Abstract
Background
Endometrial cancer (EC) risk in BReast CAncer gene 1/2 (BRCA1/2) mutation carriers is uncertain; therefore, we assessed this in a large Dutch nationwide cohort study.
Methods
We selected 5980 BRCA1/2 (3788 BRCA1, 2151 gBRCA2, 41 both BRCA1/BRCA2) and 8451 non-BRCA1/2 mutation carriers from the Hereditary Breast and Ovarian cancer study, the Netherlands cohort. Follow-up started at the date of the nationwide Dutch Pathology Registry coverage (January 1, 1989) or at the age of 25 years (whichever came last) and ended at date of EC diagnosis, last follow-up, or death (whichever came first). EC risk in BRCA1/2 mutation carriers was compared with 1) the general population, estimating standardized incidence ratios (SIRs) based on Dutch population-based incidence rates; and 2) non-BRCA1/2 mutation carriers, using Cox-regression analyses, expressed as hazard ratio (HR). Statistical tests were 2-sided.
Results
Fifty-eight BRCA1/2 and 33 non-BRCA1/2 mutation carriers developed EC over 119 296 and 160 841 person-years, respectively (SIR = 2.83, 95% confidence interval [CI] = 2.18 to 3.65; and HR = 2.37, 95% CI = 1.53 to 3.69, respectively). gBRCA1 mutation carriers showed increased risks for EC overall (SIR = 3.51, 95% CI = 2.61 to 4.72; HR = 2.91, 95% CI = 1.83 to 4.66), serous-like EC (SIR = 12.64, 95% CI = 7.62 to 20.96; HR = 10.48, 95% CI = 2.95 to 37.20), endometrioid EC (SIR = 2.63, 95% CI = 1.80 to 3.83; HR = 2.01, 95% CI = 1.18 to 3.45), and TP53-mutated EC (HR = 15.71, 95% CI = 4.62 to 53.40). For BRCA2 mutation carriers, overall (SIR = 1.70, 95% CI = 1.01 to 2.87) and serous-like EC risks (SIR = 5.11, 95% CI = 1.92 to 13.63) were increased compared with the general population. Absolute risks by 75 years remained low (overall EC = 3.0%; serous-like EC = 1.1%).
Conclusions
BRCA1/2 mutation carriers have a two- to threefold increased risk for EC, with highest risk observed for the rare subgroups of serous-like and p53-abnormal EC in BRCA1 mutation carriers.
Women with a pathogenic germline mutation in the BReast CAncer genes (BRCA1 and BRCA2) have strongly increased breast carcinoma (BC) and tubo-ovarian carcinoma (OC) risks. Penetrance studies of BRCA1/2 mutations report cumulative BC risks at age 70 years of 50%-59% for female BRCA1 mutation carriers and 42%-51% for female BRCA2 mutation carriers, together with OC risks of 34%- 45% and 13%- 21%, respectively (1).
Whether BRCA1/2 mutations also confer elevated lifetime risk for endometrial cancer (including uterine sarcomas; EC) is unclear. Studies have reported an increased EC risk in BRCA1/2 mutation carriers compared with country-specific incidence rates (standardized incidence ratios [SIRs], range = 1.9-5.3) (2-4), but others found no clearly increased EC risk (5–7) or found that increased risk was restricted to a rare but aggressive subgroup of EC: ECs with serous-like histology (eg, uterine serous carcinomas, carcinosarcomas; SIR range = 14.8-32.2; Supplementary Table 1, available online) (8–12). Furthermore, it has been suggested that the apparent increase in EC risk is not related to the BRCA1/2 mutation but to previous BC-related tamoxifen-treatment. (2,3) These conflicting data in previous cohort studies can be attributed to a limited number of ECs (n = 2-17) as a result of small cohort sizes (n = 315-4456), low mean or median age at enrolment with limited follow-up periods, or absence of outcome validation (n = 5) (2–9,13).
More recently, studies have suggested that in addition to EC of serous-like histology, a larger group of p53-abnormal ECs (1 of the 4 molecularly defined subgroups) (10,14,15) are more common in BRCA1/2 mutation carriers. EC risks for this molecular subgroup have not yet been determined.
Accurate estimation of EC risk in BRCA1/2 mutation carriers is important to counseling and clinical management. Therefore, the aim of this study was to confirm and quantify the risk of EC in a large cohort of BRCA1/2 mutation carriers compared with both the general Dutch population and non-BRCA1/2 mutation carriers.
Methods
Study Population
BRCA1/2 mutation carriers (n = 6072) were selected from the Hereditary Breast and Ovarian cancer study, the Netherlands (HEBON cohort study), an ongoing nationwide cohort study of hereditary breast and ovarian cancer (HBOC) families in the Netherlands [for details, see (16,17) and Supplementary Methods, available online]. The HEBON cohort study has been approved by the medical ethical committees of all participating centers. This study was approved by the Institutional Review Board of the Netherlands Cancer Institute.
BRCA1/2 Mutation Carriers
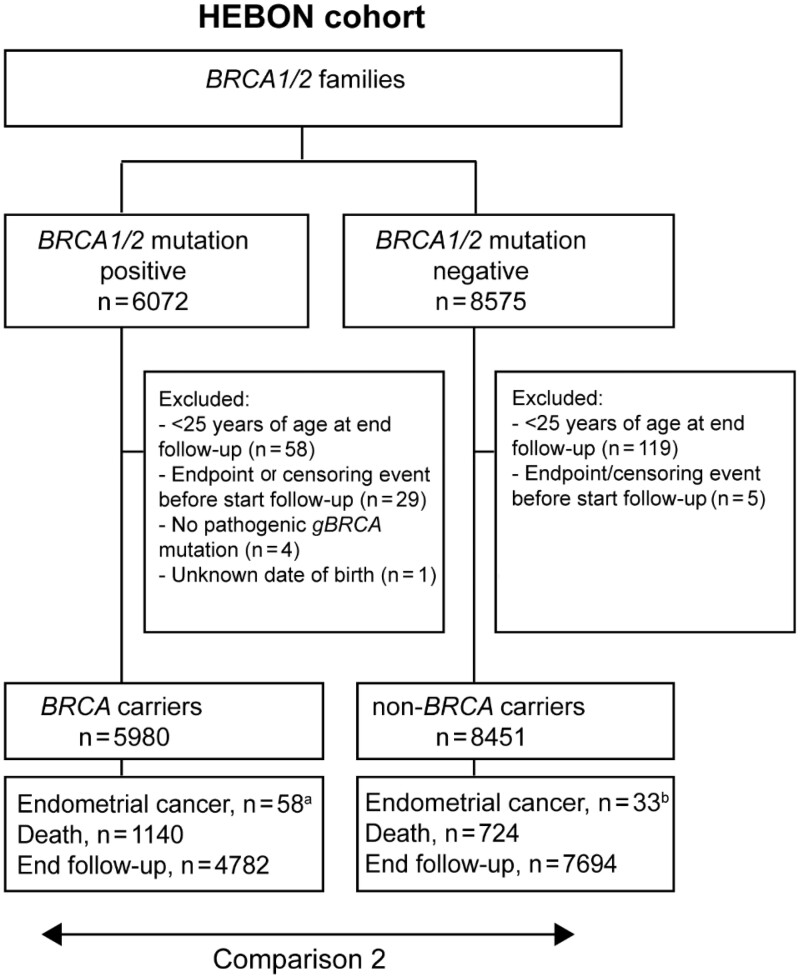
Women with a class 5 (pathogenic) or class 4 (likely pathogenic) BRCA1 or BRCA2 mutation were eligible (18). The initial cohort consisted of 6072 BRCA1/2 mutation carriers, of whom 3716 provided written informed consent allowing connection to disease registries, 876 who died before they could be invited to join the HEBON cohort, and 1480 whose connection to disease registries (see below) was approved by the medical ethical committee because they did not respond to a request to participate and did not actively deny the request after 3 invitations to do so (Figure 1).
Figure 1.
Schematic overview of the BRCA1/2 mutation carrier cohort and the non-BRCA1/2 mutation carrier cohort. aFour events were excluded as they occurred outside of the observation period: 2 before the start of follow-up (January 1, 1989) and 2 after the end of follow-up (once on January 1, 2012, and once on January 1, 2016). bSeven events were excluded: 5 events occurred after the observation period ended (January 1, 2012) and 2 events were excluded because the tumors were considered of non-endometrial origin after pathology review.
Dutch Population-Based Cancer Incidence Rates (Comparison Group 1)
Age, calendar year, and country-specific EC incidence rates (crude rates/100 000 person-years, stratified by age and calendar time) were obtained from the Netherlands Cancer Registry (NCR) for the calendar years 1989-2015 (May 2020). All tumors with an International Classification of Diseases for Oncology, Third edition, First revision (ICD-O-3.1; http://codes.iarc.fr/) topographical code of either C54 (corpus uteri) and C55 (uterus, NOS) were included.
In addition, age, calendar year, and country-specific EC incidence rates were obtained from the NCR for the following 5 histologic subgroups based on the morphological ICD-O-3.1 codes: 1) endometrioid (including mucinous); 2) serous-like (eg, uterine serous carcinoma, carcinosarcoma, mixed carcinomas); 3) clear cell carcinoma; 4) sarcoma; and 5) other (eg, neuroendocrine carcinoma) (see Supplementary Table 2, available online).
Non-BRCA1/2 Mutation Carriers (Comparison Group 2)
Non-BRCA1/2 mutation carriers (n = 8575, within-cohort comparison group) were also selected from the HEBON cohort (Figure 1). Women were eligible if they 1) were a member of a family with a proven likely pathogenic or pathogenic BRCA1 or BRCA2 mutation (not including variants of unknown statistical significance), and 2) tested negative for this likely pathogenic or pathogenic BRCA1/2 mutation.
Pathology Review and Assessment of Histologic and Molecular Subgroup
To confirm endometrial origin and define histologic and molecular subgroups, pathology reports, Hematoxylin and eosin stained (H&E) slides and Formalin Fixed Paraffin Embedded (FFPE) tumor tissue blocks of ECs of both BRCA1/2 and non-BRCA1/2-mutation carriers were collected via the Dutch Pathology Registry (PALGA) and centrally revised by at least 1 expert gynecopathologist. If pathology review was not possible, histologic subtype and grade were extracted from pathology reports or based on the morphological ICD-O-3.1 code. Although some cases of rare uterine sarcomas were included in the study, for simplicity the term “endometrial cancer” (EC) is used throughout the manuscript.
After review, ECs were classified into the same 5 histologic subgroups as described for comparison group 1 and were molecularly classified similarly to as what has been previously described: p53-abnormal or “other” (including POLE-mutant, mismatch repair-deficient, and no surrogate marker profile group) (10,14). For cases that were not available for review, assignment to molecular groups was based on histology (see the Supplementary Methods, available online).
Data Collection and Data Handling
Pseudonymized data were retrieved for BRCA1/2 and non-BRCA1/2 mutation carriers from the central HEBON database. With regular input from the NCR, the PALGA (19), and the municipal administration, the HEBON cohort study gathers data centrally, including cancer incidence, date of cancer diagnosis, risk-reducing salpingo-oophorectomy (RRSO), and date of death. In the case of BC, these data also include hormone treatment (HT; type and duration not specified). PALGA is a nationwide archive containing excerpts of all histo- and cytopathology reports in the Netherlands since 1991 (19). For details, see the Supplementary Methods (available online).
Statistical Analysis
Period at Risk for EC.
Both BRCA1/2 and non-BRCA1/2 mutation carriers were assigned a starting date for follow-up based on either nationwide PALGA coverage (January 1, 1989) or the date at which women are considered to be at risk for EC (≥25 years of age), whichever was later. Follow-up ended on the date of EC diagnosis (ICD-O-3 topographical code C54 or C55), date of death, or date of end of follow-up (January 1, 2016, for BRCA1/2 mutation carriers who provided informed consent; January 1, 2012, for all others), whichever was earlier. Women were excluded from analyses if an EC occurred before January 1, 1989, or before the age of 25 years (Supplementary Figure 1, available online). We were not informed about the extent of OC surgery and RRSO (whether or not this included a hysterectomy), and therefore the date of OC or RRSO was not used as a censoring event.
Comparison 1: BRCA1/2 Mutation Carriers vs Dutch Country-Specific Incidence Rates.
For the BRCA1/2 mutation carrier cohort, expected EC incidence was estimated based on calculated person-time at risk stratified by age and calendar time. SIRs were calculated by dividing observed ECs by expected ECs, and 95% confidence intervals (CIs) and 2-sided P values were estimated assuming a Poisson distribution. SIRs were also stratified for histologic subgroup after pathology review, mutation type (BRCA1/BRCA2), and attained age.
Comparison 2: BRCA1/2 Mutation Carriers vs Non-BRCA1/2 Mutation Carriers.
Differences in EC occurrence between BRCA1/2 and non-BRCA1/2 mutation carriers were analyzed using Cox regression and expressed as hazard ratio (HR) with accompanying 95% confidence intervals adjusted for age. Hazard ratio was also calculated after stratification for mutation type and for histologic and molecular subgroup following pathology review. Women carrying both a BRCA1 and BRCA2 mutation (n = 41, no ECs) were analyzed in both the BRCA1 and BRCA2 mutation carrier group.
The following sensitivity analyses were performed. First, to exclude potential confounding by tamoxifen use for BC, 2 separate sensitivity analyses were performed. For the first, patients were censored at the date of (first) BC diagnosis that led to HT (type and duration not specified), and for the second, patients were censored at the date of (first) BC diagnosis (both analyses included cases with Ductal Carcinoma in Situ (DCIS)). Second, to exclude testing bias (testing BRCA1/2 mutation because of EC diagnosis), person-years at risk began on the date of the BRCA1/2 DNA test. Third, to minimize potential bias due to unequal observation periods, the end date for follow-up was set to January 1, 2012, for all BRCA1/2 and non-BRCA1/2 mutation carriers.
Baseline characteristics between BRCA1/2 and non-BRCA1/2 mutation carriers were compared using the χ2 test (categorical variables) and the Mann-Whitney U-test (numerical variables). Median follow-up time was estimated using the Reverse Kaplan-Meier Method. Cumulative risk of developing EC and EC of serous-like and endometrioid histology up to the age of 75 years was estimated using competing risk analyses.
A P value of less than .05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 23.0 and STATA Statistical Software version 14.1 (StataCorp LP, College Station, TX). All data is available by contacting the corresponding author.
Results
Cohort Characteristics
A total of 5980 BRCA1/2 and 8451 non-BRCA1/2 mutation carriers were included (Figure 1). Cohort characteristics and follow-up details are described in Table 1. The total number of person-years at risk and events (overall and stratified by histologic subgroup) per 5-year age category are shown in Supplementary Table 3 (available online). Details on EC characteristics and pathology review are described in Supplementary Tables 4-6 (available online).
Table 1.
Demographic characteristics of BRCA1/2 mutation carriers and non-BRCA1/2 mutation carriers
| Demographic characteristics | BRCA1/2 carriers | non-BRCA1/2 carriers |
|---|---|---|
| Total, No. (%) | 5980 (100) | 8451 (100)a |
| BRCA1 mutation, No. (%) | 3788 (63.3) | 0 (0) |
| BRCA2 mutation, No. (%) | 2151 (36.0) | 0 (0) |
| BRCA1 and gBRCA2 mutation, No. (%) | 41 (0.7) | 0 (0) |
| Median age at start of follow-up (IQR), y | 27.4 (25.0-37.8) | 28.0 (25.0-38.2) |
| <40, No. (%) | 4737 (79.2) | 6657 (78.8) |
| 40-49, No. (%) | 775 (13.0) | 1197 (14.2) |
| 50-59, No. (%) | 321 (5.4) | 395 (4.7) |
| ≥60, No. (%) | 147 (2.5) | 202 (2.4) |
| Median age at end of follow-up (IQR), y | 51.9 (42.5-61.6) | 50.7 (42.1-60.7) |
| Median observation period (IQR), y | 22.5 (15.2-27.0) | 23.0 (16.4-23.0) |
| Total person-years at risk (SD) | 119 296 (7.1) | 160 841 (5.8) |
| Of which post gBRCA DNA test (SD)b | 56 579 (6.3) | 48 044 (5.1) |
| Ovarian cancer history, No. (%)c | 716 (12.0) | 267 (3.2) |
| Before start observation period, No. (%) | 34 (0.6) | 19 (0.2) |
| During observation period, No. (%) | 682 (11.4) | 248 (2.9) |
| EC and simultaneous/history of ovarian cancerd | 5 (0.08) | 5 (0.06) |
| BC history, No. (%)e,f | 2762 (46.2) | 2788 (33.0) |
| Before start observation period, No. (%) | 291 (4.9) | 140 (1.7) |
| During observation period, No. (%) | 2471 (41.3) | 2648 (31.3) |
| HT | ||
| HT-BC, No. (%) | 755 (12.6) | 1155 (13.7) |
| Before start follow-up, No. (%) | 14 (0.2) | 4 (0.0) |
| During follow-up, No. (%) | 741 (12.4) | 1151 (13.6) |
| HT-BC unknown, No. (%)f | 209 (3.5) | 127 (1.5) |
| Before start follow-up, No. (%) | 72 (1.2) | 39 (0.5) |
| During follow-up, No. (%) | 137 (2.3) | 88 (1.0) |
| RRSO history, No. (%)g | 3619 (60.5) | 695 (8.2) |
| Before start follow-up, No. (%) | 19 (0.3) | 25 (0.3) |
| During follow-up, No. (%) | 3600 (60.2) | 670 (7.9) |
| History RRSO unknown, No. (%) | 119 (2.0) | 4324 (51.2) |
Includes 96 women with a BRCA variant of unknown statistical significance, of whom 2 developed an endometrial carcinoma (none carried the [likely] pathogenic familial variant). BC = breast cancer; DCIS = ductal carcinoma in situ; EC = endometrial cancer; HT = hormone treatment; IQR = interquartile range; OC = tubo-ovarian carcinoma; RRSO = risk-reducing salpingo-oophorectomy.
Post BRCA DNA test; person-years from date of BRCA1/2-DNA test until end of follow-up. Date of BRCA1/2-mutation test was missing for 1682 (28.1%) carriers and 1214 (14.4%) noncarriers. For these women, the date of the BRCA1/2 DNA test was considered to be January 1, 1995. BRCA1/2 DNA tests were performed from 1995 until 2012 (median year 2007).
Date of OC diagnosis unknown for 2 non-BRCA mutation carriers.
For details, see Supplementary Tables 5 and 6 (available online).
DCIS was considered as BC. Considered the first BC if women had a history of more than 1 BC.
Date of diagnosis unknown for 1 BC in the BRCA mutation carrier group.
Includes adnexextirpation for reasons other than RRSO, for example, during hysterectomy or for OC.
EC Risk in BRCA1/2 Mutation Carriers Compared With the Dutch Country-Specific Incidence Rates
Overall EC risk in BRCA1/2 mutation carriers was increased 2.83-fold (95% CI = 2.18-fold to 3.65-fold) compared with Dutch EC incidence rates (BRCA1, SIR = 3.51, 95% CI = 2.61 to 4.72; BRCA2, SIR = 1.70, 95% CI = 1.01 to 2.87) (Table 2).
Table 2.
Observed and expected EC rates in BRCA1/2 mutation carriers compared with the Dutch country-specific incidence rates
| EC subgroups | BRCA1/2 carriers | Dutch population | SIR (95% CI) | Pa |
|---|---|---|---|---|
| Observed | Expected | |||
| All ECs | 58 | 20.53 | 2.83 (2.18 to 3.65) | <.001 |
| BRCA1 | 44 | 12.53 | 3.51 (2.61 to 4.72) | <.001 |
| BRCA2 | 14 | 8.23 | 1.70 (1.01 to 2.87) | .04 |
| Endometrioid | 35 | 16.85 | 2.08 (1.49 to 2.89) | <.001 |
| BRCA1 | 27 | 10.27 | 2.63 (1.80 to 3.83) | <.001 |
| BRCA2 | 8 | 6.77 | 1.18 (0.59 to 2.36) | .37 |
| Serous-like | 19 | 1.95 | 9.77 (6.23 to 15.31) | <.001 |
| BRCA1 | 15 | 1.19 | 12.64 (7.62 to 20.96) | <.001 |
| BRCA2 | 4 | 0.78 | 5.11 (1.92 to 13.63) | .01 |
| Sarcoma | 3 | 1.3 | 2.30 (0.74 to 7.14) | .14 |
| BRCA1 | 1 | 0.81 | 1.24 (0.17 to 8.78) | .55 |
| BRCA2 | 2 | 0.51 | 3.95 (0.99 to 15.81) | .09 |
| Clear cell | 1 | 0.29 | 3.40 (0.48 to 24.11) | .25 |
| BRCA1 | 1 | 0.18 | 5.58 (0.79 to 39.65) | .16 |
| BRCA2 | 0 | 0.12 | NA | NA |
P values were estimated assuming a Poisson distribution. CI = confidence interval; EC = endometrial cancer; NA = not applicable; SIR = standardized incidence ratio.
When ECs were stratified by histologic subgroup, BRCA1/2 mutation carriers were at increased risk for endometrioid EC (SIR = 2.08, 95% CI = 1.49 to 2.89) and for EC of serous-like histology (SIR = 9.77, 95% CI = 6.23 to 15.31) (Table 2). BRCA1 mutation carriers displayed greater risk for endometrioid EC (SIR = 2.63, 95% CI = 1.80 to 3.83) and especially for EC of serous-like histology (SIR = 12.64, 95% CI = 7.62 to 20.96). Risk for EC of serous-like histology in BRCA2 mutation carriers was lower (SIR = 5.11, 95% CI 1.92 to 13.63).
Overall EC risks were highest in the youngest age category of 25-40 years (SIR = 9.84, 95% CI = 2.68 to 25.20), although confidence intervals were broad and the majority of events occurred in older age categories (Table 3). For EC of serous-like histology, the highest risks were observed in the age category 60-80 years (SIR = 11.27, 95% CI = 5.99 to 19.27).
Table 3.
Observed and expected EC rates in BRCA1/2 mutation carriers compared with the Dutch country-specific incidence rates, according to attained age
| EC subgroup, age categories | BRCA1/2 carriers | Dutch population | SIR (95% CI) |
|---|---|---|---|
| Observed | Expected | ||
| All ECs | 58a | 20.53 | 2.83 (2.18 to 3.65) |
| 25-40 y | 4 | 0.41 | 9.84 (2.68 to 25.20) |
| 40-60 y | 25 | 10.0 | 2.50 (1.62 to 3.69) |
| 60-80 y | 28 | 9.56 | 2.93 (1.95 to 4.24) |
| Serous-like | 19 | 1.95 | 9.77 (6.23 to 15.31) |
| 25-40 y | 0 | 0.02 | 0.00 (0.00 to 149.82) |
| 40-60 y | 6 | 0.69 | 8.68 (3.19 to 18.90) |
| 60-80 y | 13 | 1.15 | 11.27 (5.99 to 19.27) |
One EC occurred after 80 years of age. Given the low number of person-years after 80 years of age, this age category is not presented in the table. CI = confidence interval; EC = endometrial cancer; SIR = standardized incidence ratio.
EC Risk BRCA1/2 Mutation Carriers Compared With Non-BRCA1/2 Mutation Carriers
In total, 58 BRCA1/2 mutation carriers developed ECs compared with 33 non-BRCA1/2 mutation carriers, over 119 296 and 160 841 at risk person-years, respectively (HR = 2.37, 95% CI = 1.53 to 3.69) (Table 4). BRCA1 mutation carriers displayed higher relative EC risk (HR = 2.91, 95% CI = 1.83 to 4.66) compared with BRCA2 mutation carriers (HR = 1.45, 95% CI = 0.75 to 2.81).
Table 4.
EC risks gBRCA1/2 mutation carriers vs non-BRCA1/2 mutation carriers
| Subgroup |
BRCA1/2 carriers |
non-BRCA1/2 carriers |
||||||
|---|---|---|---|---|---|---|---|---|
| Total, No. | Event, No. | Person-years at risk | Total, No. | Events, No. | Person-years at risk | HR (95% CI)a |
P b | |
| Main analysis | ||||||||
| All | 5980 | 58 | 119 296 | 8451 | 33 | 160 841 | 2.37 (1.53 to 3.69) | <.001 |
| BRCA1c | 3829 | 44 | 75 366 | 8451 | 33 | 160 841 | 2.91 (1.83 to 4.66) | <.001 |
| BRCA2c | 2192 | 14 | 44 809 | 8451 | 33 | 160 841 | 1.45 (0.75 to 2.81) | .27 |
| Histologic groups | ||||||||
| Endometrioid | 5980 | 35 | 119 296 | 8451 | 30 | 160 841 | 1.61 (0.97 to 2.66) | .06 |
| BRCA1c | 3829 | 27 | 75 366 | 8451 | 30 | 160 841 | 2.01 (1.18 to 3.45) | .01 |
| BRCA2c | 2192 | 8 | 44 809 | 8451 | 30 | 160 841 | 0.93 (0.41 to 2.11) | .86 |
| Serous-like | 5980 | 19 | 119 296 | 8451 | 3 | 160 841 | 8.08 (2.34 to 27.94) | .001 |
| BRCA1c | 3829 | 15 | 75 366 | 8451 | 3 | 160 841 | 10.48 (2.95 to 37.20) | <.001 |
| BRCA2c | 2192 | 4 | 44 809 | 8451 | 3 | 160 841 | 4.13 (0.83 to 20.50) | .08 |
| Molecular group | ||||||||
| p53-abnormald | 5980 | 27 | 119 296 | 8451 | 3 | 160 841 | 11.31 (3.37 to 37.95) | <.001 |
| BRCA1c | 3829 | 23 | 75 366 | 8451 | 3 | 160 841 | 15.71 (4.62 to 53.40) | <.001 |
| BRCA2c | 2192 | 4 | 44 809 | 8451 | 3 | 160 841 | 4.11 (0.83 to 20.39) | .08 |
| Sensitivity analyses | ||||||||
| Start follow-up from date of gBRCA1/2 DNA testd | ||||||||
| All histotypes | 5771 | 37 | 56 579 | 8098 | 11 | 48 044 | 3.26 (1.65 to 6.44) | .001 |
| Endometrioid | 5771 | 22 | 56 579 | 8098 | 10 | 48 044 | 2.76 (1.26 to 6.02) | .01 |
| Serous-like | 5771 | 14 | 56 579 | 8098 | 1 | 48 044 | 18.28 (2.33 to 143.34) | .01 |
| p53-abnormald | 5771 | 21 | 56 579 | 8098 | 1 | 48 044 | 26.64 (3.51 to 202.32) | .01 |
| BRCA1, all histotypesc | 3700 | 29 | 37 984 | 8098 | 11 | 48 044 | 5.57 (2.69 to 11.54) | <.001 |
| BRCA2, all histotypesc | 2108 | 8 | 18 971 | 8098 | 11 | 48 044 | 2.18 (0.80 to 5.91) | .13 |
| Additional censoring HT-BCf | ||||||||
| All | 5966 | 50 | 113 033 | 8447 | 30 | 155 002 | 2.30 (1.44 to 3.66) | <.001 |
| Endometrioid | 5966 | 32 | 113 033 | 8447 | 28 | 155 002 | 1.56 (0.93 to 2.64) | .09 |
| Serous-like | 5966 | 14 | 113 033 | 8447 | 2 | 155 002 | 8.78 (1.94 to 39.65) | .01 |
| p53-abnormald | 5966 | 22 | 113 033 | 8447 | 2 | 155 002 | 13.62 (3.15 to 59.00) | <.001 |
| BRCA1, all histotypesc | 3821 | 37 | 72 423 | 8447 | 30 | 155 002 | 2.61 (1.58 to 4.31) | <.001 |
| BRCA2, all histotypesc | 2186 | 13 | 41 461 | 8447 | 30 | 155 002 | 1.60 (0.82 to 3.12) | .17 |
All hazard ratios were adjusted for age. BC = breast cancer; CI = confidence interval; DCIS = ductal carcinoma in situ; EC = endometrial cancer; FFPE = formalin fixed paraffin embedded; HR = hazard ratio; HT = hormone treatment.
The P values assessing the null hypothesis of hazard ratio = 1.00.
Women with both a gBRCA1 and a gBRCA2 mutation were included in both analyses stratified for gBRCA1/2 mutation status.
Includes cases for which p53-status was unknown (no FFPE tumor block available) and for whom p53-status was based on most common p53-status for the histotype as described in the material and methods. When excluding cases for which p53-status was based on histotype, the number of events remained the same for gBRCA1/2 carriers, but only 2 events occurred in the non-gBRCA1/2 mutation carriers (HR = 17.07, 95% CI = 4.0 to 72.8, P < .001).
If the date of gBRCA1/2-DNA test was unknown, this date was considered to be January 1, 1995.
DCIS was considered as BC. If a woman developed a BC or DCIS for which HT status was unknown, the date of diagnoses was not considered as censoring event.
Combined BRCA1/2 histologic subgroup analysis showed strongly increased risks for EC with serous-like histology (HR = 8.08, 95% CI = 2.34 to 27.94), with BRCA1 showing higher relative risk (HR = 10.48, 95% CI = 2.95 to 37.20) than BRCA2 mutation carriers (HR = 4.13, 95% CI = 0.83 to 20.50) (Table 4). The highest HR was observed for p53-abnormal EC in BRCA1 mutation carriers (HR = 15.71, 95% CI = 4.62 to 53.40). Risk for endometrioid EC in BRCA1 mutation carriers was increased twofold (HR = 2.01, 95% CI = 1.18 to 3.45), unlike BRCA2 (HR = 0.93, 95% CI = 0.41 to 2.11).
When only follow-up after the date of BRCA1/2 DNA test is considered, EC risk among mutation carriers remained increased, with higher HRs compared with the main analyses, though with broader confidence intervals (Table 4). When excluding cases for which the BRCA1/2 DNA test date was unknown, HRs remained roughly similar, (Supplementary Table 7, available online).
To eliminate potential confounding by tamoxifen, a sensitivity analysis was performed by additionally censoring at the time of (first) HT-treated BC. This yielded a hazard ratio that was similar to the main analyses, both regarding overall EC risk and stratified for mutation type, histology and molecular subgroup (Table 4). For additional sensitivity analyses, see Supplementary Table 7 (available online).
When overall EC risk and EC risk stratified by histologic subgroup were compared between non-BRCA1/2 carriers and Dutch country-specific incidence rates, no statistically significant differences were observed (Supplementary Table 8, available online).
At the age of 75 years, the estimated cumulative risk (lifetime risk) for BRCA1/2 mutation carriers to develop EC was 3.0% (95% CI = 2.20% to 3.91%; BRCA1: 3.4%, 95% CI = 2.46% to 4.81%; BRCA2: 2.0%, 95% CI = 1.09% to 3.30%); for the subgroup of EC with serous-like histology, this was 1.1% (95% CI = 0.69% to 1.80%; BRCA1: 1.4%, 95% CI = 0.79% to 2.37%; BRCA2: 0.6%, 95% CI = 0.21% to 1.60%) (see Supplementary Table 9, available online).
Discussion
We presented data from a large cohort study that assessed EC risk among BRCA1/2 mutation carriers (n = 5980). Strengths of the study compared with earlier studies are high number of events (n = 58), long follow-up (median = 22.5 years), and pathology review to validate the outcome. We found that BRCA1 and BRCA2 mutation carriers show a two- to threefold increased EC risk, with highest increased risks found for the subgroups EC of serous-like histology (8- to 10-fold) and p53-abnormal EC (11- to 12-fold). We also showed that increased risk cannot be fully explained by previous HT use and is therefore most likely causally associated with gBRCA1/2 mutations.
Conflicting data from earlier cohort studies, most likely due to lack of power, have resulted in uncertainty regarding increased EC risk in BRCA1/2 mutation carriers (Supplementary Table 1, available online) (2–9), because only 3 of 8 reported statistically significantly increased overall EC risk (SIR range = 1.9-5.3). Those figures broadly agree with results from this study (two- to threefold increase) (2–4). A striking observation reported in 3 of the 7 studies that stratified for histotype (2,4–9) was the statistically significantly increased risk (SIR range = 14.3-32.2) for EC of serous-like histology, which seemed to be restricted to BRCA1 mutation carriers (4,8,9). Our study confirms that finding, with the highest risk indeed observed for BRCA1 mutation carriers (10- to 13-fold) but with BRCA2 mutation carriers also showing fivefold increased risk compared with the general population. By contrast, endometrioid EC risk was only increased for BRCA1 mutation carriers (two- to threefold). That BRCA mutations contribute to the development of EC is further supported by the recent study of Hughley and colleagues (20) in which they present the “etiological index”, a case-only measure of BRCA1/2 mutation associated cancer risks based on the fraction of tumors harboring biallelic BRCA1/2 inactivation. Whereas the BRCA1/2 etiological index for nonestablished BRCA1/2-associated cancers was 1.6, the respective BRCA1 etiological index of EC was 4.0, supporting an etiological role in cancer causation.
A history of tamoxifen use is considered an important confounder when assessing EC risk (21,22). These patients also seem to develop less favorable histologic subtypes such as carcinosarcomas, sarcomas, and p53-abnormal tumors (21,23). Because we were not informed about the type of HT (tamoxifen, aromatase inhibitor) women received for their BC, a potential effect was eliminated by censoring for all HT-BC in a sensitivity analysis. We nonetheless found persistent increased risk for EC overall, EC of serous-like histology, and p53-abnormal EC and can therefore conclude that increased EC risk in BRCA1/2 mutation carriers can, at best, be only partly explained by previous HT or tamoxifen use.
Highest increased EC risks were found for EC with serous-like histology and more specifically p53-abnormal EC. We have previously shown that ECs in BRCA1/2 mutation carriers are noticeably enriched for tumors of the p53-abnormal molecular subgroup, that these tumors demonstrate loss of heterozygosity (LOH) of BRCA wild-type allele (16), and that ECs of this subgroup are frequently homologous recombination deficient or show genomic scars associated with homologous recombination deficiency (15,24). Molecular alterations in these tumors are similar to those found in high-grade serous OC and basal-like BC, tumor subtypes particularly associated with the BRCA1/2-associated HBOC syndrome (10,25–27). Due to the above observations, we would argue that ECs with serous-like histology and especially ECs of the p53-abnormal molecular subgroup should be regarded as part of the BRCA1/2-associated HBOC syndrome.
A limitation of this study was the possibility of a cancer-related testing bias. EC is not an indication for BRCA1/2 DNA testing; therefore, although person-time before BRCA DNA testing was included in the main analysis, it is unlikely that this influenced the results. Only including person-time after BRCA1/2 DNA testing resulted in higher hazard ratios (though with broader confidence intervals) compared with the main analysis. This might be due to the older age of the post-BRCA1/2 DNA testing cohort, because higher SIRs were observed for older age categories (Table 3). Another potential limitation is the presence of left censoring, because the possible occurrence of EC in the period before the NCR and PALGA databases achieved nationwide coverage has naturally not been recorded but cannot be entirely excluded. However, because the majority of women were young at start of follow-up and the majority of ECs are recorded at an age older than 40 years (54 of 58 BRCA1/2 and 31 of 33 non-BRCA1/2 mutation carriers), any influence is likely minor. Data on previous hysterectomies were unavailable, but because a BRCA1/2 mutation is not an indication for hysterectomy in the Netherlands, this is unlikely to have affected our results. Pathology review could not be performed for all ECs or for the Dutch population controls; therefore, a subset of ECs might have been misclassified. This is especially relevant for high-grade EC (review resulted in histologic subgroup changes for 22% of EC in BRCA1/2 mutation carriers), which are more difficult to classify and more common in gBRCA1/2 mutation carriers (13,16). We were not informed about body weight and the use of hormone replacement therapy for the majority of cases. Especially obesity, but not modern combined hormone replacement therapy, is a well-known risk factor for EC (both endometrioid and nonendometrioid subtypes) (28–31). However, there is no reason to believe that BRCA1/2 mutation carriers are more frequently obese.
Our results provide important additional information with regard to EC risks that is essential for adequate genetic counselling of BRCA1/2 mutation carriers. Despite the observed increased overall EC risks in BRCA1/2 mutation carriers, the cumulative overall EC risk (3.0%) and risk for EC of serous-like histology (1.1%) by 75 years remains low (Supplementary Table 9, available online), because the lifetime risk of developing EC is low in the general population (approximately 1%–1.4%, with ECs of serous-like histology being even less common: 10% of all ECs) (8,32,33). Therefore, we should not routinely recommend a concurrent risk-reducing hysterectomy at the time of RRSO, especially because this will increase the complication risk of the procedure. Nevertheless, risk-reducing hysterectomy should be considered especially in the presence of other EC risk factors or when a hysterectomy is considered for other (benign) uterine pathology. Taken together, given the observed relative and absolute risks, the potential hazards and possible benefits of risk-reducing hysterectomy need to be carefully weighed, and shared decision making is crucial to conclude about individually tailored treatment advice with regard to risk-reducing surgery in BRCA1/2 mutation carriers.
Secondly, ECs that harbor BRCA1/2 mutations (germline and somatic) will likely benefit from PARP-inhibitor treatment. PARP inhibitors are proven effective maintenance treatment for BRCA-associated platinum-sensitive OC (34), and trials are currently testing efficacy in EC.
Thirdly, although previous studies have reported low incidences of BRCA1/2 mutations when screening EC patients with a history of BC (3.8%, not selected for histotype) (35) or an unselected cohort of patients with uterine serous carcinomas (2%) (36), BRCA1/2 mutation incidences in women with p53-abnormal EC, especially with a history of BC, should be studied to determine the potential value of BRCA1/2 screening in this patient population.
In summary, BRCA1/2 mutation carriers do have an important increased risk of EC. This is especially the case for the EC subgroups with unfavorable clinical outcome: serous-like EC and p53-abnormal EC. The observed increase in risk cannot be explained by previous BC-related HT. Importantly, lifetime EC risk through age 75 years remains low. This report adds critical evidence to the ongoing discussion of whether EC is a BRCA1/2-associated disease and further supports the mounting evidence that at least serous-like and p53-abnormal EC should be considered to be an integral part of the BRCA1/2-associated HBOC syndrome.
Funding
The HEBON study is supported by the Dutch Cancer Society grants NKI1998-1854, NKI2004-3088, and NKI2007-3756; the Netherlands Organisation of Scientific Research grant NWO 91109024; the Pink Ribbon grants 110005 and 2014–187.WO76; the BBMRI grant NWO 184.021.007/CP46; and the Transcan grant JTC 2012 Cancer 12–054.
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: Authors have no conflicts of interest to disclose.
Author contributions: Conception and design: MMJ, CDK, VTHBMS, MAR, GHB, FEL, TB, OD, CJA, Administrative support: MMJ, DJJ, JO, TB, OD, Collection and assembly of data: MMJ, DJJ, JO, JAH, MJEM, EBGG, MGEMA, MC, KE, IB, VTHBMS, MAR, GHB, FEL, TB, CJA, Data analysis and interpretation: MMJ, CDK, VTHBMS, TB, MJEM, JAH, MAR, OD, CJA, FEL, Manuscript writing: MMJ, CDK, VTHBMS, TB, MJEM, JAH, OD, FEL, CJA.
Acknowledgements: The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) consists of the following Collaborating Centers: Netherlands Cancer Institute (coordinating center), Amsterdam, NL: M.A. Rookus, F.B.L. Hogervorst, F.E. van Leeuwen, M.A. Adank, M.K. Schmidt, D.J. Jenner; Erasmus Medical Center, Rotterdam, NL: J.M. Collée, A.M.W. van den Ouweland, M.J. Hooning, I.A. Boere; Leiden University Medical Center, NL: C.J. van Asperen, P. Devilee, R.B. van der Luijt, T.C.T.E.F. van Cronenburg; Radboud University Nijmegen Medical Center, NL: M.R. Wevers, A.R. Mensenkamp; University Medical Center Utrecht, NL: M.G.E.M. Ausems, M.J. Koudijs; Amsterdam Medical Center, NL: T.A.M. van Os, I. van de Beek; VU University Medical Center, Amsterdam, NL: K. van Engelen, J.J.P. Gille; Maastricht University Medical Center, NL: E.B. Gómez García, M.J. Blok, M. de Boer; University of Groningen, NL: L.P.V. Berger, A.H. van der Hout, M.J.E. Mourits, G.H. de Bock; the Netherlands Comprehensive Cancer Organisation (IKNL): S. Siesling, J. Verloop; Nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA): E.C. van den Broek. HEBON thanks the study participants and the registration teams of IKNL and PALGA for contributing to data collection. Authors would like to thank Michael Schaapveld for his help with the SIR to attained age analyses. The authors would like to thank all pathology departments at the hospitals that have send pathology material for study purposes, including the NKI-AVL Biobank. Authors thank the registration team at the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry. Authors would like to thank J.J.R. Barkey Wolf for his help with writing part of the R-script.
Prior presentations: The study has been presented at the Joint Meeting of HEBON-IMPAHC-VKGN 2019 d.d. November 7, 2019.
Data Availability
Data underlying this article were collected with informed consent in the national collaborative HEBON cohort study. The HEBON steering group provided permission to share the data for this purpose with the study team, including the corresponding author (HOP2016006). Entered final sentence of the methods section which states that data is avialable.
Supplementary Material
References
- 1.Chen S, Parmigiani G.. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beiner ME, Finch A, Rosen B, et al. ; Hereditary Ovarian Cancer Clinical Study Group. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol. 2007;104(1):7–10. [DOI] [PubMed] [Google Scholar]
- 3.Segev Y, Iqbal J, Lubinski J, et al. ; Hereditary Breast Cancer Study Group. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Gynecol Oncol. 2013;130(1):127–131. [DOI] [PubMed] [Google Scholar]
- 4.Laitman Y, Michaelson-Cohen R, Levi E, et al. ; the Israeli Consortium of Hereditary Breast Cancer. Uterine cancer in Jewish Israeli BRCA1/BRCA2 mutation carriers. Cancer. 2019;125(5):698–703. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Milne RL, Lheureux S, et al. ; Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab). Risk of uterine cancer for BRCA1 and BRCA2 mutation carriers. Eur J Cancer. 2017;84:114–120.28802188 [Google Scholar]
- 6.Reitsma W, Mourits MJ, de Bock GH, Hollema H.. Endometrium is not the primary site of origin of pelvic high-grade serous carcinoma in BRCA1 or BRCA2 mutation carriers. Mod Pathol. 2013;26(4):572–578. [DOI] [PubMed] [Google Scholar]
- 7.Kitson SJ, Bafligil C, Ryan NAJ, et al. BRCA1 and BRCA2 pathogenic variant carriers and endometrial cancer risk: a cohort study. Eur J Cancer. 2020;136:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu CA, Pike MC, Kauff ND.. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2017;3(3):417–418. [DOI] [PubMed] [Google Scholar]
- 9.Saule C, Mouret-Fourme E, Briaux A, et al. Risk of serous endometrial carcinoma in women with pathogenic BRCA1/2 variant after risk-reducing salpingo-oophorectomy. J Natl Cancer Inst. 2018;110(2):213–215. [DOI] [PubMed] [Google Scholar]
- 10.Kandoth C, Schultz N, Cherniack AD, et al. ; Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94(5):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGunigal M, Liu J, Kalir T, Chadha M, Gupta V.. Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer. 2017;27(1):85–92. [DOI] [PubMed] [Google Scholar]
- 13.Thomas S, Hussein Y, Bandyopadhyay S, et al. Interobserver variability in the diagnosis of uterine high-grade endometrioid carcinoma. Arch Pathol Lab Med. 2016;140(8):836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.León-Castillo A, de Boer SM, Powell ME, et al. ; on behalf of the TransPORTEC Consortium. Molecular classification of the PORTEC-3 Trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jonge MM, Auguste A, van Wijk LM, et al. Frequent homologous recombination deficiency in high-grade endometrial carcinomas. Clin Cancer Res. 2019;25(3):1087–1097. [DOI] [PubMed] [Google Scholar]
- 16.de Jonge MM, Ritterhouse LL, de Kroon CD, et al. Germline BRCA-associated endometrial carcinoma is a distinct clinicopathologic entity. Clin Cancer Res. 2019;25(24):7517–7526. [DOI] [PubMed] [Google Scholar]
- 17.Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(5). [DOI] [PubMed] [Google Scholar]
- 18.Plon SE, Eccles DM, Easton D, et al. ; IARC Unclassified Genetic Variants Working Group. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29(11):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughley R, Karlic R, Joshi H, Turnbull C, Foulkes WD, Polak P.. Etiologic index - a case-only measure of BRCA1/2-associated cancer risk. N Engl J Med. 2020;383(3):286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman L, Beelen ML, Gallee MP, Hollema H, Benraadt J, van Leeuwen FE.. Risk and prognosis of endometrial cancer after tamoxifen for breast cancer. Comprehensive Cancer Centres' ALERT Group. Assessment of liver and endometrial cancer risk following tamoxifen. Lancet. 2000;356(9233):881–887. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow AJ, Jones ME. For the British Tamoxifen Second Cancer Study Group. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97(5):375–384. [DOI] [PubMed] [Google Scholar]
- 23.Jones ME, van Leeuwen FE, Hoogendoorn WE, et al. Endometrial cancer survival after breast cancer in relation to tamoxifen treatment: pooled results from three countries. Breast Cancer Res. 2012;14(3):R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley CW, Da Cruz Paula A, Kumar R, et al. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol Oncol. 2019;152(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord CJ, Ashworth A.. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–120. [DOI] [PubMed] [Google Scholar]
- 28.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M.. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 29.McCullough ML, Patel AV, Patel R, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17(1):73–79. [DOI] [PubMed] [Google Scholar]
- 30.Renehan AG, MacKintosh ML, Crosbie EJ.. Obesity and endometrial cancer: unanswered epidemiological questions. BJOG. 2016;123(2):175–178. [DOI] [PubMed] [Google Scholar]
- 31.Medicines and Healthcare Products Regulatory Agency. https://assets.publishing.service.gov.uk/media/5d680384ed915d53b8ebdba7/table2.pdf. Accessed October, 2020.
- 32.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E.. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. [DOI] [PubMed] [Google Scholar]
- 33.Integraal Kankercentrum Nederland. www.iknl.nl/nkr-cijfers. Accessed January, 2019.
- 34.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. [DOI] [PubMed] [Google Scholar]
- 35.Fulk K, Milam MR, Li S, et al. Women with breast and uterine cancer are more likely to harbor germline mutations than women with breast or uterine cancer alone: a case for expanded gene testing. Gynecol Oncol. 2019;152(3):612–617. [DOI] [PubMed] [Google Scholar]
- 36.Pennington KP, Walsh T, Lee M, et al. BRCA1, TP53, and CHEK2 germline mutations in uterine serous carcinoma. Cancer. 2013;119(2):332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying this article were collected with informed consent in the national collaborative HEBON cohort study. The HEBON steering group provided permission to share the data for this purpose with the study team, including the corresponding author (HOP2016006). Entered final sentence of the methods section which states that data is avialable.