Abstract
Background
Rheumatoid arthritis (RA) and periodontitis (P) are chronic inflammatory diseases characterized by joint and radiographic bone loss, respectively. IL‐23 and IL‐17 have an essential role in the immunopathogenesis of RA, and P. IL‐23 stimulates Th17 cells through which produces IL‐17, IL‐21, and RANKL. IL‐17 stimulates fibroblasts to produce RANKL, which initiates bone loss in the joints in RA and the periodontal tissue in periodontitis. The aim of this study was to determine the expression pattern of IL‐23/IL‐17 axis and soluble receptors isoforms sIL‐23R and sIL‐17RA of patients with RA presenting P (RAP).
Material and methods
Healthy subjects (HS) (n = 42), patients with P (n = 40), RA (n = 20), and patients with RAP (n = 40) were included. Plasma samples were obtained to evaluate the IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA by ELISA technique. A nonparametric Mann‐Whitney U test was used to compare the differences between groups. A Chi‐square was used to compare gender, grade and stage of periodontitis, and DAS28‐ESR between the groups. Spearman's rank correlation coefficient was used to study the association between the molecules and clinical parameters.
Results
IL‐23 levels were increased in the RAP group, and lower sIL‐23R levels were found in the RAP groups. However, IL‐17A was lower in the P and RAP group but not in RA patients. RAP group showed a decrease IL‐17A levels in advanced stages of the periodontal disease.
Conclusion
These results suggest that IL‐23 and IL‐17A tend to downregulate their expression patterns when patients present both rheumatoid arthritis and periodontitis.
Keywords: IL‐17A, IL‐23, rheumatoid arthritis‐presenting periodontitis, sIL‐17RA, sIL‐23R
Blood sample was taken to obtain plasma from all study participants. IL‐23, IL‐23R, IL‐17A, and IL‐17RA were detected with ELISA technique. Results: (a) The sIL‐23R levels were found lower in the RAP group and IL‐23 levels were increased. (b) IL‐17A was lower in the P and RAP group but not in RA patients. According to the chronicity of periodontitis, RAP group showed a decreased IL‐17A levels in advanced stages of the periodontal disease.

1. INTRODUCTION
Rheumatoid Arthritis (RA) is a chronic, systemic, and autoimmune inflammatory disease characterized by inflammation of the synovial membrane and the progressive destruction of articular cartilage and bone.1
Periodontal diseases are a group of disorders with different etiologies and clinical manifestations, including periodontitis (P), associated with a dysbiotic biofilms of periodontophatogenic bacteria Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Periodontitis is characterized by a strong inflammatory response, gingival inflammation, the formation of periodontal pockets, attachment loss, and radiographic bone loss.2, 3
It has been reported that patients with RA are more likely to develop P and that both diseases have genetic, biological, and environmental risk factors in common, including the immune response of the host.4 In this context, the support tissue destruction of the periodontium in periodontitis and the synovium in RA is generated by several mechanisms including the RANK/RANKL/OPG system. RANKL is produced by Th17 cells under the stimulation of IL‐23 or by fibroblasts stimulated by IL‐17.5 It is known that the IL‐23 produced by dendritic cells or macrophages stimulated by lipopolysaccharides binds to its specific receptor IL‐23R on Th17 cells to activate and maintain clonal expansion, as well as produce IL‐17 and RANKL.6, 7 Interestingly, IL‐23R can be released in a soluble form (sIL‐23R) and bind to IL‐23, which has been suggested to act as a signaling blocker or activator of Th17 cells.8, 9, 10 Likewise, IL‐17 activates some non‐immune cells like fibroblasts through its receptor (IL‐17RA/RC). IL‐17RA can be released in a soluble form (sIL‐17RA), and this variant could be a possible inhibitor for IL‐17A signaling.11
Once the fibroblast is activated, it can produce RANKL, and then RANKL from Th17 or fibroblasts leads to the maturation of osteoclast precursors through RANK to initiate bone loss in the joints in RA and periodontal tissue in periodontitis.12
The axis IL‐23/IL‐17 is involved in the development of chronic inflammation, host defense against bacterial infections, as well as in bone loss, and progression of periodontitis and rheumatoid arthritis.2, 4 In this context, IL‐23/IL‐17 axis in RA and P has also been studied in different samples,13, 14, 15, 16, 17, 18, 19, 20, 21, 22 as well as its receptors, IL‐23R, and IL‐17RA.23, 24, 25, 26 In patients with rheumatoid arthritis who present periodontitis, IL‐17 has been reported in the gingival crevicular fluid (GCF) and serum,27 while IL‐23 has been reported in saliva,28 likewise IL‐23 and IL‐17 in serum.29 It has also been described that IL‐23 and IL‐17 levels in GCF, saliva, serum, and plasma are elevated in both periodontitis patients and17, 18, 20, 21, 22, 24 patients with rheumatoid arthritis.13, 15, 16, 25, 27, 30, 31 Therefore, we hypothesize that IL‐23 and IL‐17A levels, as well as their soluble receptors, are elevated in patients with both pathologies. Therefore, the aim of this study was to determine the expression pattern of IL‐23/IL‐17 axis in the plasma of patients with rheumatoid arthritis‐presenting periodontitis.
2. MATERIALS AND METHODS
2.1. Study population
A cross‐sectional study was performed since March 2018 to November 2019. Patients who had clinical features typical of periodontitis and rheumatoid arthritis (described in detail below), as well as healthy subjects, were enrolled consecutively in this observational and cross‐sectional study. A total of 42 healthy subjects and 40 periodontitis patients were recruited from the Periodontology Clinic at the University of Guadalajara; twenty patients with rheumatoid arthritis and thirty‐eight rheumatoid arthritis patients with periodontitis were recruited from the “Fray Antonio Alcalde” Hospital of Guadalajara. The study was approved by the Biosecurity, Ethics, and Investigation committees from the University of Guadalajara with the number 20–97 and by the Ethic committee “Fray Antonio Alcalde” Civil Hospital with the number 155/17. The objective of the study was explained to each participant before they agreed to participate, and informed consent was written in accordance with the Declaration of Helsinki 2013.
2.2. Inclusion and exclusion criteria
2.2.1. Healthy subject group
This group included 42 patients who attended clinics for periodontal esthetic treatment with no clinical evidence of gingivitis and periodontitis.
2.2.2. Periodontitis group
This group included 40 patients diagnosed according to the new classification published in 2018 by the American Academy of Periodontics (AAP) and the European Federation of Periodontics (EFP) by a periodontology specialist.32
2.2.3. Rheumatoid arthritis groups
This patient was recruited by the Rheumatology Service of the “Fray Antonio Alcalde hospital”. A total of 60 patients were diagnosed using the 2010 Classification Criteria for Rheumatoid Arthritis of the American College of Rheumatology/European League Against Rheumatism33 by a rheumatology specialist; the periodontal diagnosis was performed by a periodontology specialist with the criteria mentioned previously. Patients were divided into two groups: 20 patients were diagnosed only with Rheumatoid Arthritis (RA) and 40 patients with Rheumatoid Arthritis who present periodontitis (RAP).
2.2.4. Exclusion criteria
No study groups included patients or subjects under 18 years of age, pregnant women, smokers, or study subjects with other diseases like gingivitis, Sjögren syndrome, psoriasis, or any systemic disease. Patients or subjects with previous periodontal treatment, antibiotics, corticosteroid, biologic therapies, or anti‐inflammatory drugs during the six months before the study were not included.
2.3. Periodontal clinical measurements
Clinical examinations were performed on all existing teeth of the study subjects, and the periodontal conditions were assessed based on the following parameters: percentage of bleeding on probing (% BoP), probing depth (PD), clinical attachment level (CAL), and percentage of radiographic bone loss (% RBL). The examination of all subjects was performed using a periodontal probe (15 mm, probe tip diameter 0.5 mm; University of North Carolina UNC‐15 Hu‐Friedy®) (Hu‐Friedy) by a single researcher, and the mean sulcular depth was calculated.
Clinical parameters were obtained on six sites per tooth, and the results were expressed as mean ± standard error (SEM) with % BoP and radiographic bone loss, PD, and CAL. Periapical radiographs were performed by the same researcher using the long‐cone paralleling technique with the same radiographic equipment, film, exposure, and development conditions for all subjects.
For the subclassification staging of P (I, II, III, and IV), CAL and the number of dental organs lost due to periodontitis were considered. Stage I to IV of periodontitis are defined based on severity and complexity of management (primarily periodontal breakdown with reference to root length and periodontitis‐associated tooth loss PD and CAL). The periodontal grade (A, B, and C) is the evidence or rapid progression and is estimated with direct or indirect evidence of progression rate in three categories: slow, moderate, and rapid progression and was evaluated with the percentage of radiographic bone loss/age ratio with Image Studio Lite software v. 5.2 in according to the new classification of periodontal diseases.32
2.4. Rheumatoid clinical parameters
The disease activity score was evaluated with 28 joints (DAS28‐ESR), with tender joint counts (TJC) and swollen joint counts (SJC), the erythrocyte sedimentation rate (ESR), and the visual analog scale (VAS). These values were divided into different degrees of disease activity: >5.1, high activity: ≤5.1 to 3.2, moderate activity; ≤3.2, low activity; and ≤2.6, remission. The Health Assessment Questionnaire Disability Index (HAQ‐DI) was evaluated for all RA and RAP patients by a rheumatology specialist. The erythrocyte sedimentation rate (ESR) was measured with Wintrobe tubes, where the rate of the fall of red blood cells was measured in millimeters after 1 hour. The C‐reactive protein (CRP) and rheumatoid factor (RF) were measured by the clinical laboratory of the “Fray Antonio Alcalde Hospital.”
2.5. Sample collection and ELISA
The peripheral blood obtained by venous puncture in EDTA tubes was centrifuged to isolate the plasma for 10 min at 700 g and stored immediately at −80°C until the enzyme‐linked immunosorbent assay (ELISA) for cytokines IL‐23, IL‐23R, IL‐17A, and IL‐17RA DuoSet® ELISA Kit (R&D Systems Minneapolis MN) was performed. Plasma was added in triplicate to the wells of microtiter plates to determine the concentrations of these molecules. Each well's optical density was determined using a microplate reader set to 450 nm with a wavelength correction to 540nm. The signal was detected using a Microplate Reader WHY101 (Poweam Medical Systems Co.). Cytokine and receptor concentrations were calculated from the standard curve according to the assay kit. The concentrations were expressed as the pg/mL in plasma.
2.6. Statistical analysis
The data distribution was evaluated using the Shapiro‐Wilk test for a small sample size. For the data that presented an abnormal distribution, a nonparametric Mann‐Whitney U test was used to compare the differences between groups. A Chi‐square was used to compare the gender, grade, stage of periodontitis, and DAS28‐ESR between the HS, P, RA, and RAP groups. The Spearman's rank correlation coefficient was selected to study the association between the IL‐23, sIL‐23R, IL‐17A, and sIL‐17RA plasma levels and the clinical findings. All results were analyzed using the SPSS version 25.0 software (SPSS.). Significance was considered when p ≤ 0.05.
3. RESULTS
3.1. Sociodemographic characteristics and clinical parameters
In the current study, we found that in the four study groups, HS, P, RA, and RAP, the female sex prevailed. The clinical characteristics of periodontitis PD, CAL, % BoP, and % RBL were increased in the P group compared with the other study groups. However, RAP patients were considered within the range of periodontitis with a CAL value ≥3. On the other hand, the erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), and C‐reactive protein (CRP) were increased in the RA group, contrary to the tender joint count (TJC), swollen joint count (SJC), and DAS28‐ESR, which were increased in patients with RAP (see Table 1).
TABLE 1.
Sociodemographic characteristics and clinical parameters
| HS | P | |
|---|---|---|
| Gender M/F | 6/22 | 8/26 |
| Age (years) | 37.26 ± 1.82 | 40.85 ± 2.06 a |
| Stage III n (%) | – | 26 (76.5) |
| Stage IV n (%) | – | 8 (23.5) |
| Grade A n (%) | – | 17 (50) |
| Grade B n (%) | – | 11 (32.4) |
| Grade C n (%) | – | 6 (17.6) |
| PD (mm) | 2.14 ± 0.13 | 4.87 ± 0.4 a |
| CAL (mm) | 1.02 ± 0.2 | 5.24 ± 0.49 a |
| % BoP | 0.52 ± 0.52 | 22.65 ± 6.77 a |
| % RBL | – | 60.55 ± 5.18 |
| PD GT (mm) | 2.31 ± 0.11 | 5.21 ± 0.44 a |
| CAL GT (mm) | 1.07 ± 0.25 | 5.47 ± 0.46 a |
| % BoP GT | 1.38 ± 1.38 | 24.47 ± 8.56 |
Data are expressed as mean and standard error or percentage.
Abbreviations: % RBL, Percentage of Radiographic Bone Loss; %BoP, Bleeding of Probing percentage; CAL, Clinical Insertion Level; GT, specific area of gingival tissue where PD, NIC, and %BoP gingival tissue were obtained; PD, Probing depth.
Significant difference between HS and P. A p ≤ 0.05 was considered as significant.
3.2. IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels
Data are represented as median (min‐max). We found a significant increase of IL‐23 plasma levels in patients with RA [170.61 (30.65–11581.25)] and RAP [106.68 (10.74–11179.34)] compared with patients with P [46.8 (8.54–854.33)] and HS [48.35 (14.06–294.35)] (see Figure 1a).
FIGURE 1.
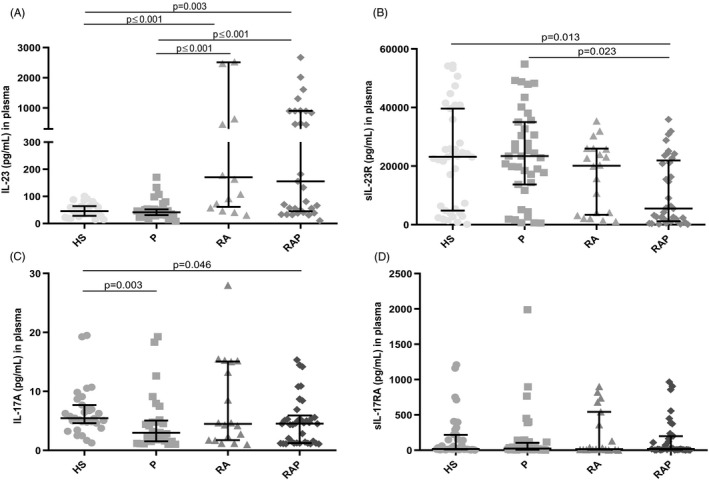
IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels in the studied groups. Data are represented with median and interquartile range. (A) IL‐23 was higher in RA and RAP compared with HS and P group, (B) sIL‐23R was higher in HS and P compared with RAP group, (C) IL‐17A was elevated in HS compared with P and RA group but not with RA group, d) no significant difference of sIL‐17RA between the study groups. HS: healthy subjects, P: periodontitis group, RA: rheumatoid arthritis group, RAP: rheumatoid arthritis with periodontitis group. p ≤ 0.05
The quantification of sIL‐23R showed lower levels in RAP [5548.15 (110.56–35938.62)] compared to patients with P [23432.58 (568.97–54846.82)] and HS [23195.15 (142.49–54486.12)]. Nonetheless, no differences were found in the RA group compared with the other study groups [20123.34 (1074.58–35387.7)] (see Figure 1b).
On the other hand, IL‐17A was higher in the HS [5.46 (1.26 −19.49)] compared with the P [2.99 (1.04 −19.26)] and RAP patients [4.61 (1.08–15.33)]. However, we did not observe a significant difference in the RA group compared with the other study groups [4.48 (0.99–27.0)] (see Figure 1c).
For sIL‐17RA, there were no significant differences among the four study groups, HS [18.54 (1.9–1209.4)] P [24.72 (0.35–1987.95)] RA [15.97 (1.08–901.82)], and RAP [18–16 (1.71–966.14)] (see Figure 1d).
With the support of the G * P3.1 software, a post hoc exploration of the effect size of the levels of IL‐23, IL‐17A, and sIL‐23R was carried out with greater interest that showed significant differences, considering a unilateral hypothesis with α = 0.05. Regarding the variable IL‐23, an effect size of d = 3.85, with a power of 1, was observed between HS and patients with RA. Moreover, between the HS and RAP patient the effect size was d = 5.49 with a power of 1. For the sIL‐23R between the HS and AR group, the effect was d = 3.01 with a power of 1. For the variable IL‐17A between HS and AR, an effect size of d = 0.96 was observed with a power of 0.98. And between HS and P, the effect size was d = 1.16 with a power of 0.99.
3.3. IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels according to stage and periodontal grade
There was an increase in P group of IL‐17A in grade B compared with grade A (p = 0.027). Similarly, sIL‐17RA was significantly higher in grades B when compared to grade A (p = 0.032) and in grade C compared to grade B (p = 0.012). In the other part, no significant differences were observed in the periodontal stage from P group. There was a significant increase of IL‐17A in stage II for the RAP group compared with stage III (p = 0.002). However, no patient from RAP group presented grade C of periodontitis, and there were no significant differences in IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA (see Table 2).
TABLE 2.
IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels according to the new periodontal classification based on stage and grade of the disease
| Periodontal stage P group (n) | IL−23 median (min–max) | sIL−23R median (min–max) | IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Stage I | – | – | – | – |
| (0) | ||||
| Stage II | – | – | – | – |
| 0 | ||||
| Stage III | 37.91 | 23964.22 | 2.66 | 31.25 |
| (31) | (8.54–854.87) | (626.23–54846.82) | (1.04–19.26) | (0.35–1987.6) |
| Stage IV | 53.79 | 18476.76 | 4 | 9.34 |
| (9) | (22.89–170.67) | (568.97–48775.21) | (1.04–7.48) | (2.32–896.02) |
| Periodontal Grade P group (n) | IL−23 median (min–max) | sIL−23R median (min–max) | IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Grade A | 35.85 | 18476.76 | 2.24 | 8.71 |
| (21) | (8.54–854.87) | (568.97–54277.85) | (1.04–7.48) | (0.35–896.02) |
| Grade B | 48.87 | 24901.28 | 5.16 | 34.19 |
| (13) | (34.56–404.75) | (661.33–48296.81) | (1.04–19.26)a | (6.75–401.53) a |
| Grade C | 44.61 | 29524.68 | 4.15 | 94.86 |
| (6) | (33.82–133.22) | (19037.1–49202.42) | (1.13–9.11) | (20.43–1987.95) a |
| Periodontal Stage RAP group (n) | IL−23 median (min–max) | sIL−23R median (min–max) | IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Stage I | 70.18 | 17782.96 | 2.95 | 10.66 |
| (5) | (10.74–2669.98) | (246.28–22662.63) | (1.19–5.09) | (2.89–966.25) |
| Stage II | 456.23 | 4232.29 | 5.59 | 22.25 |
| (21) | (33.2–10453.95) | (110.56–35938.06) | (1.08–15.339) | (3.37–905.61) |
| Stage III | 61.03 | 4161.79 | 1.53 | 9.91 |
| (12) | (32.3–11179.04) | (231.58–25103.4) | (1.1–5.5) ** | (1.71–387.95) |
| Stage IV | – | – | – | – |
| (0) |
| Periodontal Grade (n) RAP group | IL−23 median (min–max) | sIL−23R median (min–max) | IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Grade A | 106.68 | 2891.8 | 4.61 | 14.5 |
| (21) | (10.74–11179.34) | (110.56–31853.67) | (1.08–15.33) | (1.71 –966.14) |
| Grade B | 110.76 | 5931.65 | 4.75 | 32.23 |
| (17) | (33.25 –10420.7) | (231.58 –35938.62) | (1.14–14.19) | (2.12–856.99) |
| Grade C | – | – | – | – |
| (0) |
The data are presented as the mean (min–max) and frequencies. Periodontal Stage I: initial periodontitis, stage II: moderate periodontitis, Stage III: severe periodontitis with potential for additional tooth loss, Stage IV: severe periodontitis with a potential loss of the dentition. Periodontal grade A: slow rate of progression, grade B: moderate rate of progression, grade C: rapid progression rate.
Abbreviations: P, periodontitis group; RAP, rheumatoid arthritis with periodontitis group. p ≤ 0.05.
*Significant difference with stage I, ** Significant difference with stage II, *** Significant difference with stage III, (a) Significant difference compared with grade A, (b) Significant difference with stage B.
3.4. IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels according to the Disease Activity Index
According to the Disease Activity Index (DAS28‐ESR), we evaluated IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels to know whether there was a change or fluctuation concerning the disease activity score. RA patients did not have significant differences between the remission, low, moderate, or high activity score; however, in patients with RAP, sIL‐23R was higher in patients with moderate activity than with low activity (p = 0.021) (see Table 3).
TABLE 3.
IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels in DAS28‐ESR
| DAS28‐ESR RA group (n) |
IL−23 median (min–max) |
sIL−23R median (min–max) |
IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Remission | 1272.81 | 21493.09 | 2.25 | 72.97 |
| (2) | (72.66–2472.97) | (19904.05–23082.14) | (1.74–2.77) | (15.97−129.98) |
| Low | 49.18 | 18246.12 | 7.78 | 5.15 |
| (2) | (40.56–57.81) | (10721.44–25770.8) | (2.34–13.23) | (1.08–9.22) |
| Moderate | 316.53 | 21518.52 | 6.59 | 360.02 |
| (10) | (30.65–3852.26) | (1074.58–35387) | (1.18–27.99) | (8.38–901.82) |
| High | 1345.09 | 3635.75 | 4.25 | 22.64 |
| (6) | (46.29–11581.25) | (2061.05–31917) | (0.99–15.26) | (2.3–738.17) |
| DAS28‐ESR RAP group (n) | IL−23 median (min–max) | sIL−23R median (min–max) | IL−17A median (min–max) | sIL−17RA median (min–max) |
|---|---|---|---|---|
| Remission | 508.46 | 23190.71 | 6.17 | 96.66 |
| (4) | (56.05–10453.95) | (5736.12–31853.67) | (1.14–14.19) | (32.23–387.95) |
| Low | 49.58 | 2049.33 | 4.9 | 21.9 |
| (5) | (33.25–2669.98) | (522.47–23622.41) | (1.10–8.72) | (2–966.14) |
| Moderate | 268.53 | 2629.69 | 4.71 | 11.25 |
| (13) | (31.05–11179.34) | (110.56–21219.78) | (1.08–14.46) | (2.12–448.48) |
| High | 66.02 | 5739.9 | 4.5 | 15.12 |
| (16) | (10.74–905.61) | (397.17–35541.45) | (1.19–15.33) | (1.71–905.61) |
The data are presented as the mean (min‐max) and frequencies.
Abbreviations: RA, rheumatoid arthritis group; RAP, rheumatoid arthritis with periodontitis group p ≤ 0.05.
*Significant difference with remission, ** Significant difference with low activity, ***, Significant difference with moderate activity.
In addition to these results, we observe that the RAP group shows a pattern of distribution in two groups, either high or low levels in IL‐23, sIL‐23R, and IL‐17A.
Regarding IL‐23, 60% of the group with RAP has concentrations below 200pg/mL and, 42.9% of these patients have periodontal stage III with a high activity of rheumatoid arthritis. The 48.6% of patients with RAP have decreased levels of IL‐17A; 55.6% present periodontal stage III and, 44.4% have a high activity of rheumatoid arthritis; finally, 55.3% of RAP patients have low levels of sIL‐23R and, 33.3% of these patients have periodontal stage III; 42.9% have a high activity of rheumatoid arthritis.
3.5. Correlations between IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels, rheumatoid arthritis, and periodontal clinical parameters
To determine whether there is an association between IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA, a Spearman correlation test was performed. The correlations between the molecules and clinical parameters were obtained with the data of all the study groups. However, only the correlation between IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA with % RBL was obtained using the data of the group P and RAP since they are the only ones that present this characteristic. Was observed that IL‐23 and IL‐17A correlated positively (r = 0.256, p = 0.013), as did the sIL‐23R and sIL‐17RA receptors (r = 0.275, p ≤ 0.001). Similarly, IL‐23 correlated positively between sIL‐17RA (r = 0.282, p = 0.002) and IL‐17A with sIL‐17RA (r = 0.392, p ≤ 0.001). When correlating the periodontal and rheumatoid arthritis clinical parameters with the concentrations of IL‐23, IL‐17A sIL‐23R, and sIL‐17RA, we observed a significant negative correlation between IL‐23 with PD (r = −0.272, p = 0.004), IL‐17A with CAL (r = −0.226, p = 0.023), sIL‐23R with PD (r = 0.2019 p = 0.014), sIL‐23R with % BoP (r = −0.289, p = 0.003), and sIL‐23R with ESR (r = −0.292, p = 0.003).
We did not find correlations between IL‐23, IL‐17A sIL‐23R, and sIL‐17RA and the clinical parameters (CRP, FR, TJC, SJC, DAS28‐ESR, and HAQ‐DI), but we did observe significant positive correlations between these clinical parameters (see Table 4).
TABLE 4.
Correlations between IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA levels with periodontitis clinical parameters
| IL−23 | IL−17A | sIL−23R | sIL−17RA | PD | CAL | % BoP | % RBL | ESR | CRP | FR | TJC | SJC | DAS28‐ESR | HAQ‐DI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL−23 | r | 1.000 | 0.256* | −0.166 | 0.282* | −0.272* | −0.164 | −0.032 | −0.177 | 0.155 | 0.208 | 0.179 | −0.142 | 0.148 | −0.089 | −0.134 |
| p | 0.013 | 0.081 | 0.002 | 0.004 | 0.089 | 0.762 | 0.199 | 0.149 | 0.262 | 0.361 | 0.331 | 0.310 | 0.538 | 0.370 | ||
| IL−17A | r | 1.000 | −0.126 | 0.392* | −0.175 | −0.226* | −0.070 | −0.012 | 0.004 | 0.033 | −0.016 | −0.046 | −0.005 | −0.125 | −0.276 | |
| p | 0.195 | ≤0.001 | 0.077 | 0.023 | 0.497 | 0.934 | 0.973 | 0.866 | 0.936 | 0.748 | 0.974 | 0.379 | 0.055 | |||
| sIL−23R | r | 1.000 | 0.275* | 0.219* | 0.072 | −0.289* | −0.019 | −0.292* | −0.010 | 0.271 | −0.166 | −0.005 | −0.132 | −0.047 | ||
| p | ≤0.001 | 0.014 | 0.433 | 0.003 | 0.888 | 0.003 | 0.954 | 0.148 | 0.222 | 0.970 | 0.327 | 0.739 | ||||
| sIL−17RA | r | 1.000 | 0.149 | 0.029 | −0.151 | −0.030 | −0.097 | 0.126 | −0.096 | −0.042 | −0.009 | −0.052 | 0.015 | |||
| p | 0.094 | 0.752 | 0.120 | 0.825 | 0.327 | 0.476 | 0.608 | 0.758 | 0.950 | 0.702 | 0.918 | |||||
| PD | r | 1.000 | .819** | 0.321* | 0.223 | −0.205* | −0.072 | −0.088 | 0.210 | 0.174 | 0.147 | −0.050 | ||||
| p | ≤0.001 | 0.001 | 0.096 | 0.042 | 0.687 | 0.638 | 0.121 | 0.200 | 0.274 | 0.723 | ||||||
| CAL | r | 1.000 | 0.327* | 0.136 | −0.112 | −0.252 | −0.055 | 0.109 | 0.272* | 0.080 | −0.082 | |||||
| p | ≤0.001 | 0.312 | 0.279 | 0.150 | 0.767 | 0.423 | 0.043 | 0.556 | 0.561 | |||||||
| % BoP | r | 1.000 | 0.135 | 0.132 | −0.312 | −0.107 | −0.033 | −0.125 | −0.059 | −0.169 | ||||||
| p | 0.315 | 0.189 | 0.072 | 0.567 | 0.812 | 0.358 | 0.662 | 0.230 | ||||||||
| % RBL | r | 1.000 | −0.095 | −0.021 | −0.038 | 0.130 | 0.180 | 0.088 | −0.003 | |||||||
| p | 0.508 | 0.931 | 0.881 | 0.455 | 0.300 | 0.610 | 0.987 | |||||||||
| ESR | r | 1.000 | 0.267 | 0.010 | 0.291* | 0.115 | 0.488** | 0.186 | ||||||||
| p | 0.127 | 0.957 | 0.028 | 0.393 | ≤0.001 | 0.183 | ||||||||||
| CRP | r | 1.000 | 0.071 | 0.256 | 0.470* | 0.441* | 0.429* | |||||||||
| p | 0.710 | 0.144 | 0.005 | 0.009 | 0.014 | |||||||||||
| FR | r | 1.000 | −0.034 | 0.069 | −0.085 | 0.076 | ||||||||||
| p | 0.855 | 0.712 | 0.649 | 0.696 | ||||||||||||
| TJC | r | 1.000 | 0.544** | 0.852** | 0.609** | |||||||||||
| p | ≤0.001 | ≤0.001 | ≤0.001 | |||||||||||||
| SJC | r | 1.000 | 0.701** | 0.415* | ||||||||||||
| p | ≤0.001 | 0.002 | ||||||||||||||
| DAS28‐ESR | r | 1.000 | 0.603** | |||||||||||||
| p | ≤0.001 | |||||||||||||||
| HAQ‐DI | r | 1.000 | ||||||||||||||
| p |
Spearman correlation test.
Abbreviations: % BoP, bleeding of probing; % RBL, radiographic bone loss; CAL, clinical of attachment level; CRP, C‐reactive protein; DAS28‐ESR, Disease Activity Score; ESR, erythrocyte sedimentation rate; HAQ‐DI, Health Assessment Questionnaire Disability Index; PD, probe depth; RF, rheumatoid factor; SJC, swollen joint count; TJC, tender joint count.
Values in bold are significant.
*p < 0.050 ** p < 0.010.
4. DISCUSSION
In the present work, high plasma IL‐23 concentrations were observed in patients with RA compared with P and HS. These results are in accordance with different working groups that have evaluated IL‐23 in serum15, 16, 32 and plasma samples.13, 14 Likewise, we found elevated IL‐23 in RAP patients compared with HS and P groups. In this sense, it seems that the increase of IL‐23 in RA and RAP patients is due to chronic inflammation of rheumatoid arthritis itself and not due to periodontal disease.
It is known that the IL‐23R receptor can be soluble. Evidence shows that two mechanisms generate a soluble portion of IL‐23R: alternative splicing and secretion into vesicles8 or via cleavage when expressed in the cell membrane by proteases, such as ADAM10, and ADAM17.9 No significant difference was observed ADAM17 in GCF among periodontitis patients and healthy subjects.33, 34 Interestingly, we observed similar sIL‐23R levels in the plasma between healthy subjects and patients with periodontitis. In this sense, sIL‐23R, once cleaved, can bind to IL‐23, forming a complex (IL‐23‐IL‐23Rs), which has been suggested to act as a signaling blocker or activator of Th17 cells.8, 9, 10, 35 A decrease of sIL‐23R levels in patients with RAP than HS and P was found. sIL‐23R may play a protective role by capturing IL‐23, which could control the bone remodeling cycles. However, the decreased levels of sIL‐23R in RAP cannot be sufficient to block IL‐23 allowing activation of Th17 cells and, in turn, increasing the production of IL‐17 and RANKL in patients with rheumatoid arthritis, thus progressing the disease.
IL‐17A was decreased in patients with P compared with HS. These results coincide with those reported by other authors.19, 36 Nonetheless, there is a discrepancy in the levels of this cytokine that several working groups have reported.17, 18, 20, 21, 22, 27, 37, 38
We found no difference from IL‐17A in the RA group compared with the other study groups. Likewise, Rasmussen TK et al.14 did not observe a difference in the concentration of IL‐17A between HS and patients with RA. On the other hand, IL‐17A was observed to decrease in patients with RAP compared with HS. However, when comparing the groups with RAP and periodontitis, we did not observe a significant difference like that observed by Gümüs P et al.27 between GCF and serum. Regarding these findings, Ridgley LA et al.39 proposes that the expression of some cytokines, such as IL‐17A, is inversely proportional to the course and chronicity of arthritis rheumatoid and IL‐17 is expressed mainly in the preclinical phase and decreases according to the chronicity of RA. Moreover, the IL‐17A levels in RAP patients are diminished when they have high disease activity (DAS28‐ESR), according to Patschan S et al.29 Interestingly, the P and RAP group showed similar behavior since IL‐17A decreases when an advanced periodontitis stage is found (Stage III). This suggests that IL‐17 tends to modulate its expression downward as disease activity progresses in RAP.
IL‐17RA can be expressed in its soluble form via alternative splicing.11 In the present work, no significant differences were observed between the four study groups. It seems that IL‐17RA is expressed primarily in the cell membrane and not in soluble form. However, we observed a more significant increase of sIL‐17RA levels in grades B and C than in grade A in the periodontitis group. Therefore, this receptor increases its expression as the periodontal grade increases. As reported by Rigley RA et. al.,39 this soluble receptor can show kinetics according to periodontitis progression.
It is interesting to mention that only in the RAP group is there a tendency to have higher concentrations of the IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA in patients in remission.
When a Spearman correlation was performed between the IL‐23, IL‐17A sIL‐23R, and sIL‐17RA for all study groups, a significant positive correlation was found between IL‐23 and IL‐17A, which coincides with the reports by other authors.21, 37 This finding could be attributed to the fact that IL‐17 is expressed under the stimulus generated by IL‐23.40
We observed negative correlations between (IL‐23–PD) and (IL‐17A–CAL) that indicate an inversely proportional relationship between the concentration of IL‐23 with PD and IL‐17A with CAL. Indeed, PD and CAL are closely related and indicate the progression and severity of periodontitis, suggesting that the period of cytokine expression depends on periodontitis progression since, at a higher concentration of IL‐23, the PD values are lower. Moreover, when IL‐17 is elevated, the CAL values are lower.
A negative correlation was also observed between sIL‐23R with % BoP and ESR. ESR provides an indirect method to assess the level of systemic inflammation. Conversely, sIL‐23R can capture IL‐23 and thus inhibit its proinflammatory function. This negative correlation suggests that sIL‐23R could decrease systemic inflammation and, in turn, decrease the percentage of bleeding at probing (% BoP).
On the other hand, the positive correlations between the ESR and TJC and those between SJC and CRP demonstrate how ESR and CRP production reflect the clinical symptoms located in some joints, which are indicators of systemic inflammation. Similarly, the HAQ‐DI score correlated positively and significantly with CRP, TJC, SJC, and DAS28‐ESR. This suggests that the clinical and serological parameters affect disease activity rates and the degree of disability that an RA patient may have.
Is important to note that several significant differences in IL‐23, IL‐17A, and sIL‐23R levels were observed between healthy subjects and patients with P, RA, and RAP. In this sense, the size of the effect indicates the magnitude of these differences, so the effect observed in the levels of IL‐23 decreased in the HS group and increased in patients with RAP and RA is considered a large magnitude, as well as elevated sIL‐23R levels in HS and decreased in patients with RAP. Similarly, a large effect size was observed in elevated IL‐17A levels in HS compared with P and RAP. We consider these findings clinically relevant as it demonstrates a pattern of constant concentration in the different groups. Following this line of research, IL‐23, IL‐17A, and sIL‐23R could be proposed as possible biological markers for the diagnosis and progression of rheumatoid arthritis or periodontitis.
5. CONCLUSION
In conclusion, the axis IL‐23/IL‐17 is involved in developing chronic inflammation, host defense against bacterial infections, bone loss, and progression of periodontitis and rheumatoid arthritis. Regarding the Hypothesis, we observed a concordance with IL‐23 levels increased in RAP patients; however, the levels of sIL‐23R and IL‐17A are decreased. On the other hand, there are no differences in sIL‐17RA.
Regarding the disease activity index DAS28‐ESR, in patients with RAP we observed a fluctuation in the concentrations of IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA, which were elevated in remission and fluctuated between low, moderate, and high activity.
We thought that the periodontal condition would modify the expression of the molecules in patients with both pathologies; however, we did not find differences in IL‐23, IL‐17A, sIL‐23R, and sIL‐17RA between patients with RAP and RA. However, patients with RAP who have low IL‐23 and IL‐17A levels are in an advanced periodontal stage, similar to periodontitis patients. This suggests that these cytokines tend to downregulate their expression when the patients present both diseases.
We consider that if the levels of the IL‐23 and IL‐17A cytokines and their receptors in some types of biological samples become known, they could provide the molecular mechanisms of immunopathology, as well as their patterns of expression according to the chronicity and activity of the disease in patients with rheumatoid arthritis who present periodontitis. Likewise, timely biological treatments, such as anti‐IL‐17 or anti‐IL‐23, could be provided.
Finally, biological treatments, such as anti‐IL‐17 or anti‐IL‐23, have been unsuccessful in RA; nonetheless, we consider that if the pattern of these cytokines in several types of biological samples is known, this could provide timely biological treatments in the future for patients with rheumatoid arthritis and periodontitis or patients with both pathologies.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Rodríguez‐Montaño R, Bernard‐Medina AG, Oregon‐Romero E, et al. IL‐23/IL‐17 axis and soluble receptors isoforms sIL‐23R and sIL‐17RA in patients with rheumatoid arthritis-presenting periodontitis. J Clin Lab Anal. 2021;35:e23963. 10.1002/jcla.23963
Funding information
This study was carried out with financial support from the University of Guadalajara, the Mexican Association of Periodontology, and academic collaboration of the [Secretary of Public Education] of Mexico
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Funovits J, Aletaha D, Bykerk V, et al. The 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis. 2010;69(9):1589‐1595. [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 4.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(10):606‐620. [DOI] [PubMed] [Google Scholar]
- 5.Lubberts E. The IL‐23‐IL‐17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11(7):415‐429. [DOI] [PubMed] [Google Scholar]
- 6.Shapouri‐Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425‐6440. [DOI] [PubMed] [Google Scholar]
- 7.Bedoya SK, Lam B, Lau K, Larkin J. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan SH, Mancini G, Gallagher G. Identification and characterization of multiple splice forms of the human interleukin‐23 receptor alpha chain in mitogen‐activated leukocytes. Genes Immun. 2008;9(7):631‐639. [DOI] [PubMed] [Google Scholar]
- 9.Franke M, Schroder J, Monhasery N, et al. Human and murine interleukin 23 receptors are novel substrates for a disintegrin and metalloproteases ADAM10 and ADAM17. J Biol Chem. 2016;291(20):10551‐10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchar M, Vankova L, Petrokova H, et al. Human interleukin‐23 receptor antagonists derived from an albumin‐binding domain scaffold inhibit IL‐23‐dependent ex vivo expansion of IL‐17‐producing T‐cells. Proteins. 2014;82(6):975‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohda M, Misumi Y, Tashiro K, Yamazaki M, Saku T, Oda K. Identification of a soluble isoform of human IL‐17RA generated by alternative splicing. Cytokine. 2013;64(3):642‐645. [DOI] [PubMed] [Google Scholar]
- 12.Shim JH, Stavre Z, Gravallese EM. Bone loss in rheumatoid arthritis: basic mechanisms and clinical implications. Calcif Tissue Int. 2018;102(5):533‐546. [DOI] [PubMed] [Google Scholar]
- 13.Andersen T, Hvid M, Johansen C, et al. Interleukin‐23 in early disease development in rheumatoid arthritis. Scand J Rheumatol. 2015;44(6):438‐442. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen TK, Andersen T, Hvid M, et al. Increased interleukin 21 (IL‐21) and IL‐23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol. 2010;37(10):2014‐2020. [DOI] [PubMed] [Google Scholar]
- 15.Melis L, Vandooren B, Kruithof E, et al. Systemic levels of IL‐23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann Rheum Dis. 2010;69(3):618‐623. [DOI] [PubMed] [Google Scholar]
- 16.Dalila AS, Mohd Said MS, Shaharir SS, et al. Interleukin‐23 and its correlation with disease activity, joint damage, and functional disability in rheumatoid arthritis. Kaohsiung J Med Sci. 2014;7:337‐342. [DOI] [PubMed] [Google Scholar]
- 17.Cifcibasi E, Koyuncuoglu C, Ciblak M, et al. Evaluation of local and systemic levels of interleukin‐17, interleukin‐23, and myeloperoxidase in response to periodontal therapy in patients with generalized aggressive periodontitis. Inflammation. 2015;38(5):1959‐1968. [DOI] [PubMed] [Google Scholar]
- 18.Liukkonen J, Gursoy UK, Pussinen PJ, Suominen AL, Kononen E. Salivary concentrations of interleukin (IL)‐1beta, IL‐17A, and IL‐23 vary in relation to periodontal status. J periodontol. 2016;87(12):1484‐1491. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghi R, Sattari M, Dehghan F, Akbari S. Interleukin‐17 and interleukin‐23 levels in gingival crevicular fluid of patients with chronic and aggressive periodontitis. Cent Eur J Immunol. 2018;43(1):76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batool H, Nadeem A, Kashif M, Shahzad F, Tahir R, Afzal N. Salivary levels of IL‐6 and IL‐17 could be an indicator of disease severity in patients with calculus associated chronic periodontitis. Biomed Res Int. 2018;2018:8531961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awang RA, Lappin DF, MacPherson A, et al. Clinical associations between IL‐17 family cytokines and periodontitis and potential differential roles for IL‐17A and IL‐17E in periodontal immunity. Inflamm Res. 2014;63(12):1001‐1012. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz‐Gutierrez AC, de la Cruz Herrera‐Mora M, Zamora‐Pérez AL, et al. Determination of IL‐17 levels in gingival crevicular fluid in patients with chronic and aggressive periodontitis. Rev Mex Periodontol. 2014;5(2):46‐50. [Google Scholar]
- 23.van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL‐17A), IL‐17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti‐IL‐17 therapy? Arthritis Res Ther. 2014;16(4):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohyama H, Kato‐Kogoe N, Kuhara A, et al. The involvement of IL‐23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88(7):633‐638. [DOI] [PubMed] [Google Scholar]
- 25.Hillyer P, Larche MJ, Bowman EP, et al. Investigating the role of the interleukin‐23/‐17A axis in rheumatoid arthritis. Rheumatology (Oxford). 2009;48(12):1581‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruíz‐Gutiérrez AC, Rodríguez‐Montaño R, Pita‐López ML, Zamora‐Perez AL, Guerrero‐Velázquez C. Inverse behavior of IL‐23R and IL‐17RA in chronic and aggressive periodontitis. J Periodontal Implant Sci. 2021;51:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumus P, Buduneli E, Biyikoglu B, et al. Gingival crevicular fluid, serum levels of receptor activator of nuclear factor‐kappaB ligand, osteoprotegerin, and interleukin‐17 in patients with rheumatoid arthritis and osteoporosis and with periodontal disease. Journal of periodontol. 2013;84(11):1627‐1637. [DOI] [PubMed] [Google Scholar]
- 28.Correa JD, Fernandes GR, Calderaro DC, et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 2019;9(1):8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patschan S, Bothmann L, Patschan D, et al. Association of cytokine patterns and clinical/laboratory parameters, medication and periodontal burden in patients with rheumatoid arthritis (RA). Odontology. 2020;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kageyama Y, Kobayashi H, Kato N. Infliximab treatment reduces the serum levels of interleukin‐23 in patients with rheumatoid arthritis. Mod Rheumatol. 2009;19(6):657‐662. [DOI] [PubMed] [Google Scholar]
- 31.Moran EM, Mullan R, McCormick J, et al. Human rheumatoid arthritis tissue production of IL‐17A drives matrix and cartilage degradation: synergy with tumour necrosis factor‐alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11(4):R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J periodontol. 2018;89(Suppl 1):S159‐S172. [DOI] [PubMed] [Google Scholar]
- 33.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580‐1588. [DOI] [PubMed] [Google Scholar]
- 34.Bostanci N, Emingil G, Afacan B, et al. Tumor necrosis factor‐alpha‐converting enzyme (TACE) levels in periodontal diseases. J Dent Res. 2008;87(3):273‐277. [DOI] [PubMed] [Google Scholar]
- 35.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173(9):5343‐5348. [DOI] [PubMed] [Google Scholar]
- 36.Ozcaka O, Nalbantsoy A, Buduneli N. Interleukin‐17 and interleukin‐18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46(5):592‐598. [DOI] [PubMed] [Google Scholar]
- 37.Mitani A, Niedbala W, Fujimura T, et al. Increased expression of interleukin (IL)‐35 and IL‐17, but not IL‐27, in gingival tissues with chronic periodontitis. J periodontol. 2015;86(2):301‐309. [DOI] [PubMed] [Google Scholar]
- 38.Cardoso CR, Garlet GP, Crippa GE, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 39.Ridgley LA, Anderson AE, Pratt AG. What are the dominant cytokines in early rheumatoid arthritis? Curr Opin Rheumatol. 2018;30(2):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin‐23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin‐17. J Biol Chem. 2003;278(3):1910‐1914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


