Abstract
A review of parasitic plant diversity and outstanding disjunct distributions according to an updated functional classification based on these plants’ life cycles.
Introduction
Plant parasitism has evolved numerous times and in multiple different forms among photosynthetic organisms, including in green algae, rhodophytes, and land plants (Musselman and Press, 1995; Feild and Brodribb, 2005; Merckx et al., 2009; Oborník, 2019). The greatest biological diversity of parasitic plants occurs among the angiosperms that possess an organ called haustorium, which directly connects the parasite with its host vasculature (Kuijt, 1969). Commonly formed shortly after germination, the haustorium promotes attachment and invasion of host tissues, thus establishing an intimate structural and physiological bridge between parasite and host (Joel, 2013). This organ develops iteratively in a series of or physiological confirmation physiological changes, allowing the parasite to achieve optimal growth, reproduction, and to exchange nutrients and information with its host (Jiang et al., 2007; Yoshida et al., 2016; Clarke et al., 2019; Teixeira-Costa et al., 2020).
ADVANCES
Despite being a highly specialized organ and a unifying feature of several parasitic plants, haustorial parasites have evolved 12 times among angiosperms (Barkman et al., 2007; Naumann et al., 2013; Nickrent, 2020). The taxonomic diversity of these clades accounts for accounts for only 1% of flowering plant species, yet they represent broad variation in growth form, host range, geographic distribution, life history, and functionality (Figure 1 and Table 1; Heide-Jørgensen, 2008; Westwood et al., 2010). Here, we have adopted the most recent and comprehensive taxonomic account of these species (Nickrent, 2020; Table 1) and focus our attention on the functional diversity of these fascinating organisms. We also review important aspects of these plants’ life histories, which we then use to propose an amended classification system. Finally, we discuss peculiar cases of disjunct geographical distributions among parasitic species in the context of the different functional groups we propose.
Phylogenomic tools have greatly clarified the closest free-living relatives of many parasitic plant lineages.
Improved understanding of parasitic plant physiology and functional diversity have shown great variation in photosynthetic activity both among and within clades.
Figure 1.
Parasitic plant diversity. A, Krameria bicolor (Krameriaceae). B, Castilleja mexicana (Orobanchaceae). C, Comandra umbellata (Santalaceae, Santalales) D, Psittacanthus dichrous (Loranthaceae, Santalales). E, Cynomorium coccineum (Cynomoriaceae). F, Lennoa madreporoides (Lennoaceae). G, Prosopanche caatingicola (Hydnoraceae). H, Langsdorffia hypogaea (Balanophoraceae, Santalales). I, Epifagus virginiana (Orobanchaceae). J, C. filiformis (Lauraceae). K, C.campestris (Convolvulaceae). L, V.minimum (Santalaceae, Santalales). M, Rafflesia cantleyi (Rafflesiaceae). N, Bdallophytum americanum (Cytinaceae). O, Pilostyles blanchetii (Apodanthaceae). P, Mitrastemon matudae (Mitrastemonaceae). All photos by L. Teixeira-Costa, except A and N (M.H. Sandoval), E (R.G. Albaladejo), G (R. Machado), and M (C.C. Davis).
Table 1.
Number of genera and species in each parasitic plant clade, as reported by Nickrent (2020)
| Parasitic Plant Lineage | Number of Genera | Number of Species | % |
|---|---|---|---|
| Apodanthaceae (Curcubitales) | 2 | 10 | 0.2 |
| Cassytha (Laurales) | 1 | 20 | 0.4 |
| Cuscuta (Solanales) | 1 | 215 | 4.4 |
| Cynomoriaceae (Saxifragales) | 1 | 1 | 0.02 |
| Cytinaceae (Malvales) | 2 | 12 | 0.23 |
| Hydnoraceae (Piperales) | 2 | 12 | 0.23 |
| Krameriaceae (Zygophyllales) | 1 | 23 | 0.5 |
| Lennoaceae (Boraginales) | 2 | 4 | 0.08 |
| Mitrastemonaceae (Ericales) | 1 | 2 | 0.04 |
| Orobanchaceae (Lamiales) | 102 | 2,163 | 43.9 |
| Rafflesiaceae (Malpighiales) | 3 | 36 | 0.7 |
| Santalales | 179 | 2,428 | 49.3 |
| Totals | 297 | 4,926 | 100 |
Parasitic plant functional diversity and classification
Numerous parasitic plant classifications have been proposed since the 18th century, based on a range of morphological, anatomical, and physiological features (reviewed by Schrenk, 1894). Photosynthetic modality has been the most frequently used classifier, traditionally dividing parasites into hemiparasites, and holoparasites (Musselman and Press, 1995). The first group includes species that possess chlorophyll and are capable of photosynthesis, while the latter comprises parasites that lack chlorophyll and derive water, minerals, and photoassimilates entirely from their hosts (Westwood et al., 2010; Lambers and Oliveira, 2019). The specific site of parasite attachment, either to the stem or roots of host plants, has also been used as an additional form of classification, leading researchers to often recognize four types of parasitic plants: root versus stem hemiparasites, and root versus stem holoparasites (Musselman and Press, 1995; Heide-Jørgensen, 2008). To a lesser extent, climbing habit and haustorium morphology have also been recognized as useful features to classify parasitic angiosperm diversity (Schrenk, 1894).
These physiological and morphological attributes have also formed the basis for the taxonomic classification of parasitic plants prior to the widespread application of molecular phylogenetics. For instance, nearly all stem hemiparasites were previously grouped in the single large family, Loranthaceae (Engler, 1889; Kuijt, 1969). Today, this parasitic growth form is understood to have evolved 5 times independently within three different families, that is, Loranthaceae, Misodendraceae, and Santalaceae sensu lato (Vidal-Russell and Nickrent, 2008; The Angiosperm Phylogeny Group, 2016). Among root parasites, species currently placed in Orobanchaceae were previously assigned to Scrophulariaceae or Orobanchaceae, dividing a continuum of photosynthetic ability and host dependency (Boeshore, 1920; Wolfe and DePamphilis, 1998; Wolfe et al., 2005). Perhaps, the most extreme example of how or physiological confirmation functional characteristics have guided taxonomy is exemplified by the classification of Rafflesiales or Rafflesiaceae (Nickrent, 2002; Nickrent et al., 2004; Nikolov and Davis, 2017). Members of these clades show extremely reduced vegetative morphology and are only visible outside the host body during reproduction. This peculiar life form was used as an important morphological classifier since the comprehensive work by Unger (1840) strongly influencing the taxonomy of these plants for more than a century. Recent advances in molecular phylogenetics, however, have shown species previously grouped in the Rafflesiaceae to belong to at least four distinct clades with independent evolutionary origins (The Angiosperm Phylogeny Group, 2003; Nickrent et al., 2004; Barkman et al., 2007; Nikolov and Davis, 2017).
These examples highlight the challenges of classifying parasitic plants, and despite significant progress regarding their taxonomic circumscriptions (Nickrent, 2020), their functional classification remains overly simplistic. For example, certain species of Cuscuta are commonly described as hemiparasitic to holoparasitic, thus defying easy classification. While many species in this genus possess chlorophyll and are thus described as hemiparasitic (MacLeod, 1963; Choudhury and Sahu, 1999; Revill et al., 2015), even the most photosynthetically active taxa derive up to 99% of their photoassimilates from their hosts (Jeschke et al., 1994b; Hibberd et al., 1998; Clayson et al., 2014), presumably via direct parasite–host phloem connections (Haupt et al., 2001; Birschwilks et al., 2006). In this context, classifiers such as xylem- and phloem-feeder have been proposed as more informative alternatives to hemiparasite and holoparasite (Irving and Cameron, 2009). Regardless of photosynthetic capacity, all haustorial parasitic plants establish functional connections with their hosts’ xylem (Kuijt, 1969; Teixeira-Costa, 2021). Species with low to null photosynthetic capacity have been generally hypothesized to additionally withdraw nutrients directly from the host phloem. These would be classified as phloem feeders, while others exclusively connected to the host xylem would be termed xylem feeders (Irving and Cameron, 2009).
In addition to addressing the issue of Cuscuta species, this classification system is also particularly useful for the interpretation of photosynthetically efficient parasites, including several Santalales and Orobanchaceae species, which derive over 50% of their carbon from their hosts exclusively via xylem connections (Marshall and Ehleringer, 1990; Cechin and Press, 1993a; Richter et al., 1995; Tennakoon and Pate, 1996; Těšitel et al., 2011). However, the determination of parasitic plants as predominantly phloem-feeders represents a complex task, which has been detailed for only a few species. While this term can be easily applied to species such as Orobanche cernua Loefl. (Orobanchaceae), which rely almost exclusively on host phloem for both carbon and mineral nutrition (Hibberd et al., 1999), it might not be suitable to classify holoparasites that tap into the host phloem only late in their development, such as Rafflesia and Pilostyles species (Teixeira-Costa et al., 2021). Furthermore, morphological and/or physiological confirmation of parasite–host phloem connections has only been obtained for some Apodanthaceae, Balanophoraceae, Cynomoriaceae, Cuscuta, Orobanchaceae, and Rafflesiaceae species (Jeschke et al., 1994a; Hsiao et al., 1995; Hibberd et al., 1999; Fahmy and Hassan, 2020; Teixeira-Costa et al., 2021). In Hydnoraceae, Lennoaceae, and certain Balanophoraceae species, all of which lack chlorophyll, connection to the host phloem has not been detected (Kuijt, 1967; Hsiao et al., 1993; Tennakoon et al., 2007).
Considering these complexities and uncertainties, a more practical functional classification of parasitic plant diversity is needed. Moreover, such a classification needs to be reconciled against our understanding of the taxonomy and phylogeny of these plants (Nickrent, 2020). Along these lines, Těšitel (2016) combined traditionally used functional and morphological characteristics, such as photosynthetic capacity, connection to the host phloem, and location of haustoria on the host, with other aspects, such as mode of germination, growth form, and apical versus lateral haustorium. His analyses resulted in the recognition of four functional groups: root hemiparasites, stem parasites, root holoparasites, and endoparasites (Těšitel, 2016). This classification is especially suitable when characterizing the broad diversity of parasitic angiosperms. However, some adjustments to this framework are required to resolve inconsistencies and to better reflect our current understanding of the or physiological confirmation physiological nature of these symbioses.
For instance, Těšitel placed Mitrastemonaceae in his root holoparasite category. Instead, they are better classified as endoparasites (Watanabe, 1936), given that these species grow entirely within their host during most of their life cycle. The genus Phacellaria (Santalaceae), on the other hand, was originally classified by Těšitel (2016) as an endoparasite. However, species in this genus are not fully endophytic at maturity, indicating they are better classified as mistletoes (Danser, 1939; Kuijt, 2015). Other inconsistencies and lack of information regarding phloem connections and germination modality of species could have biased Těšitel’s (2016) circumscription, especially regarding endoparasites and root holoparasites. Finally, the combination of Cassytha, Cuscuta, and mistletoe species in a single group (stem parasites, sensu Těšitel 2016) clouds important differences regarding the life history and evolution of these plants.
In this context, we improve upon Těšitel's (2016) classification by using pivotal stages in the life cycle of parasitic plants as classifiers. Such pivotal stages are related to major shifts in the evolution of these plants, including the form and site of seed germination, the development of apical or lateral haustoria, and the extent of autonomous growth. While these characteristics have been partially included in other classification systems (Unger, 1840; Johow, 1891; Schrenk, 1894; Těšitel, 2016), we believe that a classification based entirely on life history could reduce artificialities inherently associated with classifying biological diversity into discrete groups (Irving and Cameron, 2009). Moreover, parasite species can display variations in photosynthesis efficiency and capacity at different developmental stages, nutrient availability conditions, or when infesting different hosts (Hull and Leonard, 1964a; Cechin and Press, 1993b; Lambers and Oliveira, 2019). Thus, a classification system that is not centered around photosynthetic activity is potentially more stable and may be useful to compare parasitic plant functionality, evolution, and life histories more broadly. Here we recognize five functional groups of parasitic plants (Table 2) to be discussed below: euphytoid parasites (Figure 2A); mistletoes (Figure 2B); parasitic vines (Figure 2C); obligate root parasites (Figure 2D); and endoparasites (Figure 2E). In the following sections, we elaborate on the general life cycle of parasitic plants, define each functional group in detail, and explore pivotal stages in the development of species classified into these groups.
Table 2.
Number of parasitic species per clade (as reported by Nickrent 2020), further subdivided according to five functional groups
| Parasitic Plant Lineages | Number of Species |
Totals | ||||
|---|---|---|---|---|---|---|
| Euphytoid Parasites | Mistletoes | Parasitic Vines | Obligate Root Parasites | Endoparasites | ||
| Apodanthaceae | – | – | – | – | 10 | 10 |
| Cassytha | – | – | 20 | – | – | 20 |
| Cuscuta | – | – | 215 | – | – | 215 |
| Cynomoriaceae | – | – | – | 1 | – | 1 |
| Cytinaceae | – | – | – | 12 | 12 | |
| Hydnoraceae | – | – | – | 12 | – | 12 |
| Krameriaceae | 23 | – | – | – | 23 | |
| Lennoaceae | – | – | 4 | – | 4 | |
| Mitrastemonaceae | – | – | – | 2 | 2 | |
| Orobanchaceae | 1,833 | – | – | 309 | – | 2,142 |
| Rafflesiaceae | – | – | 36 | 36 | ||
| Santalales | 613 | 1,647 | – | 45 | 18 | 2,323 |
| Total, n (%) | 2,469 (51.4) | 1,647 (34.3) | 235 (5.0) | 371 (7.7) | 78 (1.6) | 4,800 |
Figure 2.
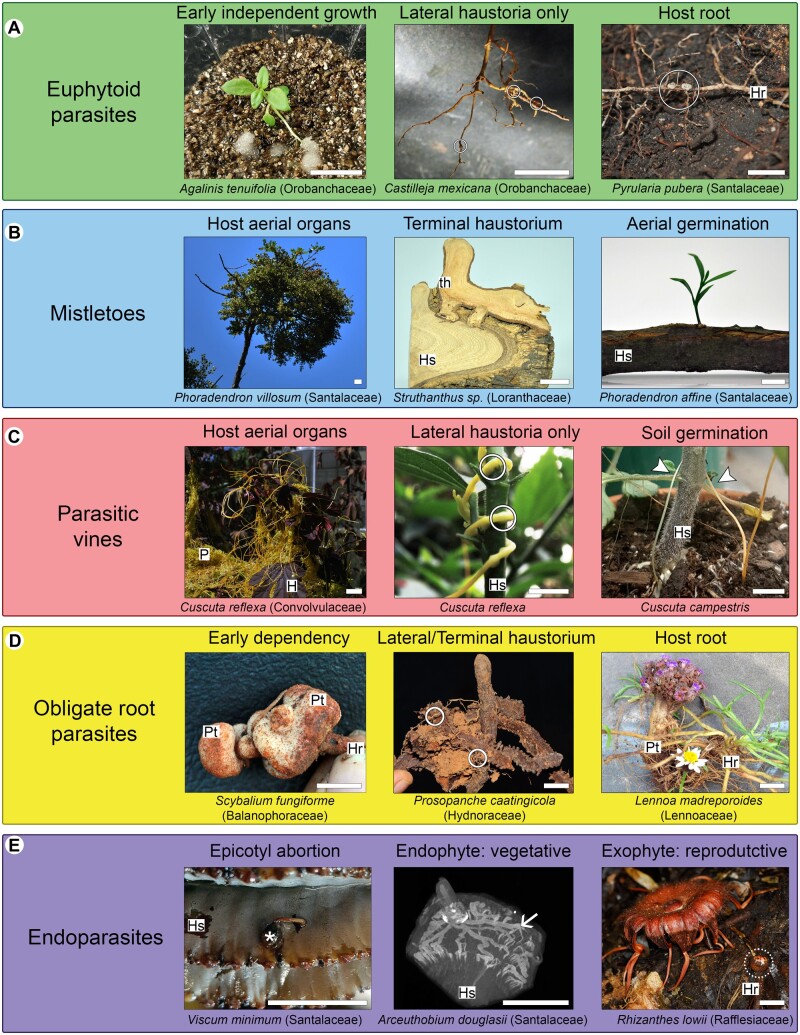
Parasitic plant classification by functional groups. A, Euphytoid parasites. B, Mistletoes. C, Parasitic vines. D, Obligate root parasites. E, Endoparasites. H, host; Hr, host root; Hs, host stem; P, parasite; Pt, parasite tuber; th, terminal haustorium; circles, lateral haustoria; dashed circle, parasite flower bud; arrow heads, parasite stems; arrow, endophytic tissue; asterisk, seedling with epicotyl abortion. All photos by L. Teixeira-Costa, except P. caatingola (R. Machado) and R. lowii (C.C. Davis). Scale bars: 2 cm.
General life history
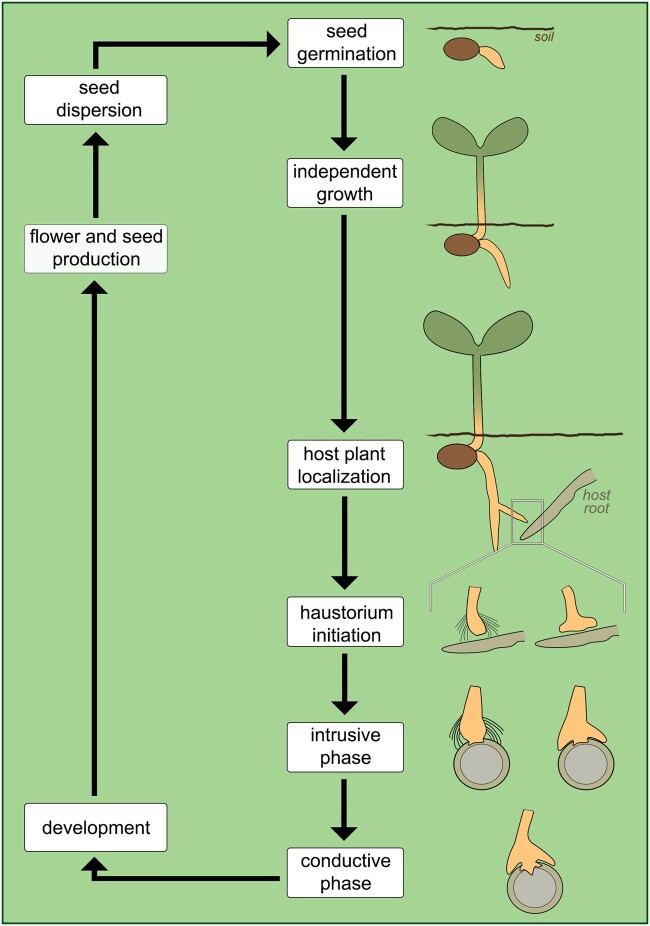
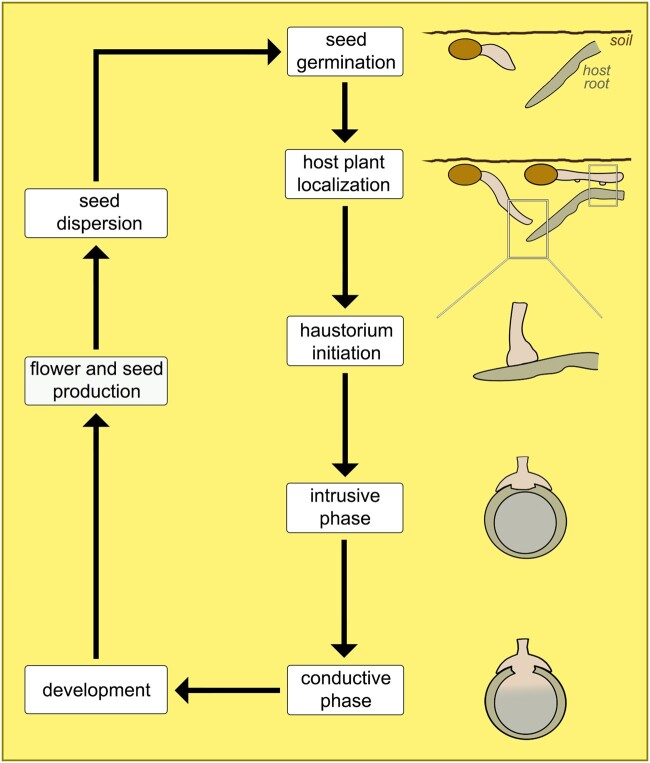
The general life cycle of parasitic plants can be divided into three stages centered around haustorium development. Each stage can be further subdivided into discrete phases. The early stage, prior to host attachment, begins with seed germination phase and ends with host plant localization. The intermediate stage corresponds to the process of haustorium development, which involves initiation, intrusive, and conductive phases. The final stage, which follows the establishment of host–parasite vascular connections, includes most of the remaining phases of plant development and reproduction. The length of each stage, and in some cases even the absence of certain phases, varies according to functional characteristics of different parasitic species.
Over 50% of all haustorial parasite species have traditionally been classified as hemiparasites (Westwood et al., 2010). Most of those that germinate on the ground, such as Nuytsia floribunda (Labill.) R. Br. ex G. Don (Loranthaceae), Krameria lanceolata Torr. (Krameriaceae), and Escobedia grandiflora (L.f.) Kuntze (Orobanchaceae), often maintain a phase of independent growth beyond radicle elongation during at least the first weeks of their life cycle (Grieve, 1975; Musselman, 1977; Cardona-Medina and Muriel Ruiz, 2015). Indeed, some hemiparasites in the Orobanchaceae and Santalales can be cultivated entirely without a host, although such facultative behavior is unlikely to occur widely in natural settings (Heide-Jørgensen, 2013). On the other hand, hemiparasites that germinate directly upon host stems and branches (i.e. aerial germination), such as Viscum album L. (Santalaceae), can grow independently only for a short duration, even when cultivated in a laboratory (Sallé, 1983). Likewise, in those parasites that germinate on the ground but form only a rudimentary root system, parasitizing the stems of their hosts (e.g. Cassytha [Lauraceae] and Cuscuta [Convolvulaceae]), independent growth is restricted to only one or 2 weeks (McLuckie, 1924; Truscott, 1966; Sherman et al., 2008).
Holoparasites, on the other hand, initially rely exclusively on the nutrients in their often-reduced endosperm until a connection with a suitable host is established (Heide-Jørgensen, 2008). For this reason, an independent growth phase is virtually absent from their life cycle. Furthermore, many holoparasites also coordinate their seed germination only in the presence of a nearby host, which increases the chance of seedling germination and establishment. This is observed in many Orobanchaceae, such as Orobanche and Phelipanche species, which require host-derived germination stimulants (de Cuyper et al., 2017; Waters et al., 2017). A similar form of germination, dependent on host-derived stimulants, has been hypothesized for other root holoparasites. It is noteworthy that a few hemiparasites, such as Striga species (Orobanchaceae), have an early development that closely resembles that of holoparasites (Spallek et al., 2013).
Upon germination, many parasites are able to actively locate a suitable host. This is most clearly documented in the genus Cuscuta (Convolvulaceae), in which species grow toward their hosts in response to chemical and/or light quality cues (Runyon et al., 2006; Yoshida and Shirasu, 2009; Furuhashi et al., 2011; Johnson et al., 2016). Mistletoes have also been shown to interact with host-derived chemical cues that could guide initial growth (Rödl and Ward, 2002; Randle et al., 2018). Similar observations have been made in Striga hermonthica (Delile) Benth. (Orobanchaceae), although a specific signaling pathway or mechanism has yet to be identified in this species (Yoshida and Shirasu, 2009; Clarke et al., 2019). Little information is available for other root parasites. These general observations highlight the broad diversity of life histories exhibited by parasitic plants and introduce some pivotal stages in the life cycle, which we will further expand in the subsequent sections.
Euphytoid parasites
Parasitic plants that are capable of photosynthesis and infest the underground system of their hosts are classified here as euphytoid parasites. The term euphytoid, meaning “resembling true (non-parasitic) plants,” comes from the classification proposed by Johow (1891), which was based mostly on photosynthetic capacity and site of seed germination. Here, we have chosen to use this term to highlight the ability of plants in this group to sustain independent growth for a few days or weeks prior to host localization (Figure 3). The term also suggests that, similar to nonparasitic plants, euphytoid parasites possess regular photosynthesis and germinate in the ground without the need for host-derived signals (Baskin and Baskin, 2014). These characteristics have been frequently used to classify these plants as facultative parasites, suggesting they could complete their life cycle without infesting another plant (Westwood et al., 2010). Considering facultative behavior is unlikely to occur in nature and that many of these plants require a host even in laboratory settings (Simpson, 1989; Heide-Jørgensen, 2013; Cardona-Medina and Muriel Ruiz, 2015; Kuijt, 2015), we have opted to not use this term.
Figure 3.
Schematic of the main phases in the life cycle of euphytoid parasites. Two types of haustorium are represented in the initiation and intrusive phases, indicating species with (left-hand side) and without (right-hand side) haustorium hairs; both have a similar appearance during the conductive phase.
In addition to the features described above, euphytoid parasites form a type of haustorium that develops as lateral extensions from young or adventitious roots (Heide-Jørgensen, 2013). Due to their position, lateral haustoria do not terminate root growth, thus allowing the formation of multiple lateral haustoria by a single parasite (Bandaranayake and Yoder, 2013). Furthermore, maintenance of apical root growth allows euphytoid parasites to expand their root system and infest multiple hosts at the same time, which often improves overall parasite growth and biomass (Marvier, 1998; Sandner and Matthies, 2018). This set of characteristics is present in all Krameriaceae (Table 2), most Orobanchaceae (Table 3), and in six families within Santalales (Table 4). Species in these clades display various life histories, ranging from annual and perennial herbs to shrubs and large trees.
Table 3.
Number of genera and species in each Orobanchaceae tribe (as reported by Nickrent 2020), further subdivided according to functional groups, including nonparasitic species
| Tribes | Functional Group | No. of Genera | No. of Species | Total |
|---|---|---|---|---|
| Brandisia Group | Euphytoid parasites | 1 | 11 | 85% (77 genera; 1,833 species) |
| Buchnereae | 34 | 352 | ||
| Cymbarieae | 5 | 15 | ||
| Pedicularideae | 21 | 1,033 | ||
| Pterygiella group | 3 | 9 | ||
| Rhinantheae | 13 | 413 | ||
| Buchnereae | Obligate root parasites | 6 | 61 | 14.3% (22 genera; 309 species) |
| Orobancheae | 15 | 243 | ||
| Rhinantheae | 1 | 5 | ||
| Lindenbergieae | Nonparasites | 1 | 7 | 0.7% (3 genera; 15 species) |
| Rehmannieae | 2 | 8 | ||
| Total | 102 | 2,157 | 102 genera; 2,157 species | |
Rhinantheae and Buchnereae appear in the table twice because they include both euphytoid and obligate root parasites.
Table 4.
Number of genera and species (as reported by Nickrent 2020) in each Santalales family (sensu APG 2016), further subdivided according to functional groups, including species that are nonparasitic and those for which trophic condition has not yet been established
| Family | Functional Group | No. of Genera | No. of Species | Total |
|---|---|---|---|---|
| Loranthaceae | Mistletoes | 70 | 1,016 | 67.9% (83 genera; 1,647 species) |
| Misodendraceae | 1 | 8 | ||
| Santalaceae | 12 | 623 | ||
| Olacaceae | Euphytoid parasites | 3 | 59 | 25.3% (54 genera; 613 species) |
| Opiliaceae | 11 | 36 | ||
| Loranthaceae | 3 | 3 | ||
| Santalaceae | 30 | 468 | ||
| Schoepfiaceae | 3 | 34 | ||
| Ximeniaceae | 4 | 13 | ||
| Coulaceae | Nonparasites | 3 | 3 | 2.9% (13 genera; 71 species) |
| Erythropalaceae | 3 | 36 | ||
| Octoknemaceae | 1 | 14 | ||
| Strombosiaceae | 6 | 18 | ||
| Balanophoraceae | Obligate root parasites | 17 | 45 | 1.8% (17 genera; 45 species) |
| Aptandraceae | Trophic mode unknown | 8 | 34 | 1.4% (8 genera; 38 species) |
| Loranthaceae | Endoparasites | 1 | 1 | 0.7% (3 genera; 18 species) |
| Santalaceae | 2 | 17 | ||
| Total | 179 | 2,428 | 179 genera; 2,432 species | |
Loranthaceae and Santalaceae appear 3 times in the table as they include mistletoes, euphytoid parasites, and endoparasites.
Following germination, euphytoid parasites can emerge above ground and start photosynthesizing even before forming haustoria. When a potential host root is located, the process of haustorium development begins. This is stimulated by physical and/or chemical cues required for haustorium initiation (Heide-Jørgensen, 2008; but see Xiang et al., 2018). In species of Krameriaceae and Santalales, physical contact with a host root stimulates the formation of a mantle, which provides mechanical anchorage during host penetration (Toth and Kuijt, 1977; Brokamp et al., 2012; Teixeira-Costa et al., 2020). In most Orobanchaceae, host-derived haustorium-inducing factors promote the development of haustorial hairs, which are modified root hairs that facilitate host root attachment by the parasite (Baird and Riopel, 1985; Heide-Jørgensen and Kuijt, 1995; Cui et al., 2016). Some Orobanchaceae may also form a type of attachment mantle via fusion of haustorial hairs (Weber, 1976; Kuijt, 1977).
Once the attachment is established, the parasite begins penetrating host tissues, often disrupting the host vascular organization due to the proliferation of parasitic tissue within the host body (Pérez-de-Luque, 2013; Wakatake et al., 2018). Xylem connections are formed at the host–parasite interface (Musselman and Dickison, 1975; Pate et al., 1990; Brokamp et al., 2012). In euphytoid Orobanchaceae, this process has been shown to be largely mediated by auxin (Wakatake et al., 2020). In all cases, the formation of host–parasite vascular connections marks the beginning of the conductive phase and the exchange of substances between the two plants, thus permitting the continuation of the parasite’s life cycle (Figure 3). Host resistance against haustorium penetration and/or establishment of vascular connections are known to cause severe reduction or death of the parasite (Cameron et al., 2006; Rispail et al., 2006).
Mistletoes
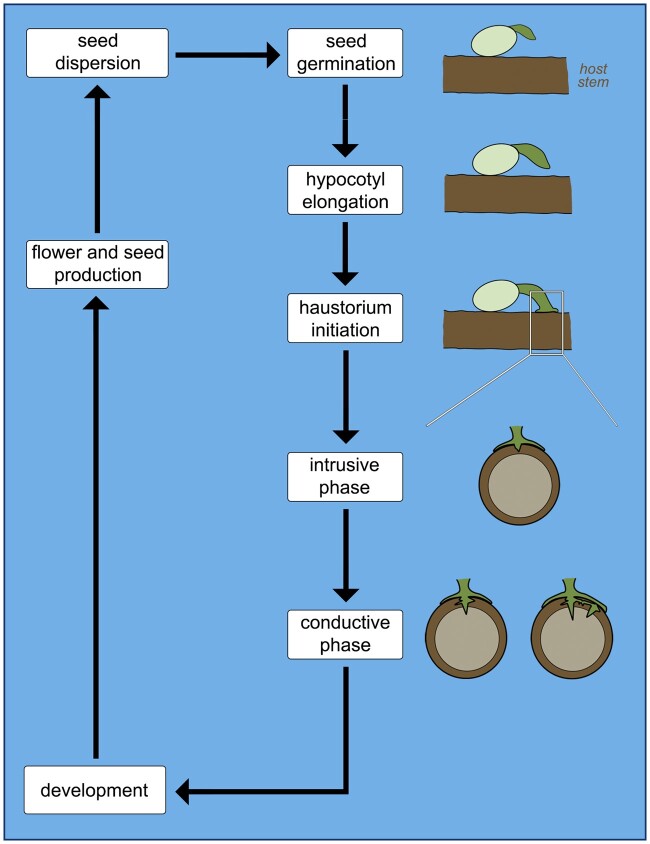
Mistletoes are shrubby plants with seeds that germinate autonomously and directly upon the branches of their hosts (Lamont, 1983; Aukema, 2003). Although photosynthetic capacity is reduced in certain species, such as those in the genus Arceuthobium (Santalaceae; Hull and Leonard, 1964a, 1964b), all mistletoes are capable of photosynthetic activity to some extent. Compared to the soil microenvironment, germination in aerial locations pose a greater risk of seed dislodgement, exposure, and predation, thus requiring a precise set of adaptations for survival (Lamont, 1983). One key adaptation along these lines is the development of a terminal haustorium, which originates from the embryo root apex (Fineran, 2001). This “revolutionary step in parasitic plant evolution” (Heide-Jørgensen, 2008) allows the parasite increased agility to access its host water supply, while also establishing a stronger mechanical connection to the host stem (Fineran, 2001). Because the terminal haustorium is differentiated at the radicle tip, thus terminating root apical meristem cells, no further growth of the primary root occurs.
Following the APG IV (2016) classification, mistletoes belong to three closely related families: Loranthaceae, Misodendraceae, and Santalaceae. The monotypic Misodendraceae are remarkably different from other mistletoes. Its members are wind-dispersed, deciduous, and exhibit high host specificity, exclusively parasitizing species of trees in the southern hemisphere genus Nothofagus (Fagaceae; Vidal-Russell and Premoli, 2015; Glatzel et al., 2017). Santalaceae includes three independently evolved mistletoe clades, with varying degrees of host specificity (Vidal-Russell and Nickrent, 2008). These members include three genera most commonly reported to cause severe damage in horticulture and forestry: Arceuthobium, Phoradendron, and Viscum (Hawksworth, 1983; Watson et al., 2020). Finally, Loranthaceae includes the greatest number of mistletoe species (Table 4), and exhibit complex developmental trajectories related to the evolution of a variety of haustorium morphologies within the family (Wilson and Calvin, 2006; Teixeira-Costa et al., 2020).
In mistletoes, hypocotyl elongation quickly leads to physical contact with the host stem, which stimulates haustorium initiation from the radicle tip (Lamont, 1983; Figure 4). The subsequent developmental step is the formation of a holdfast, a dome-shaped structure that secures attachment of the parasite to the host branch (Sallé, 1983). The borders of the holdfast frequently enlarge to form a mantle similar to that of root hemiparasites, which also helps prevent a separation between mistletoe and host during the intrusive phase (Heide-Jørgensen, 2008). Development of the terminal haustorium is a crucial step in the mistletoe life cycle. While parasites that only form lateral haustoria can attempt multiple host connections simultaneously, mistletoe growth depends entirely on the successful development of a terminal haustorium. Once a vascular connection has been established between host and parasite, thus securing water and nutrient availability, the mistletoe shoot will then emerge from the seed and development proceeds (Figure 4). This delayed shoot emergence prevents the parasite from transpiring excessively prior to securing a water source (Fineran, 2001). Following the complete establishment of a terminal haustorium, several mistletoe species may additionally form lateral haustoria (Calvin and Wilson, 2006; Teixeira-Costa et al., 2020).
Figure 4.
Schematic of the main phases in the life cycle of mistletoes. Haustorium initiation is characterized by the formation of a holdfast (white rectangle). Representation the intrusive and conductive phases focus on parasite development at the interface with the host.
Parasitic vines
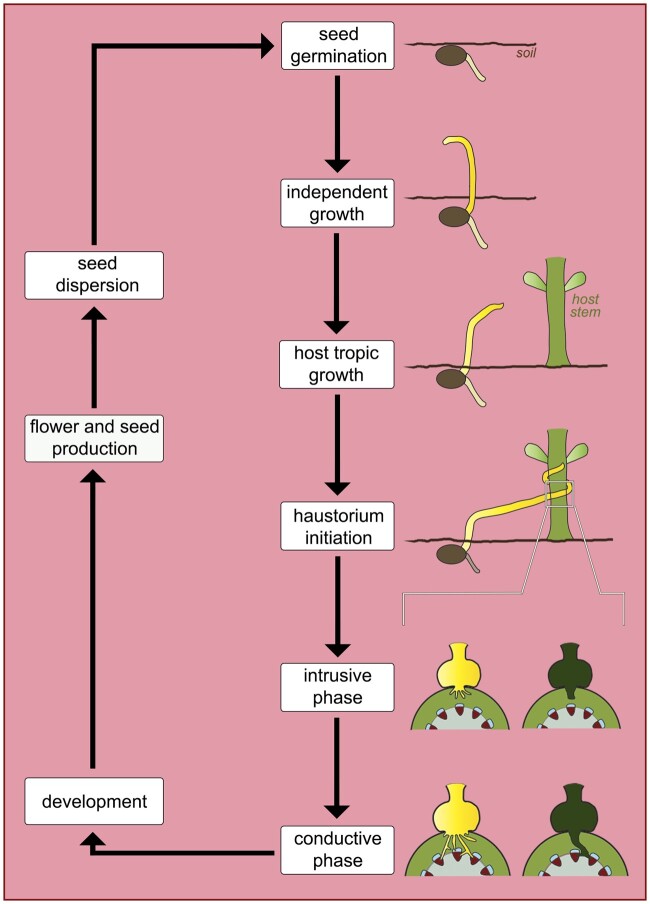
In contrast to mistletoes, parasitic vines, that is, Cassytha and Cuscuta species, have seeds that germinate autonomously on the ground, forming a rudimentary and short-lived root system (McLuckie, 1924; Truscott, 1966). The functionality of these roots with respect to water uptake is not well understood and conflicting results have been reported (Sherman et al., 2008; Behdarvandi et al., 2014). Regardless, the underground system characteristic of parasitic vines provides physical support and anchorage to the soil during their brief, free-living growth phase (Kuijt, 1969). Once a host is located, multiple lateral haustoria form quickly, allowing these parasites to be established on multiple hosts simultaneously. In contrast to euphytoid parasites, the lateral haustoria of parasitic vines originate from the stems that coil around their host. No terminal haustorium develops.
Cassytha and Cuscuta species have long been considered vines in the traditional sense due to their climbing habit and tendril-like appendages (Johow, 1891; Heide-Jørgensen, 2008; Bowling and Vaughn, 2009). Indeed, the process of host localization in these plants is facilitated by the twining of their stem and tendril-like appendages, which eventually latch onto a suitable host and coil around it (Figure 5). In Cuscuta, directional growth toward host plants is often observed in response to far-red light and host-derived volatiles (Orr et al., 1996; Runyon et al., 2006; Furuhashi et al., 2011; Johnson et al., 2016). Light quality also stimulates coiling behavior in Cuscuta, which further stimulates holdfast formation and haustorium initiation (Furuhashi et al., 1995; Tada et al., 1996). Cassytha species are assumed to behave similarly but are less well characterized (Heide-Jørgensen, 2008).
Figure 5.
Schematic of the main phases in the life cycle of parasitic vines. Note the difference in the intrusive and conductive phases between the two represented genera. Cuscuta (yellow) forms searching and conducting hypha (multiple yellow projections inside the host stem), which will connect to the host xylem (red wedges) and phloem (blue rounded rectangles). Cassytha (green) forms a typical parenchymatic endophyte (singular green projection inside the host stem).
Upon penetration, Cassytha vines develop a haustorium similar to other parasites, initially composed of parenchyma cells, which later differentiate into vessel elements (Abubacker et al., 2005). Cuscuta species, on the other hand, form an alternative type of tissue, known as searching hyphae (Kuijt, 1977). These searching hyphae grow as single cells via tip elongation, which resemble pollen tubes or fungal hyphae (Vaughn, 2003). As development progresses, searching hyphae are converted to specialized xylem and phloem hyphae (Figure 5), forming vascular connections with the host xylem and phloem conductive cells, respectively (Vaughn, 2006). In addition to their similar life histories, Cassytha and Cuscuta species are strikingly similar morphologically, bearing twining stems, small flowers, scaly leaves, and often refractory seeds. They represent a quintessential example of convergent evolution (Kuijt, 1969).
It is noteworthy that a few Orobanchaceae and Santalales species have also been described as twining or vine-like plants (Boodle, 1913; Der and Nickrent, 2008). However, these plants do not fit our classification of parasitic vines due to differences among key stages in their life cycles. Buttonia species (Orobanchaceae), for instance, parasitize the roots, and not the aerial organs of host plants (Boodle, 1913). This difference indicates that, opposed to the short-lived roots of Cassytha and Cuscuta, Buttonia species have a perennial root system from which lateral haustoria are formed. Consequently, Buttonia species are likely to grow independently for longer than Cassytha and Cuscuta, which in turn provides these climbing Orobanchaceae plants with more time to localize a suitable host. In the case of stem parasitic vine species in the Santalales, also referred to as dendroparasites (Der and Nickrent, 2008; Vidal-Russell and Nickrent, 2008), germination is aerial and followed by the development of a terminal haustorium and aerial germination, leading to a more suitable classification of these plants as mistletoes (Macklin and Parnell, 2002).
Obligate root parasites
Parasites that germinate underground, often in response to host-derived chemicals, and infest the root systems of their hosts are classified here as obligate root parasites. Although all haustorial parasites observed in nature depend on vascular connections established with a host (Heide-Jørgensen, 2013), the terminology chosen for this functional group highlights that these parasites are entirely dependent upon their hosts from the earliest stages in their development. This early dependency contrasts to both what is observed in euphytoid parasites and parasitic vines, which can sustain independent growth for a few weeks (McLuckie, 1924; Truscott, 1966; Heide-Jørgensen, 2008), and what occurs in mistletoes, which bear green hypocotyls hypothesized to provide carbon to the developing parasite (Williams, 1963; Room, 1973; Lamont and Perry, 1977; Lichter and Berry, 1991). Obligate root parasites, on the other hand, have small seeds ranging from 0.2 to 2 mm that contain little endosperm the plant can rely on prior to host attachment (Weddell, 1860; Hansen, 1980; Yatskievych and Mason Jr., 1986; Musselman and Visser, 1989; Joel and Bar, 2013). Furthermore, most species classified here as obligate root parasites are devoid of chlorophyll. The ones that are able to photosynthesize, such as Striga spp., do so at low rates and only late in their life cycle (Press et al., 1987).
Indeed, most obligate root parasites grow underground during prolong periods of time, with only their flowers/inflorescences emerging aerially from the soil (Heide-Jørgensen, 2008). In Striga species (Orobanchaceae), leafy stems are also formed from the tuberous, underground haustorium. Among parasites in this functional group, all members of Orobanchaceae (Table 3) and Balanophoraceae (Table 4) possess a terminal haustorium (Holzapfel, 2001; Joel, 2013). These plants can also develop additional lateral haustoria following terminal haustorium establishment (Attawi and Weber, 1980; Hansen, 2015). On the other hand, Cynomoriaceae, Hydnoraceae, and Lennoaceae species (Table 2) form only lateral haustoria (Kuijt, 1966; Musselman and Visser, 1989; Fahmy and Hassan, 2020). Germination and initial contact to the host root, however, remain poorly understood for species in these three families (Heide-Jørgensen, 2008; Fahmy and Hassan, 2020). In Hydnora triceps Drege and E. Mey. (Hydnoraceae), germination was shown to occur only in the presence of host-specific root exudates (Bolin et al., 2009). In Orobanchaceae, germination of obligate root parasites has been extensively documented to be dependent on host-derived chemicals (de Cuyper et al., 2017; Waters et al., 2017).
Host plant localization by obligate root parasites is often achieved by chemotropic growth of the radicle toward the host root (Figure 6; Ecroyd, 1996; Holzapfel, 2001; Joel and Bar, 2013; Clarke et al., 2019). Such directional growth is likely to accelerate the establishment of vascular connections to a suitable host. These are crucial steps for the survival of obligate root parasites, as they will otherwise die as soon as the endosperm is depleted (Heide-Jørgensen, 2008). Once the parasite reaches a host root, species with exclusively lateral haustoria will develop protuberances or lateral structures that facilitate host attachment (Figure 6). In those that develop a terminal haustorium, the tip of the radicle expands rapidly and haustorium differentiation begins (Figure 6). In most Orobanchaceae, this process is strongly dependent on the presence of haustorium-inducing factors, especially during the development of the terminal haustorium (Bandaranayake and Yoder, 2013). As haustorium development proceeds, a peculiar interface is formed between the two plants, characterized by an intensive and visually chaotic mixture of parasite and host tissues (Figure 6). For many obligate root parasites, distinguishing host from parasite tissues at this juncture can be quite difficult as has been well demonstrated in several members of the Balanophoraceae (Shivamurthy et al., 1981; Mauseth et al., 1992; Hsiao et al., 1993, 1994).
Figure 6.
Schematic of the main phases in the life cycle of obligate root parasites. Two types of haustorium are represented in the host plant localization phase, indicating species with terminal (left-hand side) and lateral (right-hand side) haustoria; both have a similar appearance during the subsequent phases.
Endoparasites
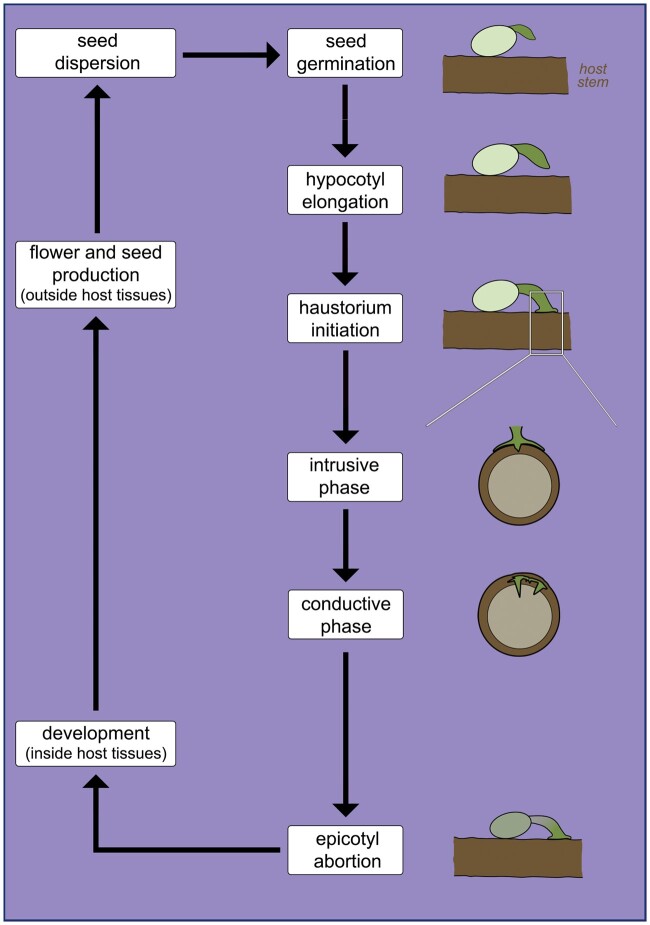
Endoparasites represent the most extreme and derived category of plant parasitism. Their vegetative body is reduced to a mycelial-like strand of cells embedded within their host roots or stems. The only organs of endoparasites to appear externally to the host are their inflorescences/flowers and fruits (Mauseth, 1990; Meijer and Veldkamp, 1993; Nikolov et al., 2014). Despite this peculiar life form, which is associated with a series of developmental adaptations, a precise definition of endoparasites as a functional group has escaped previous classifications. Unger (1840) defined this group as “parasites that arise directly above the wooden body of a host and anastomose their vascular system with that of the nutrient plant.” However, modern understanding of haustorium development and anatomy has shown that grafting between parasite and host vascular systems is the norm for species in all functional groups (Kuijt, 1977; Melnyk, 2017; Teixeira-Costa, 2021). More recently, Těšitel (2016) distinguished plants in this functional group by “the dominance of an endophytic stage in their life cycle.” Despite being more accurate, this definition also applies to a few other haustorial parasitic plants, thus requiring further refinement.
Here, we define endoparasites as species with a life cycle marked by the combination of three pivotal stages: absence of autonomous growth related to abortion of the epicotyl (embryonic shoot apex); vegetative body development restricted to endophyte; and exophyte exclusively formed by reproductive structures (Figure 7). This peculiar life cycle is characteristic of Apodanthaceae, Cytinaceae, Mitrastemonaceae, Rafflesiaceae (Table 2), and a few members of Santalales (Table 4), such as Tristerix aphyllus (Loranthaceae), Viscum minimum (Santalaceae), and Arceuthobium species (Santalaceae; Thoday and Johnson, 1930; Mauseth et al., 1985; Kuijt, 1986, 2011). Among these parasites, clear evidence of epicotyl abortion has been reported for all endoparasitic Santalalean species (Kuijt, 1986; Mauseth, 1990; Calvin and Wilson, 1996). For all other endoparasites, seed germination and initial haustorium development remain undescribed. Nevertheless, scanty reports suggest seedling development is limited (Heinricher, 1917; Wicaksono et al., 2020). Additionally, extreme plastome reduction observed in Rafflesiaceae, Apodanthaceae, and Cytinaceae renders photosynthesis, and thus autonomous growth, most likely unviable (Molina et al., 2014; Bellot and Renner, 2015; Roquet et al., 2017; Cai et al., 2021). Plastome reduction is also a hallmark of most Orobanchaceae classified as obligate root parasites (Wicke et al., 2013).
Figure 7.
Schematic of the main phases in the life cycle of endoparasites. Note the phase of epicotyl abortion, absent in other parasitic plant life cycles. Phases from seed germination to intrusive growth have only been observed in endoparasitic mistletoes.
It is noteworthy that epicotyl abortion alone is not enough to classify a plant as an endoparasite, as this feature is also observed in the mistletoe Phoradendron californicum (Santalaceae), which develops a vegetative body in the form of a dense shrub outside host tissues (Kuijt, 1989, 2003). Likewise, establishment of an extensive endophytic tissue system is not an indication of endoparasitism. This is the case of several mistletoes, such as Phoradendron leucarpum, and possibly Phacellaria species (Santalaceae), all of which grow as shrubs upon host branches (Danser, 1939; Kuijt, 2003, 2015). Finally, extension of the endophytic tissue system into the host apical meristem, leading to a special form of coordinated development known as isophasic growth, is also not necessarily a sign a species should be classified as an endoparasite. Indeed, isophasic growth is observed in the mistletoe Phoradendron perredactum (Kuijt, 2011). Among endoparasites, this isophasic growth is reported in certain Arceuthobium (Santalales) and Pilostyles (Apodanthaceae) species (Rutherford, 1970; Lye, 2006), but it remains to be confirmed in Cytinaceae, Mitrastemonaceae, and Rafflesiaceae (Teixeira-Costa et al., 2021).
Distribution and biogeographical patterns
Clarifying their taxonomic, phylogenetic, and functional diversity has increased our understanding of parasitic plant biogeography. The five functional groups discussed here are distributed across all continents (except Antarctica), suggesting that current biogeographical distribution is not obviously related to functional types. Parasitic plants are also represented in nearly all biomes, with the exception of aquatic environments (Heide-Jørgensen, 2013). However, their wide geographical distribution is deceptive because most parasitic plant clades exhibit relatively narrow distributions. Among euphytoid parasites, Krameriaceae is the most restricted clade, with species found in warm arid and semiarid areas from the southern United States to Chile (Simpson et al., 2004). Euphytoid Orobanchaceae and Santalales, on the other hand, exhibit nearly cosmopolitan distributions (Wolfe et al., 2005; Moore et al., 2010; Cameron and Phoenix, 2013; Kuijt, 2015). Still, a few genera in these clades display remarkable disjunctions. This includes the speciose Euphrasia (Orobanchaceae) inferred to have originated in Eurasia and later colonized areas in both North and South America, as well as Oceania via long-distance dispersal and vicariance (Gussarova et al., 2008). In the Santalaceae, Buckleya and Pyrularia (Figure 8A) have a characteristic eastern Asia to eastern North America distribution, hypothesized to have been achieved via the Bering land bridge (Li et al., 2001; Zhou et al., 2019).
Figure 8.
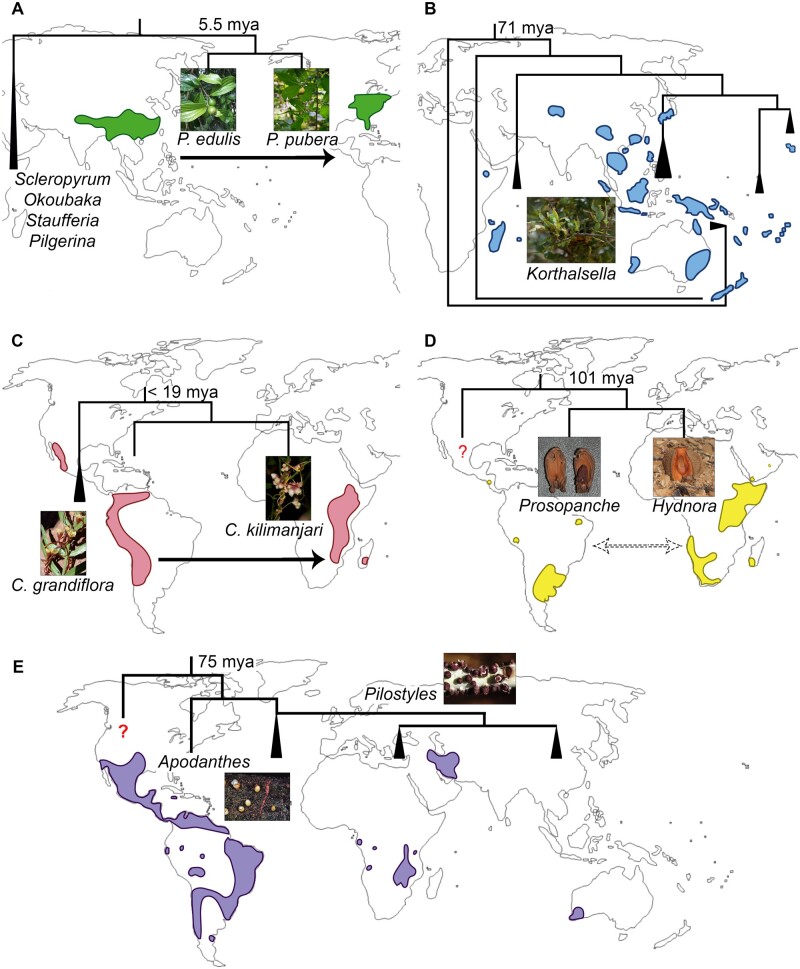
Cases of extreme disjunct distributions among different parasitic plant functional groups. A, Pyrularia (Santalaceae), including P. edulis (photo by Z. Zhou), and P. pubera (photo by L. Teixeira-Costa). B, Korthalsella (Santalaceae; photo by J. Sullivan) including multiple species. C, Cuscuta (Convolvulaceae), including C. grandiflora (photo by M. Costea) and C. kilimanjari (photo by B. Wursten). D, Hydnoraceae, including Prosopanche (photo by R. Machado) and Hydnora (photo by D. Keats). E, Apodanthaceae, including Apodanthes (photo by N. Chaisoung) and Pilostyles (photo by G. Ceccantini). Red question marks: uncertain phylogenetic position; triangles: clades with multiple species; dashed arrows: western Gondwanan breakup; black arrows: hypothesized direction of long-distance dispersion; stem group ages estimated by (A) Zhou et al. (2019), (B) Sultan (2014), (C) Neumann et al. (2020), (D–E) Naumann et al. (2013).
As a functional group, mistletoes can also be classified as nearly cosmopolitan due to the wide geographical range occupied by members of Loranthaceae and Visceae (Santalaceae). These aerial plants are found in all forested continents and several remote oceanic islands, spanning tropical and temperate zones (Watson, 2019). The strong interaction between mistletoes and bird dispersers is often invoked to explain cases of long-distance dispersal, especially in the Loranthaceae, whose diversification burst coincided with the radiation of many bird families (Liu et al., 2018). Host specificity has also been shown to play a crucial role in mistletoe speciation and distribution (Norton and Carpenter, 1998; Amico and Nickrent, 2009; Okubamichael et al., 2016). A combination of these factors is likely to have shaped the current distribution of species in the genus Korthalsella (Santalaceae; Sultan, 2014). However, despite recent advances in the biology and ecology of Korthalsella (Sultan et al., 2018; Sultan et al., 2019), the sequence of events resulting in the pattern of disjunct distribution combined with regional endemism (Figure 8B) remains challenging to retrace.
A history of long-distance dispersal events is also reported for parasitic vines, especially Cuscuta species, which migrated from Eurasia to all other continents early in the evolution of the genus (García et al., 2014). Interesting disjunction patterns are hypothesized to have occurred in several clades within the genus, such as the case of a western African species, Cuscuta kilimanjari, which is deeply nested within a South American group (Figure 8C). Nevertheless, biological explanations behind these dispersal events remain somewhat elusive, partially because Cuscuta seeds lack classical adaptations associated with dispersal syndromes (Olszewski et al., 2020). Wind is often reported as a dispersing agent, but it is unlikely to actually carry seeds between continents (Sorensen, 1986; Cain et al., 2000). Surprisingly, dispersal by water birds has been demonstrated as a possibility for Cuscuta campestris and Cuscuta pacifica (Costea et al., 2016). Additionally, fruits of Cuscuta gronovii were shown to be able to float for longer than isolated seeds, which suggests possible long-distance dispersal via water for other Cuscuta species with indehiscent fruits (Ho and Costea, 2018). Similarly, seeds of the pantropical Cassytha filiformis have been described to be both water impermeable and able to float, which would facilitate dispersal by water (Muir, 1933; Mahadevan and Jayasuriya, 2013). Further support to this hypothesis of water-mediated dispersal comes from the observation that Cassytha species are predominantly coastal (Heide-Jørgensen, 2008). Considering the presence of fleshy fruits, endozoochory is also mentioned as an important dispersal mode for some species (French and Westoby, 1996), although detailed observations appear to be missing.
Striking disjunct geographical distributions are also observed in entire parasitic families, especially those classified as obligate root parasites and endoparasites. Among the first, the case of Hydnoraceae remains puzzling due to its debated phylogenetic position within Piperales (Nickrent, 2020). The family includes two genera: Hydnora, distributed across Africa, Madagascar, and the Arabian Peninsula; and Prosopanche, occurring in Costa Rica and South America (Figure 8D; Musselman and Visser, 1989; Machado and de Queiroz, 2012). Divergence time estimates for Hydnora raise the possibility that disjunction in this clade may be consistent with vicariance between South America and Africa (Naumann et al., 2013; Nickrent, 2020).
Difficulties in determining the nonparasitic sister clade of Apodanthaceae have also limited our understanding of the historical biogeography of this endoparasite family. Long-distance dispersal may explain the current distribution of the family in the American and African continents (Figure 8E). However, the production of tiny seeds suggests the parasite might require immediate proximity of a host for survival, and perhaps even germination (Bouman and Meijer, 1994). This led Bellot (2014) to propose that Apodanthaceae could have achieved long-distance dispersal inside a host. The family is estimated to have diverged around 72 Mya (million years ago), well after the hypothesized final separation between South America and Africa around 110 Mya (Naumann et al., 2013). Apodanthaceae species are also found in restricted areas of Australia and the Arabian Peninsula, which complicates hypotheses about the history of this clade (Bellot and Renner, 2014).
The peculiar cases of geographic distribution discussed here highlight some of the recent contributions to the knowledge of parasitic plant evolution and biogeography, as well as some of the persisting questions in these areas. Our proposed classification system based on pivotal stages of these plants’ life cycles could be used to analyze and compare other cases of long-distance dispersal and disjunctions with the examples addressed here. Furthermore, we argue that a classification system focused on life history rather than on photosynthetic capacity, a trait known to vary greatly among and within clades, provides a comparative framework that best captures the broad diversity of parasitic plants.
OUTSTANDING QUESTIONS
How are shifts between different life cycles in clades of Santalales and Orobanchaceae related to the molecular evolution of species in these clades?
How do early life cycle stages, especially germination and initial infestation, occur among the most cryptic plant parasites, the endoparasites?
What is the degree of host specificity among parasitic plants and how do host associations regulate their geographical distributions and patterns of community assembly?
Acknowledgments
We thank our many colleagues who kindly provided photographs used in this work. Images in Figure 8C were obtained from PhytoImages (C. grandiflora) and Flora of Malawi (C. kilimanjari), with permission. Other images in Figure 8B (Korthalsella), Figure 8D (Hydnora), and Figure 8E (Apodanthes) are under Creative Commons license types Attribution 2.0 (creativecommons.org/licenses/by-nc/2.0/legalcode), Attribution 2.0 Generic (creativecommons.org/licenses/by/2.0/legalcode), and Attribution 3.0 Unported (creativecommons.org/licenses/by/3.0/legalcode).
Funding
L.T.C. was supported by a fellowship from the Harvard University Herbaria. Funding for parts of this research was supported by National Science Foundation Assembling the Tree of Life Grant DEB-0622764 and National Science Foundation Grant DEB-1120243 to C.C.D.
Conflict of interest statement. The authors have no conflicts of interest to declare.
Contributor Information
Luiza Teixeira-Costa, Department of Organismic and Evolutionary Biology, Harvard University Herbaria, Cambridge, Massachusetts 02138, USA.
Charles C. Davis, Department of Organismic and Evolutionary Biology, Harvard University Herbaria, Cambridge, Massachusetts 02138, USA
L.T.C. drafted the manuscript and figures. C.C.D. carefully revised the text. Both authors discussed the data and contributed to the final version of the text.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) are: Luiza Teixeira-Costa (lteixeiracosta@fas.harvard.edu) and Charles C. Davis (cdavis@oeb.harvard.edu).
References
- Abubacker MN, Prince M, Hariharan Y (2005) Histochemical and biochemical studies of parasite-host interaction of Cassyta filiformis Linn. and Zizyphus jujuba Lamk. Curr Sci 89: 2156–2159 [Google Scholar]
- Amico GC, Nickrent DL (2009) Population structure and phylogeography of the mistletoes Tristerix corymbosus and T. aphyllus (Loranthaceae) using chloroplast DNA sequence variation. Am J Bot 96: 1571–1580 [DOI] [PubMed] [Google Scholar]
- Attawi VFAJ, Weber HC (1980) Zum Parasitismus und zur morphologisch-anatomischen Struktur der Sekundarhaustorien von Orobanche-Arten (Orobanchaceae). Flora 169: 55–83 [Google Scholar]
- Aukema JE (2003) Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Front Ecol Environ 1: 212–219 [Google Scholar]
- Baird WV, Riopel JL (1985) The developmental anatomy of Conopholis americana (Orobancaceae) seedlings and tubercles. Can J Bot 64: 710–717 [Google Scholar]
- Bandaranayake PCG, Yoder JI (2013) Haustorium Initiation and Early Development. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies. Springer, Berlin, Heidelberg, Germany, pp 61–74 [Google Scholar]
- Barkman TJ, McNeal JR, Lim SH, Coat G, Croom HB, Young ND, DePamphilis CW (2007) Mitochondrial DNA suggests at least 11 origins of parasitism in angiosperms and reveals genomic chimerism in parasitic plants. BMC Evol Biol 7: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM (2014) Germination Ecology of Plants with Specialized Life Cycles and/or Habitats. Seeds: Ecology, Biogeography and, Evolulion. 2nd ed. Elsevier Science & Technology, Amsterdam, Netherlands, p: 1601 [Google Scholar]
- Behdarvandi B, Guinel FC, Costea M (2014) Despite being short-lived the rudimentary root of Cuscuta (dodders, Convolvulaceae) seedlings is functional. 50th Meeting of the Canadian Botanical Association, Montréal, Quebec, Canada p 642
- Bellot S (2014) Natural history, Taxonomy, Biogeography and Genome Evolution of the worldwide endoparasite family Apodanthaceae (Curcubitales). PhD thesis. Ludwig-Maximilians-Universität München, München, Germany
- Bellot S, Renner S (2014) The systematics of the worldwide endoparasite family Apodanthaceae (Cucurbitales), with a key, a map, and color photos of most species. PhytoKeys 36: 41–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot S, Renner SS (2015) The plastomes of two species in the endoparasite genus pilostyles (Apodanthaceae) each retain just five or six possibly functional genes. Genome Biol Evol 8: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birschwilks M, Haupt S, Hofius D, Neumann S (2006) Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J Exp Bot 57: 911–921 [DOI] [PubMed] [Google Scholar]
- Boeshore I (1920) The morphological continuity of Scrophulariaceae and Orobanchaceae. PhD thesis. University of Pennsylvania, Philadelphia, PA
- Bolin JF, Maass E, Tennakoon KU, Musselman LJ (2009) Host-specific germination of the root holoparasite Hydnora triceps (Hydnoraceae). Botany 87: 1250–1254 [Google Scholar]
- Boodle LA (1913) The root and haustorium of Buttonia natalensis. Bull Misc Inf (Royal Bot Gard Kew) 1913: 240–242 [Google Scholar]
- Bouman F, Meijer W (1994) Comparative structure of ovules and seeds in Rafflesiaceae. Plant Syst Evol 193: 187–212 [Google Scholar]
- Bowling AJ, Vaughn KC (2009) Gelatinous fibers are widespread in coiling tendrils and twining vines. Am J Bot 96: 719–727 [DOI] [PubMed] [Google Scholar]
- Brokamp G, Dostert N, Cáceres-H F, Weigend M (2012) Parasitism and haustorium anatomy of Krameria lappacea (Dombey) Burdet & B.B. Simpson (Krameriaceae), an endangered medicinal plant from the Andean deserts. J Arid Environ 83: 94–100 [Google Scholar]
- Cai L, Arnold BJ, Xi Z, Khost DE, Patel N, Hartmann CB, Manickam S, Sasirat S, Nikolov LA, Mathews S et al. (2021) Deeply Altered Genome Architecture in the Endoparasitic Flowering Plant Sapria himalayana Griff. (Rafflesiaceae). Curr Biol 31: 1–10 [DOI] [PubMed] [Google Scholar]
- Cain ML, Milligan BG, Strand AE (2000) Long-distance seed dispersal in plant populations. Am J Bot 87: 1217–1227 [PubMed] [Google Scholar]
- Calvin CL, Wilson CA (2006) Comparative morphology of epicortical roots in Old and New World Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora 201: 51–64 [Google Scholar]
- Calvin CL, Wilson CA (1996) Endophytic system. In Hawksworth FG, Wiens D, eds, Dwarf Mistletoes: Biology, Pathology and Systematics. United States Department of Agriculture Forest Service, Washington, DC, p 429 [Google Scholar]
- Cameron DD, Coats AM, Seel WE (2006) Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Ann Bot 98: 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DD, Phoenix GK (2013) Ecology of Hemiparasitic Orobanchaceae with Special Reference to Their Interaction with Plant Communities. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitism, Mechanisms and Control Strategies. Springer, Berlin, Heidelberg, Germany, pp 287–305 [Google Scholar]
- Cardona-Medina E, Muriel Ruiz SB (2015) Seed germination and plant development in Escobedia grandiflora (Orobanchaceae): Evidence of obligate hemiparasitism? Acta Biol Colomb 20: 133–140 [Google Scholar]
- Cechin I, Press MC (1993a) Nitrogen relations of the sorghum-Striga hermonthica host-parasite association: growth and photosynthesis. Plant Cell Environ 16: 237–247 [DOI] [PubMed] [Google Scholar]
- Cechin I, Press MC (1993b) Nitrogen relations of the Sorghum-Striga hermonthica host-parasite association: germination, attachment and early growth. New Phytol 124: 681–687 [DOI] [PubMed] [Google Scholar]
- Choudhury NK, Sahu D (1999) Photosynthesis in Cuscuta reflexa: a total plant parasite. Photosynthetica 36: 1–9 [Google Scholar]
- Clarke CR, Timko MP, Yoder JI, Axtell MJ, Westwood JH (2019) Molecular dialog between parasitic plants and their hosts. Annu Rev Phytopathol 57: 279–299 [DOI] [PubMed] [Google Scholar]
- Clayson C, García-Ruiz I, Costea M (2014) Diversity, evolution, and function of stomata bearing structures in Cuscuta (dodders, Convolvulaceae): from extrafloral nectar secretion to transpiration in arid conditions. Perspect Plant Ecol Evol Syst 16: 310–321 [Google Scholar]
- Costea M, Stefanović S, García MA, De La Cruz S, Casazza ML, Green AJ (2016) Waterfowl endozoochory: an overlooked long-distance dispersal mode for Cuscuta (dodder). Am J Bot 103: 957–962 [DOI] [PubMed] [Google Scholar]
- Cui S, Wakatake T, Hashimoto K, Saucet S, Toyooka K, Yoshida S, Shirasu K (2016) Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum. Plant Physiol 170: 1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuyper C, Struk S, Braem L, Gevaert K, De Jaeger G, Goormachtig S (2017) Strigolactones, karrikins and beyond. Plant Cell Environ 40: 1691–1703 [DOI] [PubMed] [Google Scholar]
- Danser BH (1939) A revision of the genus Phacellaria (Santalaceae). Blumea 3: 212–235. [Google Scholar]
- Der JP, Nickrent DL (2008) A molecular phylogeny of Santalaceae (Santalales). Syst Bot 33: 107–116 [Google Scholar]
- Ecroyd CE (1996) The ecology of Dactylanthus taylorii and threats to its survival. N Z J Ecol 20: 81–100 [Google Scholar]
- Engler A (1889) Loranthaceae. Die Natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten. Insbes. den Nutzpflanzen, Unter Mitwirkung Zahlreicher Hervorragender Fachgelehrten Begründet. Verlag von Wilhelm Engelmann, Leipzig pp 156–198 [Google Scholar]
- Fahmy GM, Hassan AERH (2020) Haustorial structure of the holoparasitic angiosperm Cynomorium coccineum L. invading host roots. Flora 274: 151731 [Google Scholar]
- Feild TS, Brodribb TJ (2005) A unique mode of parasitism in the conifer coral tree Parasitaxus ustus (Podocarpaceae). Plant Cell Environ 28: 1316–1325 [Google Scholar]
- Fineran BA (2001) Early evolution of the haustorial system in Loranthaceae mistletoes, and its relationship to the organization of the haustorium in root hemi-parasitic Santalales. Phytomorphology 51: 541–571 [Google Scholar]
- French K, Westoby M (1996) Vertebrate-dispersed species in a fire-prone environment. Austral Ecol 21: 379–385 [Google Scholar]
- Furuhashi K, Kanno M, Morita T (1995) Photocontrol of parasitism in a parasitic flowering plant, Cuscuta japonica chois, cultured in vitro. Plant Cell Physiol 36: 533–536 [Google Scholar]
- Furuhashi T, Furuhashi K, Weckwerth W (2011) The parasitic mechanism of the holostemparasitic plant Cuscuta. J Plant Interact 6: 207–219 [Google Scholar]
- García MA, Costea M, Kuzmina M, Stefanović S (2014) Phylogeny, character evolution, and biogeography of Cuscuta (dodders; Convolvulaceae) inferred from coding plastid and nuclear sequences. Am J Bot 101: 670–690 [DOI] [PubMed] [Google Scholar]
- Glatzel G, Richter H, Devkota MP, Amico G, Lee S, Lin R, Grabner M, Barlow BA (2017) Foliar habit in mistletoe-host associations. Botany 95: 219–229 [Google Scholar]
- Grieve BJ (1975) Botany in Western Australia: a survey of progress: 1900±1971. J R Soc West Aust 58: 33–53 [Google Scholar]
- Gussarova G, Popp M, Vitek E, Brochmann C (2008) Molecular phylogeny and biogeography of the bipolar Euphrasia (Orobanchaceae): recent radiations in an old genus. Mol Phylogenet Evol 48: 444–460 [DOI] [PubMed] [Google Scholar]
- Hansen B (2015) Balanophorales. In Kubitzki K, ed, Families and Genera of Vascular Plants. Vol. XII Flowering Plants. Eudicots. Santalales, Balanophorales, Springer International Publishing, Heidelberg, Germany, pp 192–208 [Google Scholar]
- Hansen B (1980) Balanophoraceae. Flora Neotropica 23: 1–80 [Google Scholar]
- Haupt S, Oparka KJ, Sauer N, Neumann S (2001) Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J Exp Bot 52: 173–177 [PubMed] [Google Scholar]
- Hawksworth FG (1983) Mistletoes as forest parasites. In Calder M, Bernhardt P, eds, Biology of Mistletoes. Academic Press, Sidney, p 348 [Google Scholar]
- Heide-Jørgensen HS (2008) Parasitic flowering plants, Brill, Leiden, the Netherlands [Google Scholar]
- Heide-Jørgensen HS (2013) Introduction: the parasitic syndrome in higher plants. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitism, Mechanisms, and Control Strategies, Springer, Berlin Heidelgerb, Germany, p 518 [Google Scholar]
- Heide-Jørgensen HS, Kuijt J (1995) The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. Am J Bot 82: 782–797 [Google Scholar]
- Heinricher E (1917) Die erste Aufzucht einer Rafflesiaceae, Cytinus hypocistis L., aus Samen. Ber Dtsch Bot Ges 35: 505–512 [Google Scholar]
- Hibberd JM, Bungard RA, Press MC, Jeschke WD, Scholes JD, Quick WP (1998) Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa. Planta 205: 506–513 [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD, Jeschke WD (1999) Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ 22: 937–947 [Google Scholar]
- Ho A, Costea M (2018) Diversity, evolution and taxonomic significance of fruit in Cuscuta (dodder, Convolvulaceae); the evolutionary advantages of indehiscence. Perspect Plant Ecol Evol Syst 32: 1–17 [Google Scholar]
- Holzapfel S (2001) Studies of the New Zealand root-parasite Dactylanthus taylorii (Balanophoraceae). Englera 22: 7–176 [Google Scholar]
- Hsiao SC, Mauseth JD, Gomez LD (1994) Growth and anatomy of the vegetative body of the parasitic angiosperm Langsdorffia hypogaea (Balanophoraceae). Bull Torrey Bot Club 121: 24–39 [Google Scholar]
- Hsiao SC, Mauseth JD, Gomez LD (1993) Growth and anatomy of the vegetative body of the parasitic angiosperm Helosis cayennensis (Balanophoraceae). Bull Torrey Bot Club 120: 295–309 [Google Scholar]
- Hsiao SC, Mauseth JD, Peng CI (1995) Composite bundles, the host/parasite interface in the holoparasitic angiosperms Langsdorffia and Balanophora (Balanophoraceae). Am J Bot 82: 81–91 [Google Scholar]
- Hull RJ, Leonard OA (1964a) Physiological aspects of parasitism in mistletoes (Arceuthobium and Phoradendron). II. The photosynthetic capacity of mistletoe. Plant Physiol 39: 1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RJ, Leonard OA (1964b) Physiological aspects of parasitism in mistletoes (Arceuthobium and Phoradendron). I. The carbohydrate nutrition of mistletoe. Plant Physiol 39: 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving LJ, Cameron DD (2009) You are what you eat: interactions between root parasitic plants and their hosts. Adv Bot Res 50: 87–138 [Google Scholar]
- Jeschke WD, Bäumel P, Räth N, Czygan FC, Proksch P (1994a) Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa roxb. and its host Lupinus albus L.: II. Flows between host and parasite and within the parasitized host. J Exp Bot 45: 801–812 [Google Scholar]
- Jeschke WD, Rath N, Baumel P, Czygan F, Proksch P (1994b) Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb and its host Lupinus albus L.I.Methods for estimating net flows. J Exp Bot 45: 791–800 [Google Scholar]
- Jiang F, Timergalina L, Kudoyarova G, Jeschke WD, Hartung W (2007) Growth and development of the facultative root hemiparasite Rhinanthus minor after removal of its host. Funct Plant Biol 34: 237–245 [DOI] [PubMed] [Google Scholar]
- Joel DM (2013) Functional structure of the mature haustorium. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitism, Mechanisms, and Control Strategies. Springer; Berlin, Heidelberg, Germany, p 518 [Google Scholar]
- Joel DM, Bar H (2013) The seed and the seedling. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitism, Mechanisms, and Control Strategies. Springer Berlin, Heidelberg, Germany, pp 147–165 [Google Scholar]
- Johnson BI, De Moraes CM, Mescher MC (2016) Manipulation of light spectral quality disrupts host location and attachment by parasitic plants in the genus Cuscuta. J Appl Ecol 53: 794–803 [Google Scholar]
- Johow F (1891) Die phanerogamen Schmarotzerpflanzen. Bot Zentralblatt 47: 279–281 [Google Scholar]
- Kuijt J (2015) Santalales. In Kubitzki K, ed, Families and Genera of Vascular Plants. Vol. XII Flowering Plants. Eudicots. Santalales, Balanophorales. Springer International Publishing, Heidelberg, Germany, p 209 [Google Scholar]
- Kuijt J (1969) The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA [Google Scholar]
- Kuijt J (1967) Parasitism in Pholisma (Lennoaceae) II. Anatomical aspects. Can J Bot 45: 1155–1162 [Google Scholar]
- Kuijt J (1977) Haustoria of phanerogamic parasites. Annu Rev Phytopathol 17: 91–118 [Google Scholar]
- Kuijt J (1966) Parasitism in Pholisma (Lennoaceae). I. external morphology of subterranean organs. Am J Bot 53: 82–86 [Google Scholar]
- Kuijt J (1986) Observations on establishment and early shoot emergence of Viscum minimum (Viscaceae). Acta Bot Neerl 35: 449–456 [Google Scholar]
- Kuijt J (2011) Isophasic parasitism in Phoradendron perredactum (Viscaceae). Acta Bot Mex 96: 11–13 [Google Scholar]
- Kuijt J (1989) A note on the germination and establishment of Phoradendron californicum (Viscaceae). Madroño 36: 175–179 [Google Scholar]
- Kuijt J (2003) Monograph of Phoradendron (Viscaceae). Syst Bot Monogr 66: 1–643 [Google Scholar]
- Lambers H, Oliveira RS (2019) Biotic Influences: Parasitic Associations. Plant Physiological Ecology, 3rd ed. Springer Nature, Cham, Switzerland, pp 597–613 [Google Scholar]
- Lamont B (1983) Germination of Mistletoes. In Calder DM, Bernhardt P, eds, Biology of Mistletoes. Academic Press, Melbourne, Australia, p 348 [Google Scholar]
- Lamont B, Perry M (1977) The effects of light, osmotic potential and atmospheric gases on germination of the mistletoe Amyema preissii. Ann Bot 41: 203–209 [Google Scholar]
- Li J, Boufford DE, Donoghue MJ (2001) Phylogenetics of Buckleya (Santalaceae) based on ITS sequences of nuclear. Rhodora 109: 137–150 [Google Scholar]
- Lichter JM, Berry AM (1991) Establishment of the mistletoe Phoradendron macrophyllum: phenology of early stages and host compatibility studies. Bot Gaz 152: 468–475 [Google Scholar]
- Liu B, Le CT, Barrett RL, Nickrent DL, Chen Z, Lu L, Vidal-Russell R (2018) Historical biogeography of Loranthaceae (Santalales): diversification agrees with emergence of tropical forests and radiation of songbirds. Mol Phylogenet Evol 124: 199–212 [DOI] [PubMed] [Google Scholar]
- Lye D (2006) Charting the isophasic endophyte of dwarf mistletoe Arceuthobium douglasii (Viscaceae) in host apical buds. Ann Bot 97: 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RF, de Queiroz LP (2012) A new species of Prosopanche (Hydnoraceae) from northeastern Brazil. Phytotaxa 75: 58–64 [Google Scholar]
- Macklin J, Parnell J (2002) Account of Santalaceae of Thailand. Thai For Bull 30: 75–115 [Google Scholar]
- MacLeod DG (1963) The parasitism of Cuscuta. New Phytol 62: 257–263 [Google Scholar]
- Mahadevan N, Jayasuriya KMGG (2013) Water-impermeable fruits of the parasitic angiosperm Cassytha filiformis (Lauraceae): confirmation of physical dormancy in Magnoliidae and evolutionary considerations. Aust J Bot 61: 322–329 [Google Scholar]
- Marshall JD, Ehleringer JR (1990) Are xylem-tapping mistletoes partially heterotrophic? Oecologia 84: 244–248 [DOI] [PubMed] [Google Scholar]
- Marvier M (1998) A mixed diet improves performance and herbivore resistance of a parasitic plant. Ecology 79: 1272–1280 [Google Scholar]
- Mauseth JD (1990) Morphogenesis in a highly reduced plant: the endophyte of Tristerix aphyllus (Loranthaceae). Bot Gaz 151: 384–353 [Google Scholar]
- Mauseth JD, Hsiao S-C, Montenegro G (1992) Vegetative body of the parasitic angiosperm Ombrophytum subterraneum (Balanophoraceae). Bull Torrey Bot Club 119: 407–417 [Google Scholar]
- Mauseth JD, Montenegro G, Walckowiak AM (1985) Host infection and flower formation by the parasite Tristerix aphyllus (Loranthaceae). Can J Bot 63: 567–581 [Google Scholar]
- McLuckie J (1924) Studies in parasitism. I. A contribution to the physiology of the genus Cassytha. Proc Linn Soc New South Wales 49: 55–78 [Google Scholar]
- Meijer W, Veldkamp JF (1993) A revision of Mitrastema (Rafflesiaceae). Blumea 38: 221–229 [Google Scholar]
- Melnyk CW (2017) Connecting the plant vasculature to friend or foe. New Phytol 213: 1611–1617 [DOI] [PubMed] [Google Scholar]
- Merckx V, Bidartondo MI, Hynson NA (2009) Myco-heterotrophy: when fungi host plants. Ann Bot 104: 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J, Hazzouri KM, Nickrent D, Geisler M, Meyer RS, Pentony MM, Flowers JM, Pelser P, Barcelona J, Inovejas SA, et al. (2014) Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol Biol Evol 31: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TE, Verboom GA, Forest F (2010) Phylogenetics and biogeography of the parasitic genus Thesium L.(Santalaceae), with an emphasis on the Cape of South Africa. Bot J Linn Soc 162: 435–452 [Google Scholar]
- Muir J (1933) The beach drift of South Africa. J Bot Soc South Africa 18: 5–10 [Google Scholar]
- Musselman LJ (1977) Seed germination and seedlings of Krameria lanceolata (Krameriaceae). Sida 7: 224–225 [Google Scholar]
- Musselman LJ, Dickison WC (1975) The structure and development of the haustorium in parasitic Scrophulariaceae. Bot J Linn Soc 70: 183–212 [Google Scholar]
- Musselman LJ, Press MC (1995) Introduction to parasitic plants. In Press MC, Graves JD, eds, Parasitic Plants. Chapman & Hall, London, p 292 [Google Scholar]
- Musselman LJ, Visser JH (1989) Taxonomy and natural history of Hydnora (Hydnoraceae). Aliso 12: 317–326 [Google Scholar]
- Naumann J, Salomo K, Der JP, Wafula EK, Bolin JF, Maass E, Frenzke L, Samain MS, Neinhuis C, DePamphilis CW, et al. (2013) Single-copy nuclear genes place haustorial Hydnoraceae within piperales and reveal a Cretaceous origin of multiple parasitic angiosperm lineages. PLoS One 8: e79204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickrent DL (2020) Parasitic angiosperms: how often and how many? Taxon 69: 5–27 [Google Scholar]
- Nickrent DL (2002) Phylogenetic origins of parasitic plants. Parasitic Plants of the Iberian Peninsula and Balearic Islands, Mundi-Prensa Libros, S. A., Madrid pp 29–56 [Google Scholar]
- Nickrent DL, Blarer A, Qiu YL, Vidal-Russell R, Anderson FE (2004) Phylogenetic inference in Rafflesiales: the influence of rate heterogeneity and horizontal gene transfer. BMC Evol Biol 4: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov LA, Davis CC (2017) The big, the bad, and the beautiful: biology of the world’s largest flowers. J Syst Evol 55: 516–524 [Google Scholar]
- Nikolov LA, Tomlinson PB, Manickam S, Endress PK, Kramer EM, Davis CC (2014) Holoparasitic Rafflesiaceae possess the most reduced endophytes and yet give rise to the world’s largest flowers. Ann Bot 114: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton DA, Carpenter MA (1998) Mistletoes as parasites: host specificity and speciation. Trends Ecol Evol 13: 101–105 [DOI] [PubMed] [Google Scholar]
- Oborník M (2019) Endosymbiotic evolution of algae, secondary heterotrophy and parasitism. Biomolecules 9: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubamichael DY, Griffiths ME, Ward D (2016) Host specificity in parasitic plants — perspectives from mistletoes. AoB Plants 8: plw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski M, Dilliott M, García-Ruiz I, Bendarvandi B, Costea M (2020) Cuscuta seeds: diversity and evolution, value for systematics/identification and exploration of allometric relationships. PLoS One 15: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr GL, Haidar MA, Orr DA (1996) Smallseed Dodder (Cuscuta planiflora) Phototropism toward Far-Red When in White Light. Weed Sci 44: 233–240 [Google Scholar]
- Pate JS, Kuo J, Davidson NJ (1990) Morphology and anatomy of the haustorium of the root hemiparasite Olax phyllanthi (Olacaceae), with special reference to the haustorial interface. Ann Bot 65: 425–436 [Google Scholar]
- Pérez-de-Luque A (2013) Haustorium invasion into host tissues. In Joel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae: Parasitism, Mechanisms, and Control Strategies. Springer; Berlin, Heidelberg, Germany, pp 75–86 [Google Scholar]
- Press MC, Shah N, Tuohy JM, Stewart GR (1987) Carbon isotope ratios demonstrate carbon flux from C4 host to C3 parasite. Plant Physiol 85:1143–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle CP, Cannon BC, Faust AL, Hawkins AK, Cabrera SE, Lee S, Lewis ML, Perez AA, Sopas J, Verastegui TJ, et al. (2018) Host cues mediate growth and establishment of oak mistletoe (Phoradendron leucarpum, Viscaceae), an aerial parasitic plant. Castanea 83: 249–262 [Google Scholar]
- Revill MJW, Stanley S, Hibberd JM, Revill MJW (2015) Plastid ability in the parasitic genus Cuscuta genome structure and of pnotosynthetic. J Exp Bot 56: 2477–2486 [DOI] [PubMed] [Google Scholar]
- Richter A, Popp M, Mensen R, Stewart GR, von Willert DJ (1995) Heterotrophic carbon gain of the parasitic angiosperm Tapinanthus oleifolius. Aust J Plant Physiol 22: 537–544 [Google Scholar]
- Rispail N, Prats E, Rubiales D (2006) Plant resistance to parasitic plants: molecular approaches to an old foe. New Phytol 173: 703–712 [DOI] [PubMed] [Google Scholar]
- Rödl T, Ward D (2002) Host recognition in a desert mistletoe: early stages of development are influenced by substrate and host origin. Funct Ecol 16: 128–134 [Google Scholar]
- Room PM (1973) Ecology of the mistletoe tapinanthus bangwensis growing on Cocoa in Ghana. J Ecol 61: 729–742 [Google Scholar]
- Roquet C, Coissac É, Cruaud C, Boleda M, Boyer F, Alberti A, Gielly L, Taberlet P, Thuiller W, Van Es J, et al. (2017) Understanding the evolution of holoparasitic plants: the complete plastid genome of the holoparasite Cytinus hypocistis (Cytinaceae). Ann Bot 118: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon JB, Mescher MC, De Moraes CM (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313: 1964–1967 [DOI] [PubMed] [Google Scholar]
- Rutherford RJ (1970) The anatomy and cytology of Pilostyles Thurberi Gray (Rafflesiaceae). Aliso 7: 263–288 [Google Scholar]
- Sallé G (1983) Germination and establishment of Viscum album L. In Calder MC, Bernhardt P, eds, Biology of Mistletoes. Academic Press, Sydney, pp 145–159 [Google Scholar]
- Sandner TM, Matthies D (2018) Multiple choice: hemiparasite performance in multi-species mixtures. Oikos 127: 1291–1303 [Google Scholar]
- Schrenk H (1894) Parasitism of Epiphegus Virginiana (broom rape, cancer root). Proc Am Microsc Soc 15: 91–127 [Google Scholar]
- Sherman TD, Bowling AJ, Barger TW, Vaughn KC (2008) The Vestigial Root of Dodder (Cuscuta pentagona) Seedlings. Int J Plant Sci 169: 998–1012 [Google Scholar]
- Shivamurthy GR, Arekal D, Swamy BGL (1981) Establishment, structure and morphology of the tuber of balanophora. Ann Bot 47: 735–745 [Google Scholar]
- Simpson B, Weeks A, Helfgott DM, Larkin L (2004) Species relationships in Krameria (Krameriaceae) based on ITS sequences and morphology: implications for character utility and biogeography. Syst Bot 29: 97–108 [Google Scholar]
- Simpson BB (1989) Krameriaceae. Flora Neotropica 49: 1–108 [Google Scholar]
- Sorensen AE (1986) Seed dispersal by adhesion. Annu Rev Ecol Syst 17: 443–463 [Google Scholar]
- Spallek T, Mutuku M, Shirasu K (2013) The genus Striga: a witch profile. Mol Plant Pathol 14: 861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A (2014) Systematics, biology and ecology of New Zealand’s pygmy mistletoes (Korthalsella: Viscaceae). PhD thesis. Massey University, Manawatu, New Zealand
- Sultan A, Robertson AW, Callmander MW, Phillipson PB, Meyer JY, Tate JA (2019) Widespread morphological parallelism in Korthalsella (Santalaceae, tribe Visceae): a molecular phylogenetic perspective. Taxon 68: 1204–1218 [Google Scholar]
- Sultan A, Tate JA, de Lange PJ, Glenny D, Ladley JJ, Heenan P, Robertson AW (2018) Host range, host specificity, regional host preferences and genetic variability of Korthalsella Tiegh. (Viscaceae) mistletoes in New Zealand. New Zeal J Bot 56: 127–162 [Google Scholar]
- Tada Y, Sugai M, Furuhashi K (1996) Haustoria of Cuscuta japonica, a holoparasitic flowering plant, are induced by the cooperative effects of far-red light and tactile stimuli. Plant Cell Physiol 37: 1049–1053 [Google Scholar]
- Teixeira-Costa L (2021) A living bridge between two enemies: haustorium structure and evolution across parasitic flowering plants. Rev Bras Bot 44: 165–178 [Google Scholar]
- Teixeira-Costa L, Davis CC, Ceccantini G (2021) Striking developmental convergence in angiosperm endoparasites. Am J Bot 108: 1–13 [DOI] [PubMed] [Google Scholar]
- Teixeira-Costa L, Ocampo G, Ceccantini G (2020) Morphogenesis and evolution of mistletoes’ haustoria. In Demarco D, ed, Plant Ontology, Nova Science Publishers, Inc., New York, NY, pp 107–157 [Google Scholar]
- Tennakoon KU, Bolin JF, Musselman LJ, Maass E (2007) Structural attributes of the hypogeous holoparasite Hydnora triceps Drège & Meyer (Hydnoraceae). Am J Bot 94: 1439–1449 [DOI] [PubMed] [Google Scholar]
- Tennakoon KU, Pate JS (1996) Heterotrophic gain of carbon from hosts by the xylem-tapping root hemiparasite Olax phyllanthi (Olacaceae). Oecologia 105: 369–376 [DOI] [PubMed] [Google Scholar]
- Těšitel J (2016) Functional biology of parasitic plants: a review. Plant Ecol Evol 149: 5–20 [Google Scholar]
- Těšitel J, Lepš J, Vráblová M, Cameron DD (2011) The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: a physiological perspective. New Phytol 192: 188–199 [DOI] [PubMed] [Google Scholar]
- The Angiosperm Phylogeny Group. (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181: 1–20 [Google Scholar]
- The Angiosperm Phylogeny Group. (2003) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linn Soc 141: 399–436 [Google Scholar]
- Thoday D, Johnson ET (1930) On Arceuthobium pusillum, peck : I. The endophytic system. Ann Bot 44: 393–413 [Google Scholar]
- Toth R, Kuijt J (1977) Anatomy and ultrastructure of the haustorium in Comandra (Santalaceae). Can J Bot 55: 455–469 [Google Scholar]
- Truscott FH (1966) Some aspects of morphogenesis in Cuscuta gronovii. Am J Bot 53: 739–750 [Google Scholar]
- Unger F (1840) Beitrage zur kenntniss der parasitischen pflanzen. Erster oder anatomisch- physiologischer theil. Ann Wiener Museum Naturgesch 2: 13–60 [Google Scholar]
- Vaughn KC (2003) Dodder hyphae invade the host: a structural and immunocytochemical characterization. Protoplasma 220: 189–200 [DOI] [PubMed] [Google Scholar]
- Vaughn KC (2006) Conversion of the searching hyphae of dodder into xylic and phloic hyphae: a cytochemical and immunocytochemical investigation. Int J Plant Sci 167: 1099–1114 [Google Scholar]
- Vidal-Russell R, Nickrent DL (2008) The first mistletoes: origins of aerial parasitism in Santalales. Mol Phylogenet Evol 47: 523–537 [DOI] [PubMed] [Google Scholar]
- Vidal-Russell R, Premoli AC (2015) Nothofagus trees show genotype difference that in fl uence infection by mistletoes, Misodendraceae. Aust J Bot 63: 541–548 [Google Scholar]
- Wakatake T, Ogawa S, Yoshida S, Shirasu K (2020) An auxin transport network underlies xylem bridge formation between the hemi-parasitic plant Phtheirospermum japonicum and host Arabidopsis. Dev 147: 1–12 [DOI] [PubMed] [Google Scholar]
- Wakatake T, Yoshida S, Shirasu K (2018) Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants. Development 145: dev164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K (1936) Morphologisch-biologische Studien uber die Gattung Mitrastemon (II). J Jpn Bot 12: 698–711 [Google Scholar]
- Waters MT, Gutjahr C, Bennett T, Nelson DC (2017) Strigolactone Signaling and Evolution. Annu Rev Plant Biol 68: 291–322 [DOI] [PubMed] [Google Scholar]
- Watson DM (2019) Mistletoes of Southern Australia, 2nd ed. CSIRO Publishing, Clayton, Australia [Google Scholar]
- Watson DM, Cook M, Fadini RF (2020) Towards best-practice management of mistletoes in horticulture. Botany 98: 489–498 [Google Scholar]
- Weber HG (1976) Studies on new types of haustoria in some central European Rhinanthoideae (Scrophulariaceae). Plant Syst Evol 125: 223–232 [Google Scholar]
- Weddell HA (1860) Memoire sur le Cynomorium coccineum, parasite de l’ordre des Balanophorées. Arch du Muséum d’Histoire Nat Paris 10: 269–308 [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15: 227–235 [DOI] [PubMed] [Google Scholar]
- Wicaksono A, Mursidawati S, Molina J (2020) A plant within a plant: insights on the development of the Rafflesia endophyte within its host. Bot Rev 87: 233–242 [Google Scholar]
- Wicke S, Müller KF, de Pamphilis CW, Quandt D, Wickett NJ, Zhang Y, Renner SS, Schneeweiss GM (2013) Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant Cell 25: 3711–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CN (1963) Development of Tapinanthus bangwensis (Engler and Krause) Danser and contact with the host. Ann Bot 27: 641–644 [Google Scholar]
- Wilson CA, Calvin C (2006) Character divergences and convergences in canopy-dwelling Loranthaceae. Bot J Linn Soc 150: 101–113 [Google Scholar]
- Wolfe AD, DePamphilis CW (1998) The Effect of Relaxed Functional Constraints on the Photosynthetic Gene rbcL in Photosynthetic and Nonphotosynthetic Parasitic Plants. Mol Biol Evol 15: 1243–1258 [DOI] [PubMed] [Google Scholar]
- Wolfe AD, Randle CP, Liu L, Steiner KE (2005) Phylogeny and biogeography of Orobanchaceae. Folia Geobot 40: 115–134 [Google Scholar]
- Xiang L, Li Y, Sui X, Li A (2018) Fast and abundant in vitro spontaneous haustorium formation in root hemiparasitic plant Pedicularis kansuensis Maxim (Orobanchaceae). Plant Divers 40: 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatskievych GA, Mason CT Jr (1986) A revision of the Lennoaceae. Syst Bot 11: 531–548 [Google Scholar]
- Yoshida S, Cui S, Ichihashi Y, Shirasu K (2016) The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 67: 643–667 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K (2009) Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol 183: 180–189 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hu JJ, Wen J, Sun H (2019) Morphometric, phylogenetic and biogeographic analyses of Pyrularia (Santalales), a parasitic disjunct lineage between eastern Asia and eastern North America. Taxon 68: 47–71 [Google Scholar]