Abstract
Purpose of Review
Acute care re-utilization, i.e., hospital readmission and post-discharge Emergency Department (ED) use, is a significant driver of healthcare costs and a marker for healthcare quality. Diabetes is a major contributor to acute care re-utilization and associated costs. The goals of this paper are to (1) review the epidemiology of readmissions among patients with diabetes, (2) describe models that predict readmission risk, and (3) address various strategies for reducing the risk of acute care re-utilization.
Recent Findings
Hospital readmissions and ED visits by diabetes patients are common and costly. Major risk factors for readmission include sociodemographics, comorbidities, insulin use, hospital length of stay (LOS), and history of readmissions, most of which are non-modifiable. Several models for predicting the risk of readmission among diabetes patients have been developed, two of which have reasonable accuracy in external validation. In retrospective studies and mostly small randomized controlled trials (RCTs), interventions such as inpatient diabetes education, inpatient diabetes management services, transition of care support, and outpatient follow-up are generally associated with a reduction in the risk of acute care re-utilization. Data on readmission risk and readmission risk reduction interventions are limited or lacking among patients with diabetes hospitalized for COVID-19. The evidence supporting post-discharge follow-up by telephone is equivocal and also limited.
Summary
Acute care re-utilization of patients with diabetes presents an important opportunity to improve healthcare quality and reduce costs. Currently available predictive models are useful for identifying higher risk patients but could be improved. Machine learning models, which are becoming more common, have the potential to generate more accurate acute care re-utilization risk predictions. Tools embedded in electronic health record systems are needed to translate readmission risk prediction models into clinical practice. Several risk reduction interventions hold promise but require testing in multi-site RCTs to prove their generalizability, scalability, and effectiveness.
Keywords: Diabetes, Readmission, Predictive models
Introduction
Acute care re-utilization, i.e., hospital readmission and post-discharge Emergency Department (ED) use, is a significant driver of healthcare costs and a marker for healthcare quality. Hospitals in the USA have been subject to financial penalties for excess readmission rates under the Hospital Readmissions Reduction Program since October of 2012 [1]. Risk contracts, often in the context of Accountable Care Organizations, incentivize hospitals and outpatient practices to reduce high-cost outcomes such as acute care re-utilization [2, 3]. Furthermore, patients view acute care re-utilization as a failure of the healthcare system that is often related to inadequate support. [4, 5] Given the prevalence of diabetes in the general population and hospitalized patients, diabetes is a major contributor to acute care re-utilization and associated costs. The annual cost of readmissions within 30 days of discharge (30-day readmission) is $20–25 billion based on the most inclusive readmission rates of 16.0 to 20.4% among patients with diabetes in the USA [6–9••, 10, 11]. To efficiently implement interventions intended to reduce readmission risk and the associated costs in this population, it is critically important to understand the causes of readmissions as well as to identify patients at higher risk. Herein, we review the growing body of literature focused on recognizing risk factors for and predicting readmission, which garners much more attention than ED use, as well as preventing acute care re-utilization among patients with diabetes.
Epidemiology of Readmissions
Diabetes Is a Risk Factor for Readmission
In the USA, more than 10 million hospital discharges of patients with diabetes accounted for 28% of all discharges in 2016 [12]. At that time, approximately 10% of the US population had diabetes, a difference that reflects the hospitalization risk associated with diabetes [13, 14]. Studies have reported that diabetes increases the risk of a 30-day readmission, the most commonly used metric, by at least 17% and up to 2.5-fold [7, 15, 16]. The increased risk of readmission is most pronounced when diabetes is the primary reason for hospitalization, with 30-day readmission rates 21 to 37% higher than discharges with diabetes as a secondary diagnosis [15, 17]. Among patients hospitalized for COVID-19, diabetes appears to be associated with a higher risk of 30-day readmission according to the one study that has examined this question to date [18].
Risk Factors for Readmission Among Patients with Diabetes
Dozens of risk factors for readmission have been identified among patients with diabetes [19–22•]. A recent systematic review and meta-analysis of 23 studies published through 2018 on more than 30 million patients provides the most complete data on a small set of commonly assessed risk factors [22•]. The strongest risk factors identified were insurance type (Medicare/Medicaid vs. private), insulin use, hospital length of stay (LOS), and certain comorbidities (heart failure, respiratory disease, depression, and renal disease). There is a modestly increased risk of readmission associated with male gender, non-white race, and higher age. One of the strongest risk factors not considered in the meta-analysis is a history of readmission or multiple hospitalizations [10, 19, 20, 23]. Abnormal values of serum sodium, creatinine, and hematocrit/hemoglobin are also reliable predictors of readmission [19, 20, 23]. Several studies have shown that comorbidity burden, including microvascular and macrovascular diabetic complications, is associated with readmission risk [19–21, 24]. Other diabetes-related factors associated with readmission are inpatient hypoglycemia, hyperglycemia, and glucose variability. Specifically, one cohort study found that inpatient blood glucose values <70 mg/dL or >180 mg/dL are associated with 30-day readmission, with more extreme values associated with greater risk [20]. In two large cohort studies, lower blood glucose values <90 mg/dL and higher glycemic variability during the 24 h before discharge were associated with a progressively greater risk of 30-day readmission [25, 26]. Whether or not hemoglobin A1c (A1C) is associated with readmission remains unclear, as studies have produced conflicting results in opposing directions or no association at all. [10, 19, 27, 28] Unfortunately, these studies are limited by a high proportion of patients missing A1C values, an issue that has been recognized elsewhere [29]. Lastly, one study found that low exercise capacity as measured by treadmill stress test is associated with readmission risk up to 1 year following discharge among patients with diabetes [30].
It is notable that very few of these readmission risk factors are modifiable, and most are probably markers of risk rather than causes of readmission per se. The primary diagnosis associated with readmission is not a particularly useful target for intervention either. When we analyzed a retrospective cohort of 44,203 discharges with a diagnosis of diabetes, the most common reasons for 30-day readmission (diabetes, cardiovascular disease [CVD], kidney disease, and post-procedure complications) only accounted for one-third of the primary diagnoses [9••]. In another retrospective cohort study of more than 100,000 patients, a secondary discharge diagnosis of diabetes was associated with a significantly increased risk of readmission for only two of the 10 most common Medicare Severity Diagnosis Related Groups (chest pain and gastrointestinal disorders) [15]. Other than diabetes, the medical causes of readmission among diabetes patients are not overwhelmingly different from the causes among patients without diabetes [15]. Furthermore, acute decompensation of chronic disease does not explain the majority of readmissions among diabetes patients. This is consistent with the observation that acute care re-utilization by any patient population tends to be a complex phenomenon with multiple causes, including hospital factors and social determinants of health in addition to health status [31]. In order to be successful, interventions intended to reduce the risk of acute care re-utilization require multiple components to address the multiple causes. In addition, more research is needed to explore how social determinants of health affect readmission risk given that current literature suggests a link but is limited. [4, 20]
Acute Care Re-Utilization Among Patients Hospitalized for Diabetic Ketoacidosis
Patients hospitalized for diabetic ketoacidosis (DKA) are distinct from the majority of inpatients with diabetes, who are not hospitalized primarily for diabetes. It has long been recognized that DKA patients are subject to acute care re-utilization, often due to repeat episodes of DKA. One study found that 12% of adults hospitalized for DKA in the USA were readmitted within 30 days and that 41% of the readmissions were attributed to recurrent DKA [32]. A classic case series of 45 patients who experienced multiple episodes of DKA described several still-relevant causes for acute care re-utilization, including poor health literacy, inadequate ambulatory follow-up care, social determinants of health, psychiatric comorbidities, and reimbursement incentives that favor hospitalization over ambulatory care [33]. Socioeconomics, psychiatric comorbidities, and drug use have been confirmed as risk factors in more recent studies [32, 34, 35]. There is some evidence that inpatient care by an endocrinologist is associated with a lower risk of readmission than care by generalists [36, 37]. A recent review covers this topic in more detail. [38]
Strategies to Predict Readmission Risk Among Patients with Diabetes
Several studies have developed models for predicting readmission risk among patients with diabetes. The most commonly used modeling approach is multivariable logistic regression. More recently, numerous machine learning techniques have been employed to develop such predictive models. Herein, we focus on models for predicting the risk of 30-day readmission among patients with diabetes published in the past 5 years (Table 1). We are not aware of any models that predict the risk of ED visits in addition to readmissions.
Table 1.
Strategies for predicting readmission risk within 30 days of discharge
| Model name | Sample size | Population | Validation | Validation C-statistic | # of variables | Variables in model |
|---|---|---|---|---|---|---|
| Logistic regression models | ||||||
| Rico, 2016 [39] |
4,879 patients 6,158 discharges |
Adults with a discharge diagnosis of T2D | None | 0.73 | 6 | Age, marital status, Charlson comorbidity index, LOS, # of admissions, discharge disposition |
|
Strengths: outcome was unplanned readmission, few variables Weaknesses: small sample size, limited to T2D, not validated |
||||||
| DERRI, 2016 [9••] |
17,284 patients 44,203 discharges 42,800 patients |
Adults on diabetes medication preadmission or discharge diagnosis of diabetes | Internal | 0.69 | 10 | Employment status, living within 5 miles of the hospital, preadmission insulin use, burden of macrovascular diabetes complications, admission serum hematocrit, creatinine, and sodium, having a hospital discharge within 90 days before admission, most recent discharge status up to 1 year before admission, and a diagnosis of anemia |
| 2018 [10] | 105,960 discharges | External | 0.63 | |||
|
Strengths: decent sample size, only 10 variables, uses only pre-discharge variables, available as a web app, externally validated, racially and ethnically diverse sample Weaknesses: single center |
||||||
| DERRI vs HOSPITAL, 2019 [17] |
200 patients 200 discharges |
Adults on diabetes medication preadmission or discharge diagnosis of diabetes | External | DERRI 0.80 HOSPITAL 0.73 |
DERRI 10 HOSPITAL 8 |
HOSPITAL: hemoglobin level at discharge, discharge from oncology service, sodium level at discharge, procedure during hospital stay, index admission type, # of admissions during the last 12 months, LOS |
|
HOSPITAL strengths: decent sample size, only 8 variables, available as a web app, outcome was potentially avoidable readmissions, developed in patients discharged from any medical service (not limited to diabetes), externally validated [40, 41] Weaknesses: single center, not usable until day of discharge [40] |
||||||
| Collins, 2017 [42] | 63,237 patients | Adult Medicare Advantage patients with a discharge diagnosis of T2D | Internal | 0.82 | 14 | Age, sex, # ED visits, LOS, diseases of urinary system, fluid and electrolyte disorders, diseases of WBCs, other nervous system disorders, diseases of the heart, other lower respiratory diseases, gastrointestinal hemorrhage, liver diseases, hemodialysis |
|
Strengths: large sample size, good performance, nearly 200 variables examined Weaknesses: did not analyze multiple hospitalizations per patient, limited target population |
||||||
| DERRI CVD, 2017 [43] | 8,189 discharges | Adults with primary discharge diagnosis of CVD and diabetes medication preadmission treatment with diabetes medication or diagnosis of diabetes | Internal | 0.68 | 10 | Living within 5 miles of the hospital; employment status; having a hospital discharge within 90 days before admission; lower educational attainment; burden of macrovascular diabetes complications; preadmission sulfonylurea therapy, preadmission metformin; higher serum creatinine; lower serum albumin; schizophrenia or mood disorders |
|
Strengths: only 10 variables, uses only pre-discharge variables, racially and ethnically diverse sample Weaknesses: single center, modest sample size, not available as a web app, not externally validated |
||||||
| Karunakaran (DERRI-Plus), 2018 [20] | 17,284 patients | Adults on diabetes medication preadmission or discharge diagnosis of diabetes | None | 0.82 | 27 | DERRI variables plus: no follow-up visit within 30 days post-index discharge, Charleston comorbidity index, LOS, insurance status, sex, race/ethnicity, preadmission glucocorticoid, preadmission thiazolidinedione, gastroparesis, WBC count, blood glucose, serum albumin, urgency of admission, cardiac dysrhythmias, schizophrenia or mood disorder, fluid or electrolyte disorder, and blood transfusion |
|
Strengths: decent sample size, racially and ethnically diverse sample Weaknesses: single center, not available as a web app, not validated, not feasible for manual POC use |
||||||
| Machine learning models | ||||||
| Alloghani, 2018 [44] | 78,363 discharges | Adults with diabetes- related hospitalization and LOS 1–14 days, treated with diabetes medications | Internal | 0.64 | 5 | # of inpatient stays, # of emergency visits, admission source id, discharge disposition, # of diagnoses |
|
Strengths: large sample size Weaknesses: few variables, administrative source of data with missing values (e.g., weight in 97% of patients), narrow inclusion criteria (only considering LOS between 1 and 14 days), not available as a web app, not usable until day of discharge | ||||||
| Alturki, 2019 [24] | 71,518 patients 101,766 discharges | Adults with diabetes- related hospitalization and LOS 1–14 days | None | 0.97 | 15 | LOS, # of procedures, # of diagnoses, # of lab procedures, # of medications, use of specific diabetes medications |
|
Strengths: highly accurate Weaknesses: not validated, administrative source of data with missing values (e.g., weight in 97% of patients), narrow inclusion criteria (only considering LOS between 1 and 14 days), not available as a web app, not feasible for manual POC use, not usable until day of discharge | ||||||
| Sarthak, 2020 [45] |
70,000 patients 100,000 discharges |
Adults with diabetes- related hospitalization and LOS 1–14 days | Internal | 0.97 | 35 | Count of medications (# of adjustments), diabetes medication, change in medication, comorbid diagnoses, insulin, # of lab procedures, medical specialty, discharge disposition, # of medications, payer, age, admission source, race, LOS, AIc, gender, admission type, # of diagnoses, # of procedures, service utilization (sum of inpatient, outpatient, and emergency visits), # inpatient, # outpatient, max glucose serum, # emergency, use of specific diabetes medication |
|
Strengths: highly accurate Weaknesses: administrative source of data with missing values (e.g., weight in 97% of patients), narrow inclusion criteria (only considering LOS between 1 and 14 days), not available as a web app, not feasible for manual POC use, not usable until day of discharge |
||||||
| Ossai, 2020 [46] | 78,363 discharges | Adults with diabetes- related hospitalization and LOS 1–14 days, treated with diabetes medications | Internal | 0.84 | 9 | Age, LOS, insulin, use of specific diabetes medications |
|
Strengths: good accuracy, removed variables from consideration that were missing greater than 90% of values Weaknesses: administrative source of data with narrow inclusion criteria (only considering LOS between 1 and 14 days), not available as a web app, not usable until day of discharge | ||||||
DERRI Diabetes Early Readmission Risk Indicator, ED emergency department, LOS length-of-stay, POC point-of-care, T2D type 2 diabetes, WBC white blood cells
Multivariable Logistic Regression Models for Predicting Readmission Risk
To our knowledge, the first model specifically designed to predict the risk of all-cause 30-day readmission among diabetes patients was the Diabetes Early Readmission Risk Indicator (DERRITM) [9••]. This model is based on 10 easily obtainable data points available at the time of admission and is converted into a point-of-care tool that is freely and publicly available in a web-based application [47]. The DERRITM was developed in a retrospective cohort of 44,203 discharges with a diagnosis of diabetes discharged from a single urban academic medical center between 2004 and 2012. The 30-day readmission rate was 20.4%. In the internal validation sample, the C-statistic (i.e., the area under the receiver operating characteristic curve) was 0.69 [9••]. Of note, a C-statistic of 0.5 is equivalent to chance, while a C-statistic of 1 represents perfect discrimination ranking cases (readmissions) as higher risk than non-cases. The C-statistic for a predictive model is typically between 0.6 and 0.85 [48]. The strongest readmission risk predictors were having a discharge within 90 days before the index admission and employment status. In a follow-up study, we externally validated the DERRITM in another retrospective cohort of 105,974 discharges with diabetes from multiple hospitals. The 30-day readmission rate in this cohort was 18.0%, and the DERRITM had a C-statistic of 0.63 [10]. We concluded that the discrepancy in predictive performance may be accounted for by the significant population differences between the internal validation and external validation samples, including sociodemographics and clinical parameters.
In contrast to our external validation study, however, the DERRITM was independently tested in another study that yielded better predictive performance than that reported in the original cohort. [23] In a case control study of 200 adults with diabetes hospitalized at an academic medical center in Arizona between 2014 and 2017, the DERRITM was compared with the HOSPITAL model, a 30-day readmission risk prediction model not specifically developed in patients with diabetes. The DERRITM had a C-statistic of 0.80, while the HOSPITAL had a C-statistic of 0.73, a difference that was nearly statistically significant (p = 0.055). Of note, the HOSPITAL model is also available as a risk prediction tool in a web-based application [49].
In an effort to improve upon the performance of the DERRITM, we developed a version among patients with diabetes specifically hospitalized for cardiovascular disease (DERRI-CVDTM) [43]. In this subset of the original DERRITM retrospective cohort, the 30-day readmission rate was 19.9%, and the DERRI-CVDTM C-statistic was 0.68. Readmission risk predictors shared between the DERRITM and DERRI-CVDTM are having a discharge within 90 days before the index admission, the number of macrovascular complications, admission serum creatinine, employment status, and living within 5 miles of the hospital. These predictors in diabetes patients with or without active CVD common to both models likely represent the more important markers of readmission risk.
In a complementary effort to improve upon the performance of the DERRITM, we developed a new model in the original DERRITM cohort without limiting the number of variables or excluding information obtained after hospital discharge [20]. This new model, DERRIplus, consists of 27 independent predictors of 30-day readmission, the strongest of which are lacking an outpatient visit after discharge, LOS, discharge status, having a prior discharge within 90 days before the index admission, employment status, race/ethnicity, type of health insurance, comorbidity burden, and abnormal admission laboratory values. The C-statistic was 0.82, considerably higher than the C-statistic of the DERRITM in the same cohort (0.69), showing that the addition of variables to the DERRITM increases the predictive accuracy of the model.
Other models have been developed for predicting readmission risk specifically among patients with a diagnosis of type 2 diabetes. One study of more than 63,000 older adults, examined nearly 200 variables and found that age, sex, number of ED visits, LOS, and several comorbidities yielded a relatively accurate model (C-statistic 0.82) [42]. A smaller study in adults with a diagnosis of T2D reported a model with age, marital status, Charlson Comorbidity Index, LOS, number of admissions, and discharge disposition that had modest accuracy for predicting unplanned readmissions [39].
Machine Learning Models
Recently, several studies have developed models for predicting readmission risk among patients with diabetes using machine learning algorithms. [24, 44–46] There is wide variation in performance among these models, which used a variety of machine learning approaches, with C-statistics ranging between 0.64 and 0.97. Notably, all four of these studies developed their models using cohorts drawn from the same database of 101,766 discharges with a diabetes diagnosis between 1998 and 2008 from 130 different US hospitals [50]. The worst performing model consisted of only 5 variables [44], while the two best performing models, both with C-statistics of 0.97, included 15 and 35 different variables [24, 45]. These exceptionally high C-statistics are beyond those reported in a systematic review of 41 studies that used electronic medical records for creating readmission risk prediction models, in which the range was 0.52 and 0.90 [51]. Neither of the high-performing models have been externally or independently validated; thus, it remains to be seen if they are generalizable.
Technical Observations About Modeling and Sampling Strategies
As reviewed above, predictive model performance varies widely by the population studied, variables included, and modeling approach. In addition, we found that different sampling strategies and modeling methods that adjust for repeat hospitalizations of individual patients (clustering) to varying degrees yield models with different results that may not be accurate [52]. The simplest way to sample hospitalizations is to include only one discharge per patient. This allows for modeling by logistic regression without consideration of additional steps to adjust for clustering of hospitalizations. However, only sampling the first discharge per patient produces a dramatically lower estimate of readmission rate than sampling all discharges per patient, about 50% lower in the original DERRITM cohort. This lower estimate inaccurately reflects the readmission rate of a population over time and affects measures of model performance. Furthermore, the use of different modeling approaches that account for clustering to varying degrees yields models with different predictive performance. It is likely that the commonly used logistic regression models without adjustment for clustering are overly optimistic. Of note, the DERRITM model uses one of the approaches for clustering adjustment. These findings reinforce the importance of analyzing all available data (all discharges) and testing models in different populations to determine generalizability, which applies broadly to all predictive models regardless of the approach used.
In addition to modeling approach and sampling strategy, the number of variables in a model tends to affect performance. In the reviewed set of readmission prediction models (Table 1), we observed a statistically significant correlation between the number of variables in a model and its C-statistic (R [2] = 0.48, p = 0.01). Unfortunately, the number of variables is inversely proportional to the ease-of-use in a point-of-care tool that requires manual user input.
There is a need for tools that make the use of comprehensive and more complex readmission risk prediction models feasible in clinical practice. Tools embedded in electronic health record (EHR) systems have the potential to address this need. It is worth noting that all of the readmission risk prediction models cited above were developed before the COVID-19 pandemic. Research is needed to validate the performance of models in diabetes patients hospitalized for COVID-19 and to explore whether COVID-19 is an independent predictor of readmission.
Strategies to Prevent Acute Care Re-Utilization Among Patients with Diabetes
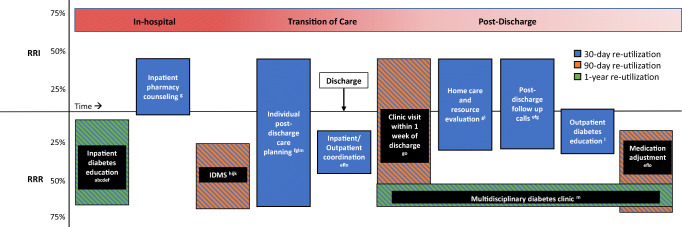
A growing number of studies have reported on the effect of interventions on readmission and ED visit risk in diabetes patients (Table 2 and Fig. 1). We only considered studies that present rates for a control or comparison group so that relative risk reduction or increase could be calculated. The majority of studies include readmission within 30 days as an outcome, some include readmission and/or ED visitation, and some have follow-up periods up to 90 days or 1 year. Most of these studies are retrospective. However, there are some randomized controlled trials (RCTs), of which 3 are adequately powered. [67–69] They tested a number of interventions, which can be sorted into 3 broad categories: inpatient diabetes education only, inpatient diabetes management by a dedicated service, and multi-component programs usually consisting of diabetes education, transition of care support and coordination, and outpatient follow-up. The effect sizes of these interventions vary considerably from zero to 71%, with most studies finding a relative risk reduction and some finding no statistically significant difference in risk.
Table 2.
Strategies to prevent acute care re-utilization
| Name, author, date | Population | Study type | Sample size | Baseline or control readmission riska | Post intervention readmission risk, RRR/RRIb | Intervention component |
|---|---|---|---|---|---|---|
| Inpatient Diabetes Education | ||||||
| Davies, 2001 [69] | Admitted patients with diagnosis of diabetes referred for education | RCT |
N = 300 Int.: 148 Control: 152 |
1-year: 25% | 1-year: 25% | Inpatient diabetes education by diabetes specialist nurse |
| Healy, 2013 [53••] | Adults with A1c>9% and discharge diagnosis of diabetes | Retrospective cohort |
30 days N = 2265 180 days N = 2069 |
30-day: 16% 180-day: 45% |
30-day: 11% (P = 0.0001), RRR 31.3%* 180-day: 37% (P = 0.002), RRR 17.8%* |
Inpatient diabetes education by diabetes educator |
| Corl, 2015 [54] | Inpatient hyperglycemia (>180 mg/dl), length of stay 2-9 days, and preexisting diabetes and/or inpatient insulin treatment | Retrospective cohort |
N = 254 Int.: 202 Control: 52 |
7-day: 6.2% 14-day: 9.2% 30-day: 13.0% |
7-day 2.5% (P = 0.004), RRR 59.7%* 14-day: 5.7 (P = 0.335) 30-day: 11.9 (P = 0.557) |
Inpatient diabetes education by staff nurse |
| Murphy, 2019 [55] | Hospitalized adults with a diagnosis of diabetes, a blood glucose >200 mg/dL on admission, and/or hemoglobin A1C >6.5% | Retrospective cohort |
Int.: 264 Control: 149 |
30-day: 21.5% | 30-day: 13.2% (P = 0.023)*, RRR 62.9% | Inpatient diabetes education by pharmacist or student pharmacist |
| Inpatient diabetes management service | ||||||
| Koproski, 1997 [56] | Admitted patients with diagnosis of diabetes | RCT |
N = 197 Int.: 85 Control: 94 |
90-day: 32% | 90-day: 15% (P = 0.01), RRR 53.1%* | Co-management by IDMS (endocrinologist, nurse, and certified diabetes educator) |
| Wang, 2016 [57] |
Patients with T2D admitted for infection or cardiac-related diagnoses Subgroup with mean BG >180 mg/dl |
Retrospective cohort |
N = 440 Int.: 91 Control: 349 Subgroup n = 116 Int.: 33 Control: 83 |
30-day: not reported Subgroup 30-day: 28.9% |
30-day: not reported, P > 0.05 Subgroup 30-day: 9.1% (P < 0.02)c, RRR 68.5%* |
Co-management by IDMS (endocrinologist and advanced practice provider) |
| Bansal, 2018 [58] | Patients with diabetes admitted to noncritical units at a single tertiary referral medical center | Retrospective cohort |
N = 262 Int.: 131 Control: 131 |
Non-surgical 30-day: 32.4% Surgical 30-day: 21.7% |
Non-surgical 30-day: 22.5% (P < 0.001), RRR 30.6%* Surgical 30-day: 26.7% (P > 0.05) |
Co-management by IDMS (endocrinologist, diabetes NP, nurse diabetes educator, and a discharge coordinator) |
| Mandel, 2019 [59] | Patients admitted with glucose <60 or >250 mg/dl, uncontrolled diabetes with recent cardiac surgery, high dose glucocorticoids, new T1D with DKAd, or insulin pump | Retrospective cohort |
N = 4650 Int.: 850 Control: 3804 |
30-day: 25% | 30-day: 14.2% (P = 0.048), RRR 43.2%* | Co-management by IDMS (endocrinologist, NP, and diabetes educator) |
| Multi-component transition of care program | ||||||
| Transitional Care Clinic, Seggelke, 2014 [60] | Patients with T2D who are medically indigent (no insurance or Medicaid without PCPe) | Pilot RCT |
N = 100 Int.: 50 Control: 50 Subgroup admitted for DM n = 30 Int.: 16 Control: 14 |
90-day: 28% Subgroup 90-day: 42.9% |
90-day: 20% (P not significant) Subgroup 90-day: 12.5%, (P < 0.05), RRR 70.9%* |
TCC visit 2 to 5 days after discharge for medication adjustment by endocrinologist, NP, or PAf |
| Sweet Transitions, Berger, 2018 [61] | Patients with poorly controlled diabetes (A1c>9%) | Prospective non-randomized trial with matched controls | Int.g: 197 patients | 30-day: 17% | 30-day: 11% (P = 0.08) | Individualized post-discharge care coordination and education, barrier identification, medication adjustment by NP h and diabetes educator, transfer of care plan to outpatient clinician |
| Diabetes transition program, Brumm, 2016 [62] | Veterans with poorly controlled diabetes (A1c≥9%) and psychosocial challenges (cognitive disorders, depression, living alone, insulin-naïve, finances, or new diagnosis) | Retrospective pre- and post-intervention |
Int.: 40 Control: historical, sample unspecified |
30-day: 14.3% | 30-day: 10% | Hospital visit by the NP-inpatient diabetes educator, weekly phone calls after discharge, 24/7 access to nurse hotline |
| Magny-Normilus, 2021 [63] | Adults with T2Di admitted to medicine or cardiovascular units with active CVDj, and prescribed insulin before admission or likely to be prescribed insulin at discharge. | RCT |
N = 180 Int.: 88 Control: 92 usual care |
30-day: 14.1% 30-day ED: 9.1%k |
30-day: 20.5%, RRI 46.1% 30-day ED: 9.8% (P=0.87) |
Inpatient pharmacist counseling, visiting nurse home evaluations, symptom screening phone calls and after-hospital care planning by NP, follow-up in post-discharge clinic within 3 days, telemonitoring of glucose, follow-up with PCP or endocrinologist within 1 week of discharge |
| Pharmacy coordination, Wright, 2019 [64] | Adults with discharge diagnosis for heart failure, acute myocardial infarction, chronic obstructive pulmonary disease, pneumonia, or diabetes (75% had DM) | Prospective pragmatic interventional study with 5:1 matched controls |
Int.: 187 Control: 935 |
30-day: 15% 30-day ED: 22% |
30-day: 9% (P = 0.02), RRR 40%* 30-day ED: 20% (P = 0.48) |
Coordination between inpatient and outpatient pharmacist |
| Diabetes Transition of Hospital Care (DiaTOHC), Rubin [65], 2020 [66] | Diabetes and high risk of 30-day readmission (≥27%) based on DERRI | Pilot RCT |
N = 91 Int.: 46 Control: 45 Subgroup with A1c>7% N = 69 |
30-day readmission or ED: 39.1% 90-day: 50% 90-day readmission or ED: 60.0% A1c>7% Subgroup 30-day readmission or ED visit: 40% |
30-day readmission or ED: 31.8% 90-day: 46.7% 90-day readmission or ED: 52.9% A1c>7% Subgroup 30-day readmission or ED: 26.5% (P = 0.23) |
Focused inpatient diabetes education, coordination of care, physician titration of diabetes therapy upon discharge based on A1c algorithm, and post-discharge phone calls by NP until 30 days after discharge |
| Multidisciplinary diabetes clinic, Bhalodkar, 2020 [27] | Adults admitted to medicine service with diagnosis of diabetes | RCT |
N = 192 Int.: 97 Control: 95 |
30-day readmission or ED: 19% 1-year readmission or ED: 38% |
30-day readmission or ED: 7% (P = 0.02), RRR 63.2%* 1-year readmission or ED: 14% (P < 0.01), RRR 63.2%* |
Outpatient visit with diabetes educator/NP, subsequent outpatient visits with NP, nutritionist, social worker or endocrinologist as needed |
*Statistically significant at P < 0.05
aOutcome is for readmission unless otherwise noted
b Relative risk reduction or relative risk increase; P value included if reported
cP value for composite outcome, individual outcome P value was not reported
dDiabetic ketoacidosis
ePrimary care provider
fPhysician assistant
gIntervention
hNurse practitioner
iType 2 diabetes
jCardiovascular disease
kPrimary outcome was diabetes medication adherence during the 90 days after discharge
Fig. 1.
Effect of interventions on the risk of acute care re-utilization within 30 days, 90 days, or 1 year of discharge. For interventions with more than 1 reference, top and bottom of each box represent the maximum and minimum values of relative risk increase (RRI) and relative risk reduction (RRR) of readmission +/− ED visit. For interventions with only 1 reference, crossing the x-axis indicates lack of statistical significance. Multiple colors represent multiple outcome time-frames. IDMS inpatient diabetes management service. a Murphy, 2019. b Healy & Dungan, 2013. c Corl, 2015. d Davies, 2001. e Rubin, 2019, 2020. f Brumm,2016. g Magny-Normilus, 2021. h Mandel, 2019. i Bansal, 2018. j Wang, 2016. k Koproski, 1997. l Berger, 2018. m Bhalodkar, 2020. n Wright, 2019. o Seggelke, 2014
Inpatient Diabetes Education Only
Four studies have examined the effect of inpatient diabetes education alone on readmission risk [53••, 54, 55, 69]. The one RCT, conducted in 300 patients with diabetes referred for inpatient education by a nurse specialist, found no difference in readmission at 1 year between patients who did and did not receive the education [69]. Two of the three retrospective cohort studies found that inpatient diabetes education was associated with a statistically significant 31 to 63% relative risk reduction (RRR) of 30-day readmission [53••, 55]. The third retrospective study reported significantly lower rates of readmission at 7 days with diabetes education, but not at 14 or 30 days after discharge [54].
Inpatient Diabetes Management Service Co-Management
There are four studies that explore the effect of a multidisciplinary inpatient diabetes management service (IDMS) on readmission risk [56–59]. The services all consisted of an endocrinologist and a nurse practitioner, and three also included a diabetes educator. In the lone RCT, which included 197 hospitalized patients with diabetes, IDMS co-management reduced the readmission rate at 90 days from 32 to 15% (RRR 53%, p = 0.01). There were three retrospective cohort studies that showed IDMS co-management was associated with a 31 to 69% RRR in readmissions at 30 days [57–59]. The smallest of these studies (N = 262) found no statistically significant difference in the subgroup of patients on a surgical service [58].
Multi-Component Transition of Care Programs
Several studies have investigated the effect of multi-component transition of care programs on acute care re-utilization [60–65, 67, 68]. Components of these programs include discharge care coordination, diabetes education, medication adjustment by a diabetes specialist upon discharge, outpatient follow-up in a diabetes clinic, follow-up by telephone for care coordination and diabetes assessment, and home care by a visiting nurse. Of the four RCTs, three reported reductions in acute care re-utilization at 30 days, 90 days, and 1 year [60, 65, 66, 68], and one found no statistically significant difference in readmissions or ED visits at 30 days [63]. Three non-randomized studies reported a 31–40% RRR in 30-day readmission rates associated with their interventions [61, 62, 64]. As a whole, these studies suggest that multi-component transition of care programs can reduce the risk of acute care re-utilization by diabetes patients. However, all of them were conducted at a single site. Reproducibility at multiple sites in an adequately powered RCT is critical to establish generalizability and effectiveness in clinical practice.
To summarize the evidence, key interventions for readmission risk reduction among patients with diabetes are inpatient diabetes education and management by a specialty team, individualized post-discharge care planning, coordination with outpatient providers, adjustment of diabetes therapy, and outpatient follow-up by diabetes specialists. The evidence supporting post-discharge follow-up by telephone is less convincing. Delivery of outpatient care by telehealth has grown dramatically with the COVID-19 pandemic. Given the benefit of healthcare access for patients, telehealth is likely to remain a substantial modality. Studies are needed, however, to determine if telehealth can deliver the same readmission benefit as that associated with in-person outpatient care.
Conclusions
Hospital readmissions and emergency department visits are common and costly among patients with diabetes, presenting an important challenge to improve quality of care and reduce healthcare costs. Major risk factors for readmission include sociodemographics, comorbidities, insulin use, LOS, and history of readmissions, most of which are non-modifiable. Several models for predicting the risk of readmission among diabetes patients have been developed. Two such models have shown reasonable accuracy in external validation and are available as tools in web-based applications. Machine learning models, which are becoming more common in this area, have the potential to harness big data from EHRs and generate more accurate readmission risk predictions. Tools embedded in EHR systems are needed to translate readmission risk prediction models into clinical practice. The accuracy of such models in patients with diabetes hospitalized for COVID-19 and the efficacy of telehealth for reducing readmission risk are areas that require additional study. Several strategies for reducing the risk of acute care re-utilization hold promise but require testing in multi-site RCTs to prove their generalizability, scalability, and effectiveness. Combining readmission risk prediction with interventions, a personalized medicine approach to population health, may be more effective at reducing readmission risk in a population than interventions delivered without regard to individualized patient selection.
Declarations
Conflict of Interest
Daniel J. Rubin and Arnav A. Shah each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Hospital Management of Diabetes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.CMS. Readmissions Reduction Program. Baltimore: Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/medicare-fee-for-service-payment/acuteinpatientPPS/readmissions-reduction-program. Published 08/24/2020. Accessed January 27, 2021
- 2.Frakt AB, Mayes R. Beyond capitation: how new payment experiments seek to find the ‘sweet spot’ in amount of risk providers and payers bear. Health Aff (Millwood) 2012;31(9):1951–1958. doi: 10.1377/hlthaff.2012.0344. [DOI] [PubMed] [Google Scholar]
- 3.Chukmaitov A, Harless DW, Bazzoli GJ, Muhlestein DB. Preventable hospital admissions and 30-day all-cause readmissions: does hospital participation in accountable care organizations improve quality of care? Am J Med Qual. 2019;34(1):14–22. doi: 10.1177/1062860618778786. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DJ, Donnell-Jackson K, Jhingan R, Golden SH, Paranjape A. Early readmission among patients with diabetes: a qualitative assessment of contributing factors. J Diabetes Complicat. 2014;28(6):869–873. doi: 10.1016/j.jdiacomp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Strunin L, Stone M, Jack B. Understanding rehospitalization risk: can hospital discharge be modified to reduce recurrent hospitalization? J Hosp Med. 2007;2(5):297–304. doi: 10.1002/jhm.206. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht JS, Hirshon JM, Goldberg R, Langenberg P, Day HR, Morgan DJ, Comer AC, Harris AD, Furuno JP. Serious mental illness and acute hospital readmission in diabetic patients. Am J Med Qual. 2012;27(6):503–508. doi: 10.1177/1062860612436576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enomoto LM, Shrestha DP, Rosenthal MB, Hollenbeak CS, Gabbay RA. Risk factors associated with 30-day readmission and length of stay in patients with type 2 diabetes. J Diabetes Complicat. 2017;31(1):122–127. doi: 10.1016/j.jdiacomp.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Ostling S, Wyckoff J, Ciarkowski SL, Pai CW, Choe HM, Bahl V, Gianchandani R. The relationship between diabetes mellitus and 30-day readmission rates. Clin Diab Endocrinol. 2017;3(1):3. doi: 10.1186/s40842-016-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.••.Rubin DJ, Handorf EA, Golden SH, Nelson DB, McDonnell ME, Zhao H. Development and validation of a novel tool to predict hospital readmission risk among patients with diabetes. Endocr Pract. 2016;22(10):1204–1215. doi: 10.4158/E161391.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin DJ, Recco D, Turchin A, Zhao H, Golden SH. External validation of the diabetes early re-admission risk indicator (DERRI()) Endocr Pract. 2018;24(6):527–541. doi: 10.4158/EP-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ADA Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HCUP Nationwide Inpatient Sample (NIS). Agency for Healthcare Research and Quality (AHRQ). 2016. https://hcupnet.ahrq.gov/ - setup. Accessed August 18, 2020.
- 13.CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services. In:2020
- 14.CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services. 2017
- 15.Sonmez H, Kambo V, Avtanski D, Lutsky L, Poretsky L. The readmission rates in patients with versus those without diabetes mellitus at an urban teaching hospital. J Diabetes Complicat. 2017;31(12):1681–1685. doi: 10.1016/j.jdiacomp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Ostling S, Wyckoff J, Ciarkowski SL, et al. The relationship between diabetes mellitus and 30-day readmission rates. Clin Diab Endocrinol. 2017;3(1):1–8. doi: 10.1186/s40842-016-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin DJ, Zhao H, Miller E. 1252-P: Readmission risk and risk factors of hospitalizations with a primary diagnosis of diabetes differ from discharges with a secondary diagnosis of diabetes. Diabetes. 2019;68(Supplement 1):1252. doi: 10.2337/db19-1252-P. [DOI] [Google Scholar]
- 18.Atalla E, Kalligeros M, Giampaolo G, Mylona EK, Shehadeh F, Mylonakis E. Readmissions among patients with COVID-19. Int J Clin Pract. 2020;75:e13700. doi: 10.1111/ijcp.13700. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DJ. Hospital readmission of patients with diabetes. Current Diabetes Rep. 2015;15(4):1–9. doi: 10.1007/s11892-015-0584-7. [DOI] [PubMed] [Google Scholar]
- 20.Karunakaran A, Zhao H, Rubin DJ. Predischarge and postdischarge risk factors for hospital readmission among patients with diabetes. Med Care. 2018;56(7):634–642. doi: 10.1097/MLR.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarthak SS, Tripathi SP EmbPred30: Assessing 30-days readmission for diabetic patients using categorical embeddings. arXiv. 2020;2002.11215v1
- 22.•.Soh JGS, Wong WP, Mukhopadhyay A, Quek SC, Tai BC. Predictors of 30-day unplanned hospital readmission among adult patients with diabetes mellitus: a systematic review with meta-analysis. BMJ Open Diabetes Res Care. 2020;8(1):e001227. doi: 10.1136/bmjdrc-2020-001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alamer AA, Patanwala AE, Aldayyen AM, Fazel MT. Validation and comparison of two 30-day re-admission prediction models in patients with diabetes. Endocr Pract. 2019;25(11):1151–1157. doi: 10.4158/EP-2019-0125. [DOI] [PubMed] [Google Scholar]
- 24.Alturki L, Aloraini K, Aldughayshim A, Albahli S. Predictors of readmissions and length of stay for diabetes related patients. Paper presented at: 2019 IEEE/ACS 16th International Conference on Computer Systems and Applications (AICCSA) 2019.
- 25.Spanakis EK, Singh LG, Siddiqui T, Sorkin JD, Notas G, Magee MF, Fink JC, Zhan M, Umpierrez GE. Association of glucose variability at the last day of hospitalization with 30-day readmission in adults with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e000990. doi: 10.1136/bmjdrc-2019-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spanakis EK, Umpierrez GE, Siddiqui T, et al. Association of glucose concentrations at hospital discharge with readmissions and mortality: a nationwide cohort study. J Clin Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed]
- 27.Engoren M, Schwann TA, Habib RH. Elevated hemoglobin A1c is associated with readmission but not complications. Asian Cardiovasc Thorac Ann. 2014;22(7):800–806. doi: 10.1177/0218492313515895. [DOI] [PubMed] [Google Scholar]
- 28.Bakeri H, Wakefield D, Dulipsingh L. Is there a role of hemoglobin A1C in predicting hospital readmission rates for patients with diabetes. Endocrinol Diab Obes. 2018;1(2):3. [Google Scholar]
- 29.Mingle D. Predicting diabetic readmission rates: moving beyond Hba1c. Curr Trends Biomed Eng Biosci. 2017;7(3):555707. doi: 10.19080/CTBEB.2017.07.555715. [DOI] [Google Scholar]
- 30.Zisman-Ilani Y, Fasing K, Weiner M, Rubin DJ. Exercise capacity is associated with hospital readmission among patients with diabetes. BMJ Open Diabetes Res Care. 2020;8(1). [DOI] [PMC free article] [PubMed]
- 31.Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist. 2008;48(4):495–504. doi: 10.1093/geront/48.4.495. [DOI] [PubMed] [Google Scholar]
- 32.Hurtado CR, Lemor A, Vallejo F, Lopez K, Garcia R, Mathew J, Galindo RJ. Causes and predictors for 30-day re-admissions in adult patients with diabetic ketoacidosis in the United States: a nationwide analysis, 2010–2014. Endocr Pract. 2019;25(3):242–253. doi: 10.4158/EP-2018-0457. [DOI] [PubMed] [Google Scholar]
- 33.Flexner CW, Weiner JP, Saudek CD, Dans PE. Repeated hospitalization for diabetic ketoacidosis: the game of “sartoris”. Am J Med. 1984;76(4):691–695. doi: 10.1016/0002-9343(84)90297-3. [DOI] [PubMed] [Google Scholar]
- 34.Everett E, Mathioudakis N. Association of area deprivation and diabetic ketoacidosis readmissions: comparative risk analysis of adults vs children with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3473–3480. doi: 10.1210/jc.2018-02232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benoit SR, Hora I, Pasquel FJ, Gregg EW, Albright AL, Imperatore G. Trends in emergency department visits and inpatient admissions for hyperglycemic crises in adults with diabetes in the U.S., 2006–2015. Diabetes Care. 2020;43(5):1057–1064. doi: 10.2337/dc19-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levetan CS, Passaro MD, Jablonski KA, Ratner RE. Effect of physician specialty on outcomes in diabetic ketoacidosis. Diabetes Care. 1999;22(11):1790–1795. doi: 10.2337/diacare.22.11.1790. [DOI] [PubMed] [Google Scholar]
- 37.Xu AC, Broome DT, Bena JF, Lansang MC. Predictors for adverse outcomes in diabetic ketoacidosis in a multihospital health system. Endocr Pract. 2020;26(3):259–266. doi: 10.4158/EP-2018-0551. [DOI] [PubMed] [Google Scholar]
- 38.Ehrmann D, Kulzer B, Roos T, Haak T, Al-Khatib M, Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diab Endocrinol. 2020;8(5):436–446. doi: 10.1016/S2213-8587(20)30042-5. [DOI] [PubMed] [Google Scholar]
- 39.Rico F, Liu Y, Martinez DA, Huang S, Zayas-Castro JL, Fabri PJ. Preventable readmission risk factors for patients with chronic conditions. J Healthc Qual. 2016;38(3):127–142. doi: 10.1097/01.JHQ.0000462674.09641.72. [DOI] [PubMed] [Google Scholar]
- 40.Donzé J, Aujesky D, Williams D, Schnipper JL. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 41.Donze JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496–502. doi: 10.1001/jamainternmed.2015.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins J, Abbass IM, Harvey R, Suehs B, Uribe C, Bouchard J, Prewitt T, DeLuzio T, Allen E. Predictors of all-cause-30-day-readmission among Medicare patients with type 2 diabetes. Curr Med Res Opin. 2017;33(8):1517–1523. doi: 10.1080/03007995.2017.1330258. [DOI] [PubMed] [Google Scholar]
- 43.Rubin DJ, Golden SH, McDonnell ME, Zhao H. Predicting readmission risk of patients with diabetes hospitalized for cardiovascular disease: a retrospective cohort study. J Diabetes Complicat. 2017. 10.1016/j.jdiacomp.2017.04.021. [DOI] [PMC free article] [PubMed]
- 44.Alloghani M, Aljaaf A, Hussain A, Baker T, Mustafina J, al-Jumeily D, Khalaf M. Implementation of machine learning algorithms to create diabetic patient re-admission profiles. BMC Med Inform Decision Mak. 2019;19(9):253. doi: 10.1186/s12911-019-0990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarthak SS, Tripathi SP. EmbPred30: Assessing 30-days readmission for diabetic patients using categorical embeddings. Smart Innov Commun Comput Sci : Proceedings of ICSICCS. 2020;2020:81. [Google Scholar]
- 46.Ossai CI, Wickramasinghe N. Intelligent therapeutic decision support for 30 days readmission of diabetic patients with different comorbidities. J Biomed Inform. 2020;107:103486. doi: 10.1016/j.jbi.2020.103486. [DOI] [PubMed] [Google Scholar]
- 47.Rubin DJ. The Diabetes Early Readmission Risk Indicator (DERRITM). https://redcap.templehealth.org/redcap/surveys/?s=3XCPCAMKWE. Published 2016. Accessed 8/23/2020.
- 48.Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 49.Donzé JD. HOSPITAL Score for Readmissions. MDCalc. https://www.mdcalc.com/hospital-score-readmissions. Published 2016. Accessed August 25, 2020.
- 50.Strack B, DeShazo JP, Gennings C, et al. Impact of HbA1c measurement on hospital readmission rates: analysis of 70,000 clinical database patient records. Biomed Res Int. 2014;2014:781670. doi: 10.1155/2014/781670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoudi E, Kamdar N, Kim N, Gonzales G, Singh K, Waljee AK. Use of electronic medical records in development and validation of risk prediction models of hospital readmission: systematic review. BMJ. 2020;369:m958. doi: 10.1136/bmj.m958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H, Tanner S, Golden SH, Fisher SG, Rubin DJ. Common sampling and modeling approaches to analyzing readmission risk that ignore clustering produce misleading results. BMC Med Res Methodol. 2020;20(1):281. doi: 10.1186/s12874-020-01162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013;36(10):2960–2967. doi: 10.2337/dc13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corl DE, Guntrum PL, Graf L, Suhr LD, Thompson RE, Wisse BE. Inpatient diabetes education performed by staff nurses decreases readmission rates. AADE Pract. 2015;3(2):18–23. doi: 10.1177/2325160314568369. [DOI] [Google Scholar]
- 55.Murphy JA, Schroeder MN, Ridner AT, Gregory ME, Whitner JB, Hackett SG. Impact of a pharmacy-initiated inpatient diabetes patient education program on 30-day readmission rates. J Pharm Pract. 2019:897190019833217. [DOI] [PubMed]
- 56.Koproski J, Pretto Z, Poretsky L. Effects of an intervention by a diabetes team in hospitalized patients with diabetes. Diabetes Care. 1997;20(10):1553–1555. doi: 10.2337/diacare.20.10.1553. [DOI] [PubMed] [Google Scholar]
- 57.Wang YJ, Seggelke S, Hawkins RM, Gibbs J, Lindsay M, Hazlett I, Low Wang CC, Rasouli N, Young KA, Draznin B. Impact of glucose management team on outcomes of hospitalizaron in patients with type 2 diabetes admitted to the medical service. Endocr Pract. 2016;22(12):1401–1405. doi: 10.4158/EP161414.OR. [DOI] [PubMed] [Google Scholar]
- 58.Bansal V, Mottalib A, Pawar TK, et al. Inpatient diabetes management by specialized diabetes team versus primary service team in non-critical care units: impact on 30-day readmission rate and hospital cost. BMJ Open Diabetes Res Care. 2018;6(1):e000460. doi: 10.1136/bmjdrc-2017-000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandel SR, Langan S, Mathioudakis NN, Sidhaye AR, Bashura H, Bie JY, Mackay P, Tucker C, Demidowich AP, Simonds WF, Jha S, Ebenuwa I, Kantsiper M, Howell EE, Wachter P, Golden SH, Zilbermint M. Retrospective study of inpatient diabetes management service, length of stay and 30-day readmission rate of patients with diabetes at a community hospital. J Commun Hosp Int Med Perspect. 2019;9(2):64–73. doi: 10.1080/20009666.2019.1593782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seggelke SA, Hawkins RM, Gibbs J, Rasouli N, Wang C, Draznin B. Transitional care clinic for uninsured and medicaid-covered patients with diabetes mellitus discharged from the hospital: a pilot quality improvement study. Hosp Pract (1995) 2014;42(1):46–51. doi: 10.3810/hp.2014.02.1091. [DOI] [PubMed] [Google Scholar]
- 61.Berger K, Corbin A, Kamal P, Bachman NE, Riddell LA, Falciglia M. Sweet transitions—improving outcomes for hospitalized patients with diabetes. In. Vol 67: Diabetes (Supplement); 2018.
- 62.Brumm S, Theisen K, Falciglia M. Diabetes transition care from an inpatient to outpatient setting in a veteran population: quality improvement pilot study. Diabetes Educ. 2016;42(3):346–353. doi: 10.1177/0145721716642020. [DOI] [PubMed] [Google Scholar]
- 63.Magny-Normilus C, Nolido NV, Borges JC, Brady M, Labonville S, Williams D, Soukup J, Lipsitz S, Hudson M, Schnipper JL. Effects of an intensive discharge intervention on medication adherence, glycemic control, and readmission rates in patients with type 2 diabetes. J Patient Saf. 2021;17(2):73–80. doi: 10.1097/PTS.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright EA, Graham JH, Maeng D, Tusing L, Zaleski L, Martin R, Seipp R, Citsay B, McDonald B, Bolesta K, Chaundy K, Medico CJ, Gunderman S, Leri F, Guza K, Price R, Gregor C, Parry DT. Reductions in 30-day readmission, mortality, and costs with inpatient–to–community pharmacist follow-up. J Am Pharm Assoc. 2019;59(2):178–186. doi: 10.1016/j.japh.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Rubin DJ, Golden S, Foster G, et al. 1251-P: The Diabetes Transition of Hospital Care (DiaTOHC) pilot study: a randomized controlled trial of an intervention designed to reduce readmission risk of patients with diabetes. Diabetes. 2019;68(Supplement 1):1251. doi: 10.2337/db19-1251-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubin DJ, Watts S, Deak A, et al. 151-LB: A pilot randomized controlled trial to reduce hospital readmission risk of patients with diabetes: 90-day outcomes. Diabetes. 2020;69(Supplement 1):151-LB. doi: 10.2337/db20-151-LB. [DOI] [Google Scholar]
- 67.Magny-Normilus C, Nolido NV, Borges JC, et al. Effects of an intensive discharge intervention on medication adherence, glycemic control, and readmission rates in patients with type 2 diabetes. J Patient Saf. 2021;17(2):73-80. [DOI] [PMC free article] [PubMed]
- 68.••.Bhalodkar A, Sonmez H, Lesser M, et al. The Effects of a Comprehensive Multidisciplinary Outpatient Diabetes Program on Hospital Readmission Rates in Patients with Diabetes: A Randomized Controlled Prospective Study. Endocr Pract. 2020;26(11):1331–1336. This is the largest RCT of an intervention designed to reduce readmission risk among hospitalized patients with diabetes. Outpatient follow up in a multidisciplinary diabetes clinic significantly reduced readmissions or ED visits within 30 days and 1 year of discharge. [DOI] [PubMed]
- 69.Davies M, Dixon S, Currie CJ, Davis RE, Peters JR. Evaluation of a hospital diabetes specialist nursing service: a randomized controlled trial. Diabet Med. 2001;18(4):301–7. [DOI] [PubMed]