Abstract
Background
Recent evidence suggests a rising incidence of cancer in younger individuals. Herein, we report the epidemiologic, pathologic, and molecular characteristics of a patient cohort with early-onset pancreas cancer (EOPC).
Methods
Institutional databases were queried for demographics, treatment history, genomic results, and outcomes. Overall survival from date of diagnosis was estimated using Kaplan-Meier method.
Results
Between 2008 and 2018, 450 patients with EOPC were identified at Memorial Sloan Kettering. Median overall survival was 16.3 (95% confidence interval [CI] = 14.6 to 17.7) months in the entire cohort and 11.3 (95% CI = 10.2 to 12.2) months for patients with stage IV disease at diagnosis. Of the patients, 132 (29.3% of the cohort) underwent somatic testing; 21 of 132 (15.9%) had RAS wild-type cancers with identification of several actionable alterations, including ETV6-NTRK3, TPR-NTRK1, SCLA5-NRG1, and ATP1B1-NRG1 fusions, IDH1 R132C mutation, and mismatch repair deficiency. A total of 138 patients (30.7% of the cohort) underwent germline testing; 44 of 138 (31.9%) had a pathogenic germline variant (PGV), and 27.5% harbored alterations in cancer susceptibility genes. Of patients seen between 2015 and 2018, 30 of 193 (15.5%) had a PGV. Among 138 who underwent germline testing, those with a PGV had a reduced all-cause mortality compared with patients without a PGV controlling for stage and year of diagnosis (hazard ratio = 0.42, 95% CI = 0.26 to 0.69).
Conclusions
PGVs are present in a substantial minority of patients with EOPC. Actionable somatic alterations were identified frequently in EOPC, enriched in the RAS wild-type subgroup. These observations underpin the recent guidelines for universal germline testing and somatic profiling in pancreatic ductal adenocarcinoma.
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related morbidity and mortality, and incidence is rising (1). PDAC is the third leading cause of cancer deaths, projected to account for 7.8% of all cancer-related mortality in 2020 (1). Tangible but incremental improvements have accrued in treatment for PDAC with FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin), gemcitabine and nab-paclitaxel, and liposomal irinotecan and 5-fluorouracil in the metastatic setting and FOLFIRINOX in the adjuvant setting (2-4). Five-year overall survival (OS) for PDAC is now 10% (5). Targeted therapy with platinum agents and poly ADP-ribose polymerase (PARP) inhibitors has established therapeutic value in patients with germline BRCA1 and BRCA2 (BRAC1/BRAC2) and other homologous repair gene alterations (6–8). However, the immunosuppressive tumor microenvironment, complex genomic alterations, and late clinical presentation with often large symptom burden along with the presence of either de novo or early acquired treatment resistance remain challenging in the care of patients with PDAC. While most patients with PDAC are diagnosed after age 60 years, rising incidence of PDAC has been noted in younger populations. Sung et al. (9) observed a notable incidence in gastrointestinal cancers, including PDAC, among people younger than age 50 years, highlighting the emerging challenge of early-onset pancreas cancer (EOPC). Tavakkoli et al. (10) have also noted rising incidence, particularly among younger populations of Black and White patients with 44% increase and 57% increase among Black and White patients aged 30-39 years, respectively. Within gastrointestinal oncology, the rising incidence of colorectal cancers, particularly distal colon and rectal cancers, in patients less than age 50 years has been clearly described with incidence rising by 1.5% in men and 1.6% in women aged 20-49 years annually between 1992 and 2005, whereas overall incidence of colorectal cancers declined during the same time period (1,11).
Several retrospective studies have evaluated clinical characteristics, outcomes, and risk factors in patients with EOPC (Supplementary Table 1, available online). Of 136 patients diagnosed with PDAC younger than 45 years between 1995 and 2008 at Memorial Sloan Kettering Cancer Center (MSK), 35 patients who underwent curative surgery had a median OS of 41.8 months, suggesting improvement in OS for EOPC in the early stage setting (12). Other retrospective reviews noted no difference in outcomes with patients with EOPC (13–16). Regarding risk factors for EOPC, alcohol intake of more than 26 grams per day, tobacco exposure, obesity, and diabetes mellitus diagnosis were associated with increased incidence of EOPC in an analysis of 1954 patients (17).
Given adoption of routine somatic and germline molecular profiling in PDAC, Ben-Aharon and our group described molecular features of patients with EOPC (n = 90, diagnosis 55 years or younger) compared with average onset pancreas cancer (AOPC) (diagnosis 70 years or older) (18). EOPC had more SMAD4 mutations, typically associated with poor prognosis, and upregulation of the TGF-beta signaling pathway (18).
Under an institutional review board–approved protocol, molecular testing has been performed routinely at MSK with MSK-IMPACT, a matched tumor-normal molecular profiling platform allowing targeted somatic analysis of cancer-associated genes and germline analysis of genes associated with cancer susceptibility. Of 336 PDAC patients undergoing somatic testing with MSK-IMPACT, the average age of patients was 64 years, and 18 (5.4%) were KRAS wild type with several actionable alterations identified in this subset (19). In an unselected population of 615 patients with PDAC at MSK, comprehensive germline molecular profiling revealed that 122 of 615 (19%) had pathogenic germline variants (PGV) (20).
In the setting of the changing epidemiology of PDAC and endorsement for routine somatic and germline molecular profiling, herein we describe the clinical characteristics, treatment history, survival outcomes, and somatic and germline molecular results from a large patient cohort with EOPC.
Methods
Patients and Data Abstraction
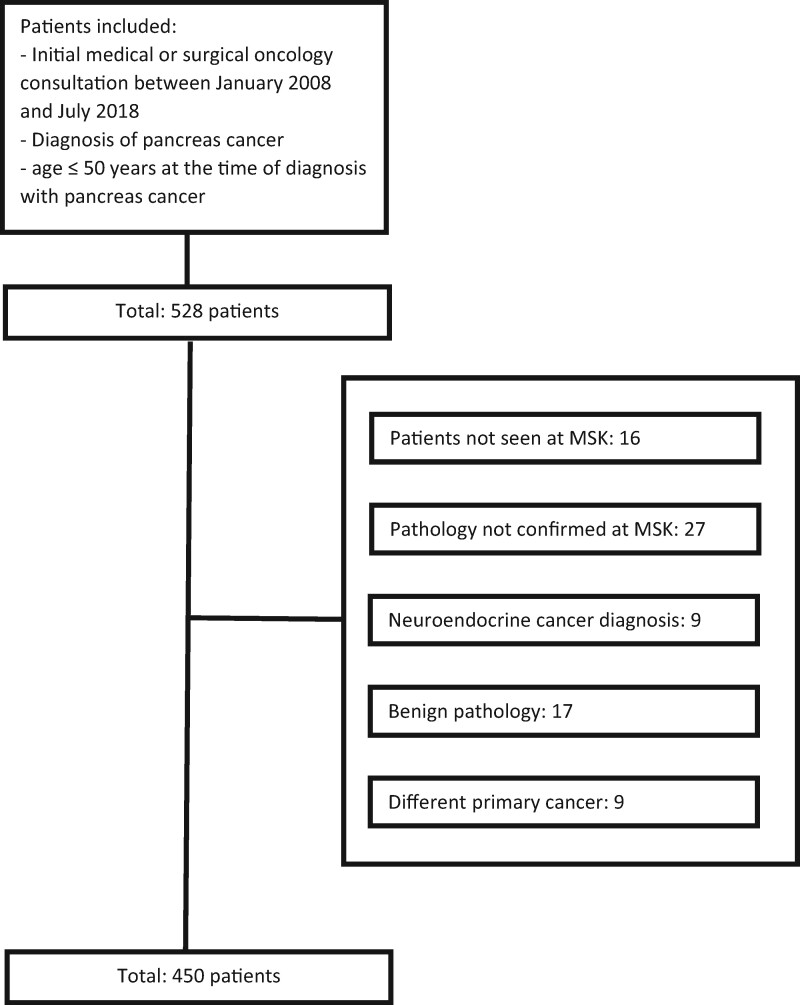
Under an institutional review board–approved retrospective research protocol with waiver of consent, an institutional database was queried for patients meeting the following criteria: 1) initial consultation at MSK between January 2008 and July 2018 with medical and/or surgical oncology; 2) diagnosis of pancreas cancer; and 3) aged 50 years or younger at the time of diagnosis with pancreas cancer. A total of 528 patients were identified (see Figure 1). Patients with pancreatic neuroendocrine tumors were excluded, and 78 patients were removed for the following reasons: patient not seen at MSK (n = 16), pathology not confirmed at MSK (n = 27), neuroendocrine cancer diagnosis (n = 9), benign pathology (n = 17), and a different primary cancer diagnosis (n = 9). Overall, 450 patients were identified. Comprehensive chart review was completed to abstract detailed demographic and clinical information, lifestyle factors, family and social history, body mass index (BMI), staging information, histopathology, treatment information, germline and somatic molecular testing results, and survival outcomes with data cutoff of October 31, 2019.
Figure 1.
Consort diagram. MSK = 450.
Statistical Analysis
Baseline demographic and clinical characteristics were summarized using median and ranges for continuous variables and frequency for categorical descriptors. Year of diagnosis was included both as dichotomized (2008-2013 vs 2014-2018 according to 2 seminal papers in 2011 and 2013 for treatment of pancreas cancer) or as continuous variable; BMI was defined as less than 25, 25-30, and 30 or more kg/m2; age at diagnosis was dichotomized as younger than 40 years vs 40 years and older; and smoking history was defined as current or former vs never (2,3). OS was calculated from date of diagnosis to date of death. Patients who were alive were censored at date of last follow-up. OS was estimated using Kaplan-Meier methods and compared between subgroups using log-rank test, and subgroups for which the number of deaths was below 10, the permutated log-rank test was employed (21). Multivariable Cox regression model was used to further examine the association between PGV status and OS while adjusting for potential confounders such as year of diagnosis (2008-2013 vs 2014-2018) and stage at diagnosis (resectable vs locally advanced unresectable vs metastatic disease). Among patients with de novo metastatic disease, association of PGV status with OS was further adjusted by year of diagnosis (2008-2013 vs 2014-2018). All statistical analyses were carried out using R version 3.6.0 with a 2-sided test. A P value of less than .05 was considered statistically significant.
Results
Clinical and Epidemiologic Characteristics of EOPC Cohort
Overall, 450 patients with EOPC diagnosed between January 2008 and July 2018 comprised the final cohort for analysis; 254 (56.4%) patients were male, and 353 (78.4%) were White (Table 1). Median age at diagnosis was 46 (range = 15-50) years. The median BMI at the time of initial consultation was 25.2 (range = 14.7-53.7) kg/m2.
Table 1.
Baseline demographic and clinical descriptors for early-onset pancreas cancer (EOPC)a
| Characteristics | No. (%) |
|---|---|
| Sex | |
| Male | 254 (56.4) |
| Female | 196 (43.6) |
| Race | |
| Asian | 26 (5.8) |
| Black | 41 (9.1) |
| White | 353 (78.4) |
| Native American, unknown, other | 30 (6.7) |
| Age at diagnosis, y | |
| Median (range) | 46 (15-50) |
| ≤30 | 9 (2.0) |
| >30 to ≤35 | 14 (3.1) |
| >35 to ≤40 | 42 (9.3) |
| >40 to ≤45 | 109 (24.2) |
| >45 to ≤50 | 276 (61.4) |
| BMI at diagnosis, kg/m2 | |
| Median (range) | 25.2 (14.7-53.7) |
| <18.5, underweight | 23 (5.4) |
| 18.5 to <25, normal weight | 182 (42.7) |
| 25 to <30, overweight | 137 (32.2) |
| 30 to <35, obesity class I | 52 (12.2) |
| 35 to <40, obesity class II | 24 (5.6) |
| ≥40, obesity class III | 8 (1.9) |
| Missing | 24 |
| Diabetes mellitus diagnosis at initial diagnosis of EOPC | |
| Yes | 76 (16.9) |
| No | 374 (83.1) |
| Tobacco history | |
| Never | 224 (51.3) |
| Former or current | 213 (48.7) |
| Unknown | 13 |
BMI = body mass index.
Of the patients, 76 (16.9%) had a history of diabetes mellitus at the time of initial consultation. One patient had a known history of hereditary pancreatitis with a germline alteration in SPINK1, and 224 (51.3%) patients were never smokers (Table 1). Because of the retrospective nature of this analysis, limited information was available regarding diabetes diagnosis and treatment history, prior diagnoses of pancreatitis, onset of tobacco use, and secondhand tobacco exposure.
Pathologic Features and Stage at Diagnosis of EOPC
On histopathologic evaluation, 433 (96.2%) patients had PDAC (Table 2). At diagnosis, 218 (49.1%) had metastatic disease, 121 (27.3%) had locally advanced unresectable disease, 105 (23.6%) had resectable disease, and 6 had an unknown stage at diagnosis; 125 (27.8%) patients underwent surgical resection with staging outlined in Table 2 using the American Joint Committee on Cancer 7th edition staging criteria (22).
Table 2.
Pathologic features, clinical stage at diagnosis, and pathologic stage after surgery
| Characteristic | No. (%) |
|---|---|
| Pathology | |
| Adenocarcinoma | 433 (96.2) |
| Acinar cell carcinoma | 5 (1.1) |
| Adenosquamous carcinoma | 4 (0.9) |
| Carcinoma NOS | 6 (1.4) |
| Othera | 2 (0.4) |
| Location of primary tumor | |
| Head | 274 (60.9) |
| Body | 90 (20.0) |
| Tail | 86 (19.1) |
| Clinical stage at diagnosis | |
| Resectable | 105 (23.6) |
| Locally advanced, unresectable | 121 (27.3) |
| Metastatic | 218 (49.1) |
| Unknown | 6 |
| ECOG performance status | |
| 0 | 102 (29.5) |
| 1 | 214 (61.8) |
| 2 | 22 (6.4) |
| 3 | 8 (2.3) |
| Unknown | 104 |
| Surgery | |
| Yes | 125 (27.8) |
| No | 325 (72.2) |
| Pathologic stage after surgeryb | 125 |
| Complete response | 1 (0.1) |
| Stage I | 15 (12.1) |
| Stage II | 97 (79.6) |
| Stage III | 1 (0.1) |
| Stage IV | 8 (6.6) |
| Unknown | 3 |
One EBV-related carcinoma; one undifferentiated carcinoma, anaplastic type. ECOG = Eastern Cooperative Oncology Group; NOS = not otherwise specified.
Staging was completed using the American Joint Committee on Cancer (AJCC) 7th edition (22); 108 surgical resection specimens were staged with the 7th edition at the time of surgery; 17 surgical resection specimens were staged with the AJCC 8th edition at the time of surgery and were converted to AJCC 7th edition staging for this analysis (23).
Personal and Family History of Cancer, Germline, and Somatic Molecular Data
Overall, 132 (29.3%) tumors had somatic molecular testing with at least 1 alteration identified; 115 tumors underwent molecular testing with MSK-IMPACT, and the remaining 17 were tested using commercial or other institutional assays (24). Additionally, 110 (83.3%) had KRAS alterations, 1 (1.0%) had NRAS alterations, and 21 (15.9%) were RAS wild type (Table 3). Eight patients had targetable alterations. Of 21 RAS wild-type tumors, 6 harbored the following targetable alterations: ETV6-NTRK3 fusion, TPR-NTRK1 fusion, SCLA5-NRG1 fusion, ATP1B1-NRG1 fusion, IDH1 R132C mutation, and mismatch repair deficiency (MRD). Two patients with RAS-altered EOPC had targetable alterations with MMR deficient cancers. Seven additional patients underwent somatic molecular testing, and no molecular alterations were identified, suggesting either poor tissue quality or an inadequate tissue specimen.
Table 3.
RAS alterations in early-onset pancreas cancer
| Mutation seen | No. (%) |
|---|---|
| KRAS alterations | 110 (83.3) |
| KRAS amp | 1 (1.0) |
| KRAS G12C | 1 (1.0) |
| KRAS G12D | 58 (43.9) |
| KRAS G12L | 1 (1.0) |
| KRAS G12R | 13 (9.8) |
| KRAS G12V | 25 (18.9) |
| KRAS G13D | 2 (1.5) |
| KRAS Q61X | 9 (6.8) |
| NRAS Q61R | 1 (1.0) |
| RAS wild type | 21 (15.9) |
| Total | 132 |
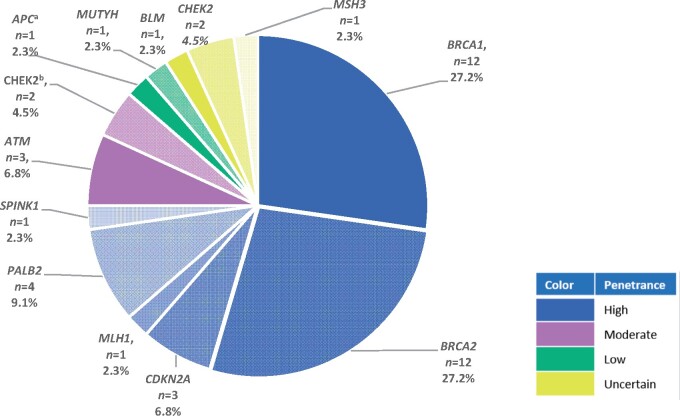
A total of 138 (30.7%) patients underwent testing to identify a PGV (Table 4). Of these patients, 97 (70.3%) had germline testing with MSK-IMPACT, and the remaining had testing using commercial assays. A PGV was identified in 44 (31.9%) patients. The most frequent PGVs were in the following genes: BRCA1 (n = 12, 27.2%), BRCA2 (n = 12, 27.2%), PALB2 (n = 4, 9.1%), and CHEK2 (n = 4, 9.1%) (Figure 2). High and moderate penetrance PGVs were present in 27.5% of patients tested for PGVs
Table 4.
Clinical and pathologic features of 138 patients with early-onset pancreas cancer who underwent germline testing
| Characteristic | All patients | Pathogenic germline variant | No pathogenic germline variant |
|---|---|---|---|
| Total | 450 | 44 | 94 |
| Age at onset, No. (%), y | |||
| Median (range) | 46 (15-50) | 46 (24-50) | 45(25-50) |
| ≤30 | 9 (2.0) | 3 (6.8) | 2 (2.1) |
| >30 to ≤40 | 56 (12.4) | 10 (22.7) | 18 (19.1) |
| >40 to <50 | 385 (85.6) | 31 (70.5) | 74 (78.7) |
| Personal history of a previous cancer, No. (%) | 23 (5.1)a | 6 (13.6) | 9 (9.6) |
| Family history of cancer in first- or second-degree family member, No. (%) | 330 (73.3)b | 37 (84.1) | 71 (75.5) |
| Family history of cancer in first-degree family member, No. (%) | 203 (45.1) | 28 (63.6) | 42 (44.7) |
| Family history of cancer in second-degree family member, No. (%) | 244 (54.2) | 26 (59.1) | 62 (66.0) |
| Clinical stage at diagnosis, No. (%) | |||
| Resectable | 105 (23.3) | 15 (34.1) | 30 (31.9) |
| Locally advanced | 121 (26.9) | 10 (22.7) | 19 (20.2) |
| Metastatic | 218 (48.4) | 19 (43.2) | 45 (47.9) |
| 12-month OS in patients with stage IV disease at diagnosis (95% CI) | 43.6 (36.8 to 50.3) | 72.2 (45.6 to 87.4) | 54.6 (38.9 to 67.9) |
Breast cancer (n = 8), melanoma (n = 4), lymphoma (n = 4), sarcoma (n = 3), pheochromocytoma (n = 2), bladder cancer (n = 1), germ cell tumor (n = 1), thyroid cancer (n = 1), and vulvar cancer (n = 1). CI = confidence interval; OS = overall survival.
56 patients with a family history of pancreas cancer, 13 with bladder cancer, 131 with breast cancer, 82 with colorectal cancer, 70 with lung cancer, 11 with melanoma, 22 with ovarian cancer, 52 with prostate cancer, and 155 with other cancers.
Figure 2.
Pathogenic germline variants and penetrance in early-onset pancreas cancer. Forty-four patients had pathologic germline variants, as shown. The APC alteration identified was APC I1307K, which has been identified as a low penetrance allele in the Ashkenazi Jewish population and without an association to familial adenomatous polyposis.a The 2 CHEK2 mutations identified were classified as moderate penetrance.b Two other patients had CHEK2 I157T identified, and the clinical significance and penetrance of CHEK2 I157T are uncertain.
Germline testing has been routinely recommended to all patients with PDAC at MSK since 2015. Of the 193 EOPC patients diagnosed between 2015 and 2018, 88 did not undergo germline testing, 105 underwent germline testing, and 30 were identified to have a pathogenic germline variant (28.6%). Assuming the 88 patients who did not undergo testing did not have a pathogenic germline variant, 30 of 193 (15.5%) had a pathogenic germline variant.
The median age at diagnosis was 46 (range = 24-50) and 45 (range = 25-50) years for patients with a PGV and those without a PGV, respectively. Personal and family history of cancers in these populations are noted in Table 4.
Treatment Details
Of the patients, 277 (61.6% of the cohort) had available systemic treatment records and known disease stage at diagnosis, including 69 (24.9%) with resectable disease, 67 (24.2%) with locally advanced disease, and 141 (50.9%) with de novo metastatic disease. Treatment details are outlined in Table 5.
Table 5.
Treatment details of 277 patients with available systemic treatment records
| Treatment | No. of patients |
|---|---|
| Resectable disease | 69 |
| Surgery | 69 |
| Neoadjuvant therapy | 12a |
| Adjuvant therapy | 57b |
| Locally advanced and metastatic disease | 208 |
| Gemcitabine-based first-line treatment | 77c |
| 5-FU based combination | 126c |
| Best supportive care | 11 |
2 gemcitabine/oxaliplatin, 10 FOLFIRINOX.
33 gemcitabine, 9 FOLFIRINOX, 15 gemcitabine-based combinations.
22 with gemcitabine and nab-paclitaxel, 111 with FOLFIRINOX.
Given changing metastatic treatment patterns between 2008 and 2018, herein we describe treatment patterns and outcomes of 79 patients with de novo metastatic PDAC diagnosed between 2014 and 2018 with full treatment history available (data not shown). Mean lines of therapy received was 2 (range = 0 to 5). Five patients did not receive cancer-directed therapy. Of 74 patients who received cancer-directed therapy, 19 received a first-line gemcitabine-based combination (15 of 19 receiving gemcitabine and nab-paclitaxel) with an average treatment duration of 4.7 months. Fifty-five patients received a 5-FU–based first-line combination (47 of 55 receiving FOLFIRINOX) with an average treatment duration of 4.8 months, and 23 patients participated in a therapeutic trial.
Of 8 patients with targetable somatic alterations, 4 patients received a targeted therapy: 1) 1 patient with an MMR-deficient pancreas cancer received immune checkpoint blockade for more than 3 years; 2) 1 patient with pancreas cancer harboring TPR1-NTRK1 fusion received 2 different NTRK-targeted therapies for approximately 1 year; 3) 1 patient with pancreas cancer harboring an NRG-1 fusion was receiving HER2/3 targeted therapy for 3 months and treatment was continuing at the time of data cutoff; and 4) 1 patient with an IDH1 R132C mutant pancreas cancer received an IDH1 inhibitor for 2 months. The following 4 patients with targetable alterations did not receive targeted therapy: 1) 1 patient whose tumor harbored an ETV6-NTRK3 fusion died before NTRK-targeted therapy was available; 2) 1 patient with an MMR-deficient pancreatic cancer has not required further systemic therapy after surgical resection and adjuvant therapy; 3) 1 patient with an SCLA-NRG-1 fusion has pursued treatment locally; and 4) 1 patient with an MMR-deficient pancreas cancer died while receiving first-line cytotoxic therapy.
For 23 patients with germline BRCA1/BRCA2 mutations, 6 had resectable disease, 4 had locally advanced disease, and 13 patients had metastatic disease. Twelve patients with metastatic disease received treatment at MSK. Eight of 12 patients received first-line therapy with a platinum-containing regimen. One patient received FOLFIRINOX for 3 months at MSK and then was lost to follow-up after pursuing care locally, 5 received treatment with cisplatin/gemcitabine with mean time on treatment of 13 (range = 2-38) months, and 2 received FOLFIRINOX (7 months and 8 months).
Survival Outcomes
Median follow-up among survivors was 20 (range = 0.3-116.4) months at the time of analysis, and 360 deaths were observed (data not shown). Median OS for the entire cohort was 16.3 months (95% confidence interval [CI] = 14.6 to 17.7). Stage at disease presentation was statistically significantly associated with OS. Median OS for patients with resectable EOPC was 28 months (95% CI = 24.3 to 44.8), 18 months (95% CI = 15.9 to 19.9) for patients with locally advanced and unresectable EOPC, and 11.3 months (95% CI = 10.2 to 12.2) for patients with metastatic disease. We did not observe any statistically significant association between OS and year of diagnosis (2018-2013 vs 2014-2018), age at diagnosis (younger than 40 vs 40 years and older), BMI status, or any KRAS or RAS alteration (data not shown). As a sensitivity analysis, we also analyzed year of diagnosis as a continuous variable, and no statistically significant association with OS was found (hazard ratio [HR] for 1 year increase = 0.98, 95% CI = 0.95 to 1.02).
Among 138 patients who underwent germline testing, patients with PGV had a statistically significant prolonged OS compared with those without PGV (1-year OS = 72.2%, 95% CI = 45.6% to 87.4% vs 54.6%, 95% CI = 38.9% to 67.9%, respectively) (Table 4). The multivariable model suggested that after controlling for the disease stage and year of diagnosis, EOPC with PGV has a statistically significant reduced risk of all-cause mortality as compared with the EOPC without a PGV (HR = 0.42, 95% CI = 0.26 to 0.69; P = .001).
Among patients with de novo metastatic disease, the presence of a PGV was also statistically significantly associated with improved OS. For patients with a PGV, the 12-month OS was 72.2% (95% CI = 45.6% to 87.4%) and 24-month OS was 31.7% (95% CI = 12.2% to 53.5%) (Table 6), and for patients without a PGV, the 12-month OS was 54.6% (95% CI = 38.9% to 67.9%) and 24-month OS was 14.3% (95% CI = 5.5% to 27.0%; P=.04) (Table 6). The association was not statistically significant after controlling for year of diagnosis (HR = 0.53, 95% CI = 0.28 to 0.99; P = .048). No statistically significant association with OS was noted with respect to smoking history, BMI, or presence of a RAS alteration among patients with de novo metastatic disease, although numbers were small in these cohorts. Given substantial changes in metastatic PDAC treatment during the time of data analysis, comparison of survival outcomes between 2008 and 2013 and 2014 and 2018 was undertaken; however, no statistically significant differences were observed (neither when year of diagnosis was dichotomized [P = .43; Table 6] nor when year of diagnosis was treated as continuous variable [P = 1.00]). Full results from univariate analyses of effects of germline alteration, smoking history, BMI, date of diagnosis, and presence of a RAS alteration for patients with operable and locally advanced unresectable EOPC are listed in Supplementary Tables 2 and 3 (available online).
Table 6.
Treatment outcomes for patients with de novo metastatic early-onset pancreas cancer
| Category | No. | 12-month, % OS (95% CI) | 24-month, % OS (95% CI) | P a |
|---|---|---|---|---|
| Overall | 218 | 43.6 (36.8 to 50.3) | 13.9 (9.4 to 19.2) | |
| Year of diagnosis | .43 | |||
| 2008-2013 | 102 | 41.5 (31.8 to 51.0) | 14.6 (8.4 to 22.3) | |
| 2014-2018 | 116 | 43.6 (36.0 to 54.8) | 13.9 (7.3 to 20.9) | |
| RAS alteration | .09 | |||
| RAS mutant | 54 | 51.0 (36.9 to 63.4) | 9.8 (3.3 to 20.6) | |
| RAS wild type | 8 | 62.5 (22.9 to 86.1) | 50.0 (15.2 to 77.5) | |
| Pathogenic germline variant | .04 | |||
| Present | 19 | 72.2 (45.6 to 87.4) | 31.7 (12.2 to 53.5) | |
| Absent | 45 | 54.6 (38.9 to 67.9) | 14.3 (5.5 to 27.0) | |
| Age at diagnosis, y | .12 | |||
| ≤40 | 36 | 47.2 (30.5 to 62.3) | 22.2 (10.5 to 36.7) | |
| >40 to ≤50 | 182 | 43.0 (36.0 to 51.0) | 12.0 (7.9 to 18.0) | |
| Body mass index, kg/m2 | .42 | |||
| <25 | 101 | 44.9 (34.7 to 54.5) | 12.8 (6.8 to 20.8) | |
| ≥ 25 to <30 | 74 | 46.2 (34.3 to 57.3) | 18.0 (10.0 to 28.0) | |
| ≥30 | 37 | 38.7 (22.7 to 54.5) | 8.9 (2.3 to 21.3) | |
| Smoking history | .07 | |||
| Never smoker | 104 | 50.9 (40.6 to 60.3) | 20.4 (12.8 to 29.3) | |
| Current or former smoker | 108 | 36.4 (27.2 to 45.6) | 8.7 (4.2 to 15.4) |
Comparison of OS distributions between subgroups was done using log-rank test, and permutated log-rank test was employed for subgroups where the number of deaths was below 10. P values were 2-sided. CI = confidence interval; OS = overall survival.
Discussion
The epidemiology of PDAC is changing with a rising incidence among patients 50 years and younger (9). Herein, we report the epidemiologic, pathologic, lifestyle, and molecular results from a cohort of 450 patients with EOPC seen at a large, tertiary referral center. The median OS for the entire EOPC cohort was 16.3 months and 11.3 months for patients with de novo metastatic EOPC. Regarding risk factors in this EOPC cohort, 48.7% were current or former smokers, 29.7% had class I-III obesity, and 16.9% had diabetes mellitus. Of 132 (15.9%) patients with EOPC, 21 underwent somatic molecular testing and were identified to be RAS wild type, and 6 of 21 (28.6%) had targetable alterations. Of 138 (31.9%) patients, 44 with EOPC who underwent germline testing had a PGV.
Regarding risk factors for EOPC, in the absence of a matched cohort of Late onset pancreatic cancer or AOPC, this analysis is limited in its ability to assess for comparative risk factors for EOPC and AOPC. However, based on the descriptive findings noted above, known risk factors such as tobacco history, obesity, or diabetes mellitus diagnosis do not clearly appear to be overrepresented in this cohort. Likewise, treatment outcomes in this population are consistent with known outcomes for PDAC with median OS of 11.3 months for patients with de novo metastatic disease.
Somatic and germline results in this EOPC cohort reveal several noteworthy and practice-endorsing findings. Of the tested EOPC cohort, 15.9% had RAS wild-type tumors. The single institution experience at MSK of somatic molecular testing of all PDACs noted that only 5.4% of tumors was RAS wild type, consistent with findings from large-scale sequencing efforts including The Cancer Genome Atlas for PDAC, which noted that greater than 90% of PDAC harbored RAS alterations (19,25,26). Although the EOPC cohort of patients with molecular testing is small, these findings suggest that EOPC may be enriched for RAS wild-type tumors. Additionally, patients with RAS wild-type EOPC were enriched for targetable alterations. Recent findings from the Pancreatic Cancer Action Network Know Your Tumor Program described that patients with targetable alterations receiving targeted therapy lived 1 year longer than those patients receiving standard cytotoxic therapies (27). Because the EOPC population is overrepresented for patients with targetable alterations, these findings highlight the importance of pursuing germline and somatic testing early in a patient’s disease course and acting on this information (28). Although comprehensive germline and somatic testing are recommended for patients in whom adequate tissue is available, another option would be to consider hierarchical testing beginning with testing of hotspot mutations in KRAS. For those patients with KRAS wild-type tumors, more comprehensive testing should be pursued, and those patients may need a further biopsy to obtain tissue for this testing. As noted by the National Comprehensive Cancer Network guidelines, consideration of cell-free DNA testing could also be considered if adequate tumor tissue is not available, although concerns remain about the adequacy of cell-free DNA as an optimal surrogate for tumor.
Germline testing results also revealed that 31.8% of patients with EOPC had a PGV, and the presence of a PGV was associated with improvement in OS for patients who underwent germline testing after controlling for stage at disease presentation and year of disease diagnosis. This observation of prolonged OS is further supported by hints in the literature underscoring this point (20,29,30). This improvement in OS is in part related to accrual of many of these patients to a recently published study defining the role of cisplatin and gemcitabine in the treatment of patients with PDAC and germline BRCA1/BRCA2 alterations (7). In this study by O’Reilly et al., patients with locally advanced and metastatic PDAC and germline BRCA1/BRCA2 and PALB2 alterations were randomly assigned to treatment with cisplatin and gemcitabine with or without the PARP inhibitor veliparib (7). OS was 15.5 months for patients receiving the triplet combination and 16.4 months for patients receiving the doublet combination of cisplatin and gemcitabine only, highlighting the importance of platinum agents in the treatment of patients with pathogenic germline BRCA1/BRCA2 and PALB2 alterations (7). Retrospective analysis of patients with both germline and somatic alterations in homologous recombinant DNA damage repair pathways also demonstrated improvement in OS when patients received first-line platinum-containing treatments (8).
The therapeutic relevance of these findings is also highlighted in the POLO study demonstrating that patients with germline BRCA1/BRCA2 alterations and with platinum sensitive PDAC had improvement in progression free survival with use of the maintenance PARP inhibitor olaparib compared with placebo (7.4 months vs 3.8 months; P = .004), illustrating both the importance of platinum therapy for patients with germline BRCA1/BRCA2 alterations and the therapeutic benefit of PARP inhibitors in the maintenance setting (6). Prospective studies are underway to better understand the role of PARP inhibitors for patients with somatic and germline alterations in DNA damage repair pathways.
Although BRCA1/BRCA2 and PALB2 comprise the majority of PGVs identified among patients with EOPC, less commonly seen PGVs were identified in many others including ATM, CDKN2A, and MLH1, underpinning the therapeutic importance of germline testing for patients and the opportunity for cancer prevention and screening in their families. Interestingly, the median age at diagnosis of patients with and without a PGV was almost the same at 46 and 45 years specifically within this population of patients with EOPC. These findings highlight the importance of universal germline testing for all patients with PDAC, not only testing for patients with EOPC.
There are notable limitations to our study. First, this analysis was retrospectively conducted, and data are abstracted from a single tertiary referral center. Patients with EOPC seen at MSK likely represent a biased population of patients with less racial and socioeconomic diversity and a relatively increased population of patients with Ashkenazi ancestry compared with the overall population of EOPC. Likewise, patients seen at a tertiary care center typically have better performance status than the broader population of EOPC. Second, 127 of 450 (28.2%) patients in this cohort were seen at MSK for only 1-2 visits, and limited follow-up treatment information is available. Third, this cohort included patients identified from 2008 to 2018, and treatment patterns have changed substantially during this time period, although survival distributions were not found to be statistically significantly different between patients diagnosed between 2008 and 2013 vs patients treated between 2014 and 2018. The etiology of these findings is not clear and may pertain to the fact that patients with EOPC compared with average age onset PDAC patients have a less favorable tumor biology and speculatively receive less benefit from current cytotoxic combinations. Fourthly, as a cancer center and tertiary care referral center, limited information is available prior to a patient’s cancer diagnosis. Consequently, there was a dearth of important information available regarding exposure history, diabetes, exercise, and other lifestyle considerations prior to cancer diagnosis. Finally, only 29.3% of patients included in this EOPC were able to undergo somatic molecular testing, although that percentage substantially increased in the later part of the cohort.
EOPC is an important subgroup of PDAC. This population is enriched for PGVs and RAS wild-type cancers with targetable alterations, providing opportunity for therapeutic actionability and improved outcomes for EOPC patients and underpinning important actionable information for patients’ families and opportunity for cancer prevention. Further prospective studies, epidemiologic analyses, and molecular analyses are required to better understand the changing epidemiology of this disease and, importantly, to better understand and exploit opportunities for cancer prevention.
Funding
This work was supported by the David M. Rubenstein Center for Pancreas Cancer, Cancer Support Grant P30 CA008748, and the Reiss Family Foundation.
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: EOR reports the following: Research Funding to Memorial Sloan Kettering: Genentech/Roche, Celgene/BMS, BioNTech, AstraZeneca, Arcus. Consulting Role: Cytomx Therapeutics (DSMB), Rafael Therapeutics (DSMB), Sobi, Silenseed, Molecular Templates, Boehringer Ingelheim, BioNTech, Ipsen, Polaris, Merck, AstraZeneca, Bayer (spouse), Genentech-Roche (spouse), Celgene-BMS (spouse), Eisai (spouse). KHY reports the following: grants and personal fees from Ipsen, grants from Halozyme, grants from BMS, outside the submitted work. RB reports the following: grant and travel credit from ArcherDx, honoraria for advisory board participation from Loxo oncology, Roche Dx and speaking fees from Illumina. WP reports the following: grants from NIH-K12 Paul Calabresi Career Development Award, grants from Parker Institute for Cancer Immunotherapy MSK Pilot Award, grants and other from SITC-TimIOs, grants and other from Merck, grants from Astellas, grants from Gossamerbio, personal fees from Ipsen, during the conduct of the study. ZKS reports the following: an immediate family member received personal fees from Genentech/Roche, personal fees from Novartis, personal fees from Adverum, personal fees from Allergan, personal fees from RegenexBio, personal fees from Gyroscope, personal fees from Optos PLC, personal fees from Regeneron, personal fees from Neurogene, outside the submitted work. AMV reports the following: research funding to MSK from Lilly, research funding to MSK from BMS, research funding to MSK from Silenseed, personal fees from Roche (immediate family member), outside the submitted work. ACW reports the following: honoraria for speaking from Shire, honoraria for speaking from Celgene, consulting fees from AstraZeneca, travel support from Intuitive Surgical, travel support from Bayer, outside the submitted work. The following authors have nothing to disclose: IS, SK, ESM, RS, LVS, OB, MC, JFC, DPK, WW, MAL, MR, DM, CAID.
Author contributions: Conceptualization: AMV, IS, RS, SK, WW, EOR. Data curation: AMV, IS, RS, SK, WW, EOR, ZKS, ESM. Formal analysis: JFC, MC, AMV, EOR, ZKS, ESM. Funding acquisition: CAID, EOR. Investigation: AMV, IS, RS, SK, WW, EOR, MAL, ZKS, ESM, LVS, ACW, MR, OB, RB, DM, DPK, WP, KHY. Methodology: AMV, IS, RS, SK, WW, EOR, JFC, MC. Project administration: AMV, EOR. Resources: AMV, EOR. Supervision: AMV, EOR. Visualization: AMV, EOR, JFC, MC. Writing—original draft: AMV, IS, RS, EOR. Writing—review & editing: AMV, IS, RS, SK, JFC, MC, WW, MAL, ZKS, ESM, LVS, ACW, MR, OB, RB, DM, CAID, DPK, WP, KHY, EOR.
Prior presentation: Part of this study was presented at Gastrointestinal Cancers Symposium 2020, San Francisco, CA.
Data Availability
All source data is available and will be archived at Memorial Sloan Kettering Cancer Center.
Supplementary Material
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed]
- 6.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Reilly EM, Lee JW, Zalupski M, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol. 2020;38(13):1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park W, Chen J, Chou JF, et al. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin Cancer Res.2020;26(13):3239–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung H, Siegel RL, Rosenberg PS, Jemal A.. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e147. [DOI] [PubMed] [Google Scholar]
- 10.Tavakkoli A, Singal AG, Waljee AK, et al. Racial disparities and trends in pancreatic cancer incidence and mortality in the United States. Clin Gastroenterol Hepatol. 2020;18(1):171–178.e110. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Jemal A, Ward EM.. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–1698. [DOI] [PubMed] [Google Scholar]
- 12.Duffy A, Capanu M, Allen P, et al. Pancreatic adenocarcinoma in a young patient population–12-year experience at Memorial Sloan Kettering Cancer Center. J Surg Oncol. 2009;100(1):8–12. [DOI] [PubMed] [Google Scholar]
- 13.Ntala C, Debernardi S, Feakins RM, Crnogorac-Jurcevic T.. Demographic, clinical, and pathological features of early onset pancreatic cancer patients. BMC Gastroenterol. 2018;18(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raissouni S, Rais G, Mrabti H, et al. Pancreatic adenocarcinoma in young adults in a Moroccan population. J Gastrointest Cancer. 2012;43(4):607–611. [DOI] [PubMed] [Google Scholar]
- 15.Lüttges J, Stigge C, Pacena M, Klöppel G.. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer. 2004;100(1):173–182. [DOI] [PubMed] [Google Scholar]
- 16.Ivy EJ, Sarr MG, Reiman HM.. Nonendocrine cancer of the pancreas in patients under age forty years. Surgery. 1990;108(3):481–487. [PubMed] [Google Scholar]
- 17.McWilliams RR, Maisonneuve P, Bamlet WR, et al. Risk factors for early-onset and very-early-onset pancreatic adenocarcinoma: a Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas. 2016;45(2):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Aharon I, Elkabets M, Pelossof R, et al. Genomic landscape of pancreatic adenocarcinoma in younger versus older patients: does age matter? Clin Cancer Res. 2019;25(7):2185–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery MA, Jordan EJ, Basturk O, et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: potential actionability and correlation with clinical phenotype. Clin Cancer Res. 2017;23(20):6094–6100. [DOI] [PubMed] [Google Scholar]
- 20.Lowery MA, Wong W, Jordan EJ, et al. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. 2018;110(10):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller G, Venkatraman ES.. Resampling procedures to compare two survival distributions in the presence of right-censored data. Biometrics. 1996;52(4):1204–1213. [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A.. AJCC Cancer Staging Manual 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 23.Amin MB, Edge SB, Greene F, et al. AJCC Cancer Staging Manual. New York, NY: Springer; 2017. [Google Scholar]
- 24.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biankin AV, Waddell N, Kassahn KS, et al. ; for Australian Pancreatic Cancer Genome Initiative. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddell N, Pajic M, Patch AM, et al. ; for Australian Pancreatic Cancer Genome Initiative. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21(4):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohal DPS, Kennedy EB, Cinar P, et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020:JCO2001364. doi: 10.1200/JCO.20.01364. [DOI] [PubMed] [Google Scholar]
- 29.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med. 2019;21(1):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav S, Kasi PM, Bamlet WR, et al. Effect of germline mutations in homologous recombination repair genes on overall survival of patients with pancreatic adenocarcinoma. Clin Cancer Res. 2020;26(24):6505–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All source data is available and will be archived at Memorial Sloan Kettering Cancer Center.