Abstract
Background
Standard screening of endometrial cancer (EC) for Lynch syndrome (LS) is gaining traction; however, the prognostic impact of an underlying hereditary etiology is unknown. We established the prevalence, prognosis, and subsequent primary cancer incidence of patients with LS-associated EC in relation to sporadic mismatch repair deficient (MMRd)-EC in the large combined Post Operative Radiation Therapy in Endometrial Carcinoma-1, -2, and -3 trial cohort.
Methods
After MMR-immunohistochemistry, MLH1-promoter methylation testing, and next-generation sequencing, tumors were classified into 3 groups according to the molecular cause of their MMRd-EC. Kaplan-Meier method, log-rank test, and Cox model were used for survival analysis. Competing risk analysis was used to estimate the subsequent cancer probability. All statistical tests were 2-sided.
Results
Among the 1336 ECs, 410 (30.7%) were MMRd. A total of 380 (92.7%) were fully triaged: 275 (72.4%) were MLH1-hypermethylated MMRd-ECs; 36 (9.5%) LS MMRd-ECs, and 69 (18.2%) MMRd-ECs due to other causes. Limiting screening of EC patients to 60 years or younger or to 70 years or younger would have resulted in missing 18 (50.0%) and 6 (16.7%) LS diagnoses, respectively. Five-year recurrence-free survival was 91.7% (95% confidence interval [CI] = 83.1% to 100%; hazard ratio = 0.45, 95% CI = 0.16 to 1.24, P = .12) for LS, 95.5% (95% CI = 90.7% to 100%; hazard ratio = 0.17, 95% CI = 0.05 to 0.55, P = .003) for “other” vs 78.6% (95% CI = 73.8% to 83.7%) for MLH1-hypermethylated MMRd-EC. The probability of subsequent LS-associated cancer at 10 years was 11.6% (95% CI = 0.0% to 24.7%), 1.5% (95% CI = 0.0% to 4.3%), and 7.0% (95% CI = 3.0% to 10.9%) within the LS, “other,” and MLH1-hypermethylated MMRd-EC groups, respectively.
Conclusions
The LS prevalence in the Post Operative Radiation Therapy in Endometrial Carcinoma trial population was 2.8% and among MMRd-ECs was 9.5%. Patients with LS-associated ECs showed a trend towards better recurrence-free survival and higher risk for second cancers compared with patients with MLH1-hypermethylated MMRd-EC.
The diagnosis of Lynch syndrome (LS) in endometrial cancer (EC) is crucial for counseling and cancer surveillance of patients and their relatives. LS is a highly penetrant, hereditary, cancer-prone syndrome caused by germline variants in the DNA mismatch repair (MMR) genes: mutL homologue 1 (MLH1), mutS homologue 2 (MSH2), mutS homologue 6 (MSH6), or postmeiotic segregation increased 2 (PMS2). The cancer risk varies per gene and is substantially lower for PMS2 (1,2). EC is often the first malignancy affecting women with LS (3), and their risk of metachronous cancer is approximately 24% at 10 years (4).
LS-associated cancers arise following MMR deficiency (MMRd) due to the somatic inactivation of the remaining wild-type MMR allele. MMRd leads to the accumulation of mismatches, insertions, and deletions in repeated sequences also known as microsatellite instability (MSI). MMRd is not an exclusive feature of LS; the vast majority (about 70%) of MMRd-ECs present with somatic inactivation of the MLH1 gene via hypermethylation of the promoter region (5,6). Most of the cases that are neither MLH1 hypermethylated nor harbor a MMR germline variant are considered sporadic due to biallelic somatic MMR gene inactivation; few are caused by an undetectable hereditary syndrome (frequently referred to as Lynch-like syndrome) (7-9). MMRd-ECs are known to have an intermediate prognosis within the molecular classification with a good response to immunotherapy (10‐13). The diagnosis of LS may allow clinicians to tailor treatment and patient information; LS-associated tumors may have a more favorable outcome (14), although there are no previous studies available on the prognostic impact of LS among MMRd-ECs.
Tumor triage by MMR-immunohistochemistry (IHC) and/or MSI analysis in combination with targeted MLH1-methylation testing can identify patients with LS. The Proportion of Endometrial Tumours Associated Lynch Syndrome study showed that IHC-based triage is most accurate, whereas clinical selection based on age and family history were imprecise predictors (15). Overall, an estimated 3% of EC cases are associated with LS (15‐17), which is similar in colorectal cancer (CRC) (18). However, these estimations were mostly based on small trials with methodological heterogeneity, often selecting their test population by age and/or family history, and incomplete testing (16).
Given its relative rarity, the prevalence and prognosis of LS should be investigated in a large population, such as the well-documented combined cohort of the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC)-1, -2, and -3 trials. These randomized controlled trials have had a major impact on guidelines for treatment in ECs (19‐21). Together they included 1336 evaluable patients comprising all risk groups with long and complete follow-up information and collected tumor blocks. The aim of our study was to investigate the prevalence and prognosis of LS-associated EC in relation to MLH1 hypermethylated MMRd-EC. Secondary objectives were to evaluate currently used age criteria for IHC-based tumor triage and the probability of developing a subsequent primary LS-associated cancer.
Methods
Study Population
In total, 1336 of 1801 ECs from the PORTEC-1, -2, and -3 clinical trials were eligible for analysis based on availability of formalin-fixed paraffin-embedded (FFPE) slides. In the PORTEC-1 trial (1990-1997), 714 patients with stage I low-intermediate and high-intermediate risk endometrioid EC were randomly assigned to receive pelvic radiotherapy or no additional treatment (19). In the PORTEC-2 trial (2002-2006), 427 endometrioid EC patients with high-intermediate risk features were randomly assigned to receive pelvic radiotherapy or vaginal brachytherapy (if stage I: ≥60 years) (20). In the international PORTEC-3 trial (2006-2013), 660 EC patients with high-risk features were randomly assigned to receive pelvic radiotherapy or chemoradiotherapy (21). In all trials, patients with a history of invasive cancer (for PORTEC-3 within the last 10 years), except for nonmelanoma skin cancer, were excluded. Full details and results of these trials have been published previously (19‐21). The study protocols were approved by the Dutch Cancer Society and the medical ethics committees at participating centers. All patients provided informed consent for participation in the trial, and for use of their tumor block for subsequent translational research. Clinicopathological data including p53-IHC and POLE-mutation status were obtained from the trial databases. Specific ethics approval was obtained for variant analysis on normal tissue among those suspected of LS. Cases from PORTEC-1 and -2 were analyzed anonymized in view of the long interval since recruitment. Cases from PORTEC-3 who were found to have LS were informed by their own physicians if LS had not been already diagnosed clinically. PORTEC-1 was conducted before time of trial registries. PORTEC-2 is registered with ISRCTN number ISRCTN16228756, and ClinicalTrials.gov number NCT00376844. PORTEC-3 is registered with ISRCTN number ISRCTN14387080, and ClinicalTrials.gov number NCT00411138.
IHC, MSI, Methylation Analysis, and Next-Generation Sequencing (NGS)
Patients were included in the current analysis if they showed loss of expression of at least 1 of the 4 MMR proteins with positive internal control (including subclonal loss defined as abrupt and complete regional loss with intervening stromal positivity) or MSI-high status when MMR-IHC failed. Details on MMR-IHC and MSI testing and scoring were described previously (5,11,12,22). Cases with MMRd phenotype are referred to as MMRd-EC in this study irrespective of POLE mutation status.
MLH1 methylation testing was performed on MLH1-deficient and/or MSI-high tumors as described previously (23). All cases with loss of MLH1 or MSI-high status without MLH1 hypermethylation; loss of MSH2 and/or MSH6; or isolated loss of PMS2 were triaged as potential LS-associated MMRd-EC. DNA isolated from matched normal/tumor FFPE tissues of these cases was amplified using long-range polymerase chain reaction followed by targeted NGS for variants in the exonic regions of MLH1, MSH2, MSH6, PMS2, POLE, and POLD1 using the Ion Proton System or Ion S5 System (Thermo Fisher Scientific) (24,25). Variants were annotated according to the following GenBank reference sequences: NM_000249.3 (MLH1), NM_000251.2 (MSH2), NM_000179.2 (MSH6), NM_000535.5 (PMS2), NM_006231.2 (POLE), and NM_001256849.1 (POLD1). All patients with germline variants (likely) affecting function (path_MMR) were verified by a clinical laboratory geneticist (C.M.T.) and considered to have LS.
Statistical Analysis
Following complete triage, cases were classified into 3 groups according to the molecular cause of their MMRd-EC: LS, methylated (including cases with MLH1 hypermethylation and subclonal MLH1 loss), and other causes (a mixed group having alternative causes of MMRd; see the Supplementary Methods and Supplementary Figure 1 for full definitions, available online). χ2 Statistics or Fisher’s exact test for categorical variables and 1-way analysis of variance or Kruskal-Wallis test for continuous variables were used to compare characteristics.
The sample size ensured sufficient power to detect an LS prevalence of 3.0% with a precision of 0.009 (95% confidence interval [CI] = 2.1% to 3.9%) within the whole population and a prevalence of 12.0% with a precision of 0.03 (95% CI = 9.0% to 15.0%) within the MMRd group (26).
Recurrence-free survival (RFS) was defined as time from random assignment to date of first relapse or death of any cause, whichever occurred first. Overall survival (OS) was defined as time from random assignment to date of death of any cause. Patients without an RFS or OS event were censored at the date of last contact. Five-year survival rates were estimated using the Kaplan-Meier method and compared with log-rank test. Cox proportional hazard models were used to estimate hazard ratios (HRs) over time; for adjusted analysis, age was included as covariate. The proportional hazard assumption was verified using Schoenfeld residuals. A competing-risk model with death as a competing event was used to estimate the cumulative incidence of developing a LS-associated second primary cancer (ie, colorectal, gallbladder, kidney, pancreas, small intestine, stomach, urinary bladder, and ureter cancer) in the different groups. A cause-specific Cox proportional hazard model was used to assess the statistical difference between the estimated probabilities. Time at risk started at random assignment and ended at date of occurrence of the first second cancer, death, or last date of study follow-up. P values less than .05 (2-tailed) were considered statistically significant. Statistical analyses were performed using R version 3.6.1.
Results
Study Population
Among the 1336 evaluable ECs, 410 (30.7%) were MMRd and eligible for further analysis. Median age of MMRd-EC patients was 65 years (interquartile range = 59-73 years). Most MMRd-ECs were early-stage tumors (74.2%) of low-grade endometrioid subtype (66.8%) and were treated with pelvic radiotherapy (51.7%). All characteristics of MMRd-ECs differed between the 3 PORTEC trials, in line with the inclusion criteria (Table 1).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | All MMRd-EC | PORTEC-1 | PORTEC-2 | PORTEC-3 | P a |
|---|---|---|---|---|---|
| Total, No. (%) | 410 (100.0) | 145 (35.6) | 114 (27.8) | 151 (36.8) | |
| Age at random assignment | <.001 | ||||
| Median (IQR), y | 65 (59-73) | 67 (61-73) | 70 (65-77) | 60 (56-66) | |
| FIGO 2009 stage, No. (%) | <.001 | ||||
| IA | 104 (25.4) | 62 (42.8) | 25 (21.9) | 17 (11.3) | |
| IB | 200 (48.8) | 83 (57.2) | 87 (76.3) | 30 (19.9) | |
| II | 36 (8.8) | 0 (0.0) | 1 (0.9) | 35 (23.2) | |
| III | 70 (17.1) | 0 (0.0) | 1 (0.9) | 69 (45.7) | |
| Histological grade and type, No. (%) | <.001 | ||||
| EEC grade 1 or 2 | 274 (66.8) | 122 (84.1) | 91 (79.8) | 61 (40.4) | |
| EEC grade 3 | 99 (24.1) | 22 (15.2) | 21 (18.4) | 56 (37.1) | |
| Serous | 11 (2.7) | 1 (0.7) | 2 (1.8) | 8 (5.3) | |
| Clear cell | 12 (2.9) | 0 (0.0) | 0 (0.0) | 12 (7.9) | |
| Other | 14 (3.4) | 0 (0.0) | 0 (0.0) | 14 (9.3) | |
| Myometrial invasion, No. (%) | .001 | ||||
| ≥50% | 274 (66.8) | 83 (57.2) | 90 (78.9) | 101 (66.9) | |
| Lymphovascular space invasion, No. (%) | <.001 | ||||
| Present | 131 (32.0) | 13 (9.0) | 16 (14.0) | 102 (67.5) | |
| Received adjuvant treatment, No. (%) | <.001 | ||||
| No treatment | 73 (17.8) | 71 (49.0) | 2 (1.8) | 0 (0.0) | |
| External beam radiotherapy | 212 (51.7) | 74 (51.0) | 58 (50.9) | 80 (53.0) | |
| Vaginal brachytherapy | 54 (13.2) | 0 (0.0) | 54 (47.4) | 0 (0.0) | |
| Chemoradiotherapy | 71 (17.3) | 0 (0.0) | 0 (0.0) | 71 (47.0) |
P values reflect χ2 statistics or Fisher’s exact test for categorical variables and Kruskal-Wallis test for age. EC = endometrial cancer; EEC = endometrioid endometrial cancer; FIGO = International Federation of Gynecology and Obstetrics; IQR = interquartile range; MMRd = mismatch repair deficient; PORTEC = Post Operative Radiation Therapy in Endometrial Carcinoma.
MMR Causes and Variant Analysis
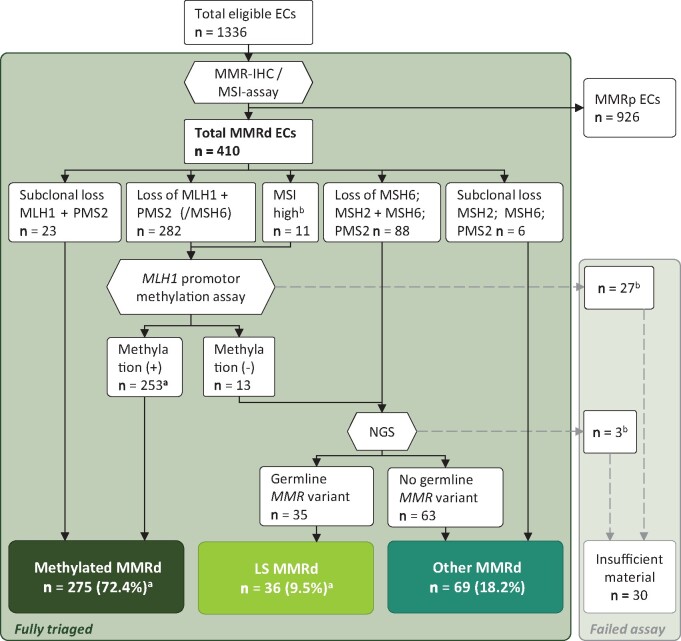
Complete triage was accomplished for 380 (92.7%) of the MMRd-ECs (Figure 1; insufficient material in 27 cases for MLH1 methylation assay and 3 for NGS). Thirty-six path_MMR variant carriers were identified, giving a 2.8% LS prevalence in the overall population and a 9.5% LS prevalence within the MMRd group. There were 18 path_MSH6, 10 path_PMS2, 6 path_MSH2, and 2 path_MLH1 variant carriers. An overview of the LS cases is displayed in Table 2. In total, 275 (72.4%) cases were classified as methylated. The remaining 69 (18.2%) MMRd cases were neither LS nor MLH1 hypermethylated and were therefore classified as “other.”
Figure 1.
Flowchart. aOne case with MLH1 promoter hypermethylation in the tumor carried a germline MLH1 variant. bInsufficient material for assay. EC = endometrial cancer; LS = Lynch syndrome; Methylation (+) = MLH1 promoter hypermethylation; Methylation (−) = no MLH1 promoter hypermethylation; MMR = mismatch repair; MMRd = mismatch repair deficient; MMRp = mismatch repair proficient; MSI = microsatellite instability; NGS = next-generation sequencing.
Table 2.
Patient and tumor characteristics of proven LS-associated ECs
| No. | Study | Age, y | FIGO (2009) | Histotype | Grade | Molecular class by TCGA surrogate | Affected MMR proteins | MLH1 promoter methylation | Gene | Coding DNA mutation | Amino acid change | Classa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PORTEC-1 | 47 | IB | Endometrioid | G2 | MMRd | MSH2 + MSH6 | NA | MSH2 | c.1351C>T | p.(Gln451*) | 5 |
| 2 | PORTEC-1 | 67 | IB | Endometrioid | G1 | MMRd | MSH2 + MSH6 | NA | MSH2 | c.363T>G | p.(Tyr121*) | 5 |
| 3 | PORTEC-1 | 52 | IA | Endometrioid | G3 | MMRd | MSH2 + MSH6 | NA | MSH2 | c.646-2A>G | p.(?) | 4 |
| 4 | PORTEC-1 | 54 | IA | Endometrioid | G1 | MMRd | MSH2 + MSH6 | NA | MSH2 | c.2458 + 1G>A | p.(?) | 4 |
| 5 | PORTEC-3 | 48 | IIIC | Endometrioid | G2 | MMRd | MSH2 + MSH6 | NA | MSH2 | c.1285C>T | p.(Gln429*) | 5 |
| 6 | PORTEC-3 | 37 | IIIC | Clear cell | G2 | MMRd | MSH2 + MSH6 | NA | MSH2 b | NA | NA | 5 |
| 7 | PORTEC-3 | 59 | IA | Clear cell | G3 | MMRd | MSH2 + MSH6 | NA | MSH6 | c.3188T>G | p.(leu1063Arg) | 5 |
| 8 | PORTEC-1 | 56 | IA | Serous | G3 | MMRd-p53abn | MSH2 subclonal + MSH6 | NA | MSH6 | c.1784delT | p.(Leu595Tyrfs*15) | 5 |
| 9 | PORTEC-1 | 67 | IB | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.1189_1190insTT | p.(Tyr397Phefs*15) | 5 |
| 10 | PORTEC-1 | 58 | IA | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.642C>A | p.(Tyr214*) | 5 |
| 11 | PORTEC-2 | 67 | IB | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.2764C>T | p.(Arg922*) | 5 |
| 12 | PORTEC-2 | 66 | IB | Endometrioid | G3 | MMRd | MSH6 | NA | MSH6 | c.1483C>T | p.(Arg495*) | 5 |
| 13 | PORTEC-2 | 73 | IB | Endometrioid | G1 | MMRd-p53abn | MSH6 | NA | MSH6 | c.1628_1629delAA | p.(Lys543Argfs*19) | 5 |
| 14 | PORTEC-2 | 82 | IIIA | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.3729_3732dupATTA | p.(Phe1245Ilefs*31) | 5 |
| 15 | PORTEC-2 | 71 | IB | Endometrioid | G2 | MMRd-p53abn | MSH6 | NA | MSH6 | c.2719_2720delGT | p.(Val907Argfs*10) | 5 |
| 16 | PORTEC-3 | 51 | IIIA | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.3477C>A | p.(Tyr1159*) | 5 |
| 17 | PORTEC-3 | 55 | IIIC | Endometrioid | G3 | MMRd-p53abn | MSH6 | NA | MSH6 | c.2906_2907delAT | p.(Tyr969Leufs*5) | 5 |
| 18 | PORTEC-3 | 61 | IB | Clear cell | G3 | MMRd | MSH6 | NA | MSH6 | c.3838C>T | p.(Gln1280*) | 5 |
| 19 | PORTEC-3 | 68 | IIIA | Endometrioid | G1 | MMRd | MSH6 | NA | MSH6 | c.467C>G | p.(Ser156*) | 5 |
| 20 | PORTEC-3 | 59 | IB | Serous | G3 | MMRd-p53abn | MSH6 | NA | MSH6 |
c.3527_3549delGACTTG GTGCCTCAGACAGAATA |
p.(Arg1176Asnfs*4) | 5 |
| 21 | PORTEC-3 | 60 | IA | Serous | G3 | POLEmut-MMRd | MSH6 | NA | MSH6 | c.2342dupC | p.(Leu782Thrfs*3) | 5 |
| 22 | PORTEC-3 | 59 | IB | Clear cell | G3 | MMRd | MSH6 | NA | MSH6 | c.3863_3865dupAAT | p.(Phe1289*) | 5 |
| 23 | PORTEC-3 | 76 | IB | Serous | G3 | MMRd | MSH6 | NA | MSH6 | c.3847_3850dupATTA | p.(Thr1284Asnfs*6) | 5 |
| 24 | PORTEC-3 | 74 | IA | Serous | G3 | MMRd-p53abn | MSH6 | NA | MSH6 | c.10C>T | p.(Gln4*) | 4 |
| 25 | PORTEC-1 | 57 | IB | Endometrioid | G3 | MMRd | PMS2 | NA | PMS2 | c.1882C>T | p.(Arg628*) | 5 |
| 26 | PORTEC-1 | 66 | IB | Endometrioid | G1 | MMRd | PMS2 | NA | PMS2 | c.1882C>T | p.(Arg628*) | 5 |
| 27 | PORTEC-1 | 64 | IB | Endometrioid | G3 | MMRd-p53abn | PMS2 | NA | PMS2 | c.247_250dupTTAA | p.(Thr84Ilefs*9) | 5 |
| 28 | PORTEC-1 | 65 | IB | Endometrioid | G1 | MMRd | PMS2 | NA | PMS2 | c.1261C>T | p.(Arg421*) | 5 |
| 29 | PORTEC-2 | 61 | IB | Endometrioid | G1 | MMRd | PMS2 | NA | PMS2 | c.904_911delGTCTGCAG | p.(Val302Thrfs*4) | 5 |
| 30 | PORTEC-2 | 61 | IB | Endometrioid | G3 | MMRd | PMS2 | NA | PMS2 | c.1831dupA | p.(Ile611Asnfs*2) | 5 |
| 31 | PORTEC-2 | 78 | IB | Endometrioid | G1 | POLEmut-MMRd | PMS2 | NA | PMS2 | c.1882C>T | p.(Arg628*) | 5 |
| 32 | PORTEC-2 | 62 | IB | Endometrioid | G2 | MMRd | PMS2 | NA | PMS2 | c.904_911delGTCTGCAG | p.(Val302Thrfs*4) | 5 |
| 33 | PORTEC-3 | 54 | IB | Endometrioid | G3 | MMRd | PMS2 | NA | PMS2 | c.137G>T | p.(Ser46Ile) | 5 |
| 34 | PORTEC-3 | 48 | II | Endometrioid | G3 | MMRd | PMS2 | NA | PMS2 | c.989-2A>G | p.(Glu330_Glu381del) | 4 |
| 35 | PORTEC-3 | 52 | IIIC | Endometrioid | G2 | Not classified | MLH1 + PMS2 | Methylated | MLH1 | c.794G>C | p.(Arg265Pro) | 4 |
| 36 | PORTEC-1 | 48 | IB | Endometrioid | G1 | MMRd | MSI-highc | Unmethylated | MLH1 | c.806C>G | p.(Ser269*) | 5 |
Classification according to the 5-tiered InSiGHT rules: class 5 is pathogenic and class 4 is likely pathogenic. G = grade; IHC = immunohistochemistry; LS = Lynch syndrome; MMRd = mismatch repair deficient; NA = not available; p53abn = p53 abnormal; POLEmut = POLE-ultramutated; PORTEC = Post Operative Radiation Therapy in Endometrial Carcinoma; TCGA = The Cancer Genome Atlas.
Loss-of-function variant in MSH2 gene identified by genetic testing (clinical data) but insufficient material for normal tissue next-generation sequencing.
No material for MLH1 and PMS2 IHC.
LS patients were younger, with a median age of 60 years (interquartile range = 54-67 years) and more often had p53 aberrant staining (20.0%) and serous (13.9%) or clear cell (8.3%) histology compared with the patients with methylated MMRd-EC (Table 3). Limiting screening of EC patients to age 50 years or younger, 60 years or younger, and 70 years or younger would have missed 31 (86.1%), 18 (50.0%), and 6 (16.7%) LS diagnoses, respectively. Figure 2 displays the distribution of the involved MMR proteins; all LS cases identified by the 4-panel approach would also have been identified by a 2-panel approach including only PMS2- and MSH6-IHC. No germline POLE/POLD1 variants affecting function were identified. LS patients with path_MSH6 and path_PMS2 variants were older than those with path_MLH1 and path_MSH2 variants (median age = 63, 62, 50, and 50 years, respectively, P = .01; Supplementary Table 1, available online).
Table 3.
Patient, tumor, and treatment characteristics according to the molecular cause of their MMRd-EC
| Characteristic | All MMRd-EC | Methylated | Other | LS | P a |
|---|---|---|---|---|---|
| Total, No. (%) | 410b | 275 (72.4) | 69 (18.2) | 36 (9.5) | |
| Age at random assignment | <.001 | ||||
| Median (IQR), y | 65 (59-73) | 67 (62-74) | 59 (55-66) | 60 (54-67) | |
| Trial, No. (%) | .002 | ||||
| PORTEC-1 | 145 (35.4) | 99 (36.0) | 22 (31.9) | 12 (33.3) | |
| PORTEC-2 | 114 (27.8) | 87 (31.6) | 8 (11.6) | 9 (25.0) | |
| PORTEC-3 | 151 (36.8) | 89 (32.4) | 39 (56.5) | 15 (41.7) | |
| FIGO 2009 stage, No. (%) | .20 | ||||
| IA | 104 (25.4) | 70 (25.5) | 17 (24.6) | 7 (19.4) | |
| IB | 200 (48.8) | 137 (49.8) | 27 (39.1) | 21 (58.3) | |
| II | 36 (8.8) | 22 (8.0) | 11 (15.9) | 1 (2.8) | |
| III | 70 (17.1) | 46 (16.7) | 14 (20.3) | 7 (19.4) | |
| Histological grade and type, No. (%) | <.001 | ||||
| EEC grade 1 or 2 | 274 (66.8) | 197 (71.6) | 40 (58.0) | 19 (52.8) | |
| EEC grade 3 | 99 (24.1) | 64 (23.3) | 18 (26.1) | 8 (22.2) | |
| Serous | 11 (2.7) | 2 (0.7) | 4 (5.8) | 5 (13.9) | |
| Clear cell | 12 (2.9) | 2 (0.7) | 6 (8.7) | 3 (8.3) | |
| Other | 14 (3.4) | 10 (3.6) | 1 (1.4) | 1 (2.8) | |
| Myometrial invasion, No. (%) | .41 | ||||
| >50% | 274 (66.8) | 187 (68.0) | 43 (62.3) | 27 (75.0) | |
| Lymphovascular space invasion, No. (%) | .96 | ||||
| Present | 131 (32.0) | 90 (32.7) | 23 (33.3) | 11 (30.6) | |
| POLEmut in tumor, No. (%) | .002 | ||||
| Exonuclease domain mutations | 19 (4.7) | 8 (2.9) | 9 (13.4) | 2 (5.7) | |
| p53 immunohistochemistry, No. (%) | <.001 | ||||
| Aberrant | 31 (7.7) | 7 (2.6) | 14 (20.9) | 7 (20.0) | |
| Received adjuvant treatment, No. (%) | .10 | ||||
| No treatment | 73 (17.8) | 47 (17.1) | 10 (14.5) | 9 (25.0) | |
| External beam radiotherapy | 212 (51.7) | 145 (52.7) | 40 (58.0) | 13 (36.1) | |
| Vaginal brachytherapy | 54 (13.2) | 39 (14.2) | 3 (4.3) | 6 (16.7) | |
| Chemoradiotherapy | 71 (17.3) | 44 (16.0) | 16 (23.2) | 8 (22.2) |
P values reflect χ2 statistics or Fisher’s exact test for categorical variables and Kruskal-Wallis test for age. EC = endometrial cancer; EEC = endometrioid endometrial cancer; FIGO = International Federation of Gynecology and Obstetrics; IQR = interquartile range; MMRd = mismatch repair deficient; POLEmut = POLE-ultramutated; PORTEC = Post Operative Radiation Therapy in Endometrial Carcinoma.
All MMRd-ECs including those with insufficient material for MLH1-methylation assay (n = 27) and normal tissue next-generation sequencing (n = 3).
Figure 2.
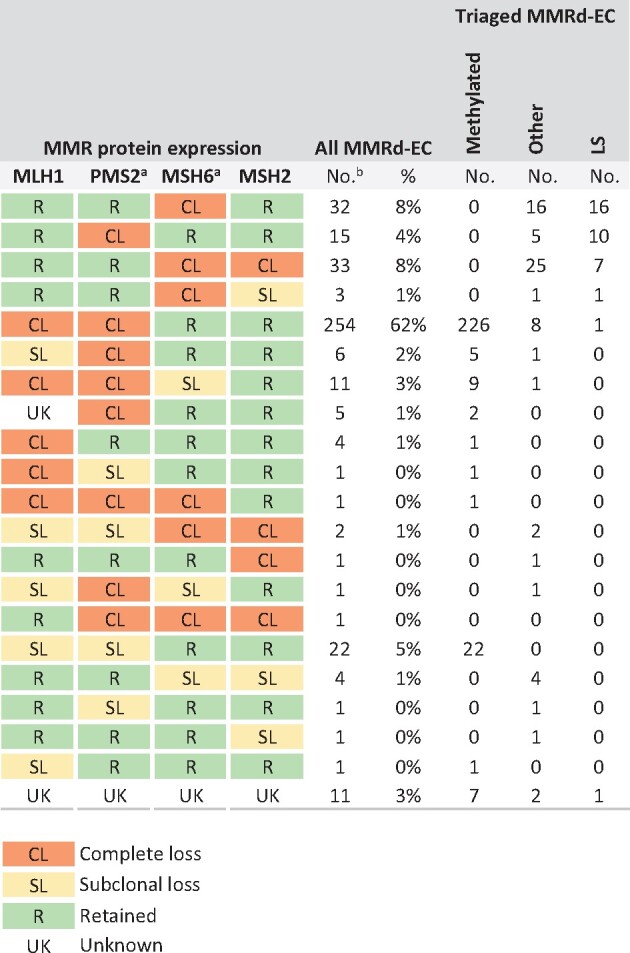
Details on the mismatch repair (MMR) protein expression according to the molecular cause of their MMR-deficient endometrial cancer (MMRd-EC). MMR protein expression was scored as following: complete loss (CL), retained (R), subclonal loss (SL), unknown/failed (UK). aThe concordance of these 2 columns shows that a 2-antibody (MSH6 and PMS2) panel is as sensitive as the full panel to detect Lynch syndrome (LS). bAll MMRd-ECs including those with insufficient material for MLH1 methylation assay (n = 27) and next-generation sequencing (n = 3).
Survival
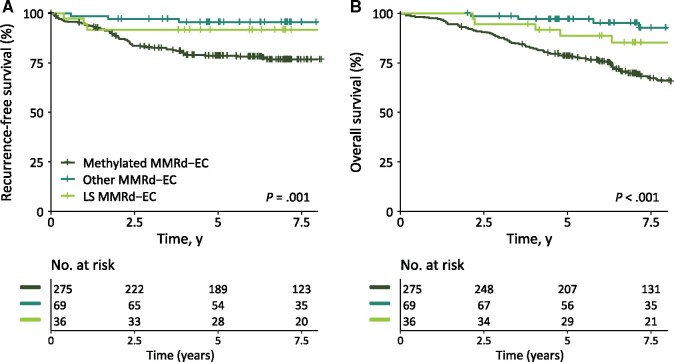
The estimated RFS for the MMRd population at 5 years was 83.7% (95% CI = 80.1% to 87.4%): 91.7% (95% CI = 83.1% to 100%) for patients with LS-associated MMRd-EC, 78.6% (95% CI = 73.8% to 83.7%) for patients with methylated MMRd-EC, and 95.5% (95% CI = 90.7% to 100%) for patients with other causes of MMRd-EC (P = .001; Figure 3, A; LS vs methylated: HR = 0.45, 95% CI = 0.16 to 1.24, P = .12; other vs methylated: HR = 0.17, 95% CI = 0.05 to 0.55, P = .003).
Figure 3.
Kaplan-Meier survival curves for recurrence-free survival (A) and overall survival (B) for patients with methylated mismatch repair deficient (MMRd), other MMRd and Lynch syndrome (LS) associated MMRd endometrial cancer (EC). All cases with MMRd phenotype are included in this analysis, including cases with a concurrent POLE variant affecting function (POLEmut-MMRd-EC). P values reflect 2-sided log-rank test.
The estimated OS for the MMRd population at 5 years was 82.8% (95% CI = 79.2% to 86.5%): 88.5% (95% CI = 78.5% to 99.8%) for patients with LS-associated MMRd-EC, 78.5% (95% CI = 73.7% to 83.5%) for patients with methylated MMRd-EC, and 97.0% (95% CI = 93.0% to 100%) for patients with other causes of MMRd-EC (P < .001; Figure 3, B; LS vs methylated: HR = 0.50, 95% CI = 0.24 to 1.02, P = .06; other vs methylated: HR = 0.27, 95% CI = 0.13 to 0.55, P < .001). After adjustment for age, the trend for better OS in the LS group was no longer observed (vs methylated MMRd-EC: HR = 0.73, 95% CI = 0.35 to 1.52, P = .40), whereas age and having another cause of MMRd were statistically significant prognostic factors (HR = 1.07, 95% CI = 1.04 to 1.09, P < .001; other vs methylated MMRd-EC: HR = 0.41, 95% CI = 0.20 to 0.85, P = .02).
Second Primary Cancers
At 10 years, the cumulative incidence of developing a second LS-associated tumor was 11.6% (95% CI = 0.0% to 24.7%) among EC patients with LS, 1.5% (95% CI = 0.0% to 4.3%) among patients with other MMRd-EC, and 7.0% (95% CI = 3.0% to 10.9%) among patients with methylated MMRd-EC (Supplementary Figure 2, available online). Three of the 4 LS-patients who developed a second primary LS-associated cancer had colon cancer (after 3.8, 4.8, and 14.9 years) and 1 had ureteral cancer (after 8.0 years; Supplementary Table 2, available online, shows cancer type distribution). The cause-specific hazard ratio for developing an LS-associated second cancer was 1.9 (95% CI = 0.63 to 5.7, P = .26) for patients with LS vs patients with methylated MMRd-EC.
Discussion
After complete IHC-based tumor triage, we found a 2.8% prevalence of LS in 1 of the largest EC trial populations worldwide, comprising all risk groups with long and complete follow-up. The prevalence of LS among patients with MMRd-EC was 9.5%. Patients with LS were relatively young, but restricted testing to women who are 60 years or younger would have missed one-half of the cases. Patients with LS tend to have a better RFS and a higher risk of developing second primary cancers compared with patients with methylated MMRd-ECs. No trend for more favorable OS was found after adjustment for age.
This is the first study to our knowledge investigating the prognostic value of LS within the MMRd-EC subgroup. Most of the recent research showed that MMRd-ECs, predominantly driven by the large number of MLH1 hypermethylated cases, have an intermediate prognosis within the molecular classification introduced by The Cancer Genome Atlas (10‐12). Our survival analysis showed that EC patients with LS tend to have a better RFS than patients with methylated MMRd-EC (HR = 0.45, P = .12), whereas LS had no statistically significant prognostic value for OS after adjustment for age (age-adjusted HR = 0.73, P = .40). The favorable prognosis has been assumed to be induced by the active local immune response (14,27). Comparable survival analysis in CRC has been published. One study showing a better OS for 85 CRC patients with LS compared with 67 sporadic MMRd patients after adjustment for age, stage, and BRAF status (HR = 0.29, 95% CI = 0.09 to 0.95, P = .04) (28). The other study also showing better OS in 37 CRC patients with LS compared with 106 methylated MMRd patients, although the difference was minimal after adjusting for age and stage (29).
The cumulative incidence for developing a second LS-associated cancer at 10 years was 11.6% (95% CI = 0.0% to 24.7%) for patients with LS vs 7.0% (95% CI = 3.0% to 10.9%) for patients with methylated MMRd-EC (HR = 1.90, 95% CI = 0.63 to 5.7, P = .26). Our analysis was underpowered due to the small number of events in the LS group. Nevertheless, the elevated risk strengthens previous reports on subsequent cancers in EC or non-CRC LS patients (15%-24%) (4,30) and is of importance for surveillance strategies.
The 2.8% prevalence of LS-EC is consistent with previous publications in which prevalences of 2.8%-3.2% were reported (15‐17). This prevalence is likely a slight underestimation. Firstly, our NGS panel did not include EPCAM and could not detect large rearrangements. To detect large rearrangement in EPCAM or the MMR genes, Multiplex Ligation-dependent Probe Amplification is most commonly used but performs poorly on FFPE tissue. Secondly, the patient selection in our trial design may have affected the prevalence. Patients younger than 60 years with stage I ECs were excluded from the PORTEC-2 trial. Nevertheless, the total PORTEC population deviates minimally from the general EC population as suggested by the similar age in the Proportion of Endometrial Tumours Associated Lynch Syndrome study, an unselected, prospective, cross-sectional study in the United Kingdom among 500 EC patients (15). Moreover, patients with a history of cancer were excluded from the PORTEC trials. The PORTEC population represents women with EC as their sentinel LS-associated malignancy, which is the case in more than one-half of those women with LS who develop cancer (3). Although this selection has potentially led to a slight underestimation of the prevalence of LS in EC, it does represent the patients in which LS could be detected by IHC-based tumor triage. The recently published meta-analysis by Ryan et al. (16) included mostly small trials with methodological heterogeneity, often selecting their test population by age and/or family history, and incomplete testing; only 1 publication included over 1000 ECs, but germline testing was limited to the minority of the triaged potential LS cases (6). Our study is the first with LS testing in an EC population consisting of more than 1000 women with almost complete MMR-IHC, targeted MLH1 methylation testing, and MMR germline testing, making our estimates more reliable.
The path_MSH6 carrier rate of 50.0% among the PORTEC patients with LS is consistent with LS testing results in other unselected EC populations (15,17), but it is remarkably high compared with LS registry data. Only 13% of path_MMR carriers in the clinically selected Prospective Lynch Syndrome Database bear path_MSH6 [1]. As mentioned above, our cohort represents patients with EC as their sentinel cancer likely to induce a lower frequency of path_MLH1 and path_MSH2. Moreover, it must be considered that most of our participants were Dutch, and the path_MSH6 rate of 30% among the Dutch LS registry patients was relatively high compared with the overall Prospective Lynch Syndrome Database (31). Lastly, path_MSH6 families are not identified efficiently by current clinical criteria for LS (32) due to the later age of onset of CRC, incomplete penetrance, and a higher risk and later age of onset of EC (1,33‐35). The same applies to path_PMS2 carriers with a substantially lower cancer risk (1,2,15,16). Correspondingly, the path_MSH6 and path_PMS2 carriers were older than the path_MLH1 and path_MSH2 carriers in our population.
Triage of incident ECs based on IHC with targeted MLH1 methylation testing, as has been adopted widely for CRC, may be a more effective strategy to identify these LS families than age- and family history–based triage. An upper age screening limit would not be recommended, because limiting screening to EC patients who are aged 70 years or younger would have missed 6 (16.7%) LS diagnoses. We confirmed that a 2-antibody panel including MSH6- and PMS2-IHC, with MSH2- or MLH1-IHC only in case of inconclusive staining, is as sensitive as the full panel to detect LS, so this could be a reliable alternative to improve cost-effectiveness (5,36).
A limitation of our study was the lack of germline LS sequencing on the whole study population. Therefore, sensitivity of the IHC-based triage to identify LS patients could not be assessed. Some patients with LS might have been diagnosed before entering the trial, although many were diagnosed after inclusion and had no prior knowledge of the germline mutation.
The diagnosis of LS in EC is crucial for counseling and cancer surveillance even though these patients might be older than those presenting with CRC (18). Moreover, LS screening in incident ECs will have consequences for the patient’s family. Cascade testing of at-risk relatives can identify path_MMR carriers who can benefit from cancer surveillance and risk-reducing treatment (37,38). The clinical impact depends on the gene-specific cancer risk and is substantially lower for path_PMS2 carriers (1,2). Finally, LS identification may have consequences by allowing clinicians to better estimate and explain prognosis and to potentially tailor treatment in the upcoming immunotherapy era (14,27,39).
Further research into the causes of the 63 cases with neither MLH1 hypermethylation nor a MMR germline variant is ongoing. It is hypothesized that the majority will be explained by a sporadic origin through biallelic somatic MMR inactivation (15,40). The determination of a sporadic explanation excludes potential undetectable LS (or Lynch-like syndrome) and will avoid a clinical management dilemma in those cases.
In conclusion, LS was identified using MMR-IHC with targeted MLH1 methylation–based triage in 2.8% of 1336 patients with EC from the combined PORTEC-1, -2, and -3 trials, corresponding to 9.5% of the MMRd tumors. LS was mainly caused by germline variants in the MSH6 and PMS2 genes. Patients with LS-associated ECs showed a trend towards better RFS and higher risk for second primary cancers compared with patients with ECs caused by MLH1 hypermethylation. Besides a prognostic impact, screening all incident ECs without an upper age limit to identify LS using tumor-based triage may benefit counseling, affect treatment decisions, and facilitate prevention strategies for current and future patients and their families.
Funding
This study was supported by the Dutch Cancer Society (UL2012-5719). Translational research was performed on tissue samples from 3 randomized clinical trials (PORTEC-1, PORTEC-2, and PORTEC-3). The trials were supported by grants from the Dutch Cancer Society (CKTO 90–01, CKTO 2001–04, and UL2006-4168/CKTO 2006–04).
Notes
Role of the funder: The Dutch Cancer society had no role in the design and conduct of the study; collection, managements, analysis, and interpretation of the data; or decision to submit the manuscript for publication.
Disclosures: CLC reports grants from Dutch Cancer Society. TB reports grants from Dutch Cancer Society. RN reports grants from Elekta, Varian and Accuray, outside the submitted work. EJC was supported by the National Institute for Health Research Manchester Biomedical Research Centre (IS-BRC-1215–20007). All remaining authors have declared no conflicts of interest.
Author contributions: Conceptualization, CCBP, ES, VTHBMS, RAN, TB, CLC; Methodology, CCBP, ES, RAN, HP, NH, TvW, TB, CLC. Validation, CMT, TvW. Formal Analysis, CCBP, HP, NH; Investigation, CCBP, ES, DR, TAR, TvW. Resources, IMJS, LCHWL, JJJ, RAN, EJC, MEP, LM, AL, CCR. Data curation, CCBP, LV, SMdB, NH; Writing—Orginal Draft, CCBP, TB, CLC; Writing—Review & Editing, CCBP, ES, VTHBMS, DR, CMT, LV, TAR, IMJS, LCHWL, JJJ, RAN, EJC, MEP, LM, AL, PB, HP, SMdB, NH, MN, TvW, TB, CLC. Visualization, CCBP; Supervision, TB, CLC. Funding Acquisition, TB, CLC.
Prior presentation: The study was presented in part at 2020 International Gynecologic Cancer Society xDigital Annual Global Meeting (September 10-12, 2020).
Acknowledgements: We thank all clinical and pathology teams at participating sites of the PORTEC and TransPORTEC study groups, as well as the women who participated in the trials and their families. We thank Alicia León-Castillo, Natalja T. ter Haar, Michelle Osse and Enno J. Dreef (Leiden University Medical Center) for excellent technical support, and Lisette M. Wiltink (Leiden University Medical Center) for verification of the second cancers in PORTEC-1 and -2 trials in the Dutch Pathology Registry (PALGA). We also thank Karen Verhoeven-Adema (Comprehensive Cancer Center, the Netherlands) for her dedicated work as central PORTEC-3 data manager and trial coordinator.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1.Dominguez-Valentin M, Sampson JR, Seppala TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med. 2020;22(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ten Broeke SW, van der Klift HM, Tops CMJ, et al. Cancer risks for PMS2-associated Lynch syndrome. J Clin Oncol. 2018;36(29):2961–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105(3):569–574. [DOI] [PubMed] [Google Scholar]
- 4.Win AK, Lindor NM, Winship I, et al. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J Natl Cancer Inst. 2013;105(4):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2016;28(1):96–102. [DOI] [PubMed] [Google Scholar]
- 6.Goodfellow PJ, Billingsley CC, Lankes HA, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group Study. J Clin Oncol. 2015;33(36):4301–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geurts-Giele WR, Leenen CH, Dubbink HJ, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-unstable cancers. J Pathol. 2014;234(4):548–559. [DOI] [PubMed] [Google Scholar]
- 8.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–646. e8. [DOI] [PubMed] [Google Scholar]
- 9.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–1316.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22(16):4215–4224. [DOI] [PubMed] [Google Scholar]
- 12.Leon-Castillo A, de Boer SM, Powell ME, et al. ; on behalf of the TransPORTEC consortium. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. [DOI] [PubMed] [Google Scholar]
- 14.Ramchander NC, Ryan NAJ, Walker TDJ, et al. Distinct immunological landscapes characterize inherited and sporadic mismatch repair deficient endometrial cancer. Front Immunol. 2019;10:3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan NAJ, McMahon R, Tobi S, et al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): a prospective cross-sectional study. PLoS Med. 2020;17(9):e1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan NAJ, Glaire MA, Blake D, et al. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. 2019;21(10):2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampel H, Pearlman R, de la Chapelle A, et al. Double somatic mismatch repair gene pathogenic variants as common as Lynch syndrome among endometrial cancer patients. Gynecol Oncol. 2021;160(1):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851–1860. [DOI] [PubMed] [Google Scholar]
- 19.Nout RA, Poll-Franse LVvd, Lybeert MLM, et al. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the Post Operative Radiation Therapy in Endometrial Carcinoma 1 (PORTEC-1) trial. J Clin Oncol. 2011;29(13):1692–1700. [DOI] [PubMed] [Google Scholar]
- 20.Wortman BG, Creutzberg CL, Putter H, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer. 2018;119(9):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20(9):1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020;250(3):312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng G, Chen A, Hong J, et al. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59(9):2029–2033. [PubMed] [Google Scholar]
- 24.Jansen AML, Tops CMJ, Ruano D, et al. The complexity of screening PMS2 in DNA isolated from formalin-fixed paraffin-embedded material. Eur J Hum Genet. 2020;28(3):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D, Hondelink LM, Solleveld-Westerink N, et al. Optimizing mutation and fusion detection in NSCLC by Sequential DNA and RNA sequencing. J Thorac Oncol. 2020;15(6):1000–1014. [DOI] [PubMed] [Google Scholar]
- 26.Daniel WW.Biostatistics: A Foundation for Analysis in the Health Sciences. 7th ed. New York: John Wiley & Sons; 1999. [Google Scholar]
- 27.Pakish JB, Zhang Q, Chen Z, et al. Immune microenvironment in microsatellite-instable endometrial cancers: hereditary or sporadic origin matters. Clin Cancer Res. 2017;23(15):4473–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu GC, Liu RY, Yan JP, et al. The heterogeneity between lynch-associated and sporadic MMR deficiency in colorectal cancers. J Natl Cancer Inst. 2018;110(9):975–984. [DOI] [PubMed] [Google Scholar]
- 29.Haraldsdottir S, Hampel H, Wu C, et al. Patients with colorectal cancer associated with Lynch syndrome and MLH1 promoter hypermethylation have similar prognoses. Genet Med. 2016;18(9):863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moller P, Seppala T, Bernstein I, et al. ; Mallorca Group (http://mallorca-group.org). Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut. 2017;66(9):1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolderink JM, De Bock GH, de Hullu JA, et al. Characteristics of Lynch syndrome associated ovarian cancer. Gynecol Oncol. 2018;150(2):324–330. [DOI] [PubMed] [Google Scholar]
- 32.Sjursen W, Haukanes BI, Grindedal EM, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet. 2010;47(9):579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22(2):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan NAJ, Morris J, Green K, et al. Association of mismatch repair mutation with age at cancer onset in lynch syndrome: implications for stratified surveillance strategies. JAMA Oncol. 2017;3(12):1702–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonadona V, Bonaiti B, Olschwang S, et al. ; French Cancer Genetics Network. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. Jama. 2011;305(22):2304–2310. [DOI] [PubMed] [Google Scholar]
- 36.Snowsill TM, Ryan NAJ, Crosbie EJ.. Cost-effectiveness of the Manchester approach to identifying Lynch syndrome in women with endometrial cancer. J Clin Med. 2020;9(6):1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burn J, Sheth H, Elliott F, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller P, Seppala T, Bernstein I, et al. ; Mallorca Group (http://mallorca-group.eu). Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66(3):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horeweg N, de Bruyn M, Nout RA, et al. Prognostic integrated image-based immune and molecular profiling in early-stage endometrial cancer. Cancer Immunol Res. 2020;8(12):1508–1519. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan DD, Clendenning M, Jayasekara H, et al. Double somatic mutations as a cause of tumor mismatch repair-deficiency in population-based colorectal and endometrial cancer with Lynch-like syndrome. Cancer Res. 2017;77(13 Suppl):Abstract nr 4266. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.