Abstract
Background
The systemic immune‐inflammation index (SII) is a recently developed indicator for systemic inflammatory response. We aimed to explore the association between SII and disease activity in patients with ankylosing spondylitis (AS).
Methods
This retrospective study included 136 patients with AS and 63 healthy controls. Patients were divided into two groups according to Bath Ankylosing Spondylitis Disease Activity Index (BASDAI); active group (n = 60) and remission group (n = 76). Clinical, laboratory, and demographic characteristics were recorded. Spearman's correlation analysis was used to determine correlations of SII with C‐reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), and BASDAI in AS patients. Binary logistic regression analysis was used to assess risk factors for AS disease activity. Receiver operating characteristic curve analysis was used to evaluate the diagnostic value of SII and the above variables for the active group compared with the remission group.
Results
Systemic immune‐inflammation index levels were higher in AS patients than in healthy controls (p < 0.001). SII levels were higher in the active group than in the remission group (p < 0.001). For patients with AS, SII correlated positively with CRP (rs = 0.483, p < 0.001), ESR (rs = 0.374, p < 0.001), and BASDAI (rs = 0.667, p < 0.001). SII (OR = 1.009, 95% CI = 1.006–1.012, p < 0.001) was an independent risk factor affecting AS disease activity. The specificity and sensitivity of SII using a cutoff value of 513.2 were 83.33% and 86.84%, respectively, for the active group.
Conclusion
Systemic immune‐inflammation index was increased in AS. SII may be a novel indicator for monitoring AS disease activity.
Keywords: ankylosing spondylitis, disease activity, inflammatory marker, inflammatory response, systemic immune‐inflammation index
The systemic immune‐inflammation index (SII) is a developed indicator for systemic inflammatory response. This study showed that SII was higher in AS. This study reveals that SII may be a novel indicator for monitoring AS disease activity.
1. INTRODUCTION
Ankylosing spondylitis (AS) is an inflammatory disease of unknown etiology characterized by chronic inflammation of the axial joints, mainly involving the sacroiliac joints, spinal processes, and paraspinal soft tissues. Peripheral joint involvement and extra‐articular manifestations can also occur, such as anterior uveitis,1 inflammatory bowel disease,2 aortic valve disease,3 and osteoporosis.4 In cases of progressive disease, joint fusion and dysfunction may also occur. Inflammatory lower back pain and morning stiffness are common symptoms of AS, which lead to the limitation of activity and aggravation of pain in the active stage of the disease.5 Therefore, evaluating disease activity in AS is crucial to further our understanding of the pathophysiology of AS and to predict prognosis. At present, C‐reactive protein (CRP) level and erythrocyte sedimentation rate (ESR), two non‐specific inflammatory biomarkers, are often used to monitor the disease activity of rheumatic diseases; however, these are not satisfactory because of their low sensitivity and specificity.6 Therefore, it is necessary to identify novel indicators that reflect the disease activity more accurately.
Recently, complete blood cell count parameters have emerged as useful biomarkers of many inflammatory diseases because of their availability and affordability. Previous studies have shown that platelet (PLT), neutrophil, lymphocyte, red blood distribution width (RDW), neutrophil‐to‐lymphocyte ratio (NLR), and platelet‐to‐lymphocyte ratio (PLR) counts are important indicators of systemic inflammation in AS.7, 8, 9 Nevertheless, studies on the relationship between these biomarkers and disease activity in patients with AS have yielded controversial results.10, 11, 12 The systemic immune‐inflammation index (SII), a novel inflammatory marker (PLT counts × neutrophil counts/lymphocyte counts), can better reflect systemic inflammation than NLR or PLR alone.13 Initially, SII was considered as a marker of poor prognosis in patients with hepatocellular carcinoma (HCC),14 and later studies mostly focused on neoplastic diseases.15, 16, 17 Recently, studies have also reported the use of SII as an indicator for autoimmune diseases, such as an index to assess the disease activity of patients with Behcet's disease18 or to predict the poor prognosis of antineutrophil cytoplasmic antibody‐associated vasculitis.19 To the best of our knowledge, the correlation between SII and AS has not been previously reported.
Hence, this study aimed to investigate the level of SII in patients with AS and its association with disease activity.
2. METHODS
2.1. Participants
In this retrospective study, we enrolled 136 AS patients that were admitted to the Qinghai University Affiliated Hospital from January 2017 to November 2020. All patients met the modified New York criteria for the classification of AS.20 Patients who had the following conditions were excluded: acute and/or chronic infections, other autoimmune diseases, diabetes mellitus, coronary artery disease, hypertension, malignancies, hematologic diseases, liver disease, kidney disease, and pregnancy. Moreover, we recruited a control group consisting of 63 age‐ and sex‐matched individuals without any disease from the medical examination center of our hospital. Our study protocol was approved by the institutional clinical research ethics committee. Owing to the retrospective nature of the study, informed consent was waived.
2.2. Disease activity index
At present, the most commonly used tool for evaluating disease activity in AS is the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),21 which includes the following six parameters: fatigue, spinal pain, peripheral joint pain, attachment point inflammation, and duration and severity of morning stiffness. A total score, ranging from 0 to 10, was calculated according to the patients' responses to six questions, with a higher score indicating more severe illness. A BASDAI score of ≥4 represents the active stage of the disease.
2.3. Demographic and laboratory data
Data on age, sex, neutrophil, lymphocyte, monocyte, white blood cell (WBC), PLT counts, RDW, NLR, PLR, SII, inflammatory markers (CRP level and ESR), human leukocyte antigen B27 (HLA‐B27), BASDAI, and disease duration were collected.
All participants fasted for at least 8 h the night before venous sample collection in the early morning.
2.4. Statistical analysis
All data analyses were performed using SPSS 19.0 statistical software and GraphPad Prism 6 software. The Kolmogorov‐Smirnov test was used to assess the normality of data distribution. Continuous variables following a normal distribution are expressed as the mean ± standard deviation, the inter‐group comparisons were analyzed by an independent sample t test, and the continuous variables that did not follow normal distribution were expressed as the median with the 1st quartile and 3rd quartile and analyzed using the Mann‐Whitney U test. The classified variables were expressed by frequency or percentage and analyzed using the chi‐square test. Spearman's correlation was used to determine the correlation between the CRP, ESR level or BASDAI, and the SII. Binary logistic regression analysis was carried out to determine the risk factors associated with disease activity in AS. The optimal critical values of SII, CRP, and ESR were calculated by analyzing the receiver operating characteristic (ROC) of subjects' working characteristics to distinguish active and remission AS patients. A p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Comparison of demographic and laboratory parameters of AS patients and controls
A total of 136 AS patients and 63 healthy controls were enrolled in this study. The basic demographic and clinical characteristics of participants in the AS and control groups are shown in Table 1. No statistically significant differences in age and sex were observed between the patients in the AS and control group (p > 0.05). Significant differences between groups were observed in counts of SII, WBC, neutrophils, lymphocytes, monocytes, PLT, RDW, NLR, and PLR. (p < 0.05).
TABLE 1.
Comparison of demographic and laboratory parameters of AS patients and controls
| Items | AS (N = 136) | Control (N = 63) | t/χ2/Z | p value |
|---|---|---|---|---|
| Age (year) | 33.00 (27.00, 42.00) | 34.00 (28.00, 44.00) | −0.741 | 0.458 |
| Sex, No. (%) | ||||
| Female | 18 (13.24) | 15 (23.81) | 3.480 | 0.062 |
| Male | 118 (86.76) | 48 (76.19) | ||
| WBC (×109/L) | 6.95 ± 1.57 | 6.36 ± 1.20 | 2.635 | 0.009 |
| Neutrophils (×109/L) | 4.05 (3.34, 5.00) | 3.42 (3.02, 4.20) | −3.854 | <0.001 |
| Lymphocytes (×109/L) | 1.97 (1.67, 2.38) | 2.27 (2.00, 2.61) | −2.814 | 0.005 |
| Monocytes (×109/L) | 0.44 (0.35, 0.54) | 0.37 (0.31, 0.43) | −4.148 | <0.001 |
| RDW (%) | 13.30 (12.70, 14.20) | 12.80 (12.60, 13.50) | −3.123 | 0.002 |
| PLT (×109/L) | 245.40 ± 56.98 | 193.17 ± 47.65 | 6.321 | <0.001 |
| NLR | 2.02 (1.57, 2.66) | 1.54 (1.31, 1.90) | −11.337 | <0.001 |
| PLR | 116.18 (92.72, 156.08) | 86.72 (70.97, 106.74) | −11.337 | <0.001 |
| SII (×109/L) | 482.86 (329.60, 661.93) | 308.00 (217.32, 365.45) | −6.801 | <0.001 |
| CRP (mg/L) | 16.45 (8.58, 32.40) | – | – | – |
| ESR (mm/h) | 17.50 (9.00, 36.50) | – | – | – |
| BASDAI | 3.80 (3.30, 4.50) | – | – | – |
| HLA‐B27 positive rate, No. (%) | 110 (80.88) | – | – | – |
| Disease duration (year) | 4.50 (2.00, 10.00) | – | – | – |
Abbreviations: AS, ankylosing spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HLA, human leukocyte antigen; NLR,neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet; RDW, red blood distribution width; SII, systemic immune‐inflammation index; WBC, white blood cell.
3.2. Comparisons of demographics, clinical data, and laboratory tests between the active group and remission group of patients with AS
Sixty patients were in the active group and 76 patients were in the remission group according to the BASDAI score. The comparisons of demographics, clinical data, and laboratory tests between the active and remission groups of patients with AS are shown in Table 2. There were no statistically significant differences in age, sex, RDW, HLA‐B27, and disease duration between the active and remission groups (p > 0.05). Compared with the remission group, the SII, WBC, neutrophils, lymphocytes, monocytes, PLT, CRP, ESR, NLR, PLR, and BASDAI were higher in the active group (p < 0.05).
TABLE 2.
Comparisons of demographics, clinical data, and laboratory tests between the active group and remission group of patients with AS
| Items | Active AS (N = 60) | Remission AS (N = 76) | t/χ2/Z | p value |
|---|---|---|---|---|
| Age (year) | 34.00 (26.25, 42.75) | 32.50 (27.00, 42.00) | −0.303 | 0.762 |
| Sex, No. (%) | ||||
| Female | 6 (10.00) | 12 (15.79) | 0.979 | 0.323 |
| Male | 54 (90.00) | 64 (84.21) | ||
| WBC (×109/L) | 7.10 (6.30, 8.30) | 6.47 (5.58, 7.68) | −2.724 | 0.006 |
| Neutrophils (×109/L) | 4.83 ± 1.33 | 3.79 ± 1.12 | 4.958 | <0.001 |
| Lymphocytes (×109/L) | 1.86 (1.57, 2.12) | 2.11 (1.71, 2.65) | −2.908 | 0.004 |
| Monocytes (×109/L) | 0.48 (0.38, 0.57) | 0.42 (0.34, 0.50) | −2.778 | 0.005 |
| RDW (%) | 13.30 (12.60, 14.13) | 13.30 (12.80, 14.45) | −0.509 | 0.611 |
| PLT (×109/L) | 273.57 ± 50.47 | 223.17 ± 52.01 | 5.684 | <0.001 |
| NLR | 2.50 (1.99, 3.12) | 1.68 (1.36, 2.07) | −5.807 | <0.001 |
| PLR | 153.33 ± 47.73 | 107.17 ± 32.93 | 6.387 | <0.001 |
| SII (×109/L) | 650.74 (555.31, 883.87) | 375.35 (294.66, 465.03) | −7.534 | <0.001 |
| CRP (mg/L) | 26.05 (15.75, 56.50) | 13.30 (6.72, 17.90) | −4.863 | <0.001 |
| ESR (mm/h) | 23.00 (14.25, 51.75) | 14.00 (7.00, 28.25) | −3.333 | 0.001 |
| BASDAI | 4.60 (4.20, 5.28) | 3.40 (3.00, 3.70) | −9.856 | <0.001 |
| HLA‐B27 positive rate, No. (%) | 48 (80.00) | 62 (81.58) | 0.054 | 0.816 |
| Disease duration (year) | 4.00 (1.00, 10.00) | 5.50 (2.00, 10.00) | −0.782 | 0.434 |
Abbreviations: AS, ankylosing spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HLA, human leukocyte antigen; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet; RDW, red blood distribution width; SII, systemic immune‐inflammation index; WBC, white blood cell.
3.3. Correlations of SII with CRP, ESR, and BASDAI in AS patients
Spearman correlations of SII with CRP, ESR, and BASDAI in AS patients are shown in Table 3 and Figure 1. SII was positively correlated with CRP (rs = 0.483, p < 0.001; Figure 1A), ESR (rs = 0.374, p < 0.001; Figure 1B), and BASDAI (rs = 0.667, p < 0.001; Figure 1C).
TABLE 3.
Correlations of SII with CRP, ESR, and BASDAI in AS patients
| Items | CRP | ESR | BASDAI | |||
|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | |
| SII | 0.483 | <0.001 | 0.374 | <0.001 | 0.667 | <0.001 |
Abbreviations: BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; SII, systemic immune‐inflammation index.
FIGURE 1.
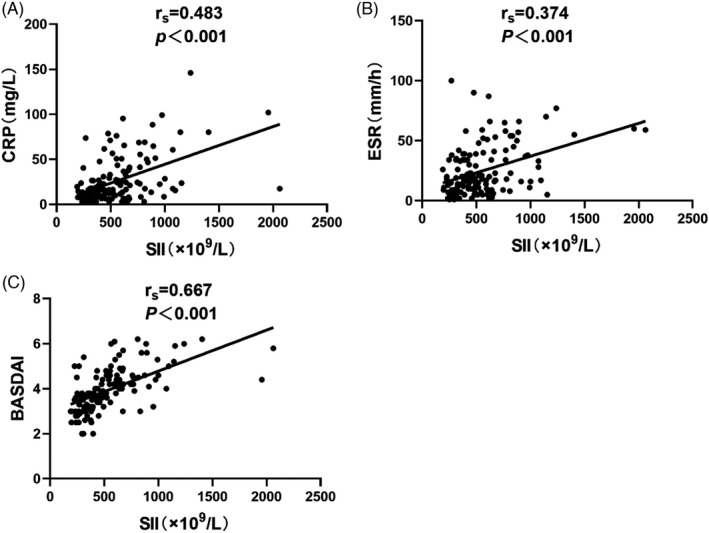
Correlations of SII with CRP, ESR, and BASDAI in AS patients to Figure 1 Correlations of SII with CRP (A), ESR (B), and BASDAI (C) in AS patients
4. Factors associated with disease activity in AS patients
We also used binary logistic regression analysis to identify the risk factors for the disease activity of AS. After univariate regression analyses, SII, CRP, and ESR were used for further multivariate regression analyses (p < 0.05). Multivariate regression analyses revealed that SII (p < 0.001; OR = 1.008; 95% CI: 1.005–1.011) was an independent risk factor for AS disease activity (Table 4).
TABLE 4.
Factors associated with disease activity in AS patients
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p value | OR (95% CI) | p value | OR (95% CI) | |
| SII | <0.001 | 1.009 (1.006–1.012) | <0.001 | 1.008 (1.005–1.011) |
| CRP | <0.001 | 1.046 (1.025–1.068) | 0.058 | |
| ESR | 0.001 | 1.032 (1.013–1.052) | 0.471 | |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; SII, systemic immune‐inflammation index.
5. Receiver operating characteristic curve analysis of SII, CRP, and ESR to evaluate disease activity in AS patients
Receiver operating characteristic curves were used to analyze the ability of SII, CRP, and ESR to indicate the disease activity of AS. The areas under the ROC curve of SII, CRP, and ESR were 0.877 (95% CI: 0.813–0.941), 0.743 (95% CI: 0.656–0.831), and 0.667 (95% CI: 0.575–0.759), respectively. SII was found to be the most effective indicator of disease activity. At the cutoff value of 513.2, the sensitivity and specificity for the diagnosis of AS disease activity were 86.84% and 83.33%, respectively (Table 5 and Figure 2).
TABLE 5.
ROC curves of the SII, CRP, and ESR for differentiating AS patients with active disease from patients in remission
| AUC | 95% CI | Optimal cutoff point | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| SII | 0.877 | 0.813–0.941 | 513.2 | 86.84% | 83.33% |
| CRP | 0.743 | 0.656–0.831 | 17.35 | 72.37% | 71.67% |
| ESR | 0.667 | 0.575–0.759 | 39.50 | 92.11% | 35.00% |
Abbreviations: AUC, area under the curve; CI, confidence intervals; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; SII, systemic immune‐inflammation index.
FIGURE 2.
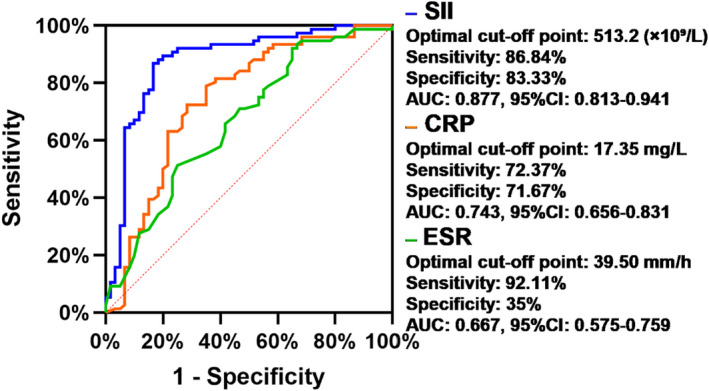
Receiver operating characteristic curves of the SII, CRP, and ESR for differentiating AS patients with active disease from patients in remission
6. DISCUSSION
To the best of our knowledge, our study is the first to report that the SII level of patients with AS was higher than that of healthy controls. Further, in patients with AS, the SII level was higher in patients with active disease than those in remission. Moreover, SII was also positively correlated with CRP, ESR, and the BASDAI. SII was also an independent risk factor for AS disease activity. Finally, compared with CRP and ESR, ROC curve analysis showed that SII had the best diagnostic value for distinguishing the disease activity of AS.
Ankylosing spondylitis is a common autoimmune disease characterized by chronic inflammation.22 The mainstay of treatment of AS is pain relief and control of inflammation. Untreated, chronic inflammation of the spinal joints and tissues can lead to stiffness,23 which can have serious adverse effects on the physical and mental health of patients.24 AS patients with the active disease tend to have more severe clinical symptoms; therefore, it is particularly important to monitor disease activity in patients with AS. At present, complete blood cell count parameters, such as NLR, PLR, and RDW, are widely used as biomarkers of inflammation. Previous studies have revealed that RDW, NLR, and PLR are associated with autoimmune diseases.25, 26, 27 Our results showed that the RDW, NLR, and PLR levels in AS patients were higher than those of healthy controls. In addition, we used the BASDAI score to classify AS patients into active and remission stages. Compared with patients in the remission stage, the NLR and PLR levels in patients with active AS were significantly higher, but there was no significant difference in RDW. Most importantly, our study was the first to demonstrate that the SII level in patients with AS was significantly higher than that in healthy individuals, and the SII level in the active stage was also higher than that in the remission stage.
There is an imbalance among neutrophils, lymphocytes, and PLTs in the peripheral blood of patients with inflammatory diseases. AS is a disease caused by factors such as immune dysfunction and inflammatory reaction. As a new type of inflammatory index, the SII is a comprehensive index based on the absolute values of neutrophils, PLTs, and lymphocytes in peripheral blood. Clinically, SII can be easily obtained from routine blood screening results, which is economical and cheap for patients. SII was first described by Hu et al14 in 2014 and identified as a useful index for the prognosis of patients with HCC. Recently, the diagnostic utility of SII (combination of SII and ferritin) has been studied in autoimmune diseases, such as for adult‐onset Still's disease.28 The increase of SII level is mainly due to the increase in neutrophils, along with thrombocytosis and lymphocytopenia caused by an inflammatory immune response. SII can represent different inflammatory and immune pathways in the body; it combines the three parameters of neutrophils, lymphocytes, and PLTs better than an evaluation of NLR or PLR alone, and it can more comprehensively reflect the balance of inflammation and immunity in the body.14
Platelets are derived from mature megakaryocytes in the bone marrow and play a vital role in hemostasis and coagulation. In addition, they are also important mediators of inflammatory response. Studies have shown that the peripheral blood PLT count in patients with AS is higher than that in healthy individuals.29, 30 Although the specific mechanisms involving PLTs in the pathogenesis of AS are not clear, it has been reported that activation of PLTs by thrombin, histamine, tumor necrosis factor‐α, and interleukin (IL)‐12 leads to adhesion between activated PLTs and neutrophils, monocytes, eosinophils, and T lymphocyte subsets, which may be a key factor in the activation of inflammatory pathways.31 Furthermore, platelet factor 4 (chemokine [C‐X‐C motif] ligand 4) is the most abundant protein in the alpha granules of PLTs, and the first member of the chemokine family to be recognized in PLTs.32 With the assistance of tumor necrosis factor and regulated upon activation, normal T‐cell expressed, and presumably secreted chemokine, PLT factor 4 stimulates neutrophils and monocytes to release inflammatory mediators and participate in the inflammatory response.33 Neutrophils, as a subgroup of leukocytes, are an important line of cellular immune defense against external microbial inflammatory stimulation and invasion of exogenous pathogens. Studies have shown that many cytokines and chemokines play an important role in the recruitment, activation, and survival of neutrophils in inflammatory sites, including IL‐17, IL‐8, interferon‐γ, tumor necrosis factor‐α, and granulocyte‐macrophage colony‐stimulating factor. As the inflammatory state continues, the body gradually develops an adaptive immune response mediated by lymphocytes. Lymphocytes are a key type of immune cell and participate in immune recognition and response. Abnormal lymphocyte signal transduction can lead to autoimmune diseases.34 The decrease in peripheral lymphocyte count may be caused by apoptosis, which reflects the dysfunction of the immune system. In systemic inflammation, the increase in neutrophil count is accompanied by a corresponding decrease in the lymphocyte count.35 This is consistent with our findings showing that the absolute lymphocyte count in patients with AS was lower than in healthy controls.
C‐reactive protein and ESR are two indicators of acute inflammation in patients with AS, and the BASDAI is a subjective tool to evaluate the disease activity of AS. Using Spearman's correlation, we found that SII in patients with AS was positively correlated with CPR, ESR, and the BASDAI. Therefore, we speculate that SII may be related to inflammatory state and disease activity in AS patients. Moreover, we could also observe that SII was an independent risk factor for AS disease activity by multivariate regression analyses. Besides, since SII had the largest area under the curve in the ROC analysis, SII can be considered as a better index to reflect AS disease activity than CRP and ESR; therefore, SII has a good guiding significance for clinicians to evaluate the disease activity of AS patients.
This study has some limitations. First, this was a single‐center, retrospective study. Second, the sample size included in this study was limited. Therefore, further prospective studies with a large sample size are warranted to further evaluate the association between SII and AS.
In conclusion, this study showed that the SII was elevated in patients with AS to both healthy controls and other patients in the remission stage and was positively correlated with disease activity. This study reveals that SII, an affordable and readily available parameter, may be a novel and reliable inflammatory marker to reflect the disease activity of AS.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Junlai Wu involved in methodology, formal analysis, writing the original draft, and software. Lifang Yan involved in investigation, formal analysis, writing the original draft, and resources. Kexia Chai involved in conceptualization, and writing, reviewing and editing.
Wu J, Yan L, Chai K. Systemic immune‐inflammation index is associated with disease activity in patients with ankylosing spondylitis. J Clin Lab Anal. 2021;35:e23964. 10.1002/jcla.23964
Junlai Wu and Lifang Yan contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Lee JH, Choi M, Rim THT, et al. Clinical characteristics and prognostic factors in ankylosing spondylitis associated uveitis. Ocul Immunol Inflam. 2019;27:64‐69. 10.1080/09273948.2017.1359630 [DOI] [PubMed] [Google Scholar]
- 2.Stolwijk C, Essers I, van Tubergen A, et al. The epidemiology of extra‐articular manifestations in ankylosing spondylitis: a population‐based matched cohort study. Ann Rheum Dis. 2015;74:1373‐1378. 10.1136/annrheumdis-2014-205253 [DOI] [PubMed] [Google Scholar]
- 3.Chetrit M, Khan MA, Kapadia S. State of the art management of aortic valve disease in ankylosing spondylitis. Curr Rheumatol Rep. 2020;22:23. 10.1007/s11926-020-00898-4 [DOI] [PubMed] [Google Scholar]
- 4.Magrey MN, Lewis S, Asim KM. Utility of DXA scanning and risk factors for osteoporosis in ankylosing spondylitis‐a prospective study. Semin Arthritis Rheum. 2016;46:88‐94. 10.1016/j.semarthrit.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 5.Domínguez CJD, Ugalde PF, Vilchez DR, et al. Positive and negative affective states and disease activity in ankylosing spondylitis. Rheumatol Int. 2015;35:519‐524. 10.1007/s00296-014-3107-y [DOI] [PubMed] [Google Scholar]
- 6.Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:1369‐1374. 10.1136/ard.2010.145995 [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Xu S, Gao X, et al. Red cell distribution width and mean platelet volume in patients with ankylosing spondylitis: a systematic review and meta‐analysis. J Clin Rheumatol. 2019. 10.1097/RHU.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 8.Sezgin M, Tecer D, Kanık A, et al. Serum RDW and MPV in ankylosing spondylitis: can they show the disease activity. Clin Hemorheol Microcirc. 2017;65:1‐10. 10.3233/CH-162067 [DOI] [PubMed] [Google Scholar]
- 9.Gökmen F, Akbal A, Reşorlu H, et al. Neutrophil/lymphocyte ratio connected to treatment options and inflammation markers of ankylosing spondylitis. J Clin Lab Anal. 2015;29:294‐298. 10.1002/jcla.21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seng JJB, Kwan YH, Low LL, et al. Role of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and mean platelet volume (MPV) in assessing disease control in Asian patients with axial spondyloarthritis. Biomarkers. 2018;23:335‐338. 10.1080/1354750X.2018.1425916 [DOI] [PubMed] [Google Scholar]
- 11.Zeb A, Khurshid S, Bano S, et al. The role of the neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as markers of disease activity in ankylosing spondylitis. Cureus. 2019;11:e6025. 10.7759/cureus.6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucuk A, Uslu AU, Ugan Y, et al. Neutrophil‐to‐lymphocyte ratio is involved in the severity of ankylosing spondylitis. Bratisl Lek Listy. 2015;116:722‐725. 10.4149/bll_2015_142 [DOI] [PubMed] [Google Scholar]
- 13.Aziz MH, Sideras K, Aziz NA, et al. The systemic‐immune‐inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2019;270:139‐146. 10.1097/SLA.0000000000002660 [DOI] [PubMed] [Google Scholar]
- 14.Hu B, Yang XR, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212‐6222. 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 15.Fest J, Ruiter R, Mulder M, et al. The systemic immune‐inflammation index is associated with an increased risk of incident cancer‐a population‐based cohort study. Int J Cancer. 2020;146:692‐698. 10.1002/ijc.32303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomrich G, Paireder M, Kristo I, et al. High systemic immune‐inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021; 273(3):532–541. 10.1097/SLA.0000000000003370 [DOI] [PubMed] [Google Scholar]
- 17.De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25:3839‐3846. 10.1158/1078-0432.CCR-18-3661 [DOI] [PubMed] [Google Scholar]
- 18.Tanacan E, Dincer D, Erdogan FG, et al. A cutoff value for the systemic immune‐inflammation index in determining activity of Behçet disease. Clin Exp Dermatol. 2021;46(2):286‐291. 10.1111/ced.14432 [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Choi H, Jung SM, et al. Systemic immune‐inflammation index could estimate the cross‐sectional high activity and the poor outcomes in immunosuppressive drug‐naïve patients with antineutrophil cytoplasmic antibody‐associated vasculitis. Nephrol (Carlton). 2019;24:711‐717. 10.1111/nep.13491 [DOI] [PubMed] [Google Scholar]
- 20.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361‐368. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 21.Garrett S, Jenkinson T, Kennedy LG. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21:2286‐2291. [PubMed] [Google Scholar]
- 22.Vanaki N, Golmohammadi T, Jamshidi A, et al. Increased inflammatory responsiveness of peripheral blood mononuclear cells (PBMCs) to in vitro NOD2 ligand stimulation in patients with ankylosing spondylitis. Immunopharmacol Immunotoxicol. 2018;40:393‐400. 10.1080/08923973.2018.1510963 [DOI] [PubMed] [Google Scholar]
- 23.Ramiro S, Stolwijk C, van Tubergen A, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow‐up of the oasis study. Ann Rheum Dis. 2015;74:52‐59. 10.1136/annrheumdis-2013-204055 [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Fan D, Xia Q, et al. The health‐related quality of life of ankylosing spondylitis patients assessed by SF‐36: a systematic review and meta‐analysis. Qual Life Res. 2016;25:2711‐2723. 10.1007/s11136-016-1345-z [DOI] [PubMed] [Google Scholar]
- 25.Gao MZ, Huang YL, Wu XD, et al. Red blood cell distribution width and neutrophil to lymphocyte ratio are correlated with disease activity of dermatomyositis and polymyositis. J Clin Lab Anal. 2018;32:e22209. 10.1002/jcla.22209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan L, Du J, Li T, et al. Platelet‐to‐lymphocyte ratio and neutrophil‐to‐lymphocyte ratio associated with disease activity in patients with Takayasu’s arteritis: a case‐control study. BMJ Open. 2017;7:e014451. 10.1136/bmjopen-2016-014451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Chen Y, Yang X, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016;36:94‐99. 10.1016/j.intimp.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 28.Kim JW, Jung JY, Suh CH, et al. Systemic immune‐inflammation index combined with ferritin can serve as a reliable assessment score for adult‐onset still’s disease. Clin Rheumatol. 2021;40:661‐668. 10.1007/s10067-020-05266-2 [DOI] [PubMed] [Google Scholar]
- 29.Qian H, Chen R, Wang B, et al. Associations of platelet count with inflammation and response to anti‐TNF‐α therapy in patients with ankylosing spondylitis. Front Pharmacol. 2020;11:559593. 10.3389/fphar.2020.559593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozan N, Alpaycı M, Aslan M, et al. Mean platelet volume, red cell distribution width, platelet‐to‐lymphocyte and neutrophil‐to‐lymphocyte ratios in patients with ankylosing spondylitis and their relationships with high‐frequency hearing thresholds. Eur Arch Otorhinolaryngol. 2016;273:3663‐3672. 10.1007/s00405-016-3980-y [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Yan CG, Xiang HY, et al. The significance of platelet activation in ankylosing spondylitis. Clin Rheumatol. 2008;27:767‐769. 10.1007/s10067-008-0847-7 [DOI] [PubMed] [Google Scholar]
- 32.Petersen F, Ludwig A, Flad HD, et al. TNF‐alpha renders human neutrophils responsive to platelet factor 4. Comparison of PF‐4 and IL‐8 reveals different activity profiles of the two chemokines. J Immunol. 1996;156:1954‐1962. [PubMed] [Google Scholar]
- 33.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15:219. 10.1186/ar4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiGangi C. Neutrophil/lymphocyte ratio: predicting cardiovascular and renal complications in patients with diabetes. J Am Assoc Nurse Pract. 2016;28:410‐414. 10.1002/2327-6924.12366 [DOI] [PubMed] [Google Scholar]
- 35.Karhade AV, Shah KC, Shah AA, et al. Neutrophil to lymphocyte ratio and mortality in spinal epidural abscess. Spine J. 2019;19:1180‐1185. 10.1016/j.spinee.2019.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


