Abstract
Background
CTR9 (Cln three requiring 9) has been reported to be implicated in protein modification and oncogenesis of several human cancers. However, the protein expression and mechanism of CTR9 in glioma progression remain unclear.
Methods
We analyzed mRNA expression of CTR9 and CTR9‐related survival curves in the public database. Then, we detected CTR9 expression in glioma tissues and constructed U251 and U87 cells with stable silencing or overexpression of CTR9. Cell function tests and Western blot were conducted to explore the effects of CTR9 on glioma proliferation, invasion and migration, and the specific mechanism. All the date was presented as means ± SEM. Two‐sample t test and one‐way analysis of variance (ANOVA) were used to identify whether there was a significant difference between each group of data.
Results
We found that CTR9 was overexpressed in glioma and inversely associated with glioma patient survival. The results manifested that knockdown of CTR9 suppressed the proliferation, migration, and invasion of glioma cells, while overexpression facilitated them. The underlying molecular mechanism may involve the regulation of JAK2/STAT3 pathway by CTR9.
Conclusion
Our present study indicates that CTR9 is highly expressed in glioma and related to glioma grading and prognosis. CTR9 regulates malignant behaviors of glioma cells by activating JAK2/STAT3 pathway. Therefore, CTR9 may be a promising biomarker for the targeted therapy and prognosis evaluation of glioma.
Keywords: CTR9, glioma, JAK2, pathway, STAT3
CTR9 regulates proliferation, migration, and invasion of glioma cells by activating JAK2/STAT3 pathway. Therefore, CTR9 is a promising biomarker for the diagnosis, targeted therapy, and prognosis evaluation of glioma.
1. INTRODUCTION
Glioma is the most common malignant tumor of the central nervous system with rapid progress, strong aggression, and high rate of relapse.1 Despite increasing progress in surgery, chemotherapy, and radiotherapy, the expected survival time is only 12 to 15 months for late diagnosis and advanced stages.2, 3 However, molecular targeted agents have therapeutic effects in the treatment of malignant tumors, such as EGFR‐ and ALK‐sensitive lung cancer and VEFG‐sensitive colorectal cancer.4, 5 Monoclonal antibodies and tyrosine kinase inhibitors have played an important role in the treatment of breast cancer, gastrointestinal stromal tumor, and melanoma as the main targeted therapy agents.6, 7, 8 Candidates for targeted therapy include tumor growth factor receptor, signal transduction molecule, apoptosis regulatory factor, proteolytic enzyme, and vascular endothelial growth factor. Hence, explorations in the molecular pathological mechanism of glioma and sequentially appropriate biomarker selection are indispensable to precise treatment of glioma.
Paf1C, composed of Paf1, Leo1, CTR9, Cdc73, and Rtf1, is involved in the malignant progression of many tumors, such as pancreatic carcinoma, ovarian cancer, Wilms tumor, and breast cancer.9, 10 Among the five subunits, CTR9 independently plays significant roles in many biological processes of tumor cells, such as transcription, ubiquitination, and cell cycle. Interestingly, CTR9 functions as a tumor suppressor in Wilms cancer,11 and however, the expression of CTR9 is up‐regulated in breast carcinoma.12, 13 Till now, whether CTR9 plays as a tumor promoter or suppressor in glioma cells awaits investigation and clarification.
Former researches have found that the activity of STAT3 in tumors was abnormally activated with high frequency, such as glioma, liver cancer, colon cancer, breast cancer, lung cancer, and stomach cancer,14, 15, 16, 17, 18, 19its activation degree is significantly related to the poor prognosis of cancer patients. The JAK2 / STAT3 pathway is a key pathway for STAT3 signal transduction and transcriptional activation. Studies have shown that the JAK and STAT were overexpressed in most malignant tumors,20, 21 especially the abnormal activation of STAT3. Normally, STAT3 functions in the cytoplasm, it was transferred into the nucleus once activated and caused abnormal transcription of target genes. STAT3 can facilitate the development of malignant tumors by regulating downstream molecules, such as c‐Myc, cyclin‐D1, MMP‐2, VEGF, Bcl‐2, and Bax.22, 23 Through its own functions and its regulation of downstream molecules, it is involved in the process of the proliferation, migration, and invasion of various tumors.24, 25, 26 Therefore, we speculate that CTR9‐mediated JAK2/STAT3 pathway may explain the malignant behaviors of glioma.
In our study, we first detected the expression of CTR9 protein in glioma patients’ tissues and glioma cell lines by Western blotting assay. Then, the role of CTR9 in glioma was studied through a variety of functional experiments. Finally, we proved that CTR9 facilitates proliferation, migration, and invasion through regulating JAK2/STAT3 pathway.
2. MATERIALS AND METHODS
2.1. Antibodies
Antibody against CTR9 was purchased from Cell Signaling Technology. JAK2, p‐JAK2, STAT3, p‐STAT3, and β‐actin antibodies were bought from Proteintech.
2.2. Tissue samples
Twenty‐two fresh brain tissue specimens from glioma and six fresh brain tissue specimens from traumatic brain injury were selected randomly from the specimen repository in Nanjing Brain Hospital. All tissues were taken from the central site of the resected tumors with typical morphology and pathologically confirmed diagnosis.
The selected samples included non‐tumor group (6 cases), grade II group (8 cases), grade III group (8 cases), and grade IV group (6 cases), according to the WHO tumor classification standard.
2.3. Cell culture
The glioma cell lines U251, T98G, and human embryonic kidney cell line HEK293T were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. All cells were cultured in DMEM with10% fetal bovine serum in a 37°C constant temperature incubator containing 5% CO2.
2.4. Construction of lentivirus
Three short hairpin RNAs (shRNAs) were designed to knock down CTR9 and synthesized as followings:
shCTR‐F1: gatccgCCTCCAGAGATTCTCAATAATTTCAAGAGAATTATTGAGAATCTCTGGAGGTTTTTTg
shCTR‐R1: aattcAAAAAACCTCCAGAGATTCTCAATAATTCTCTTGAAATTATTGAGAATCTCTGGAGGcg
shCTR‐F2: gatccgCGTGCAAATGAGACTATTCTTTTCAAGAGAAAGAATAGTCTCATTTGCACGTTTTTTg
shCTR‐R2: aattcAAAAAACGTGCAAATGAGACTATTCTTTCTCTTGAAAAGAATAGTCTCATTTGCACGcg
shCTR‐F3: gatccGCCATAATTTCATCAAGTGATTTCAAGAGAATCACTTGATGAAATTATGGCTTTTTTg
shCTR‐R3: aattcAAAAAAGCCATAATTTCATCAAGTGATTCTCTTGAAATCACTTGATGAAATTATGGCg
2.5. Establishment of U251 and T98G cell lines
Control, shCTR9#3, GFP, and GFP‐CTR9 plasmids were co‐transfected with helper plasmids pMD2.G and pSPAX2 in HEK293T cells, respectively. Virus supernatants were collected 48 h after transfection. U251 and T98G cells were infected by obtained lentiviruses and stably expressed Control, shCTR9#3, GFP, and GFP‐CTR9 after puromycin filtration.
2.6. EdU assay
Cells at the logarithmic phase were inoculated into 96‐well plates. EdU assay was conducted after overnight culture. Diluted reagent 5‐ethynyl‐20‐deoxyuridine was added into each well, and cells were incubated at 37℃ for 2 h. The cells were washed with PBS twice and fixed with 4% paraformaldehyde at room temperature for 30 min. Afterward, the cells were incubated with glycine and 0.5% Triton X‐100 for 10 and 30 min, respectively. Apollo® reagent was added into each well, and cells were incubated in dark for 30 min. After dealt with 0.5% Triton X‐100 and methanol, the nuclei of the cells were dyed with Hoechst for 20 min and photographed by a fluorescent microscopy.
2.7. Cell viability assay
Cell suspension was inoculated into 96‐well plates. CCK‐8 assay was conducted after overnight culture. 10 μl CCK‐8 reagent was applied to each well (Be careful not to form bubbles in the well), and the absorbance at 450 nm was measured with a SynergyMx Multi‐Mode Microplate Reader (Biotek) after reaction for 4 h at 37℃.
2.8. Transwell migration and invasion assays
To assess invasion ability, the upper chamber of each filter was covered with 10ug Matrigel (BD), which was evenly spread and placed in a 37°C incubator for 2 h. The serum‐free cell suspension was then added into the fliter, and the lower chamber was filled with 10% FBS. After 24 h of incubation at 37°C, the non‐invasive cells were swabbed from the upper chamber. Then, cells on the lower side of the filter were fixed with 4% paraformaldehyde for 30 min and dyed with 0.03% crystal violet solution for 10 min. Three fields of adherent cells in each well were randomly photographed and counted. The same experiment was conducted to assess migration ability except for filters pre‐coated with Matrigel.
2.9. Western blot
The same amount of proteins were placed on 10% SDS‐PAGE and then transferred to PVDF membrane after the required strips appeared. The membrane was sealed with 3% BSA for 1 h at room temperature and cut according to the molecular weight. The membranes were placed into tubes containing primary antibodies at 4°C overnight and secondary antibodies at room temperature for 1 h. The antibodies were detected by ECL or DAB. ImageJ analysis software was used to analyze the strips.
3. RESULTS
3.1. CTR9 protein is highly expressed in human glioma tissues
To explore the role of CTR9 in human gliomas, we used GEPIA Web server to analyze the expression of CTR9 mRNA in glioma and the relationship between CTR9 expression and survival of glioma patients. The results showed that CTR9 mRNA expression was significantly increased in both low‐grade glioma (LGG) and glioblastoma (GBM) tissues, compared with normal brain tissues (Figure 1A). Also, we found that high expression of CTR9 in glioma patients indicated poor prognosis (p < 0.05, Figure 1B). To verify these data, we sequentially detected CTR9 expression in non‐tumor and tumor brain tissues by Western blot. It showed that CTR9 expression level in glioma tissues was significantly increased compared with non‐tumor tissues, particularly in Grade IV gliomas (Figure 1C,D). These obtained results revealed that both mRNA and protein level of CTR9 were overexpressed in human glioma tissues, indicating that CTR9 may act as an important role in human gliomas.
FIGURE 1.
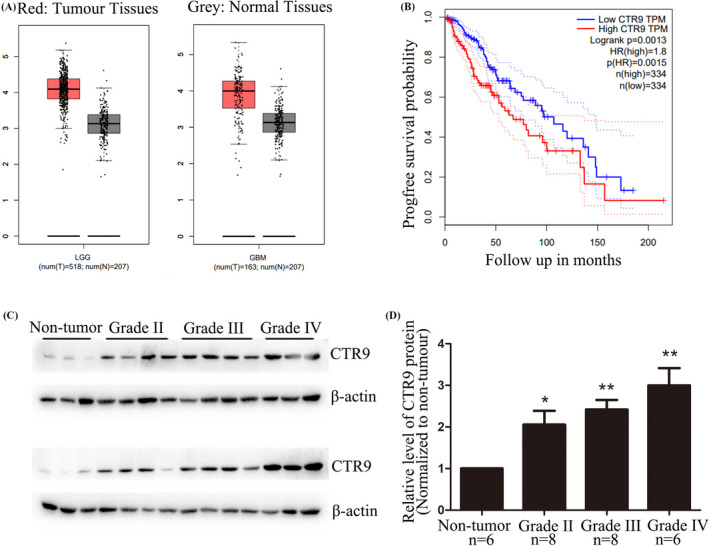
Expression of CTR9 in human glioma tissues. (A) CTR9 mRNA is overexpressed in both LGG and GBM, compared to normal brain tissues. (B) Kaplan‐Meier analysis reveals high expression of CTR9 is related to poor survival time. (C) Western blotting was used to detect the protein levels of CTR9 in non‐tumor brain tissues (6 cases) and human glioma tissues (8 cases for Grade II, 8 cases for Grade III, and 6 cases for Grade IV). (D) Statistical diagram of CTR9 expression in normal brain tissues and glioma tissues. *p < 0.05, **p < 0.01
3.2. Down‐regulation of CTR9 restrains the proliferation, migration, and invasion of glioma cells
To study the effects of CTR9 on malignant behaviors of glioma cells, we downregulated CTR9 expression in glioma cells by its specific shRNAs and reevaluated the proliferation, migration and invasion abilities of glioma cells. First, we constructed three shRNAs (shCTR9#1, shCTR9#2 and shCTR9#3) and found shCTR9#3 owned highest efficiency in suppressing CTR9 expression (Figure 2A–C). Then, we cultured the stable downregulated CTR9 and control cell lines with lentivirus packaged by shCTR9#3 and control. After that, we studied whether silencing CTR9 has a negative or positive effect on cell proliferation.
FIGURE 2.
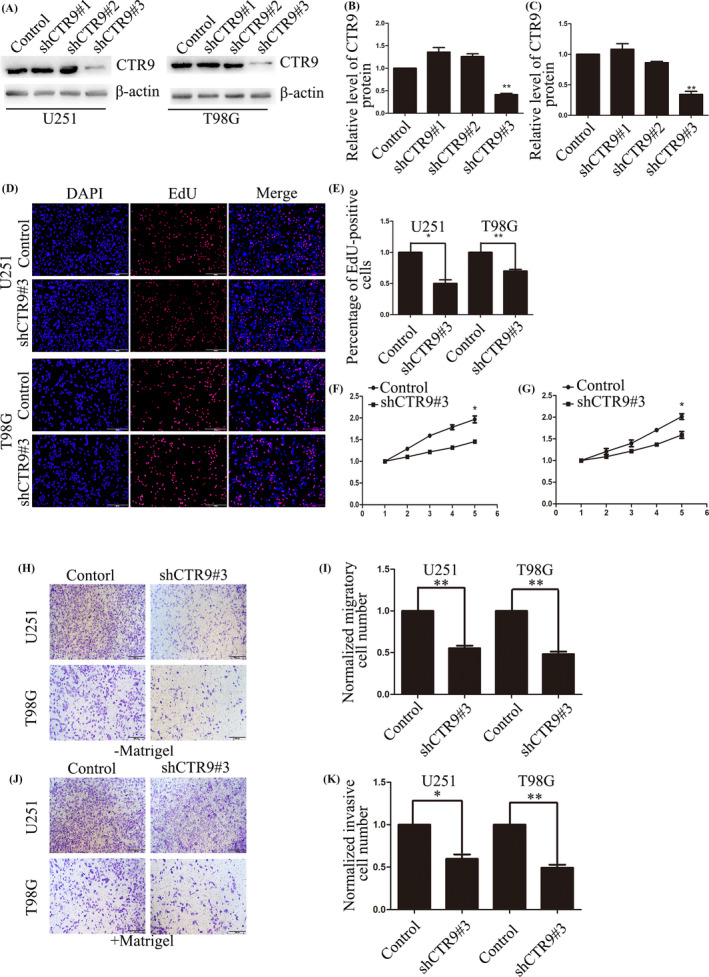
Down‐regulation of CTR9 inhibits glioma cell proliferation, migration, and invasion. (A–C) Western blot showed shCTR9#3 had the best silencing efficiency of 3 shRNAs. (D and E) EdU incorporation assay demonstrated that the proliferation of U251 and T98G cells was suppressed after down‐regulation of CTR9, compared with the control group, scale bars: 100 μm. (F and G) CCK‐8 assay reached the similar conclusion as EdU that down‐regulation of CTR9 inhibited cell viability of U251 and T98G cells. (H and I) Transwell (‐Matrigel) assay showed that silencing CTR9 suppressed migration of U251 and T98G cells, scale bars: 200 μm. (J and K) Transwell (+Matrigel) assay indicated that silencing CTR9 suppressed invasion of U251 and T98G cells, scale bars: 200 μm. *p < 0.05, **p < 0.01
The EdU incorporation assay showed that the EdU‐positive cells in CTR9‐silenced U251 cells decreased by 50%, while decreased by 25% in CTR9‐silenced T98G cells, compared with their respective control group (Figure 2D,E). Also, the CCK‐8 assay showed down‐regulation of CTR9 suppressed cell viability in glioma cells (Figure 2F,G). The results above showed that CTR9 was involved in the proliferation of human glioma cells and down‐regulation of CTR9 inhibited the proliferation of glioma cells.
To explore the role of CTR9 on the migration and invasion of glioma cells, we conducted transwell assay. The transwell migration assay (‐Matrigel) indicated that down‐regulation of CTR9 suppressed the cell migration in U251 and T98G cells (Figure 2H,I). The transwell invasion assay(+Matrigel) showed the number of invasive CTR9‐silenced U251 and T98G cells decreased by 45% and 50%, compared to the control group (Figure 2J,K). Repeated results manifested that silencing CTR9 inhibits migration and invasion of glioma cells.
3.3. Overexpression of CTR9 promotes the proliferation, migration, and invasion of human glioma cells
Additionally, in order to further clarify the effect of CTR9 on glioma cell proliferation, migration, and invasion, we overexpressed GFP‐CTR9 in U251 and T98G cells and explore the effects from another angle. The expression efficiency was testified by Western blotting (Figure 3A). EdU incorporation assay showed that EdU‐positive cells in CTR9‐overexpressed U251 and T98G cells increased by 50% and 30% compared with respective GFP group (Figure 3B,C). Also, CCK‐8 assay manifested that CTR9 promoted the proliferating ability of U251 and T98G cells compared with the control groups. The transwell migration assay (‐Matrigel) showed that overexpression of CTR9 promoted the cell migration in U251 and T98G cells (Figure 3F,G). The transwell invasion (+Matrigel) assay displayed that the number of invading U251 and T98G cells increased by 90% and 70% after CTR9 overexpression (Figure 3H,I). These results suggested that CTR9 is involved in the proliferation, migration, and invasion of human glioma cells and acts as a tumor promoter.
FIGURE 3.
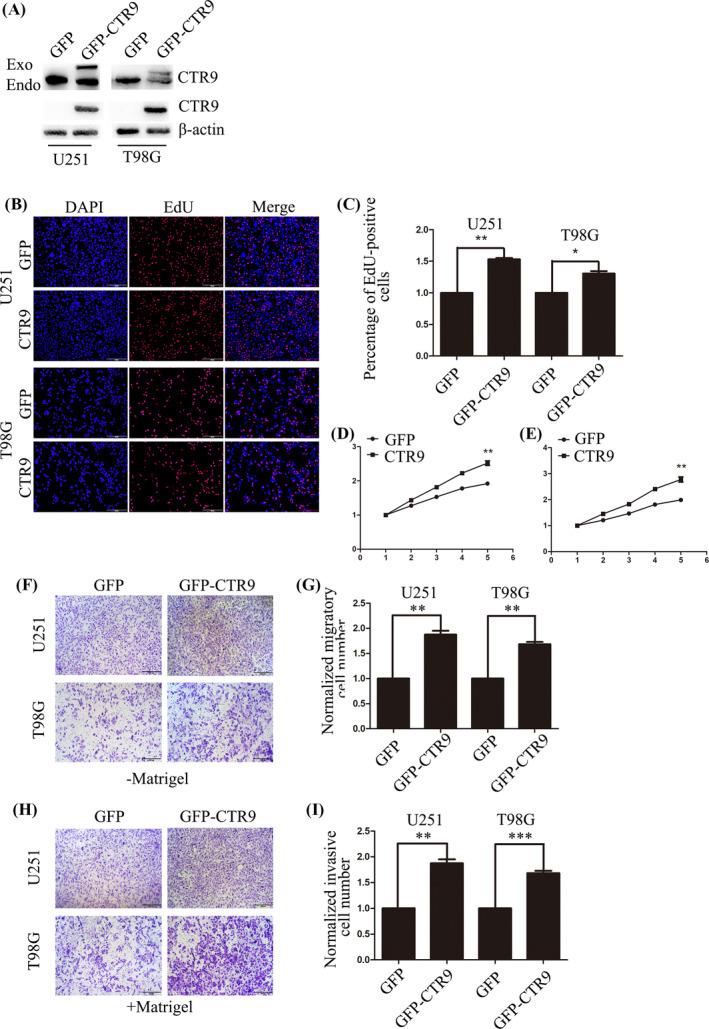
Overexpression of CTR9 promotes glioma cell proliferation, migration, and invasion. (A) Western blot confirmed successful overexpression of CTR9 in U251 and T98G cells. (B and C) EdU incorporation assay demonstrated that the proliferation of U251 and T98G cells was promoted after overexpression of CTR9, compared with the control group, scale bars: 100 μm. (D and E) CCK‐8 assay showed that overexpression of CTR9 accelerated cell viability of U251 and T98G cells. (F and G) Transwell (‐Matrigel) assay showed that overexpressing CTR9 promoted migration of U251 and T98G cells, scale bars: 200 μm. (H and I) Transwell (+Matrigel) assay indicated that overexpressing CTR9 promoted invasion of U251 and T98G cells, scale bars: 200 μm. *p < 0.05, **p < 0.01, ***p < 0.001
3.4. CTR9 promotes the proliferation, migration, and invasion of human glioma cells by regulating JAK2/STAT3 pathway
Previous reports have shown that CTR9 could activate STAT3 signaling.27 Furthermore, it is acknowledged that JAK2/STAT3 pathway is involved in growth and motility of human cancers.28, 29, 30 Based on these, we supposed that CTR9‐mediated JAK2/STAT3 pathway may play a significant role in proliferation, migration, and invasion of human glioma cells. We confirmed our inference by Western blot. The results showed that STAT3 and p‐STAT3 were decreased in CTR9 knockdown U251 and T98G cells, and however, the expression of STAT3 and p‐STAT3 was increased in CTR9‐overexpressed U251 and T98G cells (Figure 4A–C). Moreover, the expression of JAK2 and p‐JAK2, the upstream regulator of STAT3, was decreased upon silencing CTR9, but enhanced upon overexpressing CTR9 (Figure 4D–F). Furthermore, to confirm that CTR9 regulates STAT3 by activating JAK2, we applied JAK2 inhibitor SB1317 to conduct the rescue experiment. As expected, overexpression of CTR9 up‐regulated JAK2, p‐JAK2, STAT3, and p‐STAT3. However, this up‐regulation could be significantly blocked by JAK2 inhibitor SB1317 (Figure 5A–C). The results demonstrated that STAT3 and p‐STAT3 up‐regulation induced by CTR9 were attributed to JAK2 and p‐JAK2 activation. In addition, we conducted CCK‐8 and transwell assays to confirm this in the presence of the JAK2 inhibitor. As the results showed, JAK2 inhibitor SB1317 blocked cell proliferation (Figure 5D,E), migration (Figure 5F,G), and invasion (Figure 5H,I). In all, the results expounded that CTR9‐meidated JAK2/STAT3 pathway could accelerate proliferation, migration, and invasion of glioma cells.
FIGURE 4.
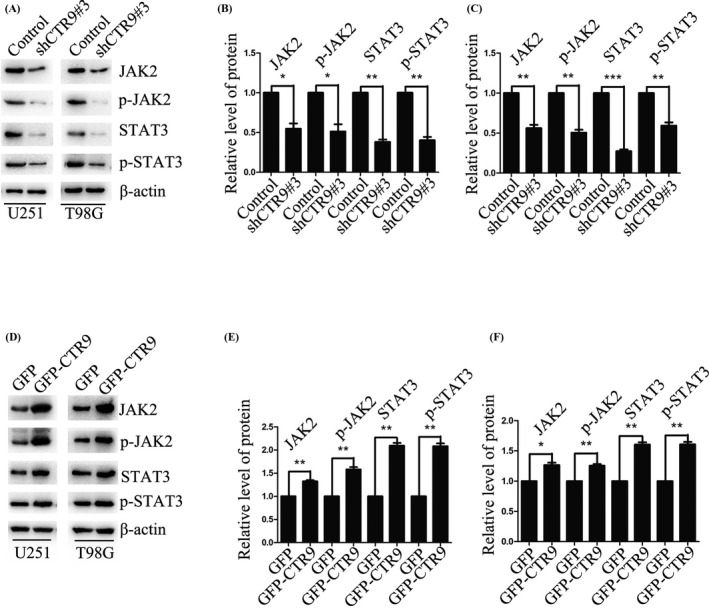
CTR9 regulates JAK2/STAT3 pathway. (A–C) Western blotting assay showed that the expression levels of JAK2, p‐JAK2, STAT3, and p‐STAT3 in U251‐shCTR9#3 and T98G‐shCTR9#3 cells were significantly suppressed than those in the control group, respectively. (D–F) Western blotting assay confirmed that the expression levels of JAK2, p‐JAK2, STAT3, and p‐STAT3 in U251 GFP‐CTR9 and T98G GFP‐CTR9 cells were significantly overexpressed than those in the control group, respectively. *p < 0.05, **p < 0.01, ***p < 0.01
FIGURE 5.
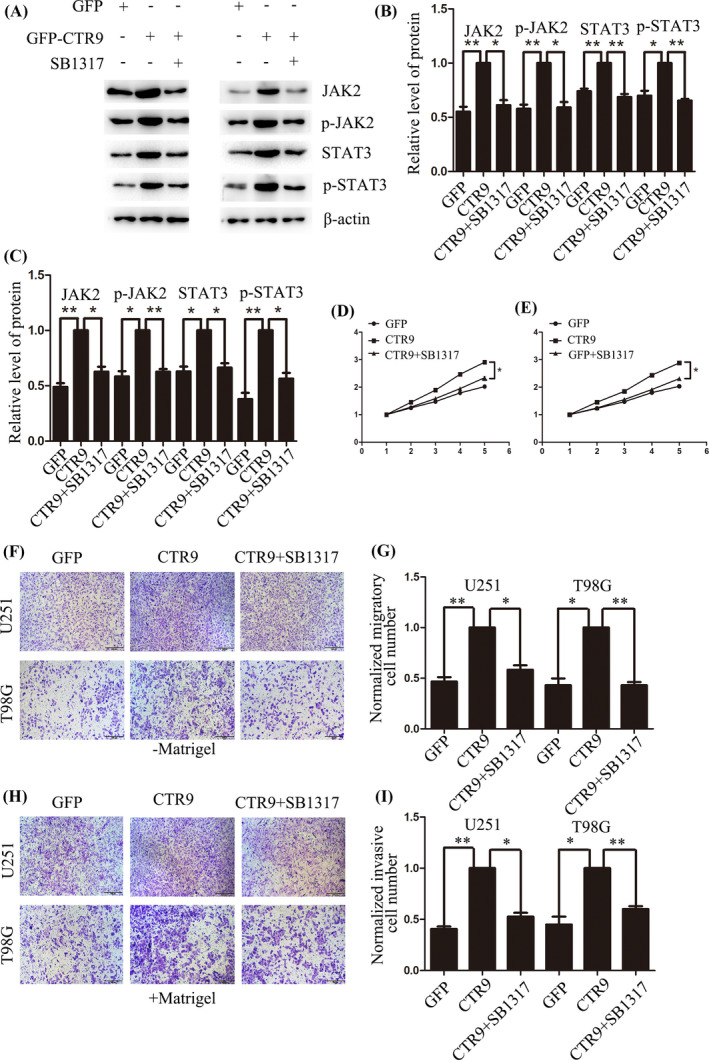
SB1317 notably blocks CTR9 overexpression‐induced up‐regulation of JAK, p‐JAK2, STAT3, and p‐STAT3, as well as proliferation, migration, and invasion of glioma cells. (A–C) Up‐regulation of JAK, p‐JAK2, STAT3, and p‐STAT3 induced by CTR9 overexpression was effectively inhibited by the JAK2 inhibitor SB1317. (D–I) Rescue experiments showed JAK2 inhibitor SB1317 significantly blocked the proliferation, migration, and invasion of glioma cells induced by CTR9 up‐regulation.*p < 0.05, **p < 0.01
4. DISCUSSION
CTR9 is a multi‐functional gene that is implicated in many cellular process such as cell cycle, transcription, neurogenesis, and embryonic organogenesis.10, 11, 31, 32 Former researches have shown that CTR9 functions as a tumor suppressor in Wilms tumor while drives ERα‐positive breast tumorigenesis,12 which indicates that CTR9 is a potential candidate for human tumor biomarkers. However, the expression of CTR9 and its clinical relevance in glioma patients have never been clearly elaborated. In our present study, we detected that CTR9 was up‐regulated in human glioma tissues compared with tissues derived from normal brain. In addition, we drew a conclusion that high expression of CTR9 was strongly associated with poor prognosis of glioblastoma patients through public database analysis. We clarified that up‐regulation of CTR9 in glioma cells could accelerate proliferation, migration, and invasion of glioma cells by regulating the JAK2/STAT3 pathway. These findings suggest that CTR9 exerts as a tumor‐promoting role in glioma and is a promising therapeutic strategy in human brain glioma.
It is widely acknowledged that silencing of specific tumor suppressor genes and activation of certain oncogenes are responsible for the occurrence and malignant progression of glioblastoma.33, 34 In particular, JAK2/STAT3 pathway is deeply involved in the survival and motion of tumor cells.35 The excessive activation of JAK2 signaling in glioma is accelerated by abnormal signals from upstream regulators.36, 37 This usually involves any gain‐of‐function mutations or up‐regulation of upstream inhibitors, and, on the other hand, any loss‐of function mutation or down‐regulation of the upstream inhibitors. In this study, we found that CTR9 could promote glioma cell proliferation, migration, and invasion by modulating JAK2/STAT3 pathway. That is to say, up‐regulation of CTR9 may be a significant event in the development and malignant progression of human glioma.
STAT3 plays a strong inhibitory role on cell apoptosis through JAK2/STAT3 pathway, and its continuous activation of cells is deeply involved in the occurrence and progression of human malignant tumors.38 Phosphorylated Stat3, once transferred into the nucleus, can regulate the transcription of some target genes involved in basic physiological functions and induce the abnormal expression of genes closely related to cell proliferation, differentiation, and apoptosis, such as Bcl‐XL and c‐Myc, promoting cell proliferation and malignant transformation.39 In the present study, we found that Stat3 and p‐STAT3 were significant up‐regulated upon CTR9‐induced activation of JAK2 pathway. Besides, the overexpression of STAT3 and acceleration of glioma cell proliferation, migration, and invasion induced by up‐regulation of CTR9 could be distinctly blocked by JAK2 inhibitor.
Taken together, we affirmed the association between the CTR9‐mediated JAK2/STAT3 pathway and the malignant behaviors of glioma. Our findings provided a basis for potential therapeutic target and prognosis evaluation in glioma. However, more sequent researches are urgent to be conducted to clarify the precise mechanisms of CTR9‐mediated activation of JAK2/STAT3 pathway in human glioma.
CONFLICT OF INTERESTS
The authors declare no competing interests.
AUTHORS CONTRIBUTIONS
Study design: Yuhai Zhang and Yang Xu. Experiments: Yang Xu and Jiaguo Chen. Data analysis: Jiaguo Chen and Gao He. Manuscript preparation: Yang Xu. Manuscript review: Yuhai Zhang. The manuscript was approved by the authors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Informed consents were acquired from each patient or their families and passed by the Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. Case number: 2020‐KY076‐05.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from all participants.
ACKNOWLEDGEMENTS
We would like to thank Dr. Liang Zhao for critical review of this manuscript.
Xu Y, Chen J, He G, Zhang Y. CTR9‐mediated JAK2/STAT3 pathway promotes the proliferation, migration, and invasion of human glioma cells. J Clin Lab Anal. 2021;35:e23943. 10.1002/jcla.23943
Yang Xu and Jiaguo Chen have contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data can be required from the corresponding author.
REFERENCES
- 1.Arevalo OJ, Valenzuela R, Esquenazi Y, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a practical approach for gliomas, part 1. Basic tumor genetics. Neurographics. 2017;7(5):334‐343. [Google Scholar]
- 2.Wei N, Chu E, Wipf P, Schmitz JC. Protein kinase d as a potential chemotherapeutic target for colorectal cancer. Mol Cancer Ther. 2014;13(5):1130‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahar T, Rozovski U, Hess KR, et al. Percentage of mesenchymal stem cells in high‐grade glioma tumor samples correlates with patient survival. Neuro Oncol. 2016;19(5):660‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles DW, Weickhardt A, Jimeno A. Afatinib for the treatment of patients with EGFR‐positive non‐small cell lung cancer. Drugs Today. 2013;49(9):523‐535. [DOI] [PubMed] [Google Scholar]
- 5.Kuifeng HE, Binbin C, Guangliang LI, Haohao W, Ketao J. The effect of anti‐VEGF drugs (bevacizumab and aflibercept) on the survival of patients with metastatic colorectal cancer (mCRC). OncoTargets Ther. 2012;5:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carloni S, Fabbri F, Brigliadori G, et al. Tyrosine Kinase Inhibitors Gefitinib, Lapatinib and Sorafenib Induce Rapid Functional Alterations in Breast Cancer Cells. Curr Cancer Drug Targets. 2010;10(4):422‐431. [DOI] [PubMed] [Google Scholar]
- 7.Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother. 2014;15(14):1979‐1989. [DOI] [PubMed] [Google Scholar]
- 8.Chi M, Puzanov I. Targeted drug development in melanoma and nonsmall cell lung cancer: BRAF, MEK, and ALK inhibitors. memo ‐ Magazine of European. Med Oncol. 2012;5(4):302‐308. [Google Scholar]
- 9.Tomson BN, Arndt KM. The many roles of the conserved eukaryotic Paf1 complex in regulating transcription, histone modifications, and disease states. Biochem Biophys Acta. 2013;1829(1):116‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Zheng M, Chu X, et al. Paf1 and Ctr9 subcomplex formation is essential for Paf1 complex assembly and functional regulation. Nat Commun. 2018;9(1):3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanks S, Perdeaux ER, Seal S, et al. Germline mutations in the PAF1 complex gene CTR9 predispose to Wilms tumour. Nat Commun. 2014;5:4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H, Xu W. Ctr9, a key subunit of PAFc, affects global estrogen signaling and drives ERα‐positive breast tumorigenesis. Genes Dev. 2015;29(20):2153‐2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, Lu L, Chan NT, et al. Systematic identification of Ctr9 regulome in ERα‐positive breast cancer. BMC Genom. 2016;17:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguly D, Fan M, Yang CH, et al. The critical role that STAT3 plays in glioma‐initiating cells: STAT3 addiction in glioma. Oncotarget. 2018;9(31):1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z‐Z, Huang L, Wu Y‐H, Zhai W‐J, Zhu P‐P, Gao Y‐F. LncSox4 promotes the self‐renewal of liver tumour‐initiating cells through Stat3‐mediated Sox4 expression. Nat Commun. 2016;7:12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang R, Wang H, Deng L, et al. IL‐22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SF, Hou MF, Chen FM, et al. Prognostic value of protein inhibitor of activated STAT3 in breast cancer patients receiving hormone therapy. BMC Cancer. 2016;16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian YY, Chai S, Liang Z, et al. KIF5B‐RET fusion kinase promotes cell growth by multilevel activation of STAT3 in lung cancer. Mol Cancer. 2014;13(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui H, Zhang X, Sun C, et al. IL‐22 produced by cancer‐associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br J Cancer. 2014;111(4):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa T, Miyamoto M, Aoyama T, et al. JAK2/STAT3 pathway as a therapeutic target in ovarian cancers. Oncol Lett. 2018;15:5772‐5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Hu F, Li G, et al. Human colorectal cancer‐derived mesenchymal stem cells promote colorectal cancer progression through IL‐6/JAK2/STAT3 signaling. Cell Death Dis. 2018;9(2):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isomäki P, Junttila I, Vidqvist K‐L, Korpela M, Silvennoinen O. The activity of JAK‐STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology. 2015;54(6):1103‐1113. [DOI] [PubMed] [Google Scholar]
- 23.Darnell J, Kerr I, Stark G. Jak‐STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415‐1421. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Dong Z, Wang F, Peng H, Liu J‐Y, Zhang J‐T. A small molecule compound targeting STAT3 DNA‐binding domain inhibits cancer cell proliferation, migration, and invasion. ACS Chem Biol. 2014;9(5):1188‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen N, Huang X, Li J. Upregulation of miR‐129‐5p affects laryngeal cancer cell proliferation, invasiveness, and migration by affecting STAT3 expression. Tumor Biol. 2016;37(2):1789‐1796. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Yan R, He X, He J. Constitutive activation of STAT3 and cyclin D1 overexpression contribute to proliferation, migration and invasion in gastric cancer cells. Am J Transl Res. 2017;9(12):5671‐5677. [PMC free article] [PubMed] [Google Scholar]
- 27.Youn MY, Yoo HS, Kim MJ, et al. hCTR9, a component of Paf1 complex, participates in the transcription of Interleukin 6‐responsive genes through regulation of STAT3‐DNA interactions. J Biol Chem. 2007;282(48):34727‐34734. [DOI] [PubMed] [Google Scholar]
- 28.Marotta LLC, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24– stem cell–like breast cancer cells in human tumors. J Clin Invest. 2011;121(7):2723‐2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv C, Huang Y, Liu Z‐X, et al. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomarkers. 2016;17(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Ji Q, Ye N, et al. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX‐2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10(5):e0123478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyun‐Seok Y, Jung‐Hwa S, Joo‐Yeon Y, Ki KY. CTR9, a component of PAF complex, controls elongation block at the c‐Fos locus via signal‐dependent regulation of chromatin‐bound NELF dissociation. PLoS One. 2013;8(4):e61055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahrampour S, Thor S. Ctr9, a key component of the Paf1 complex, affects proliferation and terminal differentiation in the developing drosophila nervous system. G3 Genesgenetics. 2016;6(10):3229‐3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie M, Ji Z, Bao Y, et al. PHAP1 promotes glioma cell proliferation by regulating the Akt/p27/stathmin pathway. J Cell Mol Med. 2018;22(7):3595‐3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Zhang X, Wang L, et al. Loss of SH3GL2 promotes the migration and invasion behaviours of glioblastoma cells through activating the STAT3/MMP2 signalling. J Cell Mol Med. 2017;21(11):2685‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byun HJ, Darvin P, Kang DY, et al. Silibinin downregulates MMP2 expression via Jak2/STAT3 pathway and inhibits the migration and invasive potential in MDA‐MB‐231 cells. Oncol Rep. 2017;37(6):3270‐3278. [DOI] [PubMed] [Google Scholar]
- 36.Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL‐6R through JAK2/STAT3 mediate epithelial–mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100(1):134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Ding H, Han Y, Sun D, Wang H, Zhai X. The significance of microRNA‐184 on JAK2/STAT3 signaling pathway in the formation mechanism of glioblastoma. Oncol Lett. 2015;10(6):3510‐3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nizam U, Rae‐Kwon K, Ki‐Chun Y, et al. Persistent activation of STAT3 by PIM2‐driven positive feedback loop for epithelial‐mesenchymal transition in breast cancer. Cancer Sci. 2019;106(6):718‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Schütte D, Wulf G, et al. Stem‐cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl‐XL pathway. Hum Mol Genet. 2006;15(2):201‐211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be required from the corresponding author.


