Abstract
Joint inflammation is a key player in the pathogenesis of osteoarthritis (OA). Imperatorin, a plant-derived small molecule has been reported to have anti-inflammatory properties; however, its effect on chondrocytes is not known. Here, we investigated the effects of Imperatorin on IL-1β induced expression of inducible nitric oxide synthase (iNOS) and nitric oxide production in primary human OA chondrocytes and cartilage explants culture under pathological conditions and explored the involved signaling pathways. We pretreated chondrocytes or explants with Imperatorin (50μM) followed by IL-1β (1ng/ml), and the culture supernatant was used to determine the levels of nitrite production by Greiss assay and chondrocytes were harvested to prepare cell lysate or RNA for gene expression analysis of iNOS by Western blot or qPCR and in explants by IHC. Pretreatment of primary chondrocytes and cartilage explants with Imperatorin suppressed IL-1β induced expression of iNOS and NO production. Imperatorin blocked the IL-1β-induced phosphorylation of ERK/MAPK signaling pathway to suppress iNOS expression. The role of ERK in the regulation of iNOS expression was verified by using ERK inhibitor. Interestingly, we also found that Imperatorin binds to iNOS protein and inhibits its activity in vitro. Our data demonstrated that Imperatorin possess strong anti-inflammatory activity and may be developed as a therapeutic agent for the management of OA.
Keywords: Imperatorin, Osteoarthritis, iNOS, inflammation, oxidative-stress, plant-derived-polyphenol
1. Introduction
Nitric oxide (NO) is an important inflammatory mediator which is involved in the pathogenesis of Osteoarthritis (OA) [1–3]. The excessive production of nitric oxide in cartilage inhibits matrix synthesis and enhances its degradation [4]. Additionally, NO reacts with oxidants such as superoxide anion, thereby promoting cellular injury. NO is produced by conversion of L-arginine to L-citrulline and is catalyzed by nitric oxide synthase (NOS) enzyme. NO is a gaseous free radical with a very short half-life of a few seconds, so it’s level in biological fluids is measured indirectly by estimating the level of nitrite (NO2)− , which is the more stable metabolite of NO by using Greiss assay [5]. NO production in OA cartilage has been illustrated by immunostaining with anti-nitrotyrosine antibodies [6]. The expression of iNOS is upregulated in OA chondrocytes, resulting in excess NO production and eventual release of other catabolic and inflammatory cytokines [7]. iNOS expression is preferentially upregulated in the superficial zone of OA cartilage, suggesting active production of NO in that area [8]. The importance of iNOS in OA pathogenesis can be emphasized by the observation that iNOS knock out mice are resistant to developing experimental OA [9]. These experiments suggest that iNOS is a potential therapeutic target for the management of OA.
Imperatorin, known as 9-[(3-methyl-2-buten-1-yl)oxy]-7H-furo[3,2-g]chromen-7-one or 8-(1,1-dimethylallyloxy)-psoralen, is a plant secondary metabolite belonging to the family of furanocoumarins [10]. Imperatorin is mainly found among plants belonging to the Apiaceae and Rutaceae families, particularly in Angelica dahurica, A. archangelica, Notopterygium, Peucedani, and Radix Glehniae [11] and is known for many traditional applications. It is a noted constituent in many traditional medications, especially in Traditional Chinese Medicine system [12]. It has been reported that Imperatorin has a wide range of pharmacological properties, that include anti-inflammatory, anti-bacterial, anti-convulsant and anti-tumor activities [13, 14]. However, its anti-inflammatory effect has not been explored in OA and the cell type directly affected in OA.
In this study, we used an in vitro and ex vivo model of OA pathogenesis to investigate the effects of Imperatorin on IL-1β induced expression of iNOS, production of NO and associated signaling pathways. We have demonstrated that Imperatorin treatment significantly suppressed the expression of iNOS in primary human OA chondrocytes and cartilage explants and the levels of nitrite in the culture supernatant. Interestingly, in addition to suppressing the expression of iNOS, we also found that Imperatorin can also bind to iNOS protein and inhibit its activity. Our data also showed that Imperatorin suppressed iNOS by inhibiting the IL-1β induced activation of ERK-MAPK signaling pathway, but was independent of the NF-κB pathway.
2. Materials and Methods
2.1. Reagents
Imperatorin (99% pure by HPLC, #0519S) was purchased from Alkemist labs. Gene-specific TaqMan assays were purchased from Integrated DNA Technologies. DMEM/F12 Media (#11320082) and FBS used for cell culture were purchased from HyClone Laboratories. Pronase (# 10165921001) and collagenase (# 10103578001) enzymes were purchased from Sigma-Aldrich. Recombinant human IL-1β (#201-LB) was purchased from R&D Systems. Antibody against iNOS (#ab3523) was from Abcam and antibodies against p38 (#sc-7972), and β-Actin (#sc-81178) were from Santa Cruz Biotechnologies and c-Fos (#4384), p-c-Fos (#5348), p-ERK (#4370), ERK (#4695), IκBα (#4812), p-p38 (#4092), SAPK/JNK (#9252) and p-JNK (#9251) antibodies were from Cell Signaling Technology.
2.2. Primary chondrocyte isolation and culture
All the methods used in this study were carried out in accordance with the approved guidelines and all protocols were approved by the IRB of Northeast Ohio Medical University, Rootstown, OH and SUMMA Health, Barberton, OH as a “non-human subject study under 45 CFR”. Primary human OA articular chondrocytes were isolated from discarded and de-identified cartilage of OA patients who underwent knee/hip replacement surgery at the SUMMA Health Hospitals as described before in several of our publications [15–18]. Normal cartilage samples (with no known history of any rheumatic disease) were provided by National Disease Research Institute (NDRI). Cartilage was scraped off the surface of the knee/hip joint with the help of sterile scalpels. The cartilage pieces were incubated at 37°C for 1 hour in a digestion media containing 1.5 mg Pronase /ml in Dulbecco’s modified Eagle medium (DMEM) + F12 (1:1) supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin and 100μg/ml streptomycin. This was followed by overnight incubation at 37°C in a digestion media containing 1.5 mg Collagenase/ml in DMEM/F12 + 10% FBS. Next day, the sample was passed through a 70 μm cell strainer. The cells were collected by centrifugation at 180g for 5 minutes followed by washing with PBS. Finally, the chondrocytes were suspended in complete DMEM/F12 and seeded at a density of 1×106 cells per well in 6-wells plate and incubated at 37°C in humidified CO2 incubator for attachment for 2–3 days. For explant culture, cartilage was aseptically scraped off from the surface of knee joint and 3mm thick cartilage explants were produced by using a punch biopsy tool. Explants were maintained in multi-well plates in DMEM/F-12 (1:1) supplemented with 10% FBS at least for 4–5 days before any treatment.
2.3. Chondrocyte/Cartilage explant treatment with Imperatorin and IL-1β
All the experiments were done using primary human OA chondrocytes from at least five independent donors. Chondrocytes or cartilage explants were maintained in DMEM/F-12 (1:1) media and were serum-starved overnight before every experiment. Next day, chondrocytes were pretreated with Imperatorin or 0.1 % DMSO (as control) for two hours, followed by stimulation with IL-1β (1ng/ml) for 24 hours. The culture supernatant was collected to estimate nitrite levels and chondrocytes were harvested for cell lysate or total RNA preparation and cartilage explants were harvested and processed for histology as described below.
2.4. Evaluation of cell viability by MTT assay
Primary human OA chondrocytes were plated (20,000 cells/well) in 96-well plate and their viability was determined using MTT assay as described previously [15].
2.5. Estimation of Nitrite
NO is a gaseous free radical with a very short half-life of a few seconds, so it’s level in biological fluids is measured indirectly by estimating the level of nitrite (NO2)− , which is the more stable metabolite of NO by using Greiss assay as described previously [19].
2.6. Western Blotting
Estimation of protein expression in chondrocytes was estimated by Western blotting using validated antibodies as described previously [20, 21].
2.7. RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated using Trizol-chloroform reagent. cDNA was synthesized using 1 μg of total RNA using a cDNA synthesis kit (# 4368814, Life Technologies Corp). mRNA expression was quantified using TaqMan Gene Expression Assays as described previously [22] . β-actin was used as endogenous expression control.
2.9. Histology and Immunohistochemistry (IHC)
For histological and immunohistochemical analysis, human OA cartilage (n=5) or human cartilage explants were harvested and fixed in 4% paraformaldehyde and processed for paraffin embedding. The extent of damage to the cartilage was determined by Safranin O/Fast Green (S/F) staining and expression of iNOS was determined by IHC as described previously [23].
2.10. Molecular docking of Imperatorin with iNOS
Two-dimensional (2D) structure of Imperatorin was drawn at ChemDraw/ChemSketch editor. This 2D structure was saved in .mol file format and was further converted to 3D structure and its structural geometry was optimized to the lowest energy state. Finally, stable 3D structure was saved in .pdb format and PDB file was converted to charged file format PDBQT. Protein crystal structure of iNOS was collected from protein databank as PDB 4cx7. PDB 4cx7 contains 04 chains A, B, C, D and ligands SO4, GOL, HEM, S71 & H4B. Out of this chain-A only was considered for docking. Co-crystallized ligand S71 at chain-A was used as reference for identification of binding site center. Binding site pocket of radius of 25 Angstrom around binding site center was considered for docking studies. Before docking, PDB files were converted to charged file format PDBQT. After preparation of ‘.pdb’ files of Imperatorin as well as iNOS, docking studies were performed with AutoDock Vina software [24].
2.11. Human Phospho-Kinase Proteome Profiling
Relative phosphorylation of the kinases was determined using Proteome Profiler human phosphor kinase array kit (#ARY003B) according to the manufacturer’s instructions. In brief, primary human OA chondrocytes were treated with IMP and IL-1β as described above and chondrocytes were harvested to prepare cell lysate. The lysate was incubated with the antibody-coated membrane and incubated at 4°C for overnight. Next day, the membranes were washed and incubated with biotin-labeled anti-phospho antibodies. The signal produced was proportional to the phosphorylation in the bound analyte and was quantified using ImageJ software.
2.12. Immunofluorescence
Chondrocytes (0.1×106) were seeded in 8-well chamber slide and treated with Imperatorin and IL-1β as described above. At the end of experiment, cells were fixed and permeabilized and were probed with anti-c-Fos or c-Jun antibody followed by anti-rabbit Alexa-Fluor 488/594 secondary antibody (Life Technologies) as described previously [25] . Nuclei were counterstained with DAPI and chondrocytes were mounted using antifade mounting medium (Vectashield) and visualized by Olympus FV1000 confocal microscope.
2.13. Statistical analyses
All the data were analyzed by GraphPad Prism (Version 8.2.1) software for statistical significance. Statistical significance of nonparametric data between two groups was analyzed by Wilcoxon-Mann-Whitney U test and Student’s t-test was used for the parametric data set. Statistical significance between more than two groups was determined by one-way analysis of variance (ANOVA) followed by Tukey test for post hoc analysis. P-value ≤ 0.05 was considered as significant.
3. Results
3.1. iNOS expression was upregulated in human OA cartilage and chondrocytes under pathological conditions
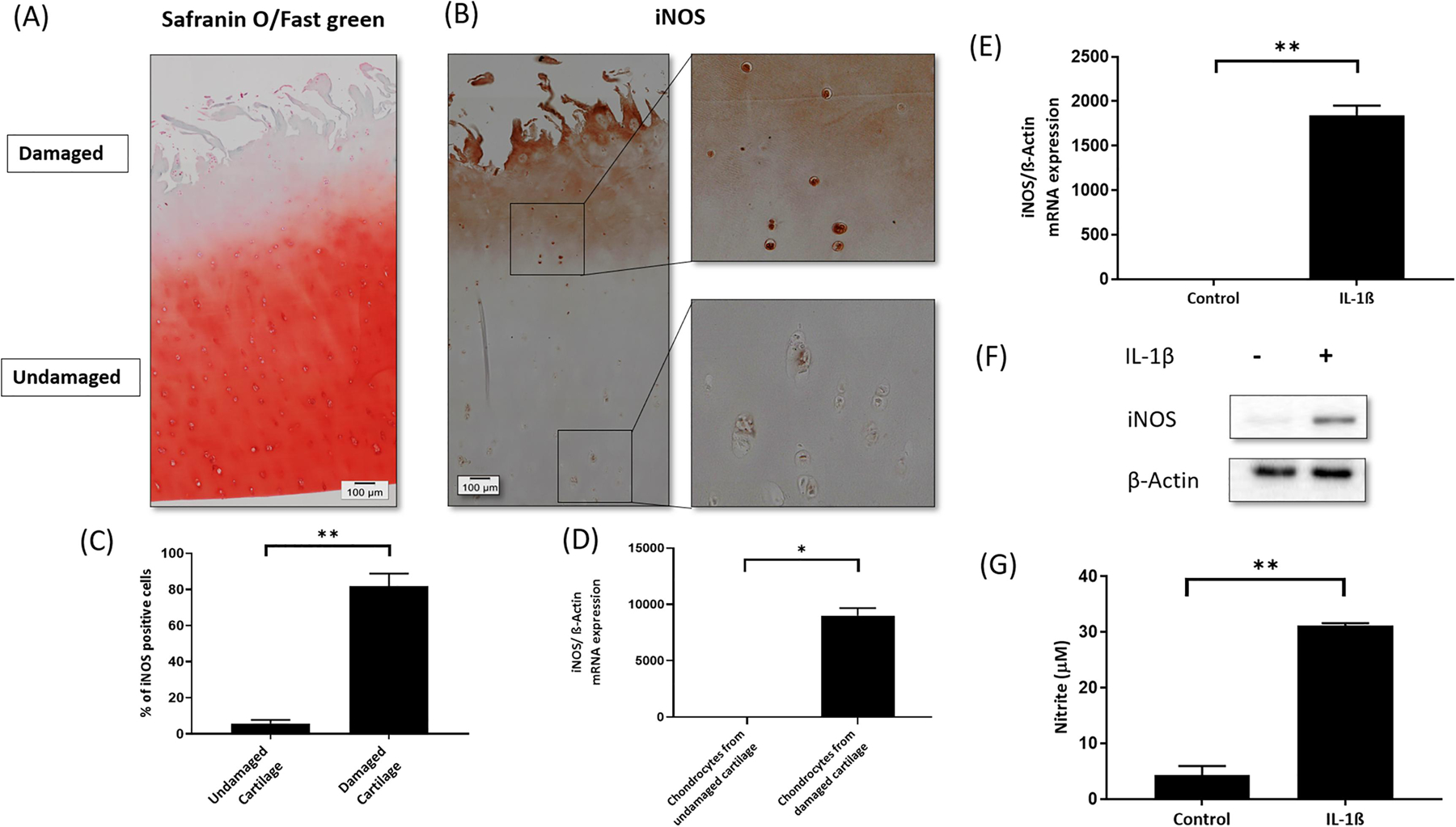
The human OA cartilage sections were stained with S/F to determine the extent of ECM degradation. Compared to the deep zone, the surface area of OA cartilage exposed to synovial cavity showed higher level of proteoglycan degradation (Figure 1A). Investigation of iNOS expression by IHC in human OA cartilage (Mankin score >3) showed high level of iNOS in the damaged area compared to the undamaged area of cartilage from the same patient (Figure 1B&C). We quantified the expression of iNOS in the damaged versus undamaged OA cartilage from the same patient by isolating total RNA from chondrocytes isolated directly from the damaged and undamaged area of the OA cartilage from the same patients and found a significant increase in the expression of iNOS in chondrocytes isolated from the damaged area (n=5) (Figure 1D). As previously reported by others and us, IL-1β stimulation of chondrocytes increased the expression of iNOS at mRNA (Figure 1E) & protein level (Figure 1F) and NO production (Figure 1G) in vitro [26, 27].
Figure 1. iNOS expression was upregulated in human OA cartilage and chondrocytes, and IL-1β-induced the expression of iNOS in OA chondrocytes.
(A) Safranin O/Fast green staining to visiualize damaged (superficial) and undamaged (deep zone) area of OA cartilage (n=5) (20X magnification). (B) Expression of iNOS in damaged and undamaged region of OA cartilage determined by IHC (20X magnification) and (C) Quantification of the IHC data. (D) iNOS expression at mRNA level in damaged and undamaged human OA cartilage (n=5). β-Actin was used as a normalization control. (E) iNOS expression in chondrocytes treated with IL-1β at mRNA level and (F) protein level. β-actin was used as endogenous normalization or loading control. (G) Culture supernatant was used to determine the level of NO production. (*p≤0.05, **p ≤0.005).
3.2. Imperatorin was not toxic to human OA chondrocytes
We determined the effect of Imperatorin on chondrocyte viability by MTT assay. Imperatorin (10, 25, 50 μM) had no adverse effects on chondrocytes viability at all the concentrations used in this study (Figure 2A).
Figure 2. Imperatorin suppressed IL-1β-induced iNOS expression and production of NO in OA chondrocytes.
(A) Effect of Imperatorin (50, 25 & 10 μM) on chondrocyte viability was determined by MTT assay. Chondrocytes treated with 0.1% DMSO served as control. (B) iNOS mRNA expression in OA chondrocytes treated with IL-1β in the presence or absence of Imperatorin. β-actin was used as endogenous expression control (* p≤0.05). (C) Expression of iNOS at protein level in OA chondrocytes treated with IL-1β in the presence or absence of Imperatorin. (D) Quantification of band intensity by ImageJ. (E) Chondrocyte culture supernatant from the above experiment was used to NO levels. The values are mean ± SD of five independent experiments (**p ≤0.005). The values are mean ± SD of three independent experiments.
3.3. Imperatorin suppressed the IL-1β-induced iNOS expression and production of NO in OA and normal chondrocytes.
To analyze the effect of Imperatorin on iNOS expression in chondrocytes under pathological condition, we pretreated OA chondrocytes with Imperatorin (50μM) for 2h, followed by stimulation with IL-1β for 24 h. IL-1β stimulation significantly upregulated the expression of iNOS, which was suppressed by Imperatorin at mRNA (Figure 2B) and protein levels (Figure 2C). Densitometry of the Western blot was carried out to quantify the relative protein expression of iNOS (Figure 2D). We also determined the effect of Imperatorin on IL-1β induced NO production and found that Imperatorin significantly reduced the production of NO in IL-1β treated OA chondrocytes (Figure 2E). Importantly, Imperatorin also inhibited IL-1β induced NO levels in normal chondrocytes (Supplementary Figure 1A). Calculation of percentage inhibition of NO by Imperatorin revealed that Imperatorin 50μM, 25μM & 10μM caused 87%, 67.5% and 46.3% inhibition of NO production, respectively (Supplementary Figure1B). Imperatorin also suppressed the production of NO in chondrocytes post-stimulation with IL-1β indicating its therapeutic potential (Supplementary Figure 1C).
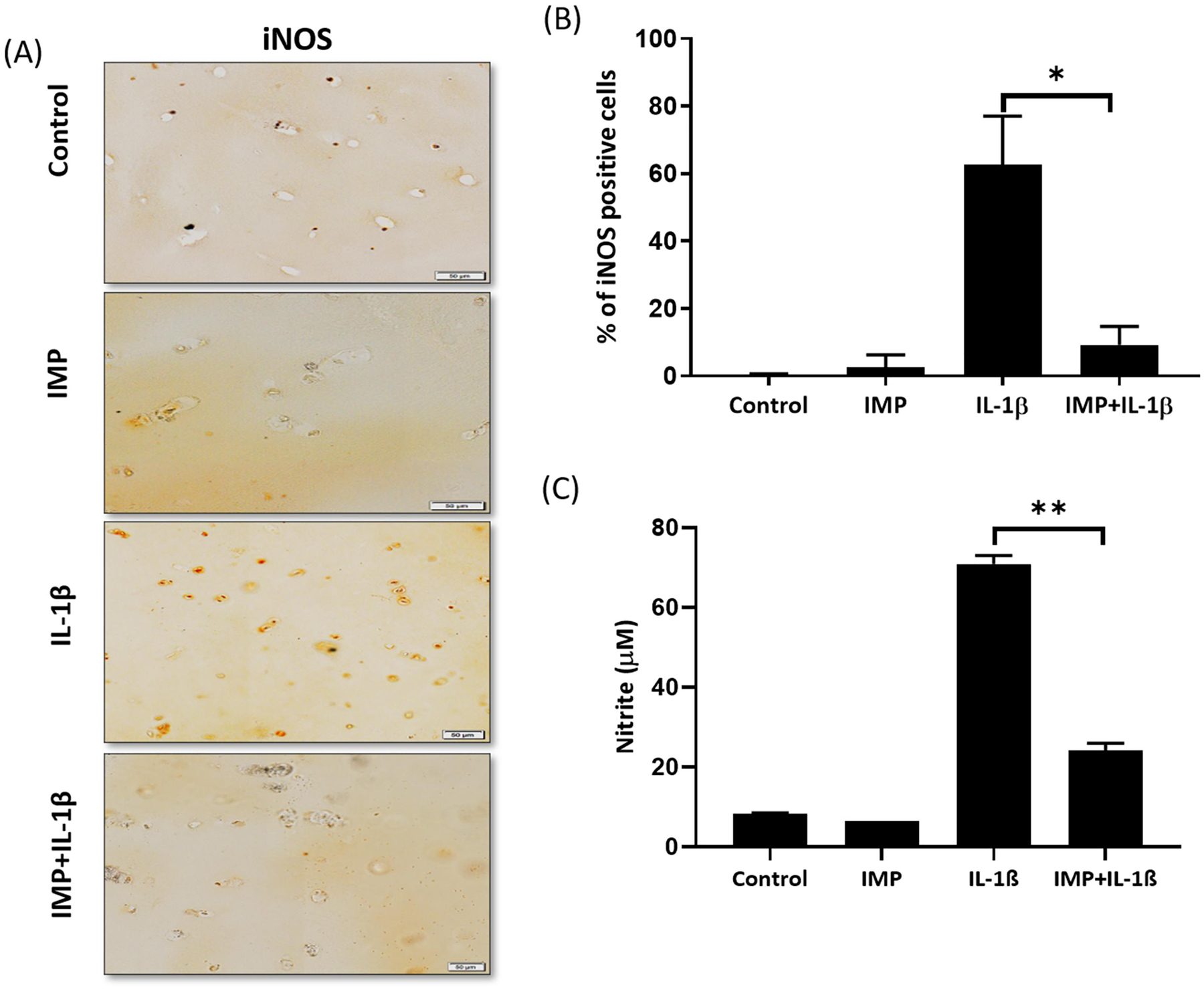
3.4. Imperatorin inhibited the IL-1β-induced NO levels and expression of iNOS in human OA cartilage explants
Human OA cartilage explants were treated with Imperatorin followed by IL-1β as described above. It was found that the expression of iNOS in IL-1β stimulated cartilage explants was suppressed by pretreatment with Imperatorin (Figure 3A). Quantification of the immunohistochemical staining was carried out by counting the percentage of iNOS positive cells. A significantly lower level of iNOS positive cells was seen in the IMP+IL-1β treated explants as compared with the IL-1β treated explants (Figure 3B). In addition, presence of Imperatorin significantly reduced the production of NO upon IL-1β stimulation (Figure 3C). These results show that Imperatorin has anti-inflammatory activity and it suppressed IL-1β induced expression of iNOS and production of NO in OA and in normal human chondrocytes.
Figure 3. Imperatorin inhibited IL-1β-induced NO production and expression of iNOS in human cartilage explants.
(A) iNOS expression in human OA cartilage explants treated with IL-1β in the presence or absence of Imperatorin was determined by IHC (20X magnification). (B) Quantification of IHC data. For each explant, 5 sections were scored (N=5). (C) Supernatants of the explants were collected to estimate the levels of NO. The values are mean ± SD of three independent experiments. (** p≤0.005). The values are mean ± SD of three independent experiments.
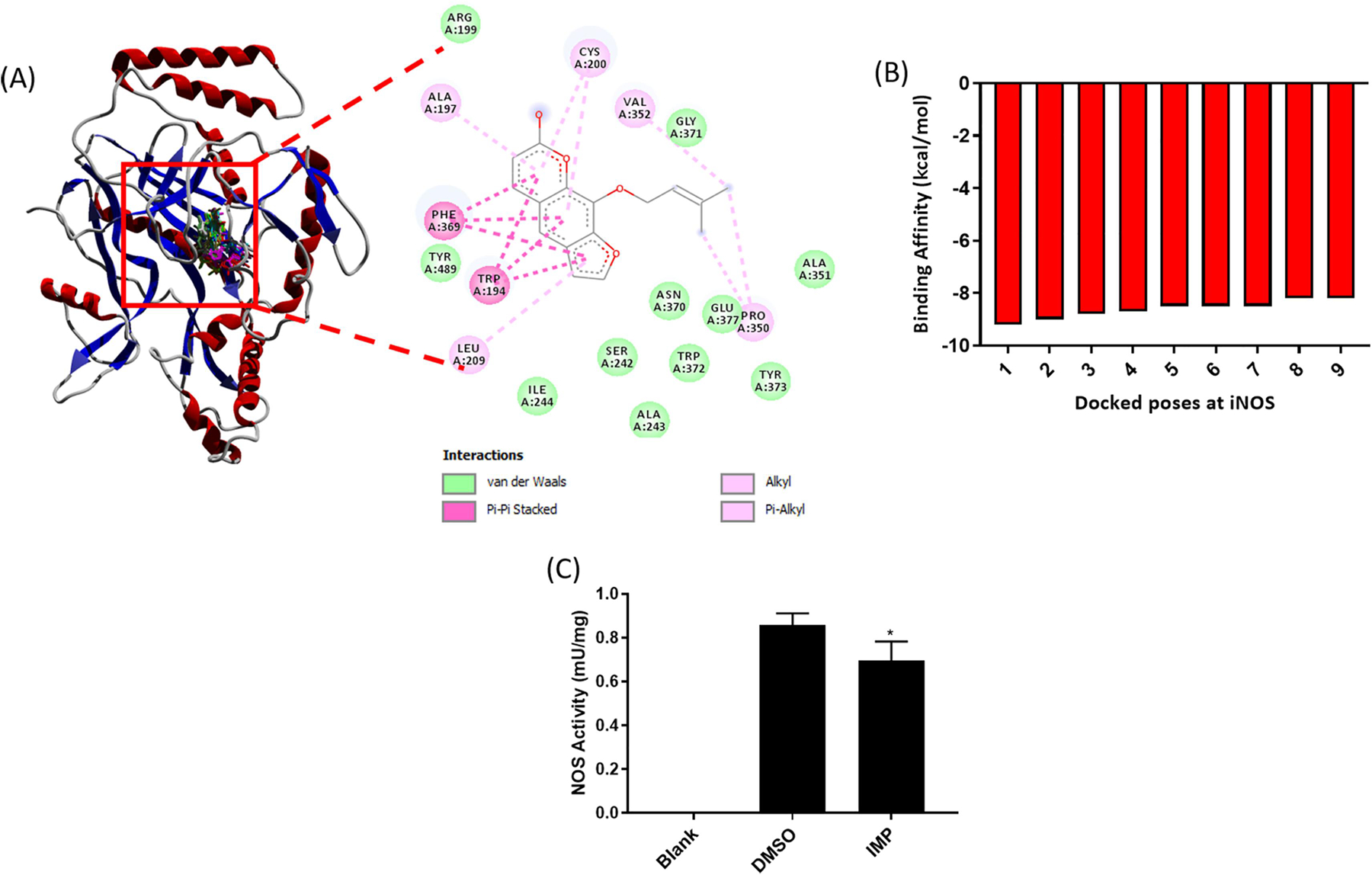
3.5. Imperatorin binds to iNOS and inhibited its activity as evident by molecular docking
We performed molecular docking analyses of Imperatorin against iNOS protein and generated nine docking poses (Figure 4A). Each of nine conformations can be observed at single binding location that supports the feasible binding affinity of Imperatorin towards iNOS. Possible molecular interactions between iNOS and Imperatorin can be seen as Van der Waals, hydrogen bonds and pi-interactions. Binding affinity (kcal/mol) was shown for each docked pose (Figure 4B). Negative value of binding affinity shows the stability of the Imperatorin-iNOS interaction. To experimentally confirm that Imperatorin inhibits NOS activity by binding to iNOS, we measured the iNOS activity in the chondrocytes treated with Imperatorin using an in vitro iNOS activity assay and found that Imperatorin indeed inhibited the iNOS activity (Figure 4C). These results indicate that Imperatorin suppressed the production of NO not only by decreasing the expression of iNOS, but also by binding to iNOS and inhibiting its activity.
Figure 4. Imperatorin binds to iNOS protein and inhibits its activity.
(A) Docking of Imperatorin on iNOS protein was done to predict its binding with iNOS. (B) Binding affinity values for nine different poses of Imperatorin against iNOS. (C) Endogenous NOS activity in OA chondrocyte lysate was determined in the presence and absence of Imperatorin using the BioVision NOS activity assay.
3.6. Imperatorin suppressed iNOS by inhibiting the IL-1β induced activation of ERK-MAPK/AP1 pathway
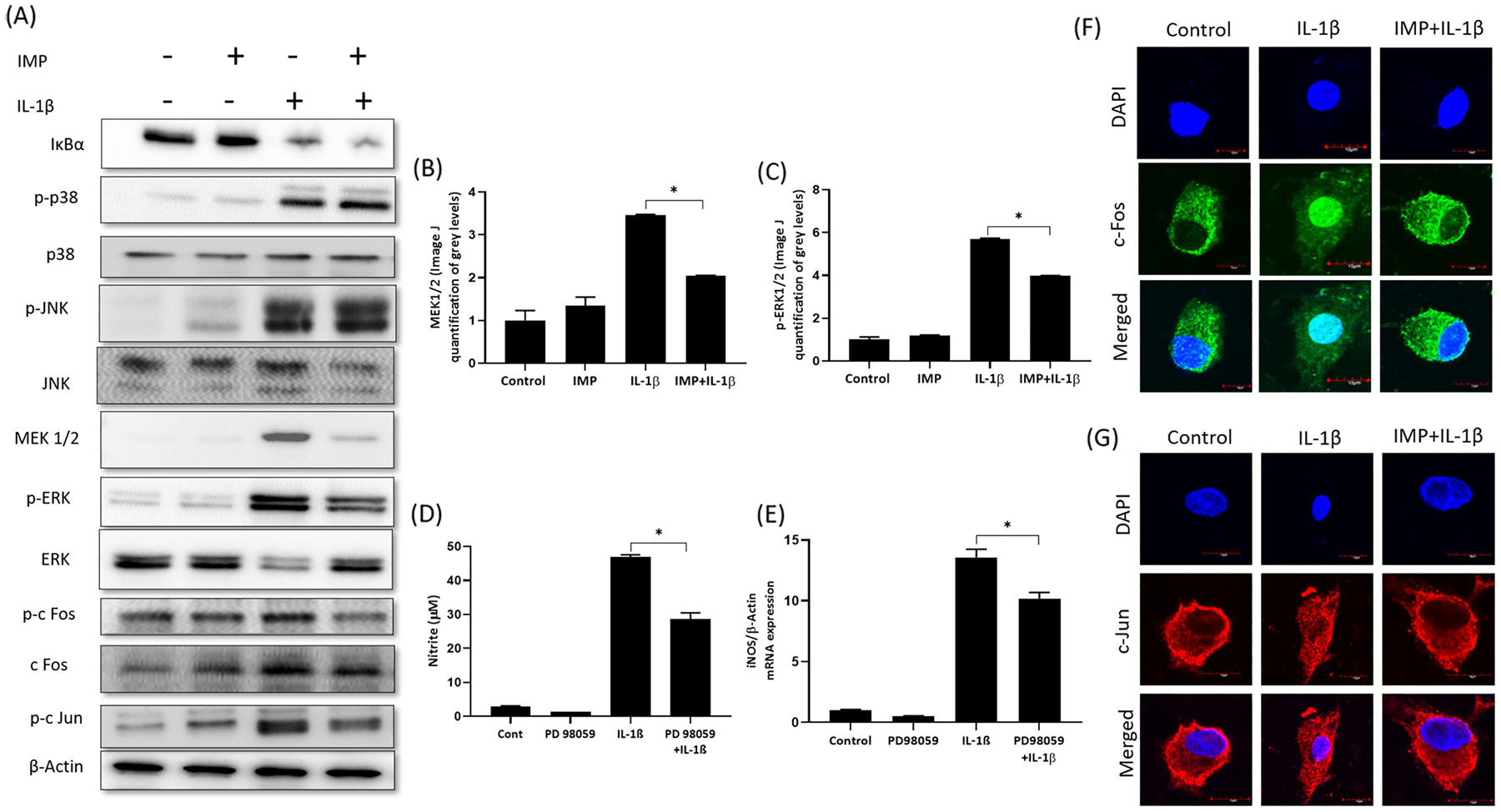
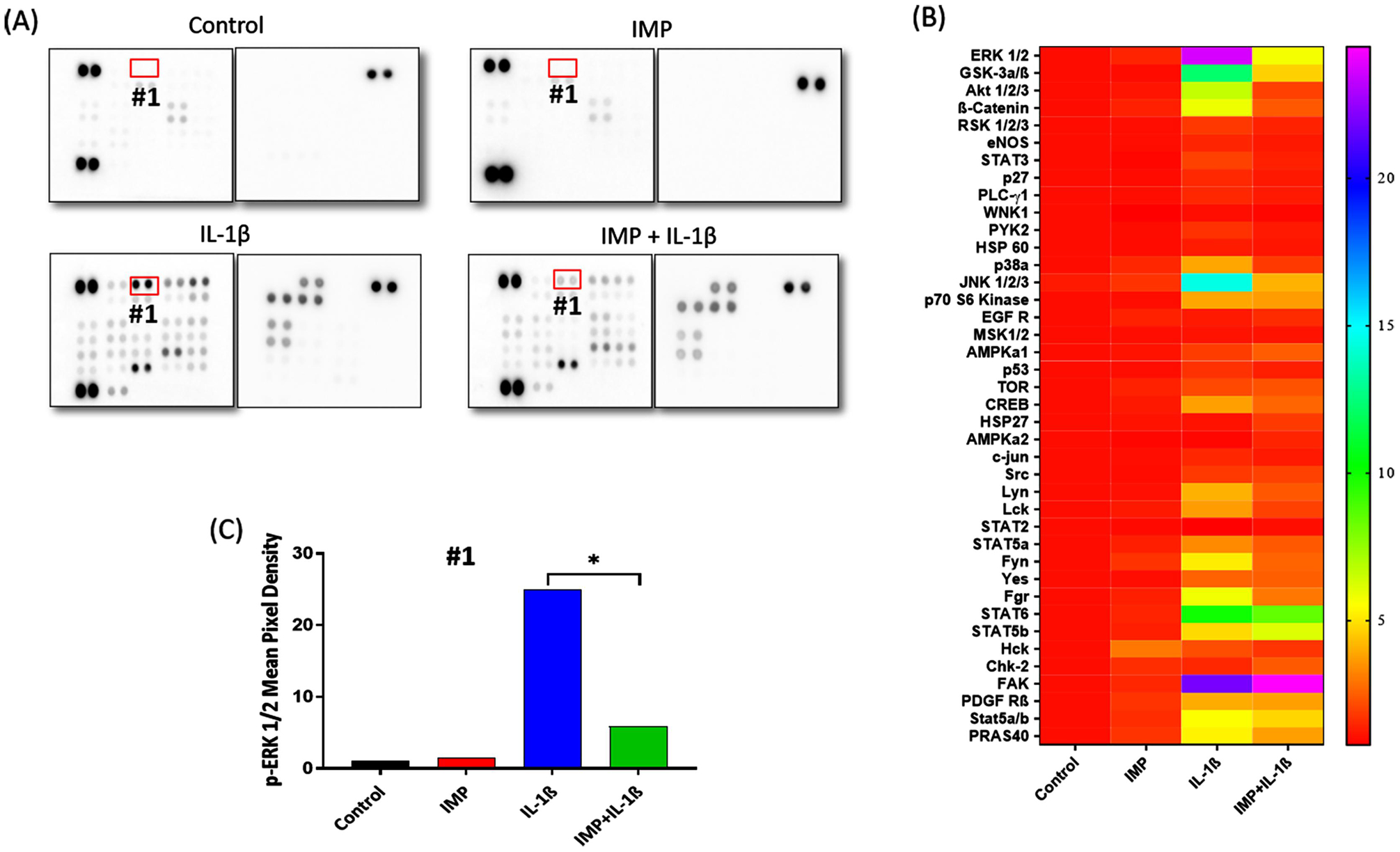
To find out the signaling pathway suppressed by Imperatorin, we performed Proteome Profiler Human Phospho-Kinase Array on cell lysates obtained from the following treatment groups: DMSO (Control), IMP, IL-1β and IMP+ IL-1β (Figure 5A). The pixel densities of every dot were quantified by ImageJ and a heat map of densitometric analyses of dot-blot image signals from treated chondrocytes normalized to those from control was generated and presented as fold change in protein levels (Figure 5B). The signaling molecule whose phosphorylation was most affected due to Imperatorin pretreatment was ERK1/2. The mean pixel density for p-ERK1/2 spots was plotted, and it was found that Imperatorin inhibited IL-1β induced phosphorylation of ERK1/2 (Figure 5C). The result from proteome profiler array was confirmed by immunoblotting (Figure-6A). Our results showed that Imperatorin suppressed the activation of ERK1/2 and its upstream kinase, MEK (Figure 6A, B&C). Next, we examined the effect of Imperatorin on the activation of other members of MAPK family. As indicated by phosphorylation, p-38 and JNK were activated by IL-1β, but Imperatorin had no effect on the activation of p-38 and JNK (Figure 6A). We also examined the effect of Imperatorin on the activation of NF-κB signaling pathway and found no effect of Imperatorin on IL-1β induced degradation of IκBα (Figure 6A). The role of ERK1/2 in the regulation of iNOS was validated by using PD98059, an inhibitor of MEK/ERK pathway. We found that PD98059 treatment suppressed the IL-1β induced production of iNOS (Figure 6D) and NO (Figure 6E). One of the substrates that is downstream of ERK1/2 is Activator protein 1 (AP-1), a heterodimer of c-Fos and c-Jun [28]. We determined the effect of Imperatorin treatment on the activation of c-Fos and c-Jun transcription factors in IL-1β stimulated OA chondrocytes and found that Imperatorin inhibited the IL-1β induced phosphorylation of c-Fos and c-Jun (Figure 6A). The activation of c-Fos and c-Jun was further confirmed by determining their nuclear translocation by immunofluorescence staining. c-Fos and c-Jun translocated to nucleus as early as 20 minutes post-IL-1β stimulation which was suppressed by Imperatorin (Figure 6F&G). Thus, Imperatorin mediated inhibition of iNOS was dependent on the ERK-MAPK pathway, but independent of NF-κB pathway.
Figure 5. Imperatorin inhibited ERK-MAPK pathway in human OA chondrocytes.

(A) Proteome Profiler Human Phospho-Kinase Array was performed on cell lysates obtained from chondrocytes treated with DMSO (Control) or IMP or IL-1β or IMP + IL-1β. (B) Heat map of densitometric analyses of dot-blot image signals from treated chondrocytes normalized to those from control and presented as fold change in protein levels. (C) The pixel densities of the single dots of p-ERK ½ were quantified by ImageJ software (*p≤0.05).
Figure 6. Imperatorin suppressed iNOS by inhibiting ERK-MAPK pathway.
(A) Human OA chondrocytes were treated with IL-1β in the presence or absence of Imperatorin and the activation of NF-κB, p-38, JNK, MEK1/2, ERK1/2, c-Fos and c-Jun was investigated by Immunoblotting. Immunoblot (B and C) Band intensities of MEK1/2 and ERK1/2 were quantified by ImageJ. (D) Primary human OA chondrocytes were pre-treated with PD98059 (5μM) to inhibit MEK/ERK followed by treatment with IL-1β (1 ng/ml). Culture supernatants were harvested to determine NO level and (E) Chondrocytes were harvested for RNA isolation followed by qPCR analysis for iNOS expression. β-actin was used as endogenous normalization control. (F) Translocation of c-Fos to nucleus was analyzed by immunofluorescence using c-Fos-specific antibody. (G) Translocation of c-Jun to nucleus was analyzed by immunofluorescence using c-Jun-specific antibody. Nuclei were counterstained with DAPI and images were captured by Olympus FV1000 confocal microscope (60X magnification).
4. Discussion
This study reports the inhibitory effects of Imperatorin on NO production and expression of iNOS in OA chondrocytes and cartilage explants. To our knowledge, this is the first study reporting the inhibition of IL-1β induced expression of iNOS by Imperatorin and demonstrating that this was achieved by inhibiting the activation of ERK-MAPK/AP1 signaling pathway in IL-1β stimulated OA chondrocytes. In the present study, we showed that pretreatment of OA chondrocytes with Imperatorin significantly inhibited both the production of NO and iNOS expression in response to stimulation with IL-1β. Imperatorin exhibited a dose-dependent inhibitory effect on iNOS transcript level and a corresponding dose-dependent inhibition in the NO production. We found consistent results in normal human chondrocytes as well. Since cultured primary chondrocytes do not mimic the exact cartilage matrix environment, it is crucial to study the inhibitory response in explant culture in which extracellular matrix accurately mimics the in vivo environment. We observed that Imperatorin suppressed the IL-1β induced NO levels and inhibited the expression of iNOS in human OA cartilage explants.
Plant-derived small molecules with NO inhibitory potential are particularly important as they have minimum side effects and are cost-effective [29]. We have demonstrated that Imperatorin was not toxic to primary human chondrocytes. Many compounds that inhibit iNOS are being researched for their potential protective effects in Osteoarthritis [8]. L-NIL, a selective inhibitor of iNOS, attenuates the progression of experimental OA in dogs [30] and notably, SD-6010, a selective inhibitor of iNOS has been beneficial in osteoarthritic and is currently in clinical trials [31]. Previous studies have demonstrated that Imperatorin possesses potent anti-inflammatory activity [11]. It has been previously demonstrated that Isoimperatorin, an isoform of Imperatorin, reduced the expression levels of MMP-13, Runx2, Col X and VEGF in murine chondrocytes [32]. It is also demonstrated that in mice treated with Isoimperatorin, the expression levels of MMP13, Runx2, Col X and VEGF were reduced and Isoimperatorin ameliorated pathological alterations in OA by delaying cartilage degradation. In another study, it was shown that Imperatorin significantly reduced collagen-induced arthritis by reducing synovial hyperplasia [33].
Furthermore, to elucidate the mechanism of Imperatorin mediated inhibition of iNOS, we investigated common signaling pathways involved in iNOS regulation. IL-1β has been shown to induce NO production through activation of NF-κB, MAPK, and JAK-STAT pathways in chondrocytes [34]. To find out the exact pathway affected by Imperatorin, we used human phospho-kinase array. Analysis of the array data revealed that Imperatorin inhibited phosphorylation of ERK1/2. We further confirmed the array data using Western Blotting. As activation of NF-κB has mostly been implicated in the pathological expression of iNOS and production of NO [34], we first investigated the activation of NF-κB by analyzing protein expression of IκBα and we observed that Imperatorin did not inhibit the IL-1β induced activation of NF- κB. Next, we investigated the activation of p-38 and JNK by analyzing protein expression of P-p38, p38, P-JNK, JNK and found that Imperatorin did not inhibit the IL-1β induced activation of p-38 and JNK. In a previous study it was reported that Imperatorin inhibited the expression of iNOS by suppressing the activity of NF- κB in RAW 264.7 cells [11] and taken together with our data indicates that the anti-inflammatory activity of Imperatorin is through different signaling pathways in different cell types.
AP-1 is one of the downstream substrate of ERK that controls many cellular processes including cell proliferation, differentiation and apoptosis [35]. AP-1 is a heterodimer that is mainly composed of c-Fos and c-Jun, so we looked at the response of Imperatorin treatment on the activation of c-Fos and c-Jun transcription factors in IL-1β stimulated OA chondrocytes. We found that Imperatorin inhibited the activation of ERK1/2 via inhibiting the IL-1β induced nuclear translocation of c-Fos and c-Jun. It has been shown in the past that inhibition of ERK has the potential to slow OA progression [36]. We used PD98059 to inhibit ERK/MAPK pathway in chondrocytes and found that inhibition of ERK1/2 suppressed the expression of iNOS and production of NO confirming its role in the regulation of iNOS in chondrocytes.
We calculated the % inhibition of the expression of iNOS and production of NO, respectively, in IL-1β vs. IMP+ IL-1β treated chondrocytes and found that % inhibition in the production of NO (87%) was much greater than the inhibition in expression of iNOS (39%), suggesting that this inhibition may not be only because of the inhibition of iNOS expression, but may also be due to decreased in iNOS activity. To investigate this, we performed molecular docking of Imperatorin against iNOS and found that Imperatorin showed strong binding towards iNOS. We confirmed this finding by checking the effect of Imperatorin on iNOS activity and we found that Imperatorin inhibited the iNOS activity. Thus, Imperatorin mediated inhibition of iNOS was dependent on ERK-MAPK/AP1 pathway, but independent of NF-κB and p-38, JNK signaling pathways. Also, imperatorin binds to iNOS protein and inhibits its enzymatic activity.
Conclusion
In conclusion, these results revealed that Imperatorin treatment inhibited the expression of iNOS and effectively suppressed the production of NO in OA chondrocytes under pathological conditions. These results have potential clinical significance considering the role of NO in joint metabolism and cartilage homeostasis. Although further studies are required to analyze the observed inhibitory response in mouse OA model, these results for the first time demonstrate that Imperatorin can counteract the production of NO in OA joint has thus, has the potential to be developed as a therapeutic agent for OA management.
Supplementary Material
Supplementary figure 1 (A) Effect of IMP on IL-1β-induced NO in normal healthy chondrocytes: Primary human chondrocytes were treated or not with Imperatorin (10, 25 & 50 μM) in the presence or absence of IL-1β (1 ng/mL) for 24 h. The nitrite levels in the chondrocyte culture medium were assessed by Griess assay. (B) Calculation of % Inhibition of NO at different concentrations of Imperatorin (C) Therapeutic effect of IMP on IL-1β-induced NO in normal chondrocytes: Primary human chondrocytes were pretreated with Imperatorin (50 μM) for 2 hours and then treated with IL-1β (1 ng/mL) for 24 h or Imperatorin and IL-1β treatment given at the same time or Imperatorin treatment given after 2 hours of pretreatment of IL-1β (*p≤0.05, **p ≤0.005).
Funding:
This work was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases and the NIH/National Center for Complementary and Integrative Health of the National Institutes of Health under Award Numbers R01AR067056 and AT007373 respectively.
Abbreviations:
- IMP
Imperatorin
- ERK
extracellular signal-regulated kinase
- IL-1β
interleukin-1β
- MAPK
mitogen-activated protein-kinase
- NF-κB
nuclear-factor-κB
- IHC
Immunohistochemistry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- [1].Vuolteenaho K, Moilanen T, Knowles RG, Moilanen E, The role of nitric oxide in osteoarthritis, Scand J Rheumatol 36(4) (2007) 247–58. [DOI] [PubMed] [Google Scholar]
- [2].Amin AR, Abramson SB, The role of nitric oxide in articular cartilage breakdown in osteoarthritis, Curr Opin Rheumatol 10(3) (1998) 263–8. [DOI] [PubMed] [Google Scholar]
- [3].Jang D, Murrell GA, Nitric oxide in arthritis, Free Radic Biol Med 24(9) (1998) 1511–9. [DOI] [PubMed] [Google Scholar]
- [4].Abramson SB, Attur M, Amin AR, Clancy R, Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis, Curr Rheumatol Rep 3(6) (2001) 535–41. [DOI] [PubMed] [Google Scholar]
- [5].Tsikas D, Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids, Free Radic Res 39(8) (2005) 797–815. [DOI] [PubMed] [Google Scholar]
- [6].Loeser RF, Carlson CS, Del Carlo M, Cole A, Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1, Arthritis Rheum 46(9) (2002) 2349–57. [DOI] [PubMed] [Google Scholar]
- [7].Abramson SB, Osteoarthritis and nitric oxide, Osteoarthritis Cartilage 16Suppl 2 (2008) S15–20. [DOI] [PubMed] [Google Scholar]
- [8].Leonidou A, Lepetsos P, Mintzas M, Kenanidis E, Macheras G, Tzetis M, Potoupnis M, Tsiridis E, Inducible nitric oxide synthase as a target for osteoarthritis treatment, Expert Opin Ther Targets 22(4) (2018) 299–318. [DOI] [PubMed] [Google Scholar]
- [9].Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA, Progress in the development of selective nitric oxide synthase (NOS) inhibitors, Curr Pharm Des 8(3) (2002) 177–200. [DOI] [PubMed] [Google Scholar]
- [10].Wang KS, Lv Y, Wang Z, Ma J, Mi C, Li X, Xu GH, Piao LX, Zheng SZ, Jin X, Imperatorin efficiently blocks TNF-alpha-mediated activation of ROS/PI3K/Akt/NF-kappaB pathway, Oncol Rep 37(6) (2017) 3397–3404. [DOI] [PubMed] [Google Scholar]
- [11].Zhang X, Li W, Abudureheman A, Cheng T, Peng P, Imperatorin possesses notable antiinflammatory activity in vitro and in vivo through inhibition of the NFkappaB pathway, Mol Med Rep 16(6) (2017) 8619–8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang WQ, Zhu ZX, Song YL, Qi BW, Wang J, Su C, Tu PF, Shi SP, Dimeric furanocoumarins from the roots of Angelica dahurica, Nat Prod Res 31(8) (2017) 870–877. [DOI] [PubMed] [Google Scholar]
- [13].Sun J, Chi G, Soromou LW, Chen N, Guan M, Wu Q, Wang D, Li H, Preventive effect of Imperatorin on acute lung injury induced by lipopolysaccharide in mice, Int Immunopharmacol 14(4) (2012) 369–74. [DOI] [PubMed] [Google Scholar]
- [14].Zheng YM, Lu AX, Shen JZ, Kwok AH, Ho WS, Imperatorin exhibits anticancer activities in human colon cancer cells via the caspase cascade, Oncol Rep 35(4) (2016) 1995–2002. [DOI] [PubMed] [Google Scholar]
- [15].Ansari MY, Ahmad N, Haqqi TM, Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes, Cell Physiol Biochem 49(3) (2018) 932–946. [DOI] [PubMed] [Google Scholar]
- [16].Khan NM, Ahmad I, Ansari MY, Haqqi TM, Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1beta stimulated osteoarthritis chondrocytes, Chem Biol Interact 274 (2017) 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Haseeb A, Khan NM, Ashruf OS, Haqqi TM, A Polyphenol-rich Pomegranate Fruit Extract Suppresses NF-kappaB and IL-6 Expression by Blocking the Activation of IKKbeta and NIK in Primary Human Chondrocytes, Phytother Res 31(5) (2017) 778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Akhtar N, Khan NM, Ashruf OS, Haqqi TM, Inhibition of cartilage degradation and suppression of PGE2 and MMPs expression by pomegranate fruit extract in a model of posttraumatic osteoarthritis, Nutrition 33 (2017) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rasheed Z, Akhtar N, Khan A, Khan KA, Haqqi TM, Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-kappaB in activated human mast cells: suppression of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-8, J Pharmacol Exp Ther 333(2) (2010) 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ansari MY, Khan NM, Ahmad I, Haqqi TM, Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes, Osteoarthritis Cartilage 26(8) (2018) 1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khan NM, Ansari MY, Haqqi TM, Sucrose, But Not Glucose, Blocks IL1-beta-Induced Inflammatory Response in Human Chondrocytes by Inducing Autophagy via AKT/mTOR Pathway, J Cell Biochem 118(3) (2017) 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ansari MY, Haqqi TM, Interleukin-1beta induced Stress Granules Sequester COX-2 mRNA and Regulates its Stability and Translation in Human OA Chondrocytes, Sci Rep 6 (2016) 27611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ansari MY, Khan NM, Ahmad N, Green J, Novak K, Haqqi TM, Genetic Inactivation of ZCCHC6 Suppresses Interleukin-6 Expression and Reduces the Severity of Experimental Osteoarthritis in Mice, Arthritis Rheumatol 71(4) (2019) 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Trott O, Olson AJ, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J Comput Chem 31(2) (2010) 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Haseeb A, Ansari MY, Haqqi TM, Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes, J Orthop Res 35(2) (2017) 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM, Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes, Free Radic Biol Med 106 (2017) 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, Lee DA, Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs, Arthritis Res Ther 10(2) (2008) R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hess J, Angel P, Schorpp-Kistner M, AP-1 subunits: quarrel and harmony among siblings, J Cell Sci 117(Pt 25) (2004) 5965–73. [DOI] [PubMed] [Google Scholar]
- [29].Akhtar N, Haqqi TM, Current nutraceuticals in the management of osteoarthritis: a review, Ther Adv Musculoskelet Dis 4(3) (2012) 181–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J, Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level, Arthritis Rheum 43(6) (2000) 1290–9. [DOI] [PubMed] [Google Scholar]
- [31].Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, Abramson SB, Manning PT, Miller CG, Vignon E, A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee, Ann Rheum Dis 72(2) (2013) 187–95. [DOI] [PubMed] [Google Scholar]
- [32].Ouyang J, Jiang H, Fang H, Cui W, Cai D, Isoimperatorin ameliorates osteoarthritis by downregulating the mammalian target of rapamycin C1 signaling pathway, Mol Med Rep 16(6) (2017) 9636–9644. [DOI] [PubMed] [Google Scholar]
- [33].Zhai KF, Duan H, Chen Y, Khan GJ, Cao WG, Gao GZ, Shan LL, Wei ZJ, Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways, Food Funct 9(4) (2018) 2070–2079. [DOI] [PubMed] [Google Scholar]
- [34].Schmidt N, Pautz A, Art J, Rauschkolb P, Jung M, Erkel G, Goldring MB, Kleinert H, Transcriptional and post-transcriptional regulation of iNOS expression in human chondrocytes, Biochem Pharmacol 79(5) (2010) 722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ameyar M, Wisniewska M, Weitzman JB, A role for AP-1 in apoptosis: the case for and against, Biochimie 85(8) (2003) 747–52. [DOI] [PubMed] [Google Scholar]
- [36].Loeser RF, Erickson EA, Long DL, Mitogen-activated protein kinases as therapeutic targets in osteoarthritis, Curr Opin Rheumatol 20(5) (2008) 581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1 (A) Effect of IMP on IL-1β-induced NO in normal healthy chondrocytes: Primary human chondrocytes were treated or not with Imperatorin (10, 25 & 50 μM) in the presence or absence of IL-1β (1 ng/mL) for 24 h. The nitrite levels in the chondrocyte culture medium were assessed by Griess assay. (B) Calculation of % Inhibition of NO at different concentrations of Imperatorin (C) Therapeutic effect of IMP on IL-1β-induced NO in normal chondrocytes: Primary human chondrocytes were pretreated with Imperatorin (50 μM) for 2 hours and then treated with IL-1β (1 ng/mL) for 24 h or Imperatorin and IL-1β treatment given at the same time or Imperatorin treatment given after 2 hours of pretreatment of IL-1β (*p≤0.05, **p ≤0.005).


