Abstract
Purpose
The present study aimed to assess the prevalence of vascular lake (VL), its associated factors and correlation with prognosis in hepatocellular carcinoma (HCC) patients treated with drug-eluting bead transarterial chemoembolization (DEB-TACE).
Patients and Methods
A total of 286 primary HCC patients (with 384 treated nodules) receiving DEB-TACE treatment were recruited, and their clinical characteristics were documented. The occurrence of VL was recorded, and treatment responses were assessed according to the modified response evaluation criteria in solid tumor (mRECIST).In terms of treatment response, the total response status (including CR, PR, SD and PD), objective response rate (ORR) and disease control rate (DCR) were elevated in VL patients compared to non-VL patients as well as in VL nodules compared to non-VL nodules. Liver function indexes and adverse events were assessed. Progression-free survival (PFS) and overall survival (OS) were evaluated with the last follow-up date of March 2020.
Results
The patient-based and nodule-based VL occurrence rates were 17.1% and 16.4%, respectively. Larger tumor size, pseudocapsules and smaller bead size were independently associated with VL occurrence. PFS and OS were more prolonged in VL patients than in non-VL patients, and VL independently correlated with better PFS and OS. For liver function, the liver function indexes before and after DEB-TACE were of no difference between VL patients and non-VL patients. Additionally, the incidences of adverse events were similar between VL patients and non-VL patients.
Conclusion
VL occurs in 17.1% of HCC patients treated with DEB-TACE, and it is correlated with larger tumor size, pseudocapsule, smaller bead size, more favorable treatment response and better survival.
Keywords: hepatocellular carcinoma, drug-eluting bead transarterial chemoembolization, vascular lake, related factors, efficacy, safety
Introduction
Hepatocellular carcinoma (HCC), which accounts for most liver cancer cases, is one of the most lethal cancers and is predicted to result in 1 million related deaths worldwide in 2030.1,2 Patients with HCC are treated mostly according to the Barcelona clinical stage of liver cancer (BCLC) staging system. Potential curative therapies, such as resection and ablation, are recommended for patients at BCLC stage 0 or A; however, patients at more advanced stages can only receive noncurative treatments, such as sorafenib, regorafenib, chemoembolization and supportive care.3,4 Unfortunately, patients at middle and advanced stages occupy the majority of HCC patients because the disease is mostly asymptomatic at early stages, which largely delays the duration before diagnosis. In addition, some early-stage HCC patients are not qualified for resection or other potentially curative therapies because of inadequate physical function or unique tumor features.5,6 Therefore, the management of these patients is crucial for improving the overall prognosis of HCC patients.
In recent years, chemoembolization has been increasingly applied in HCC patients at various stages, but the BCLC staging system only recommends it as a first-line therapy for intermediate-stage patients.7 Conventional transarterial chemoembolization (cTACE) is the leading type of chemoembolization until the existence of drug-eluting bead TACE (DEB-TACE), which allows HCC patients to have more choices of treatment. More recently, studies have revealed that DEB-TACE not only exceeds cTACE in reducing systemic toxicity but also realizes more favorable efficiency in HCC patients.8,9 For instance, a previous study has reported that DEB-TACE achieves a better treatment response and survival profile than cTACE in HCC patients with a history of multiple cTACE treatments.8 Furthermore, other studies have stated that DEB-TACE displays acceptable efficacy and tolerance when treating advanced HCC patients, indicating a potential wider application of DEB-TACE in the clinical setting.10,11
Vascular lake (VL) is a phenomenon that occasionally occurs in the embolization of HCC, and it normally presents as blocking in the tumor-feeding artery and pooling of the contrast agent during embolization.12 The occurrence of VL is not uncommon in HCC patients during DEB-TACE treatment according to several reports, with an estimated prevalence of approximately 10%-20%; however, the causes that generate VL are still under investigation.12,13 Several researchers have suggested that the generation of VL may be correlated with the rupture of the targeted tumor caused by a sudden stasis in the tumor-feeding artery, resulting in a rapid increase in local pressure.12 However, reports about the precise mechanism of VL in DEB-TACE or other chemoembolization procedures in HCC patients are quite scarce. More importantly, the most concerning issue of VL occurrence is whether it has an impact on the efficacy and safety of DEB-TACE in HCC patients. However, this issue is rarely investigated as previous literature mostly focuses on case reports of VL during DEB-TACE in HCC patients, and studies about the effect of VL on the efficacy and safety of DEB-TACE treatment in HCC are scarce. In addition, previous studies all have small sample sizes, and no study has been conducted to explore the correlation between VL and long-term prognosis in HCC patients treated with DEB-TACE.
Hence, the occurrence of VL, its associated factors and its correlation with prognosis in HCC patients treated with DEB-TACE were investigated in this study.
Methods
Patients
In total, 286 newly diagnosed primary HCC patients who were treated with DEB-TACE as first-line treatment in our hospital between August 2016 and December 2019 were recruited for this study. The inclusion criteria were as follows: (1) diagnosed with primary HCC; (2) age within 18 to 75 years; (3) at least one measurable lesion as defined by the diameter of the lesion on helical computed tomography (CT) scan ≥10 mm according to the modified response evaluation criteria in solid tumors (mRECIST);14 (4) well-to-moderately preserved liver function as defined by the Child-Pugh stage A or B; (5) normal renal function and normal coagulation function (or coagulation dysfunction corrected after treatment); (6) proportion of liver tumors less than 75% of total liver volume; (7) no previous therapy for HCC and suitable for DEB-TACE treatment; (8) life expectancy more than 12 weeks; (9) Eastern Cooperative Oncology Group (ECOG) performance score 0~1; and (10) regular follow-up. The exclusion criteria were as follows: (1) Child-Pugh stage C; (2) severe coagulation dysfunction that could not be corrected after treatment; (3) serum bilirubin >51 μmol/L, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >5 upper limit of normal (ULN); (4) portal venous shunt; (5) left ventricular ejection fraction (LVEF) <50%; (6) serious comorbidities (eg, hypertension and coronary heart disease) or history of psychiatric disease or severe allergies; (7) HCC patients candidate for liver transplantation, resection, or local thermal ablation; (8) pregnant or lactating women.
Ethical Approval
This study was approved by the Ethics and Scientific Trial Committee of the First Affiliated Hospital of Zhengzhou University and was conducted in accordance with the principles expressed in the Declaration of Helsinki and adhered to the standards set by the International Conference on Harmonization and Good Clinical Practice. All patients signed informed consents. The study was registered in the Chinese Clinical Trial Registry with a registration number of ChiCTR-IOR-17012159.
Clinical Data Collection
The clinical characteristics of the patients were documented, including age, sex, etiology, tumor number, portal vein invasion, Child-Pugh stage and Barcelona Clinic Liver Cancer (BCLC) stage. In addition, nodule-based features, including tumor size, tumor location and pseudocapsule, were also collected and recorded. Importantly, due to the confidentiality of our hospital’s policy, the raw data of our study was not allowed to be public, therefore the data would only be accessible via contacting the corresponding author (for scientific research use only).
DEB-TACE Procedures
The DEB-TACE procedure was performed under local anesthesia. The right femoral artery was punctured by the Seldinger technique, and a 5F arterial sheath was then placed. A 5F RH catheter or a 5F Yashiro catheter was selectively inserted into the superior mesenteric and celiac trunk for undergoing hepatic artery angiography, outlining the hepatic artery anatomy, identifying the feeding arteries of tumors and evaluating portal vein patency status. The feeding arteries of the tumor were catheterized with a microcatheter using the superselective catheterization method followed by perfusion chemotherapy. Afterwards, CalliSpheres® Beads (100–300 μm or 300–500 μm, Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) loaded with arsenic trioxide or adriamycin were injected into the feeding arteries of tumors for chemoembolization. The preparation of CalliSpheres® Beads and drug loading were performed according to the method described in previous studies.15,16 The embolization was stopped when the near stasis observed in the feeding arteries of tumors. If a tumor blush was still observed after injection of the loaded CalliSpheres® beads, additional polyvinyl alcohol (PVA) particles with diameters of 350–560 μm were added. The embolization endpoint was the disappearance of the tumor blush under angiography. Postoperative routine liver protection and symptomatic treatments were administered for patients without other special treatments.
Management of VL
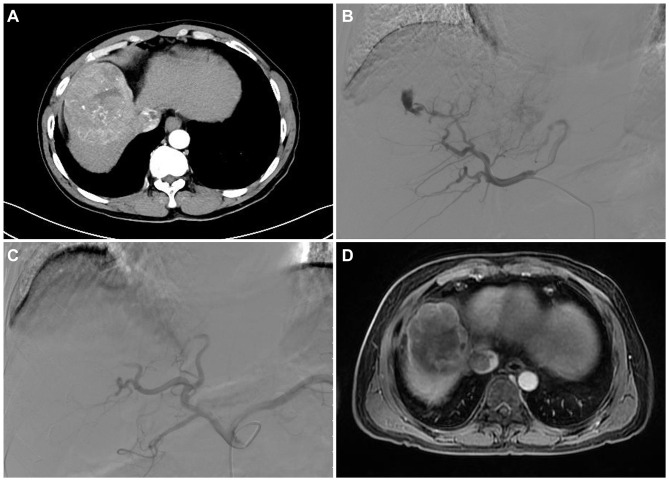
According to a previous study, the VL was defined as focal, well-circumscribed and persistent retention of contrast material in various forms on the venous phase of angiography and was distinguished from a tumor blush.12 Figure 1A displayed the HCC tumor of a 52-year-old male patient, and a partial response (PR) after the DEB-TACE treatment (Figure 1D). In the current study, the VL phenomenon occurred in the early arterial phase of angiography after embolization with drug-loaded CalliSpheres® Beads. The VL phenomenon changed its size progressively with microsphere embolization, disappeared slowly and was still observed after the venous phase (example shown in Figure 1B). For the tumor-feeding arteries with the VL phenomenon, drug-loaded CalliSpheres® Beads were continuously used for embolization. If the drug-loaded CalliSpheres® beads were depleted, PVA particles were further added for embolization until the VL phenomenon disappeared (example shown in Figure 1C). Routine blood examination was performed at 1 day after the operation in patients with VL to confirm whether the PVA particles led to further rupture and hemorrhage of the tumor. According to the occurrence of the VL phenomenon during DEB-TACE, patients were divided into VL patients and non-VL patients, and the nodules were divided into VL nodules and non-VL nodules.
Figure 1.
Image of VL and its management in a 52-year-old male patient. Arterial phase in the CT was significantly enhanced, and the middle hepatic vein and inferior vena cava were invaded by tumor (A). In the first DEB-TACE, CalliSpheres® Beads with a diameter of 100–300 μm loaded with 15 mg of arsenic trioxide were used to embolize the right hepatic artery, and the VL phenomenon occurred in the re-examined angiography (B). PVA particles with a diameter of 350–560 μm were subsequently added for embolization until the VL phenomenon disappeared in the re-examined angiography (C). MRI at 4 weeks after first DEB-TACE revealed partial contrast enhancement in the peripheral of intrahepatic lesion, suggesting a partial response according to the mRECIST criteria (D).
Abbreviations: VL, vascular lake; CT, computed tomography; DEB-TACE, drug-eluting bead transarterial chemoembolization; PVA, polyvinyl alcohol; MRI, magnetic resonance imaging; mRECIST, modified response evaluation criteria in solid tumors.
Assessments and Follow-Up
All patients underwent contrast-enhanced CT or Magnetic Resonance Imaging (MRI) at 4~6 weeks after first DEB-TACE, and the treatment response for each nodule and patient was evaluated according to the mRECIST criteria,14 where the response was classified as complete response (CR), PR, stable disease (SD), and progressive disease (PD). Objective response rate (ORR) was defined as CR+PR, and disease control rate (DCR) was defined as CR+PR+SD. Liver function was determined before DEB-TACE, one day after DEB-TACE, 7 days after DEB-TACE and 1 month after DEB-TACE. Adverse events after DEB-TACE, such as nausea, vomiting, abdominal pain and ascites, were recorded in detail and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). In addition, patients were followed up every 4–6 weeks by imaging examination and biochemical examination, and the survival status was documented at each visit. Progression-free survival (PFS) and overall survival (OS) were evaluated based on follow-up records, with the last follow-up date of March 2020. Additionally, the median follow-up time in our study was 17 (12–21) months. The PFS was defined as the time interval from the date of first DEB-TACE to the date of disease progression; the OS was defined as the time interval from the date of first DEB-TACE to the date of patient’s death.
Statistical Analysis
SPSS 21.0 statistical software (IBM, Chicago, Illinois, USA) was used for data processing and statistical analysis. Quantitative data were described as mean ± standard deviation (SD), and qualitative data were described as number with percentage (No. (%)). Comparison of quantitative data between two groups was determined by Student’s t test, and comparison of qualitative data between two groups was determined by Chi-square test, Fisher’s exact test or Wilcoxon rank sum test. PFS and OS were demonstrated using Kaplan-Meier curves, and the difference in PFS and OS between the two groups was determined by the Log rank test. Factors related to VL occurrence were assessed by multivariate logistic regression model analysis, and factors related to PFS and OS were analyzed by multivariate Cox’s proportional hazards regression. P value <0.05 was considered as statistically significant.
Results
Characteristics of HCC Patients and the Treated Nodules
In the 286 HCC patients, the number of patients aged < 60 years and patients aged ≥60 years was 211 (73.8%) and 75 (26.2%), respectively (Table 1). There were 199 (69.6%) males and 87 (30.4%) females. In addition, the number of patients with unifocal tumors and patients with multifocal tumors was 205 (71.7%) and 81 (28.3%), respectively. The number of patients with portal vein invasion and patients with no portal vein invasion was 205 (71.7%) and 81 (28.3%), respectively. The number of patients in BCLC stage A, BCLC stage B and BCLC stage C was 30 (10.5%), 172 (60.1%) and 84 (29.4%), respectively. In addition, no difference was found between VL patients and non-VL patients regarding age, sex, etiology, tumor number, portal vein invasion, Child-Pugh stage, BCLC stage or loading drugs (all P > 0.05). The other information of HCC patients’ characteristics could be seen in Table 1.
Table 1.
Clinical Characteristics of Patients
| Items | Total Patients (N=286) | VL Patients (n=49) | Non-VL Patients (n=237) | P value |
|---|---|---|---|---|
| Age, No. (%) | 0.681 | |||
| <60 years | 211 (73.8) | 35 (71.4) | 176 (74.3) | |
| ≥60 years | 75 (26.2) | 14 (28.6) | 61 (25.7) | |
| Gender, No. (%) | 0.709 | |||
| Male | 199 (69.6) | 33 (67.3) | 166 (70.0) | |
| Female | 87 (30.4) | 16 (32.7) | 71 (30.0) | |
| Etiology, No. (%) | 0.906 | |||
| Hepatitis B | 183 (64.0) | 33 (67.3) | 150 (63.3) | |
| Hepatitis C | 51 (17.8) | 9 (18.4) | 42 (17.7) | |
| Alcoholic cirrhosis | 31 (10.8) | 4 (8.2) | 27 (11.4) | |
| Alcohol/hepatitis | 4 (1.4) | 1 (2.0) | 3 (1.3) | |
| Others | 17 (6.0) | 2 (4.1) | 15 (6.3) | |
| Tumor number, No. (%) | 0.966 | |||
| Unifocal | 205 (71.7) | 35 (71.4) | 170 (71.7) | |
| Multifocal | 81 (28.3) | 14 (28.6) | 67 (28.3) | |
| Portal vein invasion, No. (%) | 0.151 | |||
| No | 205 (71.7) | 31 (63.3) | 174 (73.4) | |
| Yes | 81 (28.3) | 18 (36.7) | 63 (26.6) | |
| Child-Pugh stage, No. (%) | 0.662 | |||
| A | 173 (60.5) | 31 (63.3) | 142 (59.9) | |
| B | 113 (39.5) | 18 (36.7) | 95 (40.1) | |
| BCLC stage, No. (%) | 0.359 | |||
| A | 30 (10.5) | 5 (10.2) | 25 (10.5) | |
| B | 172 (60.1) | 33 (67.3) | 139 (58.7) | |
| C | 84 (29.4) | 11 (22.5) | 73 (30.8) | |
| Loading drugs, No. (%) | 0.819 | |||
| Doxorubicin | 156 (54.5) | 26 (53.1) | 130 (54.9) | |
| Arsenic trioxide | 130 (45.5) | 23 (46.9) | 107 (45.1) |
Note: Comparison between the two groups (VL group and non-VL group) was determined by Chi-square test or Wilcoxon rank sum test.
Abbreviations: VL, vascular lake; BCLC, Barcelona Clinic Liver Cancer.
With regard to the treated nodules (N=384), 268 (69.8%) nodules were located in the right liver, and 116 (30.2%) nodules were located in the left liver (Table 2). The number of nodules with a size of <3 cm, 3–5 cm, 5–10 cm and ≥10 cm was 26 (6.8%), 58 (15.1%), 295 (76.8%), and 5 (1.3%), respectively. One hundred ninety-nine (51.8%) nodules presented with pseudocapsule and 185 (48.2%) nodules did not present with pseudocapsule. Additionally, the number of nodules treated with 100–300 μm CalliSpheres® beads and 300–500 μm CalliSpheres® beads was 297 (77.3%) and 87 (22.7%), respectively. Moreover, there was no difference in tumor location (P = 0.771) or tumor size (P = 0.054) between VL nodules and non-VL nodules; however, the occurrence of pseudocapsule was elevated in VL nodules compared to non-VL nodules (P < 0.001), and the proportion of treatments with 100–300 μm CalliSpheres® beads was increased in VL nodules compared to non-VL nodules (P = 0.039).
Table 2.
Characteristics of Treated Nodules
| Items | Total Treated Nodules (N=384) | VL Nodules (n=63) | Non-VL Nodules (n=321) | P value |
|---|---|---|---|---|
| Tumor location, No. (%) | 0.771 | |||
| Right | 268 (69.8) | 43 (68.3) | 225 (70.1) | |
| Left | 116 (30.2) | 20 (31.7) | 96 (29.9) | |
| Tumor size, No. (%) | 0.054 | |||
| <3 cm | 26 (6.8) | 2 (3.2) | 24 (7.5) | |
| 3–5 cm | 58 (15.1) | 6 (9.5) | 52 (16.2) | |
| 5–10 cm | 295 (76.8) | 54 (85.7) | 241 (75.1) | |
| ≥10 cm | 5 (1.3) | 1 (1.6) | 4 (1.2) | |
| Pseudocapsule, No. (%) | <0.001 | |||
| No | 199 (51.8) | 15 (23.8) | 184 (57.3) | |
| Yes | 185 (48.2) | 48 (76.2) | 137 (42.7) | |
| CalliSpheres® diameter | 0.039 | |||
| 100–300 μm | 297 (77.3) | 55 (87.3) | 242 (75.4) | |
| 300–500 μm | 87 (22.7) | 8 (12.7) | 79 (24.6) |
Note: Comparison was determined by Chi-square test or Wilcoxon rank sum test.
Abbreviation: VL, vascular lake.
Incidence of VL and Related Factors
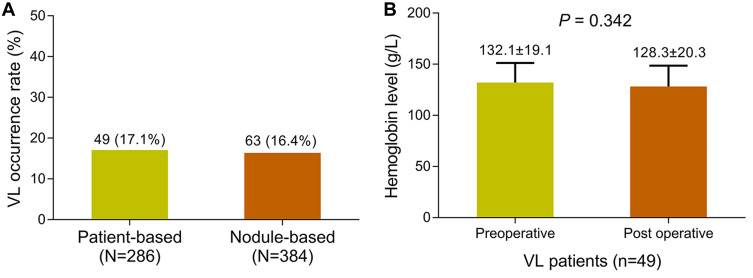
The patient-based VL occurrence rate was 17.1% and the nodule-based VL occurrence rate was 16.4% (Figure 2A). In addition, the postoperative hemoglobin level did not vary compared to the preoperative level, indicating that treatment of VL using PVA was tolerable (P = 0.342) (Figure 2B). Furthermore, multivariate logistic regression model analysis elucidated that larger tumor size (P = 0.005), pseudocapsule (P < 0.001) and smaller bead size (P = 0.007) were independently associated with VL occurrence in HCC patients treated with DEB-TACE (Table 3). In addition, the category of chemotherapy drugs used in our study was not correlated with VL occurrence in patients (P = 0.819) (Supplementary Table 1).
Figure 2.
VL occurrence. VL occurrence rate in patients and nodules (A). Preoperative hemoglobin level and postoperative hemoglobin level (B).
Abbreviation: VL, vascular lake.
Table 3.
Nodule-Based Analysis of Factors Related to VL Occurrence
| Items | Multivariate Logistic Regression Model | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| Tumor size* | 0.005 | 2.417 | 1.303 | 4.482 |
| Pseudocapsule (yes vs no) | <0.001 | 6.117 | 3.205 | 11.675 |
| CalliSpheres® diameter (100–300 μm vs 300–500 μm) | 0.007 | 3.124 | 1.373 | 7.105 |
Notes: *Tumor size was categorized as <3 cm=1, 3–5 cm=2, 5–10 cm=3 and ≥10 cm=4. Factors affecting VL were analyzed by multivariate logistic regression model.
Abbreviations: VL, vascular lake; OR, odds ratio; CI, confidence interval.
Correlation Between VL and Treatment Response
In the total treated nodules, the rates of nodules assessed as CR, PR, SD and PD were 18.5%, 42.4%, 22.7% and 16.4%, respectively (Table 4). Moreover, the ORR and DCR were 60.9% and 83.6%, respectively. In VL nodules, the CR, PR, SD, PD, ORR and DCR were 22.2%, 66.7%, 9.5%, 1.6%, 88.9% and 98.4%, respectively, and the CR, PR, SD, PD, ORR and DCR in non-VL nodules were 17.8%, 37.7%, 25.2%, 19.3%, 55.5% and 67.4%, respectively. In addition, the total response rate (P < 0.001), ORR (P < 0.001) and DCR (P < 0.001) were all increased in VL nodules compared to non-VL nodules. In the total HCC patients, the percentages of patients who achieved CR, PR, SD and PD were 18.5%, 47.9%, 21.0% and 12.6%, and the ORR and DCR were 66.4% and 87.4, respectively (Table 5). In VL HCC patients, the CR, PR, SD, PD, ORR and DCR were 20.4%, 71.4%, 6.2%, 2.0%, 91.8% and 98.0%, respectively. In non-VL HCC patients, the CR, PR, SD, PD, ORR and DCR were 18.2%, 43.0%, 24.0%, 14.8%, 61.2% and 85.2%, respectively. The total response rate (P =0.002), ORR (P < 0.001) and DCR (P = 0.014) were all elevated in VL HCC patients compared to non-VL HCC patients.
Table 4.
Nodule-Based Analysis of Treatment Response After First DEB-TACE Therapy
| Items | Total Treated Nodules (N=384) | VL Nodules (n=63) | Non-VL Nodules (n=321) | P value |
|---|---|---|---|---|
| Total response | <0.001 | |||
| CR, No. (%) | 71 (18.5) | 14 (22.2) | 57 (17.8) | |
| PR, No. (%) | 163 (42.4) | 42 (66.7) | 121 (37.7) | |
| SD, No. (%) | 87 (22.7) | 6 (9.5) | 81 (25.2) | |
| PD, No. (%) | 63 (16.4) | 1 (1.6) | 62 (19.3) | |
| ORR, No. (%) | 234 (60.9) | 56 (88.9) | 178 (55.5) | <0.001 |
| DCR, No. (%) | 321 (83.6) | 62 (98.4) | 259 (67.4) | <0.001 |
Note: Comparison was determined by Wilcoxon rank test or Chi-square test.
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; VL, vascular lake; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Table 5.
Patient-Based Analysis of Treatment Response After First DEB-TACE Therapy
| Items | Total Patients (N=286) | VL Patients (n=49) | Non-VL Patients (n=237) | P value |
|---|---|---|---|---|
| Total response | 0.002 | |||
| CR, No. (%) | 53 (18.5) | 10 (20.4) | 43 (18.2) | |
| PR, No. (%) | 137 (47.9) | 35 (71.4) | 102 (43.0) | |
| SD, No. (%) | 60 (21.0) | 3 (6.2) | 57 (24.0) | |
| PD, No. (%) | 36 (12.6) | 1 (2.0) | 35 (14.8) | |
| ORR, No. (%) | 190 (66.4) | 45 (91.8) | 145 (61.2) | <0.001 |
| DCR, No. (%) | 250 (87.4) | 48 (98.0) | 202 (85.2) | 0.014 |
Note: Comparison was determined by Wilcoxon rank test or Chi-square test.
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; VL, vascular lake; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Correlation Between VL and Survival
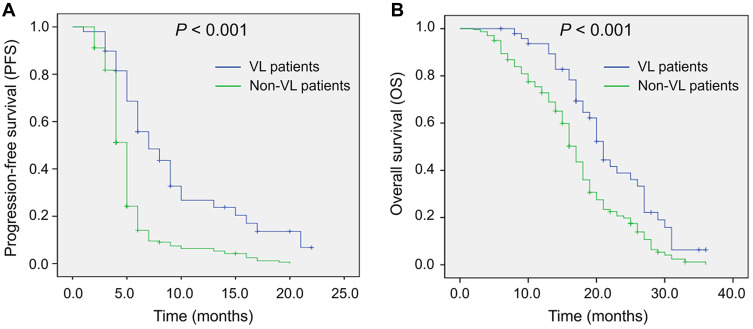
PFS (P < 0.001) (Figure 3A) was more favorable in VL HCC patients than in non-VL HCC patients, and OS (P < 0.001) (Figure 3B) was also more prolonged in VL HCC patients than in non-VL HCC patients. Furthermore, multivariate Cox’s proportional hazards regression analysis showed that VL was an independent factor for predicting longer PFS (P < 0.001) and OS (P < 0.001) (Table 6). However, portal vein invasion was independently correlated with worse PFS (P < 0.001) and OS (P < 0.001).
Figure 3.
Comparison of PFS and OS between VL patients and non-VL patients. Comparison of PFS between VL patients and non-VL patients (A). Comparison of OS between VL patients and non-VL patients (B).
Abbreviations: PFS, progression-free survival; OS, overall survival; VL, vascular lake.
Table 6.
Multivariate Cox’s Proportional Hazards Regression Analysis of Factors Related to PFS and OS
| Items | PFS | OS | ||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Non-VL vs VL | <0.001 | 1.988 (1.389–2.847) | <0.001 | 4.442 (2.938–6.717) |
| Portal vein invasion (yes vs no) | <0.001 | 2.714 (2.034–3.621) | <0.001 | 4.815 (3.441–6.739) |
Abbreviations: PFS, progression-free survival; OS, overall survival; VL, vascular lake; HR, hazard ratio; CI, confidence interval.
Correlation Between VL and Liver Function
Between VL patients and non-VL patients, the ALT levels before (P = 0.711), 1 day after (P = 0.364), 7 days after (P = 0.534), and 1 month after (P = 0.152) DEB-TACE was of no difference (Table 7). The AST levels before (P = 0.082), 1 day after (P = 0.832), 7 days after (P = 0.223) and 1 month after (P = 0.581) DEB-TACE did not vary between VL patients and non-VL patients. In addition, the ALB levels before (P = 0.577), 1 day after (P = 0.212), 7 days after (P = 0.433) and 1 month after (P = 0.579) DEB-TACE were similar between VL patients and non-VL patients. These data suggested that VL did not result in decreased liver function in HCC patients receiving DEB-TACE.
Table 7.
Liver Function Indexes Before and After First DEB-TACE Therapy
| Liver Function Indexes | VL Patients (n=49) | Non-VL Patients (n=237) | P value |
|---|---|---|---|
| ALT (U/L), mean±SD | |||
| Before DEB-TACE | 47.4±41.2 | 49.7±29.1 | 0.711 |
| 1 day after DEB-TACE | 171.5±87.2 | 182.9±78.4 | 0.364 |
| 7 days after DEB-TACE | 75.2±33.6 | 71.4±39.9 | 0.534 |
| 1 month after DEB-TACE | 39.1±18.9 | 45.9±32.0 | 0.152 |
| AST (U/L), mean±SD | |||
| Before DEB-TACE | 49.50±27.2 | 44.2±21.7 | 0.082 |
| 1 day after DEB-TACE | 163.6±56.5 | 161.7±67.2 | 0.832 |
| 7 days after DEB-TACE | 81.1±36.1 | 89.4±44.7 | 0.223 |
| 1 month after DEB-TACE | 57.4±35.1 | 54.1±38.7 | 0.581 |
| ALB (g/L), mean±SD | |||
| Before DEB-TACE | 35.7±4.9 | 36.1±4.5 | 0.577 |
| 1 day after DEB-TACE | 31.9±5.5 | 30.9±5.0 | 0.212 |
| 7 days after DEB-TACE | 33.4±5.2 | 32.8±4.8 | 0.433 |
| 1 month after DEB-TACE | 37.5±5.0 | 37.9±4.5 | 0.579 |
Note: Comparison was determined by Student’s t test.
Abbreviations: DEB-TACE, drug-eluting beads transarterial chemoembolization; VL, vascular lake; ALT, alanine aspartate aminotransferase; SD, standard deviation; AST, aspartate aminotransferase; ALB, albumin.
Correlation Between VL and Adverse Events
The incidences of adverse events, including fever (P = 0.171), pain (P = 0.199), ascites (P = 0.681), nausea and vomiting (P = 0.130), and gastrointestinal bleeding (P = 1.000) were of no difference between VL patients and non-VL patients (Table 8). Moreover, the majority of the adverse events were grade 1 and grade 2, and a small proportion of adverse events were grade 3. In addition, no grade 4 adverse events existed.
Table 8.
Adverse Events After First DEB-TACE Therapy
| Items | VL Patients (n=49) | Non-VL Patients (n=237) | P value |
|---|---|---|---|
| Fever, No. (%) | 21 (42.9) | 127 (53.6) | 0.171 |
| Grade 1 | 16 (32.7) | 92 (38.8) | |
| Grade 2 | 5 (10.2) | 33 (13.9) | |
| Grade 3 | 0 (0.0) | 2 (0.8) | |
| Pain, No. (%) | 26 (53.1) | 102 (43.0) | 0.199 |
| Grade 1 | 7 (14.3) | 30 (12.7) | |
| Grade 2 | 15 (30.6) | 58 (24.5) | |
| Grade 3 | 4 (8.2) | 14 (5.9) | |
| Ascites (4 weeks after DEB-TACE), No. (%) | 14 (28.6) | 61 (25.7) | 0.681 |
| Grade 1 | 10 (20.4) | 47 (19.8) | |
| Grade 2 | 3 (6.1) | 9 (3.8) | |
| Grade 3 | 1 (2.0) | 5 (2.1) | |
| Nausea and vomiting, No. (%) | 8 (16.3) | 63 (26.6) | 0.130 |
| Garde 1 | 8 (16.3) | 63 (26.6) | |
| Grade 2 | 0 (0.0) | 0 (0.0) | |
| Grade 3 | 0 (0.0) | 0 (0.0) | |
| Gastrointestinal bleeding, No. (%) | 0 (0.0) | 1 (0.4) | 1.000 |
| Grade 1 | 0 (0.0) | 1 (0.4) | |
| Grade 2 | 0 (0.0) | 0 (0.0) | |
| Grade 3 | 0 (0.0) | 0 (0.0) |
Note: Comparison was determined by Fisher’s exact test or Chi-square test.
Abbreviations: VL, vascular lake; DEB-TACE, drug-eluting beads transarterial chemoembolization.
Discussion
More than a decade has passed since the first application of DEB-TACE in the treatment of HCC patients (mostly middle-stage patients), and newer data suggest that it might also be applicable for other categories of patients, including its use in early-stage patients before surgery as bridge therapy, advanced patients and patients with liver metastasis.17–19 Thus, further investigation of DEB-TACE in a wider group of HCC patients is warranted. For HCC, mounting evidence has shown that DEB-TACE is efficient and tolerable and that it is superior to cTACE in HCC patients. For instance, a recent multicenter cohort study has revealed that DEB-TACE realizes better CR and ORR as well as similar liver function laboratory index levels and adverse event incidences compared to cTACE in HCC patients.20 Another retrospective cohort study has elucidated that the incidences of adverse events, including abdominal pain, nausea and vomiting, fever and fatigue, are markedly reduced in HCC patients treated with DEB-TACE compared to patients treated with cTACE.21
Apart from the favorable efficiency and safety of DEB-TACE in HCC patients, other aspects have rarely been investigated, such as the role of VL during the DEB-TACE procedure. Therefore, the present study was conducted and it displayed that the incidence of VL was 17.1% in HCC patients and 16.4% in treated nodules during the DEB-TACE procedure. A previous retrospective cohort study conducted in HCC patients treated with cTACE reports cases of VL that occurred before embolization with an incidence of 21.9% in patients (151 HCC patients) and 16.8% in all treated nodules (232 treated nodules).12 With regard to VL during DEB-TACE, a previous cohort study illuminates that in HCC patients who have no vascular invasion or metastasis treated with DEB-TACE, the incidence of VL is 26.0% in the total treated nodules and that the VL is subsequently managed with gelatin sponge particles until it disappears.12 In addition, a retrospective cohort study illustrates that VL occurs in 12.1% all treated nodules (there are 323 treated nodules in 200 HCC patients) during DEB-TACE treatment.13 Compared with these previous studies, the VL incidence in our study was slightly different in number but was within the range of the previous reports regarding VL incidence during TACE treatment. The slight difference may be attributed to the variance in sample size, patient clinicopathological features and treatment modalities. With regard to the mechanisms responsible for the occurrence of VL during DEB-TACE treatment in our HCC patients, there is still a lack of established evidence. One possible explanation is that the microvasculature is often weak in HCC tumors, hence, when injecting the contrast agent, normal saline and DEBs are into the tumor-feeding artery, the microvasculature is easily broken due to the sudden increase in pressure, which will consequently cause the VL during DEB-TACE.12
Furthermore, several factors related to VL occurrence during DEB-TACE were identified in our study, including larger tumor size, pseudocapsule and smaller bead size. A retrospective cohort study elucidates that poor tumor differentiation is an independent predictive factor for the occurrence of VL in HCC patients who receive cTACE.22 Another cohort study reveals that in HCC patients treated with DEB-TACE, tumor size larger than 3 cm, the presence of a pseudocapsule, and higher alpha fetal protein (AFP) levels are independently associated with the occurrence of VL.13 These two prior studies present VL-related factors similar to ours. With regard to the factors independently correlated with VL during DEB-TACE in our study, their correlation with VL may be explained by the following theories: (1) larger tumor size: we presumed that the VL was more likely to occur in a larger tumor because more microspheres would be used if the tumor was larger, which increased the risk of VL due to an increase pressure in the vascular bed. (2) Pseudocapsule: the existence of them in a tumor mostly resulted from the tumor growth causing pressure on the surrounding tissue, which mainly composed of fibrous tissue and liver tissue (if the pseudocapsule occurred in a liver cancer), and this could prevent the growth of tumor to some extent. Therefore, we presumed that pseudocapsule might enhance the embolization effect during DEB-TACE and contribute to the occurrence of VL. However, this is merely a presumption that should be validated by animal experiments in the future. (3) With regard to smaller bead size, smaller microbeads more easily entered the microvasculature; therefore, the accumulation of smaller microbeads elevated the pressure inside the vessels, led to the rupture of vessels and/or targeted tumors, resulting in VL occurrence.
In this study, we also found that VL occurrence was associated with a more favorable treatment response and was an independent predictive factor for prolonged DFS and OS in HCC patients. Predictive factors other than VL have been evaluated by previous studies, such as, a cohort study elucidates that vascular endothelial growth factor (VEGF) level could be a prognostic factor in unresectable HCC patients receiving DEB-TACE and cTACE.23 As for VL, a retrospective cohort study reported that the occurrence of VL is correlated with better ORR in HCC patients who are treated with DEB-TACE, while, the association of VL with survival is not investigated in this study.13 Another retrospective cohort study shows that in advanced HCC patients (BCLC C stage) treated with DEB-TACE, the occurrence of VL is independently associated with more favorable radiological response, and the other independent predictive factor is homogeneous tumor enhancement on cone-beam computed tomography; however, this previous study predominantly aims at exploring the predictive factors for treatment responses but not the correlation between VL and long-term patient prognosis.24 These prior investigations on the prognostic role of VL in HCC patients treated with DEB-TACE are quite limited in the literature, and the elemental mechanism has not yet been studied. More importantly, these previous studies are in accordance with ours regarding the correlation of VL with better outcomes in HCC patients treated with DEB-TACE. With regard to our finding, it not only uncovered the correlation of VL during DEB-TACE with a better treatment response but also its association with a more favorable long-term survival. As for possible explanations, these results might derive from the fact that the occurrence of VL indicates a better embolization of the tumor-feeding artery; in addition, tumor rupture, a cause of VL, also indicates a better treatment effect of DEB-TACE.12 Nevertheless, the detailed underlying mechanism should be explored by further experiments. However, the results still provide valuable information that VL may be a prognostic factor in the management of HCC patients treated with DEB-TACE. Moreover, we also discovered that the occurrence of VL was not correlated with more liver function damage or adverse events in HCC patients treated with DEB-TACE. This result probably indicates that VL has no impact on the safety of DEB-TACE in HCC patients, which, however, needs to be validated by more large-scale studies and experimental studies.
Compared with previous studies, one predominant advantage of the present study was that the sample size was larger, which was beneficial for a more sufficient statistical power. However, there were also some limitations in this study. First, the percentage of patients or nodules with VL was small, which might interfere with the statistical analysis; however, this was normal because VL was rare in the clinical setting. Second, we included only HCC patients and not patients with other types of liver cancer, suggesting that our findings may not be applicable to patients with other liver cancers. Third, the patients with portal vein invasion were included in our study, however, TACE is recommended as standard therapy for BCLC stage B patients but not BCLC stage C patients (with portal vein invasion). Nonetheless, in the clinical setting, TACE is applied in patients with portal vein invasion due to that these patients do not have too many treatment options, and there is also study reporting that TACE could be applied in patients with portal vein invasion.10 Hence, we included the patients with portal vein invasion in order to include as many patients as possible.
In conclusion, VL occurs in 17.1% HCC patients treated with DEB-TACE, and it is correlated with larger tumor size, pseudocapsule, smaller bead size, more favorable treatment response as well as better survival.
Acknowledgments
This study was supported by China Health Promotion Foundation (No. XM_2018_011_0006_01) and Henan Medical Science and Technology Research Project (LHGJ2019162). Hao Li and Manzhou Wang are co-first authors.
Data Sharing Statement
The data would be accessible via contacting the corresponding author (for scientific research use only).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.World Health Organization. Projections of mortality and causes of death, 2016 to 2060. Available from:http://www.who.int/healthinfo/global_burden_disease/projections/en/. Accessed May03, 2021.
- 2.Xu J. Trends in liver cancer mortality among adults aged 25 and over in the United States, 2000–2016. Hyattsville (MD): National Center for Health Statistics; 2018. [Google Scholar]
- 3.Tsilimigras DI, Bagante F, Moris D, et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: a multi-institutional analysis of 1010 patients. Surgery. 2019;166(6):967–974. doi: 10.1016/j.surg.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Pinero F, Marciano S, Fernandez N, et al. Adherence to Barcelona Clinic Liver Cancer therapeutic algorithm for hepatocellular carcinoma in the daily practice: a multicenter cohort study from Argentina. Eur J Gastroenterol Hepatol. 2018;30(4):376–383. doi: 10.1097/MEG.0000000000001049 [DOI] [PubMed] [Google Scholar]
- 5.Oweira H, Petrausch U, Helbling D, et al. Early stage hepatocellular carcinoma in the elderly: a SEER database analysis. J Geriatr Oncol. 2017;8(4):277–283. doi: 10.1016/j.jgo.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Akada K, Koyama N, Taniguchi S, et al. Database analysis of patients with hepatocellular carcinoma and treatment flow in early and advanced stages. Pharmacol Res Perspect. 2019;7(4):e00486. doi: 10.1002/prp2.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34(2):153–159. doi: 10.1053/j.semdp.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Li H, Wu F, Duan M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: a comparison of efficacy and safety. Medicine (Baltimore). 2019;98(21):e15314. doi: 10.1097/MD.0000000000015314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang H, Long L, Yao Y, et al. CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma. Technol Cancer Res Treat. 2019;18:1533033819830751. doi: 10.1177/1533033819830751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prajapati HJ, Dhanasekaran R, El-Rayes BF, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24(3):307–315. doi: 10.1016/j.jvir.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Xu J, Zhang W, et al. Safety and efficacy of drug-eluting bead transarterial chemoembolization combined with apatinib in patients with advanced hepatocellular carcinoma. Acad Radiol. 2020;27(5):704–709. doi: 10.1016/j.acra.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 12.Seki A, Hori S, Shimono C. Management of vascular lake phenomenon on angiography during chemoembolization with superabsorbent polymer microspheres. Jpn J Radiol. 2015;33(12):741–748. doi: 10.1007/s11604-015-0486-2 [DOI] [PubMed] [Google Scholar]
- 13.Cavalcante RN, Nasser F, Motta-Leal-Filho JM, et al. Occurrence of vascular lake phenomenon as a predictor of improved tumor response in hcc patients that underwent DEB-TACE. Cardiovasc Intervent Radiol. 2017;40(7):1044–1051. doi: 10.1007/s00270-017-1678-1 [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 15.Liang B, Zhao D, Liu Y, et al. Chemoembolization of liver cancer with doxorubicin-loaded CalliSpheres microspheres: plasma pharmacokinetics, intratumoral drug concentration, and tumor necrosis in a rabbit model. Drug Deliv Transl Res. 2020;10(1):185–191. doi: 10.1007/s13346-019-00672-9 [DOI] [PubMed] [Google Scholar]
- 16.Duan XH, Li H, Ren JZ, et al. Hepatic arterial chemoembolization with arsenic trioxide eluting callispheres microspheres versus lipiodol emulsion: pharmacokinetics and intratumoral concentration In A rabbit liver tumor model. Cancer Manag Res. 2019;11:9979–9988. doi: 10.2147/CMAR.S199188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y. Drug-eluting bead transarterial chemoembolization is efficient and well-tolerated in treating elderly Chinese hepatocellular carcinoma patients. Int J Clin Exp Pathol. 2018;11(10):4867–4878. [PMC free article] [PubMed] [Google Scholar]
- 18.Cao WZ, Zhou ZQ, Jiang S, et al. Efficacy and safety of drug-eluting beads for transarterial chemoembolization in patients with advanced hepatocellular carcinoma. Exp Ther Med. 2019;18(6):4625–4630. doi: 10.3892/etm.2019.8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rostas J, Tam A, Sato T, et al. Image-guided transarterial chemoembolization with drug-eluting beads loaded with doxorubicin (DEBDOX) for unresectable hepatic metastases from melanoma: technique and outcomes. Cardiovasc Intervent Radiol. 2017;40(9):1392–1400. doi: 10.1007/s00270-017-1651-z [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Zhao C, Zhao H, et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres((R)) microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am J Transl Res. 2019;11(12):7456–7470. [PMC free article] [PubMed] [Google Scholar]
- 21.Karalli A, Teiler J, Haji M, et al. Comparison of lipiodol infusion and drug-eluting beads transarterial chemoembolization of hepatocellular carcinoma in a real-life setting. Scand J Gastroenterol. 2019;54(7):905–912. doi: 10.1080/00365521.2019.1632925 [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Zhong BY, Zhang L, et al. Occurrence of vascular lake phenomenon before embolization for the prediction of lipiodol uptake for intermediate-stage hepatocellular carcinoma patients that underwent cTACE. Cardiovasc Intervent Radiol. 2020;43:1460–1467. doi: 10.1007/s00270-020-02501-w [DOI] [PubMed] [Google Scholar]
- 23.Farid K, Elalfy H, Abo El-Khair SM, et al. Prognostic value of vascular endothelial growth factor in both conventional and drug eluting beads transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma in HCV patients. Expert Rev Gastroenterol Hepatol. 2020;14(12):1203–1214. doi: 10.1080/17474124.2020.1823215 [DOI] [PubMed] [Google Scholar]
- 24.Chang KH, Hwang ZA, Chang PY, et al. Predictive imaging for tumor response to drug-eluting microsphere transarterial chemoembolization in patients with BCLC-C advanced hepatocellular carcinoma. Sci Rep. 2019;9(1):20032. doi: 10.1038/s41598-019-56545-1 [DOI] [PMC free article] [PubMed] [Google Scholar]