Abstract
Aim
To test a novel method of assessment of platelet adhesion to a fibrinogen‐coated surface in whole blood under flow conditions.
Methods
We developed a fluidic device that mimics blood flow in vessels. The method of detection of platelet adhesion is based on recording of a scattered laser light signal from a fibrinogen‐covered surface. Testing was performed in platelet‐rich plasma (PRP) and whole blood of healthy volunteers. Control measurements were performed, followed by tests with inhibition of platelet GPIIa/IIIb and GPIb receptors. Then, the same testing sequence was performed in whole blood of persons with autoimmune thrombocytopenia and type 3 von Willebrand disease.
Results
The change in intensity of scattered light was 2.7 (2.4; 4.1) times higher in whole blood (0.2 ± 0.08V, n = 7) than in PRP (0.05 ± 0.02 V, n = 7), p < 0.01. The blocking of GP IIb/IIIa receptors decreased the intensity of scattered light to 8.5 (6.5;12)%; the blocking of GPIb receptors decreased it to 34 (23;58)%, p < 0.01. In the whole blood of a person with autoimmune thrombocytopenia, the inhibition of GPIb receptors decreased platelet adhesion, but no effect was observed in type 3 von Willebrand disease. Inhibition of platelet GPIIb/IIIa receptors alone or combined inhibition of GPIb and GPIIb/IIIa receptors resulted in almost total suppression of adhesion in both cases.
Conclusion
Our system effectively registers platelet adhesion to a fibrinogen‐coated surface under controlled‐flow conditions and may successfully be applied to the investigation of platelet adhesion kinetics.
Keywords: fibrinogen‐coated surface, flow conditions, platelet adhesion, recording of a scattered laser light signal from a fibrinogen‐covered surface, whole blood
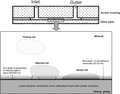
The laser optical system for recording of platelet adhesion to protein‐coated surfaces under flow conditions. The illustration of the flow chamber with the flat glass surface coated with a monolayer of cell‐adhesive molecules. The glass surface was coated with fibrinogen. The reflected light evanesces into the blood sample beyond the reflection zone to a depth of approximately 400–600 nm. Platelets or other blood cells in the flow do not interact with this laser radiation, which evanesces to such a small depth. The laser light can interact with platelets only when they are strongly attached to or spread onto the glass surface.
1. INTRODUCTION
Functional evaluation of platelet adhesion to protein‐coated surfaces under flow conditions is fundamental for science and laboratory diagnostics. Multiple studies described the process of platelet adhesion to surface‐adsorbed proteins.1, 2, 3, 4 The current practice of quantitative assessment of platelet adhesion is based on morphometric analysis of adherent platelets by microscopy5, 6 or counting of platelets before and after the interaction with a testing surface.7, 8 A pre‐requisite for the majority of existing methods of quantitative evaluation of platelet adhesion is static testing conditions, which is a major drawback, considering that platelet adhesion is largely dependent on flow conditions.9, 10, 11, 12 Microfluidic assays and new biosensors are alternatives that may provide more accurate evaluation of platelet adhesion to surfaces under flow conditions.13, 14, 15, 16, 17 De Zanet et al. introduced a new biosensor for the real‐time analysis of blood thrombus formation under flow conditions.18 It is based on measuring the global electrical impedance of the blood sample between a pair of specifically designed gold microelectrodes placed on a collagen‐coated surface. However, this technique possesses significant inaccuracy in evaluation of blood clot formation because of the a priori presumption of characteristics that describe the relationship between the growing clot and the magnitude of its impedance, which significantly influences the detected signal.
Recently, we developed a novel laser optical testing system for evaluation of platelet adhesion to protein‐coated surfaces under flow conditions using special laser optical and microfluidic techniques. The method allows performing real‐time assessment of the kinetics of platelet adhesion to protein‐coated surfaces under controlled‐flow conditions. We validated the method by measuring platelet adhesion to fibrinogen‐coated surfaces in platelet‐rich plasma from healthy volunteers.19 In this work, we measured platelet adhesion to a fibrinogen‐coated surface under precisely controlled‐flow conditions in whole blood of healthy volunteers before and after inhibition of platelet GPIb and GPIIb/IIIa receptors. Blood samples of a person with autoimmune thrombocytopenia and a person with von Willebrand disease were also investigated.
2. MATERIALS AND METHODS
2.1. Blood sample preparation
Venous blood was collected from the cubital vein into 3.2% sodium citrate S‐Monovette tubes (Germany) at the anticoagulant to blood ratio of 1 to 9. Platelet‐rich plasma (PRP) was prepared by centrifuging whole blood at 200g for 5 min, whereupon 2/3 of the supernatant was collected for further studies. Platelet‐poor plasma (PPP) was prepared by centrifugation of remaining PRP at 2000 g for 15 min. Platelet adhesion measurement experiments were carried out within 4 h of blood sampling. Blood was not recalcified prior to any of the tests. The platelet count in blood samples was measured on the Abacus Junior B haematology analyser (Diatron). Seven healthy volunteers and 2 patients 30–55 years old participated in this study. Healthy volunteers were recruited from laboratory staff and their relatives. All of them had normal full blood count tests, erythrocyte sedimentation rate and ECG patterns. Volunteers with cardiovascular, haematologic, metabolic diseases, any known inflammatory or autoimmune disease, surgery in the previous 6 months, acute or chronic inflammation and/or immunomodulatory medication were excluded from the study. Volunteers did not take any antiplatelet, anticoagulation and anti‐iflammation (including aspirin) therapy. Informed written consent was obtained from patients and healthy volunteers according to the Declaration of Helsinki, and this study was approved by the Ethics Committee of the National Medical Research Centre of Cardiology, Moscow.
2.2. The laser optical system for recording of platelet adhesion to protein‐coated surfaces under flow conditions
To measure kinetics of platelet adhesion to protein‐coated surfaces, we developed a laser optical testing system, which consists of a cell assembly with a disposable flow chamber, a specialised optical prism system with semiconductor laser KLM‐E650‐5–3 with λ = 650 nm (FTI Optronic, Russia) and photodetector (photodiode S1133, Hamamatsu), a computer‐controlled peristaltic pump P625 (Instech Laboratories, Inc.) and an analogue‐to‐digital converter (ADC) (L‐Card) connecting the system to a computer (Figure 1). The kinetics of platelet adhesion were recorded, stored and analysed by L‐Graph2 software (L‐Card). In previous work, we described the design and application of our microfluidic system for recording the kinetics of platelet adhesion to fibrinogen‐coated glass at well‐defined shear rates in platelet‐rich plasma (PRP).19
FIGURE 1.
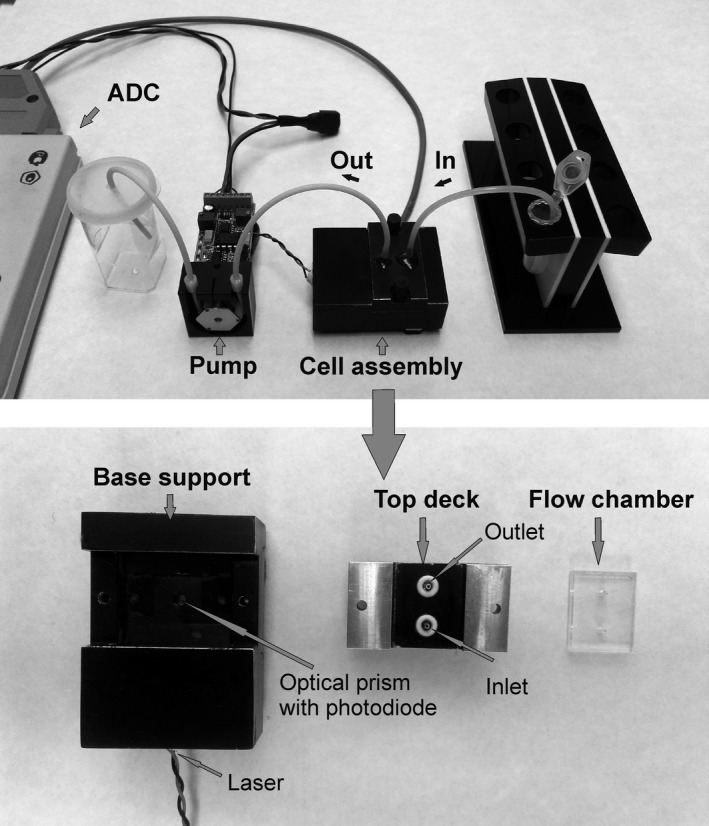
The laser optical system for recording of platelet adhesion to protein‐coated surfaces under flow conditions. Complete assembly of the laser test system and its components: fluid is drawn from the reservoir through the inlet tubing into the cell through a computer‐controlled peristaltic pump. The analogue‐to‐digital converter (ADC) is used for connection of the system to a computer
To study platelet adhesion to fibrinogen, we used a disposable optical microfluidic flow chamber that mimics blood flow in blood vessels. The disposable plain‐flow chamber was designed from a glass plate and an acrylic housing in a way that laser radiation incident on the boundary between two optical media (the glass surface and blood plasma) at the angle of incidence greater than the critical incidence angle is reflected completely from the optical boundary under the law of total internal reflection (Figure 2). Nevertheless, the reflected light evanesces into the blood sample beyond the reflection zone to a depth of approximately 400–600 nm. According to the law of total internal reflection, evanescent wave decays exponentially, that is, it decreases in proportion to its current value. Given the wavelength of the laser λ = 650 nm, we shall expect stronger evanescence in the vicinity of the optical boundary and weaker evanescence farther away from the boundary, but within the given wavelength. Platelets or other blood cells in the flow do not interact with this laser radiation. The light can interact with platelets only when they spread tightly onto the glass surface. This strong adherence causes the interaction of attached platelets with evanescent light and leads to an increase in the intensity of scattered light, which is detected by a photodetector. The extent of platelet adhesion is measured as the time‐dependent increase in the intensity of scattered light and expressed in Volts (V).
FIGURE 2.
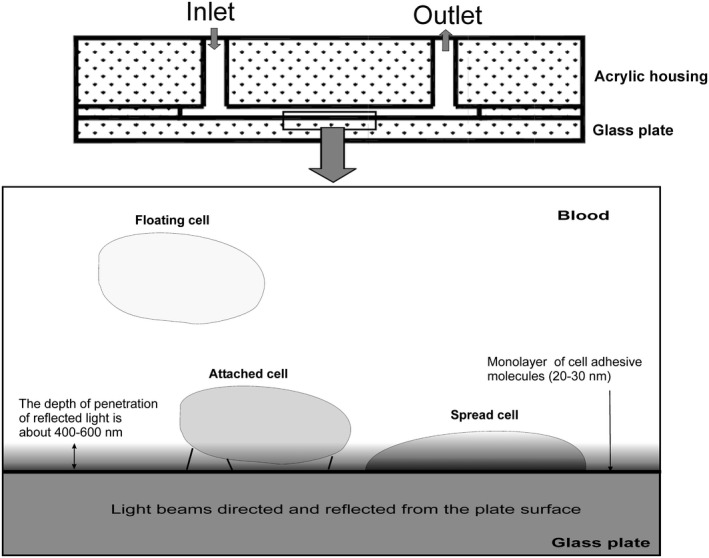
The illustration of the flow chamber with the flat glass surface coated with a monolayer of cell‐adhesive molecules. The reflected light evanesces into the blood sample beyond the reflection zone to a depth of approximately 400–600 nm. Platelets or other blood cells in the flow do not interact with this laser radiation, which evanesces to such a small depth. The laser light can interact with platelets only when they are strongly attached to or spread onto the glass surface
In our testing, blood was set to move through the flow chamber at shear rate of 1300 s−1. Shear rates were calculated by the formula , where Q stands foe blood sample consumption per unit of time, W for the flow chamber cross‐sectional width (3 mm) and H for the flow chamber cross‐sectional height (0.1 mm). The target shear rates were set with the peristaltic pump rotation speed, by charging the sample consumption per unit of time.
2.3. Materials
Fibrinogen (SKU #3879, Lot#SLBR9592V, purity 50–70%, clottability >95%), adenosine diphosphate (ADP, SKU#A5285, Lot #SLBM3854V, purity ≥95%), phosphate‐buffered saline (PBS, SKU #P4417, Lot #SLBH8389V) and paraformaldehyde (SKU #P6148, purity >95%) were purchased from Sigma, monoclonal antibody (mAb) AK2 to GP Ib (a gift by dr. A. Mazurov from National Medical Research Center of Cardiology) and mAb Fab2mAb CRC64 to GP IIb/IIIa (reg. #N003348/02, Monafram), produced and tested according to previously published articles.20, 21 Fibrinogen and ADP solutions were stored in aliquots at –20 ºC, and mAbs were stored at –70 ºC. Glass surfaces were coated with fibrinogen by incubation with 50 µg/ml fibrinogen for 10 min. After the incubation, surfaces were dried and stored at room temperature. In our experience, surfaces prepared this way may be used up to 12 months after coating.
2.4. Microscopy of adherent cells
Post‐flow fixation of blood cells adherent to a fibrinogen‐coated surface after 15 min of perfusion was performed in a flow chamber by rinsing with PBS and fixation with 1% formaldehyde solution. Giemsa staining of adherent cells was performed according to the manufacturer's instructions. Stained samples were investigated with Leica DM 5000 B (Leica Biosystems) fluorescence microscope in the bright field mode.
2.5. Aggregometry
Platelet aggregation tests were performed in PRP by optical turbidimetric method with ALAT‐2 laser aggregation analyzer (Biola). Aggregation tests were performed for at least 5 min.
2.6. Statistics
Data are presented as median (lower quartile; upper quartile). The Mann‐Whitney U test was used to compare two groups of data, which were as follows: whole blood and PRP in the study of difference between intensity of platelet adhesion in whole blood and PRP; control measurements and measurements with inhibition of GPIb receptors, or control measurements and measurements with inhibition of GP IIb/IIIa receptors. Differences were regarded as statistically significant if the null hypothesis was rejected with probability >95%. STATISTICA software version 6.0 (StatSoft Inc.) was used for analysis. Throughout the article, the number of measurements (n = 7) refers to the number of healthy volunteers, one measurement per each person.
3. RESULTS
3.1. Platelet adhesion to fibrinogen in PPP, PRP and whole blood under flow conditions
No signal from a fibrinogen‐coated surface was recorded after perfusion of PPP (platelet concentration 11 ± 2.3 × 103) of 7 healthy volunteers (data is not shown). We investigated platelet adhesion to a fibrinogen‐coated surface in PRP and whole blood obtained from seven healthy volunteers. The extent of platelet adhesion to the optical surface was measured by the increase in the intensity of scattered light detected by the photodiode. The change in intensity of scattered light was 2.7 (2.4; 4.1) times higher in whole blood (0.2 ± 0.08 V, n = 7) than in PRP (0.05 ± 0.02 V, n = 7), p < 0.01. The blocking of GP IIb/IIIa receptors decreased the intensity of scattered light to 8.5 (6.5;12)%; the blocking of GPIb receptors decreased it to 34 (23;58)%, p < 0.01. The intra‐donor coefficient of variability (CV) in whole blood was measured in blood of one healthy volunteer 4 times at the first visit (4.85% CV) and 3 times at the second visit 2 months later (2.85% CV).
In Figure 3, we show typical curves of platelet adhesion to the fibrinogen‐coated surface of the flow chamber in PRP and whole blood samples of the same healthy subject. The platelet concentration was 329 ± 32.8 × 103/μl and 261 ± 35.4 × 103/μl in PRP and whole blood samples, respectively. Platelets in PRP and whole blood were activated with 5 μM ADP before the flow started. Preactivation with ADP was done in all tests due to the concern that red blood cells in whole blood samples may leak ADP during blood perfusion in unequal amounts, which would non‐uniformly affect platelet activation in different samples. Hence, the preactivation was done to achieve uniform activation of platelets between samples. The shear rate at the surface of optical bedding was 1300 s−1. A greater change in the intensity of scattered light occurred in whole blood than in PRP (curves 1 and 2, respectively). Platelet GPIIb/IIIa inhibition by anti‐GP IIb/IIIa mAb resulted in blocking of platelet adhesion to the fibrinogen coating and absence of optical signal changes on the photodiode in both whole blood and PRP (curves 3 and 4, respectively). In experiments with platelet‐poor plasma, at the platelet concentration less than 10 × 103/μl, no change in the intensity of scattered light was observed. Also, the interaction of PRP or whole blood with the flow chamber surface without fibrinogen coating for 20–25 min produced no changes in the intensity of scattered light in both cases (data are not shown).
FIGURE 3.
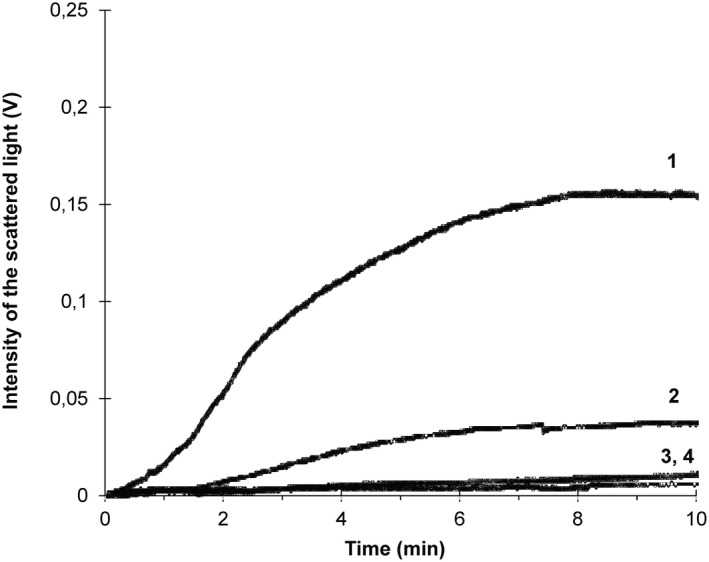
Typical curves of kinetics of platelet adhesion to a fibrinogen‐coated glass surface of the flow chamber in platelet‐rich plasma and whole blood samples of the same healthy subject. The platelet concentration is 329 × 103/μl and 261 × 103/μl in the platelet‐rich plasma and whole blood sample, respectively. Platelets in whole blood and PRP were activated with 5 μM adenosine diphosphate before the flow was started. The shear rate was 1300s−1. Curve 1—in whole blood; Curve 2—in PRP; Curve 3—in whole blood with anti‐GPIIb/IIIa mAb; Curve 4—in PRP with anti‐GPIIb/IIIa mAb
After whole blood perfusion was completed, the flow chambers were rinsed, fixed with 1% formaldehyde and stained with Giemsa stain. The microscopy in the bright field mode revealed that in control samples (Figure 4A), the fibrinogen‐coated surface was covered with long strands, presumably of protein origin (Figure 4B). All over, and in close proximity to the strands, mononuclear and polymorphonuclear cells were visible. The strands and the whole visible area were covered with small anucleate cells, presumably, platelets. In samples where GP IIb/IIIa receptors were inhibited (Figure 4A), the visible field was clear, with only few stray cells visible (Figure 4C).
FIGURE 4.
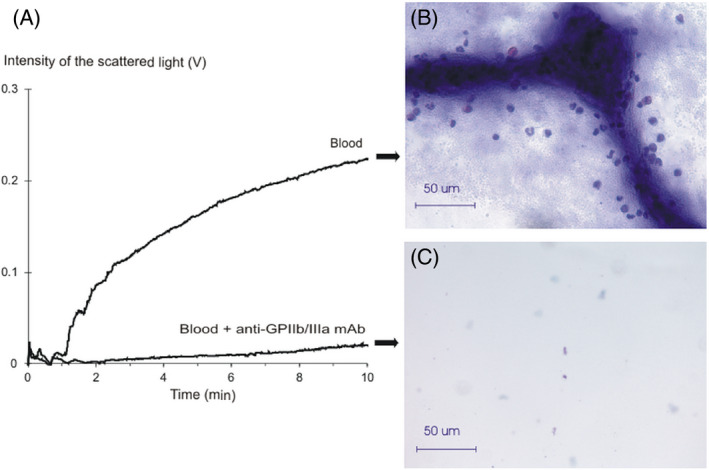
The comparison of the adhesion curves to the microscopy findings obtained after the perfusion of whole blood samples. A, The curves of kinetics of platelet adhesion to a fibrinogen‐coated glass surface recorded in a whole blood sample of a healthy volunteer. Blood—the curve from perfusion of a control sample; Blood+anti‐GPIIb/IIIa mAb—the curve from a perfusion of a whole blood sample with inhibited GPIIb/IIIa receptors; B, The microscopy of a fibrinogen‐coated glass after 10 min of perfusion of the control whole blood sample. Stained with Giemsa. Magnification ×40; C, The microscopy of a fibrinogen‐coated glass after 10 min of the GPIIb/IIIa‐inhibited whole blood sample. Stained with Giemsa. Magnification ×40
3.2. The role of GPIb and GPIIb/IIIa in platelet aggregation in PRP and in platelet adhesion to fibrinogen in whole blood under flow conditions
Figure 5A shows typical curves of platelet aggregation in PRP before and after inhibition of platelet GPIb and GPIIb/IIIa receptors. The platelet count was 217 ± 27.3 × 103/μl. Platelets were activated with 5 μM ADP. In the control measurement, the figure shows the increase in the intensity of light transmission (curve 1) after platelet activation. Inhibition of the platelet GPIb receptors did not affect platelet aggregation (curve 2). Inhibition of platelet GPIIb/IIIa receptors resulted in the pronounced decrease of platelet aggregation (curve 3). Figure 5B shows the results obtained from seven healthy volunteers. Platelet aggregation was registered over 5 min. The extent of platelet aggregation was calculated as the maximum increase in the intensity of light transmission and in the control experiments was taken as 100%. The extent of platelet aggregation with anti‐GPIb mAb was 100 (103; 98)%; with anti‐GPIIb/IIIa mAb, it was 0.0 (0.0; 0.0)% comparative to that of the control. In all cases, platelet aggregation was totally blocked by anti‐GPIIb/IIIa mAb (p < 0.01, n = 7).
FIGURE 5.
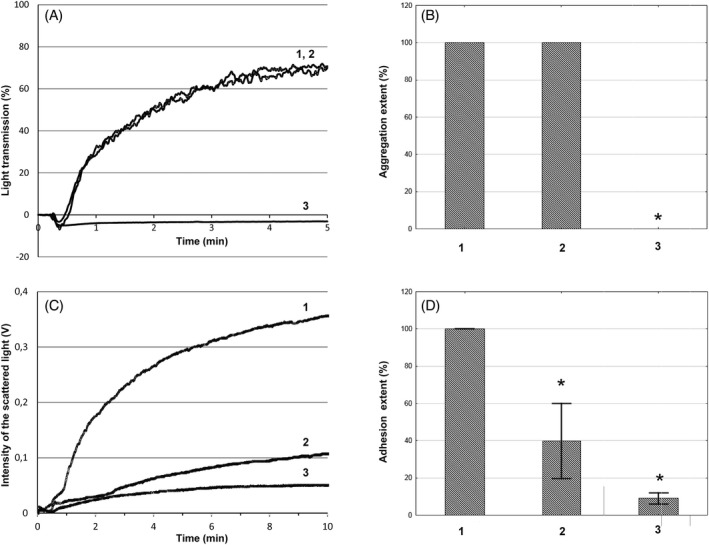
The kinetics of platelet aggregation in PRP (A) and adhesion to fibrinogen‐coated surface under flow conditions in whole blood (C) samples of the same healthy subject after inhibition of platelet GPIb and GPIIb/IIIa receptors. The extent of platelet aggregation (B) and platelet adhesion in whole blood (D) of 7 healthy volunteers. Platelets were activated with 5 μM adenosine diphosphate (ADP). The shear rate in platelet adhesion experiments was 1300s−1. A, C) Curve 1—platelet aggregation and adhesion in the control experiments; Curve 2—platelet aggregation and adhesion with anti‐GPIb mAb; Curve 3—platelet aggregation and adhesion with anti‐GPIIb/IIIa mAb. B, D) The extent of platelet aggregation and adhesion in the control experiments was taken as 100%. 1—control experiments; 2—after addition of anti‐GPIb mAb mAb; 3—after addition of anti‐GPIIb/IIIa mAb. *—p < 0.01, n = 7, Mann‐Whitney U test. Error bars represent standard deviation. In Figure 5B error bars are not given, because there was no deviation in aggregation. It was either 100% in control and GPIb‐inhibited measurements or 0.0% in GPIIb/IIIa‐inhibited measurements
Figure 5C shows typical curves of platelet adhesion to a fibrinogen‐coated surface under flow in whole blood before and after inhibition of platelet GPIb and GPIIb/IIIa receptors. The platelet concentration was 190 ± 34.2 × 103/μl. Platelets were activated with 5 μM ADP before the flow was started. The shear rate at the surface of optical bedding was 1300 s−1. In the control measurement, the figure shows a strong increase in the intensity of scattered light (curve 1). Inhibition of the platelet GPIb receptors in whole blood resulted in a decrease of platelet adhesion to the fibrinogen coating, thus lowering the optical signal (curve 2). Inhibition of platelet GPIIb/IIIa receptors resulted in the pronounced decrease of platelet adhesion to the fibrinogen coating (curve 3). Combined inhibition of GPIb and GPIIb/IIIa receptors resulted in a stronger effect and almost total suppression of the signal of scattered light from the optical surface (data is not shown). Figure 5D shows the results obtained from seven healthy volunteers. Adhesion in whole blood was registered over 10 min at a shear rate of 1300 s−1. The peak intensity of scattered light after 10 min of constant flow in the control experiments was 0.2 ± 0.08 V (n = 7) in whole blood and 0.05 ± 0.02 V (n = 7) in PRP, p < 0.01. In each experiment, this level of control measurement was taken as 100%. The intensity of scattered light of whole blood samples with anti‐GPIb mAb was 34 (23; 58)%; with anti‐GPIIb/IIIa mAb, it was 8.5 (6.5; 12.0)%, comparative to that of control. When combined anti‐GPIb and anti‐GPIIb/IIIa mAbs were added, the intensity of scattered light fell to 7.4 (5.0;9.8)%. In all cases, the intensity of scattered light was significantly different from the control measurements (p < 0.01, n = 7).
3.3. Platelet adhesion to fibrinogen in whole blood under flow condition in persons with autoimmune thrombocytopenia and type 3 von Willebrand disease
We investigated platelet adhesion to a fibrinogen‐coated surface in whole blood of a person with autoimmune thrombocytopenia (Figure 6A) and a person with type 3 von Willebrand disease (Figure 6B). Platelet concentrations were 85 × 103/μl and 269 × 103/μl, respectively. Platelets were activated with 5 μM ADP before the flow was started. The shear rate at the surface of the optical bedding was 1300 s−1.
FIGURE 6.
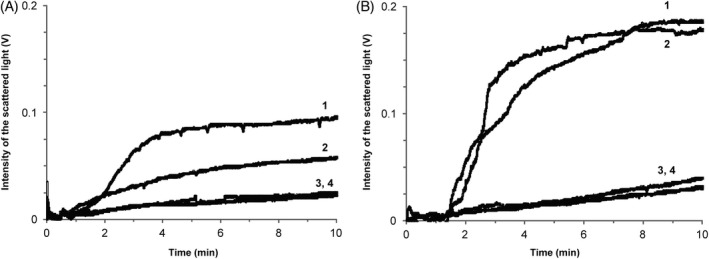
The kinetics of platelet adhesion to fibrinogen‐coated glass surface in whole blood under flow conditions in persons with autoimmune thrombocytopenia (A) and type 3 von Willebrand disease (B). Platelet concentration is 85 × 103/μl and 269 × 103/μl, respectively. Platelets were activated with 5 μM adenosine diphosphate (ADP) before the flow was started. The shear rate was 1300s−1. Curve 1—platelet adhesion in the control experiments; Curve 2—platelet adhesion with anti‐GPIb mAb; Curve 3—platelet adhesion with anti‐GPIIb/IIIa mAb; Curve 4—platelet adhesion with anti‐GPIb mAb and anti‐GPIIb/IIIa mAb
The figure of control measurement shows pronounced increase in the intensity of scattered light (curve 1). Inhibition of the platelet GPIb receptors decreased platelet adhesion to fibrinogen coating in the case of autoimmune thrombocytopenia (curve 2, Figure 6A), but no effect was observed in the type 3 von Willebrand disease (curve 2, Figure 6B). Inhibition of platelet GPIIb/IIIa receptors alone or combined inhibition of the two receptors resulted in almost total suppression of adhesion in both cases (curves 3 and 4, Figure 6).
4. DISCUSSION
Earlier, we reported platelet adhesion assay using a novel device for the study of platelet adhesion to protein‐coated surfaces under controlled‐flow conditions. To test the device and to validate its application to platelet adhesion assays, we first measured adhesion of platelets to a fibrinogen‐coated surface in PRP.19 Our system allows simulating flow conditions in different types of vessels by changing shear rates over a wide range. In our experiments, shear rate was set at 1300 s−1, which corresponds to the flow conditions of major arteries.22 The results demonstrate that the device reliably registers the changes in the intensity of scattered light detected on the photodiode in PRP under the set flow conditions. It is important that the dynamics of the changes depend strongly on the concentration of platelets in the sample.19 We prepared fibrinogen‐coated surfaces manually. In this work, we compare results obtained in PRP and whole blood samples of the same subjects. We found that the increase in the intensity of scattered light was greater in whole blood than in PRP. In both cases, platelet GPIIb/IIIa receptor inhibition resulted in blocking of platelet adhesion and absence of optical signal change. This proves that the device properly registers platelet interaction with fibrinogen, which is strongly dependent on the specific binding of fibrinogen to GPIIb/IIIa receptors. Thus, we conclude that the change in the intensity of scattered light correctly reflects the process of platelet adhesion to a fibrinogen‐coated surface.
The stronger intensity of adhesion in whole blood samples as compared to PRP samples requires an additional explanation. Here, we encounter two basic phenomena that occur in flowing blood. The first is that, unlike blood plasma, whole blood behaves as a non‐Newtonian fluid. This means that under the same flow velocity, the shear rate of whole blood is different from that of blood plasma.23 As an estimate, if the shear rate of the blood plasma is 1300 s−1, the value for whole blood should be 7–10 times higher, that is approximately 9000–13 000 s−1. Another important point is that when whole blood moves along a flat surface at high shear rates, platelets are forced out to the surface by larger red blood cells, which concentrate in the centre of the flow.24 This phenomenon was described earlier. It was shown that the higher shear rates in blood flow promote this margination of platelets.25 Enhanced platelet margination pushes platelets to the vessel wall; the main result of this is an increase in the local platelet concentration in close proximity to the optical surface. Both of these phenomena lead to a significant increase in the intensity of platelet interaction with a protein‐coated surface in a flow chamber. The microscopy findings (Figure 4) demonstrate that the enhancement of light scattering in whole blood may also come from adherent nucleate cells. This finding needs further investigation. The result of these phenomena observed in our tests is a greater increase in the intensity of scattered light during the interaction of platelets with a surface in whole blood as compared to PRP. Although the margination pushes platelets closer to the evanescent field at the optical boundary, it does not cause light scattering. We performed tests with PRP and whole blood perfusion through chambers with an albumin‐coated glass surface at shear rate of 1300 s−1 and did not register any significant change in the optical signal (data is not shown).
An important feature of our work is the opportunity to study the role of GPIb and GPIIb/IIIa receptors in platelet adhesion to fibrinogen under flow conditions. Numerous studies have described interactions of platelets with surface‐adsorbed proteins, such as fibrinogen, von Willebrand factor (VWF) and albumin. Under static conditions or conditions of low shear rate, platelets adhere mostly to fibrinogen, whereas under high shear rates, platelets are preferentially bound by VWF.26 The process of VWF‐mediated platelet adhesion to fibrinogen at high shear rates is not clear, but it is possible that platelets adhere to fibrinogen first (because VWF in blood do not adhere to fibrinogen on its own) and present binding sites for VWF. As soon as VWF is bound, it unfurls and provides multiple binding sites for platelets, thus increasing adhesion.27 We shall also keep in mind that platelets bear their own VWF in α‐granules, which they can release and directly participate in formation of VWF threads on fibrinogen‐coated surfaces. Using a microfluidic device, Schneider et al. were able to visualise directly the conformational changes of VWF under shear flow. In particular, they found that VWF demonstrates a reversible globule‐stretch transition at the critical shear rate in the absence of any adsorbing surface. They identified VWF fibres as hydrodynamically activated biopolymers, being haemostatically active only under high shear rates. The authors found that the observed critical activation threshold falls in the same range as the wall shear rate found in small arteries and equals to about 5000 s−1.28 In this work, we found that two platelet glycoprotein receptors, GPIb and GPIIb/IIIa, mediated platelet adhesion in whole blood under flow conditions. A shift from fibrinogen‐mediated to VWF‐mediated adhesion could be shown experimentally by our test system, when we compare the results obtained in PRP and whole blood. We showed that platelet adhesion in whole blood at arterial shear rates depends on the platelet binding to VWF via the GPIb receptor. Inhibition of the platelet GPIb receptor mAb resulted in a 66% decrease in the intensity of platelet adhesion in whole blood, while under the same conditions in PRP these antibodies did not provide a significant effect. Combined inhibition of both GPIb and GPIIb/IIIa receptors resulted in almost total suppression of the platelet adhesion in PRP and in whole blood. Endenburg S.C. et al. report that in blood of patient with Bernard‐Soulier syndrome, who has deficient GPIb receptors, platelet adhesion to a fibrin‐coated surface was markedly decreased, especially at a high shear rate (1600s−1). The same was also true for the patient with severe Von Willebrand disease in this and another study.27, 29
Finally, we investigated platelet adhesion in whole blood in persons with autoimmune thrombocytopenia and type 3 von Willebrand disease. We demonstrated that our new technique can be successfully applied for investigation of platelet adhesion kinetics under controlled‐flow conditions in both cases. Moreover, in the person with type 3 von Willebrand disease, in whose blood VWF was completely absent, inhibition of platelet GPIb receptor did not affect platelet adhesion. In this case, only inhibition of platelet GPIIb/IIIa receptor resulted in a significant decrease in the intensity of platelet adhesion.
Our novel optical detection system effectively registers platelet adhesion to a fibrinogen‐coated surface in whole blood under controlled‐flow conditions and may be successfully applied to the investigation of platelet adhesion kinetics.
CONFLICTS OF INTEREST
None of the authors declares any conflicts of interest.
ACKNOWLEDGEMENTS
We are grateful to Dr. Aleksey Mazurov for the helpful comments and suggestions regarding the article. The study is funded by Russian Scientific Foundation (project # 21‐15‐00029).
Gabbasov ZA, Avtaeva YN, Melnikov IS, et al. Kinetics of platelet adhesion to a fibrinogen‐coated surface in whole blood under flow conditions. J Clin Lab Anal. 2021;35:e23939. 10.1002/jcla.23939
Contributor Information
Zufar A. Gabbasov, Email: zufargabbasov@yandex.ru.
Peter Kruzliak, Email: kruzliakpeter@gmail.com.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Wu H, Zhao G, Zu H, Wang JH, Wang QM. Real‐time monitoring of platelet activation using quartz thickness‐shear mode resonator sensors. Biophys J. 2016;110(3):669‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gergei I, Kälsch T, März W, Krämer BK, Kälsch AI. Platelet and Monocyte activity markers and mortality in patients with end‐stage renal disease. Clin Lab. 2020;66(3). 10.7754/Clin.Lab.2019.190903 [DOI] [PubMed] [Google Scholar]
- 3.Kwak D, Wu Y, Horbett TA. Fibrinogen and von Willebrand's factor adsorption are both required for platelet adhesion from sheared suspensions to polyethylene preadsorbed with blood plasma. J Biomed Mater Res A. 2005;74(1):69‐83. [DOI] [PubMed] [Google Scholar]
- 4.Fu Q, Ye C, Han B, et al. Designing and validating autoverification rules for hematology analysis in sysmex XN‐9000 hematology system. Clin Lab. 2020;66(4). 10.7754/Clin.Lab.2019.190726 [DOI] [PubMed] [Google Scholar]
- 5.Jamiolkowski MA, Pedersen DD, Wu WT, Antaki JF, Wagner WR. Visualization and analysis of biomaterial‐centered thrombus formation within a defined crevice under flow. Biomaterials. 2016;96:72‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guha Thakurta S, Miller R, Subramanian A. Adherence of platelets to in situ albumin‐binding surfaces under flow conditions: role of surface‐adsorbed albumin. Biomed Mater. 2012;7(4): 45007. [DOI] [PubMed] [Google Scholar]
- 7.Hisasue M, Ai T, Kimura K, et al. Modification of the algorithm used by automated hematology analyzer XN‐3000 improves specificity in the detection of schistocytes. Clin Lab. 2021;67(1). 10.7754/Clin.Lab.2020.200227 [DOI] [PubMed] [Google Scholar]
- 8.Lopez‐Alonso A, Jose B, Somers M, et al. Individual platelet adhesion assay: measuring platelet function and antiplatelet therapies in whole blood via digital quantification of cell adhesion. Anal Chem. 2013;85(13):6497‐6504. [DOI] [PubMed] [Google Scholar]
- 9.Faxälv L, Bolin MH, Jager EW, Lindahl TL, Berggren M. Electronic control of platelet adhesion using conducting polymer microarrays. Lab Chip. 2014;14(16):3043‐3049. [DOI] [PubMed] [Google Scholar]
- 10.Lei KF, Chen KH, Tsui PH, Tsang NM. Real‐time electrical impedimetric monitoring of blood coagulation process under temperature and hematocrit variations conducted in a microfluidic chip. PLoS One. 2013;8(10):e76243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai WB, Grunkemeier JM, McFarland CD, Horbett TA. Platelet adhesion to polystyrene‐based surfaces preadsorbed with plasmas selectively depleted in fibrinogen, fibronectin, vitronectin, or von Willebrand's factor. J Biomed Mater Res. 2002;60(3):348‐359. [DOI] [PubMed] [Google Scholar]
- 12.Grunkemeier JM, Tsai WB, McFarland CD, Horbett TA. The effect of adsorbed fibrinogen, fibronectin, von Willebrand factor and vitronectin on the procoagulant state of adherent platelets. Biomaterials. 2000;21(22):2243‐2252. [DOI] [PubMed] [Google Scholar]
- 13.Brouns SLN, van Geffen JP, Heemskerk JWM. High‐throughput measurement of human platelet aggregation under flow: application in hemostasis and beyond. Platelets. 2018;29(7):662‐669. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Neelamegham S. Application of microfluidic devices in studies of thrombosis and hemostasis. Platelets. 2017;28(5):434‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branchford BR, Ng CJ, Neeves KB, Di Paola J. Microfluidic technology as an emerging clinical tool to evaluate thrombosis and hemostasis. Thromb Res. 2015;136(1):13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez E, Petrich BG, Shattil SJ, Ginsberg MH, Groisman A, Kasirer‐Friede A. Microfluidic devices for studies of shear‐dependent platelet adhesion. Lab Chip. 2008;8(9):1486‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij B. J. M., Závodszky G., Hoekstra A. G., Ku D. N.. Biorheology of occlusive thrombi formation under high shear: in vitro growth and shrinkage. Scientific Reports. 2020;10 (1). 10.1038/s41598-020-74518-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Zanet D, Battiston M, Lombardi E, et al. Impedance biosensor for real‐time monitoring and prediction of thrombotic individual profile in flowing blood. PLoS One. 2017;12(9):e0184941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avtaeva YN, Mel'nikov IS, Gabbasov ZA. Real‐time recording of platelet adhesion to fibrinogen‐coated surface under flow conditions. Bull Exp Biol Med. 2018;165(1):157‐160. [DOI] [PubMed] [Google Scholar]
- 20.Berndt MC, Du XP, Booth WJ. Ristocetin‐dependent reconstitution of binding of von Willebrand factor to purified human platelet membrane glycoprotein Ib‐IX complex. Biochemistry. 1988;27(2):633‐640. [DOI] [PubMed] [Google Scholar]
- 21.Byzova TV, Vlasik TN, Mazurov AV. Inhibition of platelet aggregation by monoclonal antibodies against glycoprotein IIb–IIIa complex. Bull Exp Biol Med. 1994;118(10):402‐405. [PubMed] [Google Scholar]
- 22.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525‐1541. [PubMed] [Google Scholar]
- 23.Neeves KB, Maloney SF, Fong KP, et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear‐resistance of platelet aggregates. J Thromb Haemost. 2008;6(12):2193‐2201. [DOI] [PubMed] [Google Scholar]
- 24.Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8(6):819‐824. [DOI] [PubMed] [Google Scholar]
- 25.Casa LDC, Ku DN. Thrombus Formation at High Shear Rates. Annu Rev Biomed Eng. 2017;19:415‐433. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda Y, Handa M, Kawano K, et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J Clin Invest. 1991;87(4):1234‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Endenburg SC, Hantgan RR, Lindeboom‐Blokzijl L, et al. On the role of von Willebrand factor in promoting platelet adhesion to fibrin in flowing blood. Blood. 1995;86(11):4158‐4165. [PubMed] [Google Scholar]
- 28.Schneider SW, Nuschele S, Wixforth A, et al. Shear‐induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A. 2007;104(19):7899‐7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hantgan RR, Hindriks G, Taylor RG, et al. Glycoprotein Ib, von Willebrand factor, and glycoprotein IIb:IIIa are all involved in platelet adhesion to fibrin in flowing whole blood. Blood. 1990;76(2):345‐353. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


