with local treatments for high-risk localized prostate cancer. Eur Urol. 2019;75:44–60. doi: 10.1016/j.eururo.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Gleave ME, Goldenberg SL, Chin JL, et al. Randomized comparative study of 3- vs. 8-month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. J Urol. 2001;166:500–7. doi: 10.1016/S0022-5347(05)65971-X. [DOI] [PubMed] [Google Scholar]
- 36.Van der Kwast TH, Têtu B, Candas B, et al. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: Three vs. six months of endocrine therapy. Urology. 1999;53:523–9. doi: 10.1016/S0090-4295(98)00542-1. [DOI] [PubMed] [Google Scholar]
- 37.Sterling J, Chua K, Patel H, et al. Interim analysis of phase 2 randomized prospective study on neoadjuvant apalutamide/abiraterone acetate with prednisone and the feasibility of performing nerve-sparing radical prostatectomy in men with high-risk prostate cancer ( NCT02949284) J Urol. 2020;203:e249. doi: 10.1097/JU.0000000000000844.09. [DOI] [Google Scholar]
- 38.Bolla M, De Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 39.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 40.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs. radiation alone for prostate cancer: A randomized trial. JAMA. 2008;299:289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 41.Schröder F, Bangma C, Angulo JC, et al. Dutasteride treatment over 2 years delays prostate-specific antigen progression in patients with biochemical failure after radical therapy for prostate cancer: Results from the randomised, placebo-controlled Avodart After Radical Therapy for Prostate Cancer Study (ARTS) Eur Urol. 2013;63:779–87. doi: 10.1016/j.eururo.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Andriole G, Lieber M, Smith J, et al. Treatment with finasteride following radical prostatectomy for prostate cancer. Urology. 1995;45:491–7. doi: 10.1016/S0090-4295(99)80021-1. [DOI] [PubMed] [Google Scholar]
- 43.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): A randomized, multicenter, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–37. doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 44.Eastham JA, Heller G, Halabi S, et al. CALGB 90203 (Alliance): Radical prostatectomy with or without neoadjuvant chemohormonal therapy in men with clinically localized, high-risk prostate cancer. J Urol. 2019;201:e997. doi: 10.1097/01.JU.0000557504.00464.d6. [DOI] [Google Scholar]
- 45.Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine vs. androgen deprivation therapy alone for high-risk localized prostate cancer (GETUG 12): A phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–94. doi: 10.1016/S1470-2045(15)00011-X. [DOI] [PubMed] [Google Scholar]
- 46.Patel D, McKay R, Parsons JK. Immunotherapy for localized prostate cancer: the next frontier? Urol Clin North Am. 2020;47:443–56. doi: 10.1016/j.ucl.2020.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.