Abstract
Background
Abnormal spindle‐like microcephaly (ASPM) has been proved to participate in tumor progression. However, the underlying mechanism of ASPM in liver hepatocellular carcinoma (LIHC) remains elusive.
Methods
The mRNA and protein expression were determined using Western blot and qRT‐PCR, and the capacities of cells proliferation, migration, and invasion were evaluated by CCK‐8, colony formation, wound healing, and transwell. MeRIP was performed to validate the interaction between ASPM and methyltransferase‐like 3 (METTL3).
Results
Herein, we found that ASPM was significantly upregulated in LIHC, and the high expression of ASPM was associated with poor LIHC prognosis. Furthermore, ASPM knockdown could suppress LIHC cells proliferation, migration, and invasion, while ASPM overexpression exerted reverse effect. Mechanistically, we revealed that the N6‐methyladenosine (m6A) modification of ASPM mRNA mediated by METTL3 promoted its expression in LIHC. More importantly, silencing METTL3 suppressed LIHC cells proliferation, migration, and invasion, which could be retained by ASPM overexpression.
Conclusion
Collectively, our findings suggested that METTL3/ASPM axis could serve as a novel promising therapeutic candidate for LIHC.
Keywords: ASPM, cells proliferation, hepatocellular carcinoma, invasion, LIHC, m6A, migration
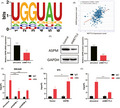
METTL3 mediated m6A modification of ASPM in LIHC.A. SRAMP database was performed to predict m6A sequences motif of METTL3 and ASPM. B. STARbase database was performed to analyze the correlation between the expression of METTL3 and ASPM. For further confirmation, SNU449 cells were stably infected with METTL3 knockdown, and ASPM protein and mRNA expression level were measured by qPCR(C) and Western blotting (D). E. ASPM was silenced in LIHC cells, and MeRIP‐qPCR was adopted to test m6A levels of ASPM. F. ASPM was overexpressed in LIHC cells, and MeRIP‐qPCR was adopted to test m6A levels of ASPM. G. MeRIP‐PCR was utilized to assess the m6A levels of ASPM when METTL3 was knockdown. **p < 0.01, ***p < 0.001. Data represent at least three independent sets of experiment.
1. INTRODUCTION
Liver cancer is the fourth common trigger of inducing cancer‐related mortality globally.1 Liver hepatocellular carcinoma (LIHC) is the most pathological genre of liver cancer, occupying 75%–85% of all cases globally in liver cancer.2 Currently, LIHC can be alleviated by therapeutic strategies, including liver transplantation surgery, interventional therapy, surgical resection, chemotherapy, and radiotherapy,3 whereas the poor 5‐year survival rate, distant metastasis, and frequent recurrence contribute to poor prognosis of LIHC patients.4, 5 Therefore, there is an urgent need to develop the diagnosis and treatment of LIHC and explore the molecular mechanism underlying tumorigenesis; moreover, clarifying the novel biomarkers predicting LIHC recurrence has far‐reaching significance for ameliorating prognosis.
Abnormal spindle‐like microcephaly (ASPM) is initially identified as a microcephaly‐associated protein,6 which primarily involved in determining the neurogenesis7 and brain size.8, 9 Recently, ASPM has been proved to participate in the cancers progression through diverse mechanisms. Wang J et al. suggested that ASPM served an crucial oncogenic part in regulating LUAD cell migration by facilitating epithelial‐mesenchymal transition via the PI3K/AKT pathway,10 and Yuan Y J et al. clarified that ASPM contributed to the development of LSCC by mediating CDK4.11 However, whether and how ASPM regulates LIHC progression remain elusive.
RNA methylation modifications approximately account for 60% of RNA modifications, and N6‐methyladenosine (m6A) is the most popular modification in eukaryotic mRNA and IncRNAs.12 Mechanistically, m6A methylation mainly occurs on adenine of the RRACH sequence,13 and its biological function primarily determined by m6A methylases, which consisting of writers (METTL3, METTL14, and WTAP), erasers (FTO, ALKBH5), and readers (YTHDFs).14 Moreover, m6A methylases system has been proved to mediate the biological functions, such as RNA transcription, processing, splicing, degradation, translation, and stability.15, 16 Among these m6A methylases, previous studies have demonstrated that METTL3 has participated in regulating cancers progression; for example, METTL3‐mediated mA modification upregulated Sec62 which promoted the chemoresistance to CRC though mediating β‐catenin and enhancing Wnt pathways.17 Nevertheless, the mechanism through which METTL3 mediates the m6A methylation of ASPM to regulate LIHC remains elusive.
Therefore, we examined the expression level of ASPM in LIHC tissues and analyzed the correlation between ASPM expression with prognosis of LIHC patients. Moreover, we investigated the functional effect of ASPM on LIHC cells proliferation, migration, and invasion and revealed the m6A methylation modification of ASPM mediated by METTL3. To summarize, our study reveals a novel mechanism of METTL3/ASPM axis in LIHC cells growth and metastasis, which may be considered as novel biomarkers for LIHC recurrence prediction and potential therapeutic targets.
2. MATERIALS AND METHODS
2.1. Tissue collection
LIHC tissues and corresponding normal LIHC tissues were collected from Ningbo First Hospital. The use of clinical samples was approved by the ethics committee of Ningbo First Hospital. The research was performed consistence with the guiding principles of the World Medical Association Declaration of Helsinki.
2.2. Cell culture and transfection
Human liver cancer cell lines SNU449, Hepg3b, and Hepg2 were purchased from the American Type Culture Collection (ATCC, USA), and MHCC‐97H cell line was obtained from the Liver Cancer Institute (Fudan University, Shanghai, China). SNU449 cells were incubated in ATCC‐formulated RPMI‐1640 Medium with 10% fetal bovine serum (FBS); in addition, ATCC‐formulated Eagle's Minimum Essential Medium containing 10% FBS was used to culture Hepg3b and Hepg2 cells. Dulbecco's modification of Eagle's medium (DMEM) (Thermo Fisher Scientific, USA) with 10% FBS was used to culture MHCC‐97H cells.
The overexpression vector of ASPM (pcDNA‐ASPM), shRNAs‐against ASPM, and METTL3 was purchased and designed by GeneChem Corporation (Shanghai, China) (Table S1). SNU449, Hepg3b, MHCC‐97H, and Hepg2 cells were transfected with indicated plasmids consistence with references of lipofectamine (Vision 2000, 11668‐019, Invitrogen, USA) and were cultured for 48 h.
2.3. Cell counting kit‐8 (CCK‐8)
The SNU449, Hepg3b, MHCC‐97H, and Hepg2 cells were added into a 96‐well plate containing 10% CCK‐8 solution (cell counting kit) (Sigma, USA). After incubation for 24, 48, 72, and 96 h, respectively, Cell counting kit reagent (Beyotime Biotechnology, Shanghai, China) was performed to detect the optical density (OD) value at 450 nm for the absorbance.
2.4. Colony formation assay
With a 1 × 103 cells per well density, SNU449, Hepg3b, MHCC‐97H, and Hepg2 cells at a density of 1 × 103 were maintained in a 6‐well plate for 2 weeks at 37°C with 5% CO2. Followed by fixing using 4% paraformaldehyde and staining using 1% crystal violet. Then, the number of colonies was calculated and the images were recorded.
2.5. Transwell
Transwell Chamber (Corning Glass Works, Corning, NY, USA) was employed to assess cell invasive ability. In brief, SNU449, Hepg3b, MHCC‐97H, and Hepg2 cells were added into the upper chambers containing Matrigel (BD, Franklin Lakes, United States). After 24 h, the invaded cells were fixed with 4% paraformaldehyde and staining with 0.1% crystal violet. The number of cells was counted, and images were recorded in inverted fields under a microscope (Zeiss, Germany).
2.6. Wound scratch assays
SNU449, Hepg3b, MHCC‐97H, and Hepg2 cells were seeded in 12‐well plate for 1 day, and then a sterile pipette tip was used to perform scratch, followed by incubating for another 48 h. Then, microscope was adopted to capture the images, and ImageJ software was used to measure the migration percentage.
2.7. RNA immunoprecipitation (RIP)
To assess the interaction of METTL3 and ASPM, transfected SNU449 cells were utilized for the extraction of total RNA. Magna methylated RNA immune precipitation (MeRIP) m6A Kit (17–10499, Merck Millipore, USA) was introduced for the evaluation of ASPM m6A modification, following manufacturer's indications. The immunoprecipitated RNA was examined utilizing qRT‐PCR.
2.8. Western blotting (WB)
The lysis buffer (Beyotime, China) was used to collect the protein, and protein assay kit of BCA (Beyotime, China) was performed for quantification. Then, the extracted protein samples were isolated by SDS‐PAGE gels (Jinsirui, China) and were transferred on the membranes of PVDF (Merck Millipore, USA). After then, these membranes were maintained in PBS containing 10% skim milk for 1 h and were mixed with the primary antibodies at 4°C overnight, and the blots were cultured with secondary antibody conjugated by horseradish peroxidase (HRP) (Cell Signaling Technology, USA). For evaluation of the protein expression level, Odyssey Infrared Imaging System (LI‐COR Biosciences) was adopted to visualize the blots and the Image J software (National Institutes of Health) was used to calculate the protein level. GAPDH (ab8245, 1:1000, abcam, UK) was used to normalize the loading. The primary antibodies ASPM (ab238106, 1:1000), PCNA (ab92552, 1:5000), N‐cadherin (ab76011, 1:10000), Vimentin (ab16700, 1:1000), and E‐cadherin (ab76055, 1:500) were purchased from Abcam (UK).
2.9. qRT‐PCR
For the assess of the mRNA expression level, the total RNA was obtained from tissues and cells by TRIzol reagent (Takara, Japan). RNA reverse‐transcription was performed using PrimeScript RT Reagent Kit (Takara, Japan). Then, the mRNA level of ASPM was evaluated by Real‐time PCR (RT‐PCR) consistent with manufacturer's references. GAPDH was used to normalize the mRNA level. The expression of the mRNA was analyzed using 2−ΔΔCt formula. The primer sequences are listed in Table S2.
2.10. Statistical analysis
SPSS (Version 20, Chicago, USA) and GraphPad Prism (Version 7, USA) were employed for the statistical analysis. Student's t test and one‐way analysis of variance (ANOVA) were adopted to evaluate the differences between two or multiple groups, and the Kaplan‐Meier method was performed to estimate overall survival. The data are represented as the mean ± SD in our study. p < 0.05 presents statistically significant.
3. RESULTS
3.1. ASPM is highly expressed in LIHC
To explore the correlation between ASPM and LIHC, we first performed online analysis using GEPIA2 database and found that ASPM was highly expressed in LIHC (Figure 1A). In addition, the overall survival analysis based on TCGA (https://portal.gdc.cancer.gov/) and ICGC (http://www.icgc.org/) database exhibited that LIHC patients with high ASPM expression indicated poor overall survival (Figure 1B). To further confirm the expression levels of ASPM, we utilized Western blotting (Figure 1C) and qPCR (Figure 1D) methods, and demonstrated that the expression levels of ASPM in LIHC group were considerably higher than in normal group. Collectively, these data suggested that ASPM was highly expressed in LIHC, and the high expression of ASPM was correlated with poor LIHC prognosis, implying that ASPM may participate in LIHC progression.
FIGURE 1.
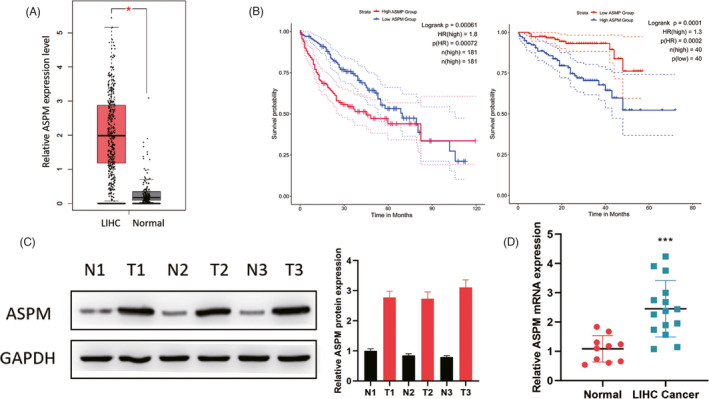
High ASPM expression in LIHC. (A) GEPIA2 database was performed to analyze the ASPM expression levels in LIHC. (B) Kaplan‐Meier survival curves of OS based on ASPM mRNA expression in LIHC patients. The data were analyzed in TCGA(left) and ICGC(right) database. ASPM protein and mRNA expression in LIHC tissues and corresponding normal tissues were measured by Western blotting (C) and qPCR (D). *p < 0.05, ***p < 0.001. Data represent at least three independent sets of experiment
3.2. ASPM knockdown inhibits LIHC cells growth and metastasis
To evaluate the functional effect of ASPM on LIHC cells, we established SNU449 and Hepg3b cells with stable ASPM knockdown (Figure 2A,B). Downregulation of ASPM significantly suppressed LIHC cells proliferation, as exhibited by CCK‐8 and colony formation results (Figure 2C,D). Similarly, transwell and wound scratch assays (Figure 2E,F) demonstrated that ASPM knockdown inhibited the migratory and invasive capacities of SNU449 and Hepg3b cells. Western blot assay exhibited that ASPM depletion caused the downregulation of PCNA, N‐cadherin, and Vimentin, and the up‐regulation of E‐cadherin, suggesting the suppressive effect on EMT process (Figure 2G). These findings indicated that silencing ASPM could remarkably suppress LIHC cells proliferation, invasion, and EMT process.
FIGURE 2.
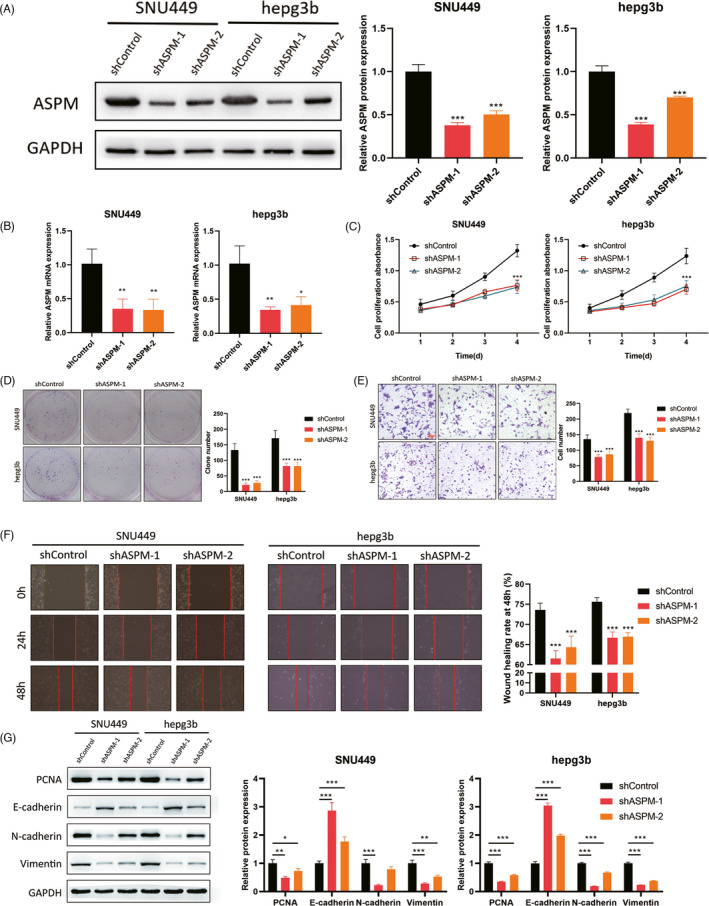
ASPM silence suppresses LIHC cells growth and metastasis. For functional experiments, SNU449 and Hepg3b cells were stably infected with ASPM shRNA, and the transfection efficacy was detected by Western blotting (A) and qPCR (B). CCK‐8 (C) and colony formation (D) were used to evaluate the proliferation rate. The transwell invasion (E) and wound scratch and (F) assays were adopted to examine the migratory and invasive capacity. G. Western blotting was utilized to detect EMT‐related proteins.*p < 0.05, **p < 0.01, ***p < 0.001. Data represent at least three independent sets of experiment
3.3. ASPM overexpression facilitates LIHC cells growth and metastasis
To further explore the effect of overexpressing ASPM on LIHC cells growth and metastasis, we upregulated ASPM expression in MHCC‐97H and Hepg2 cells (Figure 3A,B). CCK‐8 and colony formation assays exhibited that the proliferation rate of LIHC cells with overexpressed ASPM was much faster than the control group (Figure 3C,D). Moreover, the migratory and invasive capacities of LIHC cells were significantly enhanced by ASPM overexpression (Figure 3E,F). Additionally, we observed that ASPM overexpression upregulated the expression levels of PCNA, N‐cadherin, and Vimentin, while inhibited E‐cadherin (Figure 3G). These results clarified that ASPM overexpression could facilitate LIHC cells proliferation, migration, invasion, and EMT process.
FIGURE 3.
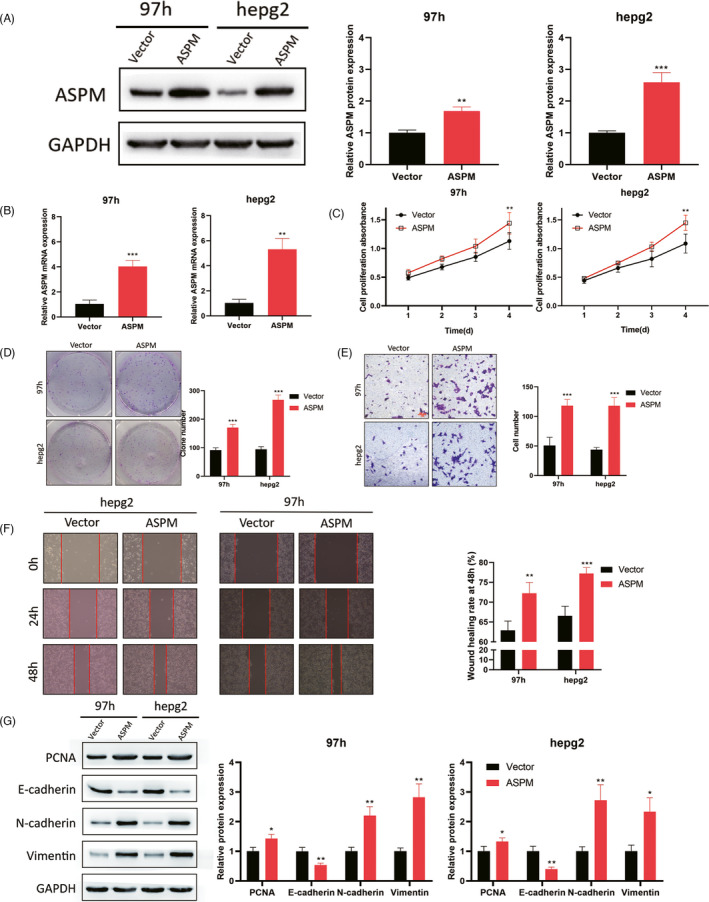
ASPM overexpression promotes LIHC cells growth and metastasis. For further investigation, MHCC‐97H and Hepg2 cells were infected with ASPM overexpression plasmid, and the transfection efficacy was detected by Western blotting (A) and qPCR (B). CCK‐8 (C) and colony formation (D) were used to measure the proliferation rate. The transwell invasion (E) and wound scratch (F) assays were used to examine the invasion and migration. G. Western blotting was employed to detect EMT‐related proteins. **p < 0.01, ***p < 0.001. Data represent at least three independent sets of experiment
3.4. METTL3‐mediated m6A modification of ASPM in LIHC
We next explored the underlying mechanism of ASPM in mediating LIHC cells biological function. The bioinformatics tool SRAMP revealed that there was an m6A sequences motif which could be considered as the binding site of METTL3 on ASPM (Figure 4A). In addition, STARbase (http://starbase.sysu.edu.cn/) database exhibited that the expression of ASPM was positively correlated with METTL3 in LIHC (Figure 4B). For further confirmation, we performed METTL3 knockdown in LIHC cells and observed that silencing METTL3 inhibited the expression level of ASPM (Figure 4C,D). Furthermore, MeRIP‐qPCR showed that the m6A modification was enriched in ASPM mRNA sequence (Figure 4E,F) and METTL3 depletion significantly decreased the m6A abundant levels of ASPM in LIHC cells (Figure 4G). These results implied that METTL3 positively regulated ASPM expression via m6A modification in LIHC.
FIGURE 4.
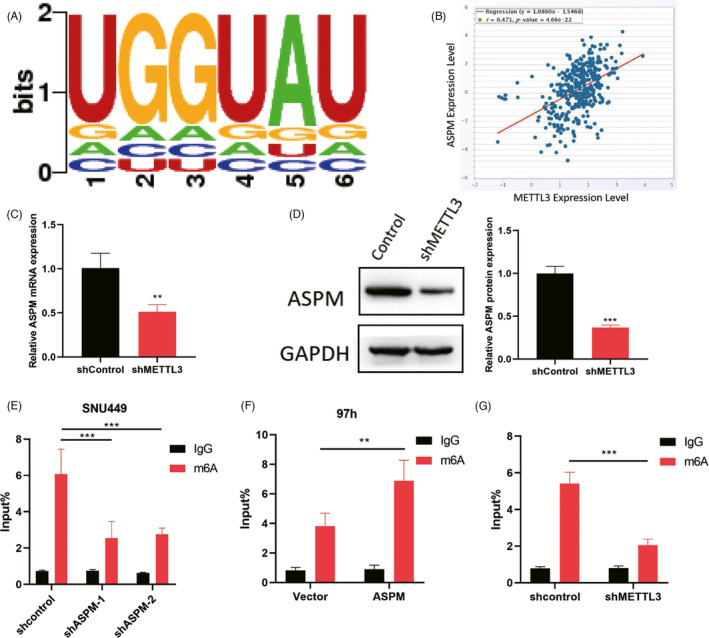
METTL3 mediated m6A modification of ASPM in LIHC. (A) SRAMP database was performed to predict m6A sequences motif of METTL3 and ASPM. (B) STARbase database was performed to analyze the correlation between the expression of METTL3 and ASPM. For further confirmation, SNU449 cells were stably infected with METTL3 knockdown, and ASPM protein and mRNA expression level were measured by qPCR (C) and Western blotting (D). (E) ASPM was silenced in LIHC cells and MeRIP‐qPCR was adopted to test m6A levels of ASPM. (F) ASPM was overexpressed in LIHC cells and MeRIP‐qPCR was adopted to test m6A levels of ASPM. (G) MeRIP‐PCR was utilized to assess the m6A levels of ASPM when METTL3 was knockdown. **p < 0.01, ***p < 0.001. Data represent at least three independent sets of experiment
3.5. METTL3 promotes LIHC cells growth and metastasis via ASPM
To validate whether METTL3 regulates LIHC cells progression in a ASPM‐dependent manner, SNU449 and Hepg3b cells were cotransfected with METTL3 shRNA plasmid and ASPM overexpression plasmid, and Western blot and qPCR assays were performed to examine the transfection efficacy (Figure 5A). We found that METTL3 knockdown significantly suppressed LIHC cells proliferation, which could be rescued by ASPM overexpression (Figure 5B,C). Similarly, the migratory and invasive capacities of LIHC cells were inhibited by METTL3 knockdown, which could be retained by ASPM overexpression (Figure 5D,E). Taken together, our results demonstrated that METTL3 could promote LIHC cells proliferation, migration, and invasion via upregulating ASPM.
FIGURE 5.
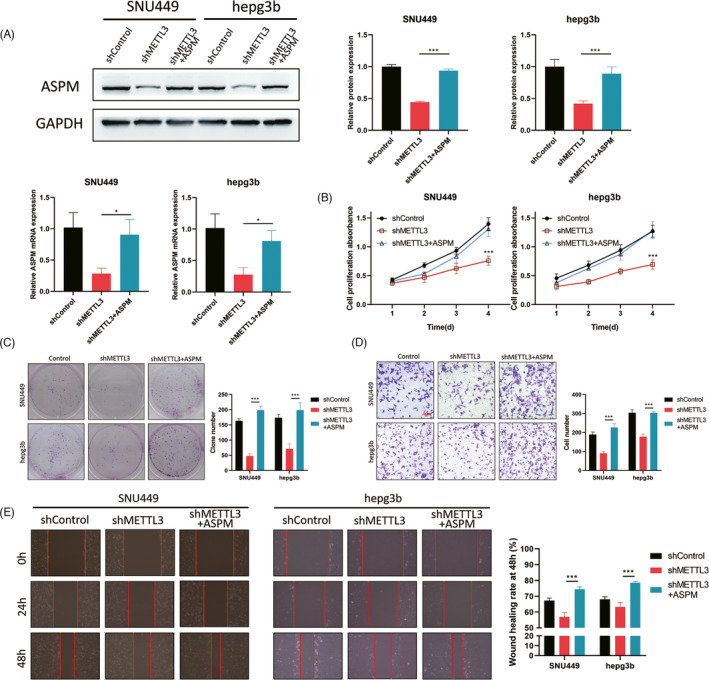
METTL3 facilitates LIHC cells growth and metastasis via ASPM. SNU449 and Hepg3b cells were cotransfected with METTL3 shRNA plasmid and ASPM overexpression plasmid. (A) The expression level of ASPM was examined using Western blot and qPCR. The CCK‐8 (B) and colony formation (C) were used to analyze the proliferation rate. The transwell invasion (D) and wound scratch (E) experiments were performed to examine the migratory and invasive capacities. *p < 0.05, ***p < 0.001. Data represent at least three independent sets of experiment
4. DISCUSSION
In this study, our results illustrated that ASPM was highly expressed in LIHC, and the high expression of ASPM was correlated with poor LIHC prognosis. In addition, ASPM knockdown suppressed LIHC cells proliferation, migration, and invasion, while overexpressing ASPM exerted the inhibited effects. Moreover, METTL3 promoted LIHC cells growth and metastasis via m6A modification of ASPM.
Previous studies have suggested that ASPM mediated the spindle organization, spindle orientation, and cytokinesis18; in particular, it was proved that its cytoplasmic expression levels vary remarkably among different types of tumor.19, 20 These reports implied that ASPM exerts diverse biological functions in malignant tumor. In addition, extensive studies have shown that ASPM was highly expressed in cancer and participated in promoting the development of diverse cancers, such as diffuse large B‐cell lymphoma,21 bladder cancer,22 glioblastoma,19 and prostate cancer.23 Consistent with previous reports, we found that ASPM was highly expressed in LIHC tissues and could facilitate LIHC cells proliferation, migration, and invasion.
As referred above, m6A methylation is the widely popular modification in approximately 100 types of chemical modifications, and m6A modification has made significant contribution to the cancers progression. For example, as a crucial mA "reader," YTHDF1 aggravated the progression of cervical cancer through mA‐mediated up‐regulation of RANBP2,24 HNRNPA2B1 induced multiple myeloma development by mediating ILF3 mRNA stabilization in a m6A‐dependent manner,25 and METTL14 inhibited the tumor metastasis of hepatocellular carcinoma via modifying the m6A methylation of GFR/PI3K/AKT.26 As a crucial m6A methylase, METTL3 has been indicated to involve in cancers progression via mediating cancer‐related proteins methylation level. ChenF et al. suggested that METTL3 mediated N6‐methyladenosine in KRT7 mRNA and regulated its translation efficiency to promote breast cancer lung metastasis27; in addition, Chen C et al. demonstrated that METTL3‐mediated circ1662 accelerated YAP1 nuclear localization to enhance the capacity of tumor metastasis in colorectal cancer.28 However, whether METTL14 regulates m6A methylases modification of ASPM in LIHC remains elusive. Herein, we found that there was an m6A sequences motif which could be considered as the binding site of METTL3 on ASPM, as well as a positive correlation between the expression level of METTL3 and ASPM. Furthermore, METTL3 could facilitate LIHC cells growth and metastasis via m6A modification of ASPM.
To summarize, our study demonstrated that ASPM was upregulated in LIHC and the high expression of ASPM indicated poor LIHC prognosis. In addition, ASPM could promote LIHC cells proliferation, migration, and invasion. Moreover, we suggested that METTL3 mediated m6A methylation of ASPM to facilitate LIHC cells growth and metastasis. These findings provided the rationale for considering METTL3/ASPM axis as a novel therapeutic strategy against progression of LIHC.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Tab S1
Tab S2
ACKNOWLEDGEMENTS
None.
Wang A, Chen X, Li D, Yang L, Jiang J. METTL3‐mediated m6A methylation of ASPM drives hepatocellular carcinoma cells growth and metastasis. J Clin Lab Anal. 2021;35:e23931. 10.1002/jcla.23931
Contributor Information
An Wang, Email: Wangan_ZJ@163.com.
Jianshuai Jiang, Email: Jiangshuai67@163.com.
DATA AVAILABILITY STATEMENT
My manuscript has no associated data.
REFERENCES
- 1.Llovet J, Montal R, Sia D, Finn R. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599‐616. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Ma H, Li X, et al. DNASE1L3 as an indicator of favorable survival in hepatocellular carcinoma patients following resection. Aging. 2020;12(2):1171‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975‐2014, featuring survival. J Natl Cancer Inst. 2017;109(9):1975‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji J, Rong Y, Luo C, et al. Up‐regulation of hsa‐miR‐210 promotes venous metastasis and predicts poor prognosis in hepatocellular carcinoma. Front Oncol. 2018;8:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong X, Liu L, Zhao A, Pfeifer G, Xu X. The abnormal spindle‐like, microcephaly‐associated (ASPM) gene encodes a centrosomal protein. Cell cycle (Georgetown, Tex). 2005;4(9):1227‐1229. [DOI] [PubMed] [Google Scholar]
- 7.Williams S, Garcia I, Crowther A, et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development (Cambridge, England). 2015;142(22):3921‐3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponting C. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics (Oxford, England). 2006;22(9):1031‐1035. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J. Evolution of the human ASPM gene, a major determinant of brain size. Genetics. 2003;165(4):2063‐2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Liang J, Li H, et al. Oncogenic role of abnormal spindle‐like microcephaly‐associated protein in lung adenocarcinoma. Int J Oncol. 2021;58(5):23‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Y, Sun Y, Gao R, Yin Z, Yuan Z, Xu L. Abnormal spindle‐like microcephaly‐associated protein (ASPM) contributes to the progression of Lung Squamous Cell Carcinoma (LSCC) by regulating CDK4. J Cancer. 2020;11(18):5413‐5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer K, Saletore Y, Zumbo P, Elemento O, Mason C, Jaffrey S. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149(7):1635‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang Z, Hu Y, Hu J, Huang Y, Zheng S, Guo C. The crucial roles of N‐methyladenosine (mA) modification in the carcinogenesis and progression of colorectal cancer. Cell Biosci. 2021;11(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B‐B, Wang X‐Y, Gu X‐Y, et al. N 6‐methyladenosine (m 6 A) RNA modification in gastrointestinal tract cancers: roles, mechanisms, and applications. Mol Cancer. 2019;18(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Choe J, Park O, Kim Y. Molecular mechanisms driving mRNA degradation by mA modification. Trends Genet. 2020;36(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 16.Wei W, Ji X, Guo X, Ji S. Regulatory role of N ‐methyladenosine (m A) methylation in RNA processing and human diseases. J Cell Biochem. 2017;118(9):2534‐2543. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Su K, Sun X, et al. Sec62 promotes stemness and chemoresistance of human colorectal cancer through activating Wnt/β‐catenin pathway. J Exp Clin Cancer Res. 2021;40(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Midgley C, Bergh A, et al. Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol. 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Huang L, Yang Y, et al. ASPM promotes glioblastoma growth by regulating G1 restriction point progression and Wnt‐β‐catenin signaling. Aging. 2020;12(1):224‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Zhuo Y, Zheng Y, et al. High expression of ASPM correlates with tumor progression and predicts poor outcome in patients with prostate cancer. Int Urol Nephrol. 2017;49(5):817‐823. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, He Z, Zhu Y, Jiang C, Deng Y, Wei B. ASPM predicts poor clinical outcome and promotes tumorigenesis for diffuse large B‐cell lymphoma. Curr Cancer Drug Targets. 2021;21(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 22.Gao Z, Yu F, Jia H, Ye Z, Yao S. ASPM predicts poor prognosis and regulates cell proliferation in bladder cancer. Kaohsiung J Med Sci. 2020;36(12):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 23.Pai V, Hsu C, Chan T, et al. Correction: ASPM promotes prostate cancer stemness and progression by augmenting Wnt‐Dvl‐3‐β‐catenin signaling. Oncogene. 2019;38(8):1354. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Luo Q, Kang J, et al. YTHDF1 aggravates the progression of cervical cancer through mA‐mediated up‐regulation of RANBP2. Front Oncol. 2021;11:650383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang F, Tang X, Tang C, et al. HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A‐dependent stabilization of ILF3 mRNA. J Hematol Oncol. 2021;14(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Zhuang Y, Zhang J, Chen M, Wu S. METTL14 inhibits hepatocellular carcinoma metastasis through regulating EGFR/PI3K/AKT signaling pathway in an m6A‐dependent manner. Cancer Manag Res. 2020;12:13173‐13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Chen Z, Guan T, et al. N6‐methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Can Res. 2021;81:2847‐2860. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Yuan W, Zhou Q, et al. N6‐methyladenosine‐induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11(9):4298‐4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Tab S2
Data Availability Statement
My manuscript has no associated data.


