Abstract
Objective
To investigate the significance of lymphocyte‐to‐monocyte ratio (LMR) combined with carbohydrate antigen (CA) 19‐9 for predicting postoperative recurrence of colorectal cancer (CRC) in patients with type II diabetes.
Methods
We conducted a retrospective analysis of 106 postoperative patients with stage II–III CRC and with type II diabetes. Their clinical indexes such as LMR and CA19‐9 were collected, and the patients were followed up for 5 years.
Results
The CA19‐9 level was 119.7 U/ml at baseline in the relapsed group, while this was 24.81 U/ml in non‐relapsed group (p = 0.001). On the contrary, the LMR level was 5.10 and 2.57 for non‐relapsed and relapsed group (p < 0.001), respectively. Kaplan‐Meier survival curves stratified by CA19‐9 and LMR suggested that patients with lower CA19‐9 had higher survival probability (p < 0.001), while patients with high LMR level had higher survival probability (p < 0.001). The multivariable Cox proportional hazard regression analysis with CA19‐9 and LMR indicated that although the baseline CA19‐9 is significantly associated with increasing risk of disease recurrence, the HR (HR = 1.0, 95% CI 1.00–1.01) was small and close to 1, whereas the high baseline LMR (HR = 0.44, 95% CI 0.32–0.61) was associated with decrease in disease recurrence. Model with continuous CA19‐9 and LMR was able to better predict (AUC 73.17%) the disease recurrence.
Conclusion
LMR combined with CA19‐9 may become a new index for predicting postoperative recurrence of CRC in patients with diabetes.
Keywords: CA19‐9, colorectal cancer, diabetes, lymphocyte‐to‐monocyte ratio
In the present study, a total of 106 patients with CRC and type II diabetes before surgery were included in the analysis, the median follow‐up was 60 months, and 38 had a disease recurrence. We found that both LMR and CA19‐9 are significantly related to prognosis, and the combination of the above two is expected to become a new index for predicting postoperative recurrence of CRC in patients with diabetes.

1. INTRODUCTION
Colorectal cancer (CRC) is a common malignant tumor, and its morbidity and mortality rank third among cancers worldwide.1 In China, the incidence of CRC is gradually increasing.2 Surgical resection is the main radical treatment for CRC, but some tumors may relapse and metastasize after surgery, shortening overall survival.3 Diabetes is a metabolic disease characterized by high blood glucose level. There are many common inducing factors for diabetes and CRC, such as hyperlipidemia, obesity, and chronic inflammation. According to relevant reports, patients with diabetes have an obvious increased risk of CRC,4 and they have worse prognosis than CRC patients without diabetes.5 Therefore, researchers are striving to find predictors of postoperative recurrence of CRC in patients with diabetes.
The common markers used for diagnosis and monitoring of malignant tumors include carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19‐9, and CA125.6, 7, 8 Recent studies have shown that inflammatory responses play a crucial role in the occurrence and development of malignant tumors.9 New inflammatory response indexes include neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), hemoglobin‐to‐platelet ratio (HPR), lymphocyte‐to‐monocyte ratio (LMR), fibrinogen‐to‐prealbumin ratio (FPP), fibrinogen‐to‐albumin ratio (FAR), albumin‐to‐lymphocyte ratio (ALR) and fibrinogen‐to‐lymphocyte ratio (FLR). These inflammatory response indexes are currently widely used in the early diagnosis, disease monitoring, and prognostic evaluation of malignant tumors.6, 10, 11, 12 Inflammatory response is an inducing factor of CRC and a risk factor for disease progression.9 Previous studies have shown that inflammatory response indexes are correlated with the prognosis of advanced CRC.13 In this study, we aimed to explore the predictive indexes of postoperative recurrence of CRC in patients with diabetes.
2. MATERIALS AND METHODS
2.1. Study population
CRC‐operated patients between January 1, 2011, and December 31, 2014, were included in this retrospective study from Beijing Chao‐Yang Hospital, Capital Medical University. In this study, we only include patients with (1) pathologically confirmed CRC after radical resection; (2) diagnosis type II diabetes before CRC; (3) stage II–III CRC; and (4) no radiotherapy, chemotherapy, targeted therapy, or immunotherapy before surgery. Individuals with the following conditions were deemed ineligible for the study: (1) diagnosed with other tumors before surgery; (2) severe infection (life‐threatening infection that can cause single or multiple organ dysfunction); (3) hematological diseases; and (4) autoimmune disease.
Study was approved by the Ethics Committee of Beijing Chao‐Yang Hospital. Patients were not required to give signed informed consent.
2.2. Assessments
The blood test indexes in all patients were collected within 2 weeks before surgery, including lymphocytes, monocytes, and CA19‐9. After surgery, patients were monitored by CT or MRI re‐examination at six‐month intervals. All patients were followed for 5 years or until disease recurrence, whichever is the first. Disease recurrence was defined as the occurrence of new evaluable lesions at the surgical site or at a distant site, as seen by imaging examination. Disease‐free survival (DFS) was defined as the time from operation to CRC recurrence.
2.3. Statistical analysis
Data were presented as the mean ± standard deviation for continuous variables or percentage for category variables. For the comparison between groups, t test or Mann‐Whitney U test for continuous variables and chi‐square test or Fisher's exact test for categorical variable were used wherever appropriate. Survival was evaluated with the Kaplan‐Meier method, and significant differences in survival curves were evaluated with the logrank test. To investigate the association of LMR and CA19‐19 with DFS and if adding these two variables increases the discrimination ability, a Cox proportional hazard regression model with and without LMR and CA19‐19 was used adjusting for well‐known risk factors. Another model with dichotomized LMR and CA19‐19 by median value was also assessed. The adjusted risk factors include age, gender, smoking, TNM stage, tumor location, and chemotherapy. The model performance metrics of calibration and discrimination were studied. The discrimination ability of the models was evaluated using the area under the receiver operating curve (ROC). All statistical tests were two‐sided, and a p‐value of <0.05 was considered statistically significant. All analyses were carried out with R 4.0.5.
3. RESULTS
3.1. Patents and baseline characteristics
A total of 778 patients were screened, 628 patients were without type II diabetes and excluded; 15 patients were stage I; 4 patients were synchronous malignancy; 12 patients had preoperative therapy; 3 patients had other tumor; 7 patients had severe infection; 1 patient had hematological diseases; 2 patients had autoimmune disease. Finally, 106 patients were included in the analysis (Figure 1). The median follow‐up was 60 months, ranging from 5 to 60 month. During the follow‐up period, a total of 38 had a disease recurrence.
FIGURE 1.
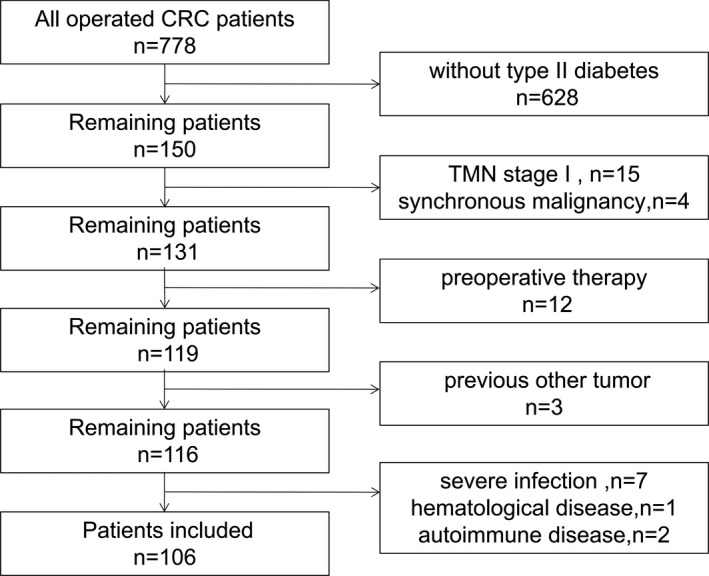
Flow diagram of patients included and excluded in this study
Table 1 summarizes the baseline characteristics of the study population by recurrence status. The average age at baseline in relapsed group was 65.84 years, which was close to the non‐relapsed group (64.72 years). This was the same for gender, smoking status, tumor location, and chemotherapy status. According to the TNM staging system, a higher T3 stage was observed (78.9%) in the relapsed group, while the percentage of TNM stage was close in the non‐relapsed group. The CA19‐9 level was 119.7 U/ml at baseline in the relapsed group, which was 24.81 U/ml for non‐relapsed group. But to the contrary, the LMR level was 5.10 and 2.57 for non‐relapsed and relapsed group. This was the same for both variables after dichotomizing by median value.
TABLE 1.
Baseline characteristics of the CRC patients with diabetes
| Var | Level | No | Yes | p |
|---|---|---|---|---|
| n | 68 | 38 | ||
| Age (mean (SD)) | 64.72 (8.57) | 65.84 (9.10) | 0.529 | |
| Gender (%) | Female | 25 (36.8) | 10 (26.3) | 0.378 |
| Male | 43 (63.2) | 28 (73.7) | ||
| Smoking (%) | No | 40 (58.8) | 24 (63.2) | 0.818 |
| Yes | 28 (41.2) | 14 (36.8) | ||
| TNM stage (%) | 2 | 33 (48.5) | 8 (21.1) | 0.010 |
| 3 | 35 (51.5) | 30 (78.9) | ||
| Tumor location (%) | Rectum | 29 (42.6) | 10 (26.3) | 0.193 |
| Left colon | 20 (29.4) | 12 (31.6) | ||
| Right colon | 19 (27.9) | 16 (42.1) | ||
| Chemotherapy (%) | No | 7 (10.3) | 4 (10.8) | 1.000 |
| Yes | 61 (89.7) | 33 (89.2) | ||
| CA199 (mean (SD)) | 24.81 (65.46) | 119.70 (220.18) | 0.001 | |
| CA199a (%) | Low | 43 (63.2) | 9 (24.3) | <0.001 |
| High | 25 (36.8) | 28 (75.7) | ||
| LMR (mean (SD)) | 5.10 (1.96) | 2.57 (1.11) | <0.001 | |
| LMRa (%) | Low | 21 (30.9) | 31 (83.8) | <0.001 |
| High | 47 (69.1) | 6 (16.2) | ||
| DFS (mean (SD)) | 60.00 (0.00) | 22.68 (16.24) | <0.001 |
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CRC, colorectal cancer; DFS, disease‐free survival; LMR, lymphocyte‐to‐monocyte ratio.
Categorized with median.
3.2. KM survival curves analysis by CA19‐9 and LMR
To illustrate independent discrimination ability of CA19‐9 and LMR, Kaplan‐Meier survival curves were stratified by CA19‐9 and LMR (Figure 2). The results suggested that patients with high CA19‐9 had lower survival probability, while the patients with lower CA19‐9 had higher survival probability. But in contrast, patients with high LMR level had higher survival probability, whereas the patients with lower LMR level had lower survival probability. Statistically significant results were observed in both stratifications.
FIGURE 2.
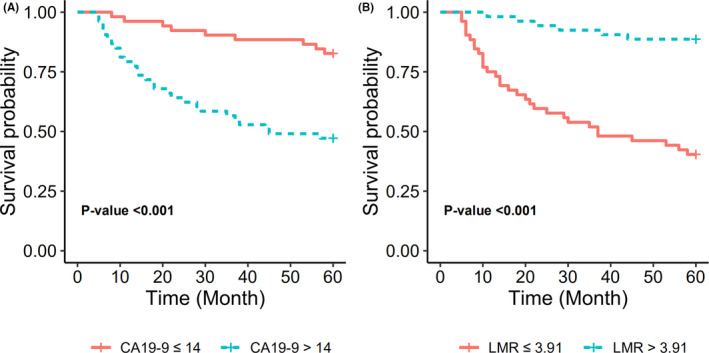
DFS stratified by CA19‐9 (A) and LMR (B) levels
3.3. Multivariable analysis with and without CA19‐9 and LMR
Table 2 shows the multivariable Cox proportional hazard regression analysis with and without CA19‐9 and LMR. The results indicated that CA19‐9 and LMR were significantly associated with disease recurrence. The HR was 1.00 (1.00, 1.01) and 0.44 (0.32, 0.61) for CA19‐9 and LMR, respectively. This was the same for the dichotomized CA19‐9 and LMR, though the HR was 4.24 (1.80, 9.98) and 0.14 (0.06, 0.35) compared to the low level of CA19‐9 and LMR, respectively. Model with continuous CA19‐9 and LMR was able to better predict (AUC 73.17%) the disease recurrence compared to model without CA19‐9 and LMR (AUC 64.98%) and of those dichotomized (AUC 64.75%), see Figure 3.
TABLE 2.
Multivariable analysis with and without CA19‐9 and LMR
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.02 (0.98, 1.06) | 0.45 | 1.02 (0.98, 1.06) | 0.40 | 1.02 (0.98, 1.07) | 0.26 |
| Gender | ||||||
| Female (ref) | – | – | – | – | – | – |
| Male | 1.54 (0.68, 3.51) | 0.30 | 0.91 (0.39, 2.14) | 0.83 | 0.77 (0.30, 1.96) | 0.58 |
| Smoking | ||||||
| No (ref) | – | – | – | – | – | – |
| Yes | 0.63 (0.30, 1.34) | 0.23 | 0.90 (0.41, 1.96) | 0.79 | 1.28 (0.54, 3.00) | 0.57 |
| TNM stage | ||||||
| 2 (ref) | – | – | – | – | – | – |
| 3 | 2.95 (1.29, 6.71) | 0.01 | 1.48 (0.61, 3.55) | 0.38 | 1.51 (0.59, 3.87) | 0.39 |
| Tumor location | ||||||
| Rectum (ref) | – | – | – | – | – | – |
| Left colon | 1.40 (0.60, 3.29) | 0.44 | 0.94 (0.39, 2.29) | 0.89 | 1.38 (0.57, 3.35) | 0.48 |
| Right colon | 1.51 (0.64, 3.53) | 0.34 | 0.82 (0.34, 1.99) | 0.66 | 1.16 (0.45, 3.02) | 0.76 |
| Chemotherapy | ||||||
| No (ref) | – | – | – | – | – | – |
| Yes | 0.72 (0.24, 2.10) | 0.54 | 1.18 (0.35, 3.95) | 0.79 | 1.23 (0.33, 4.58) | 0.76 |
| CA199 | 1.00 (1.00, 1.01) | <0.001 | ||||
| LMR | 0.44 (0.32, 0.61) | <0.001 | ||||
| CA199a | ||||||
| Low (ref) | – | – | ||||
| High | 4.24 (1.80, 9.98) | <0.001 | ||||
| LMRa | ||||||
| Low (ref) | – | – | ||||
| High | 0.14 (0.06, 0.35) | <0.001 | ||||
Model 1: Age, Gender, Smoking, TNM stage, Tumor location, Chemotherapy. Model 2: Model 1 plus LMR and CA19‐19. Model 3: Model 1 plus categorized LMR and CA19‐9.
Categorized with median.
FIGURE 3.
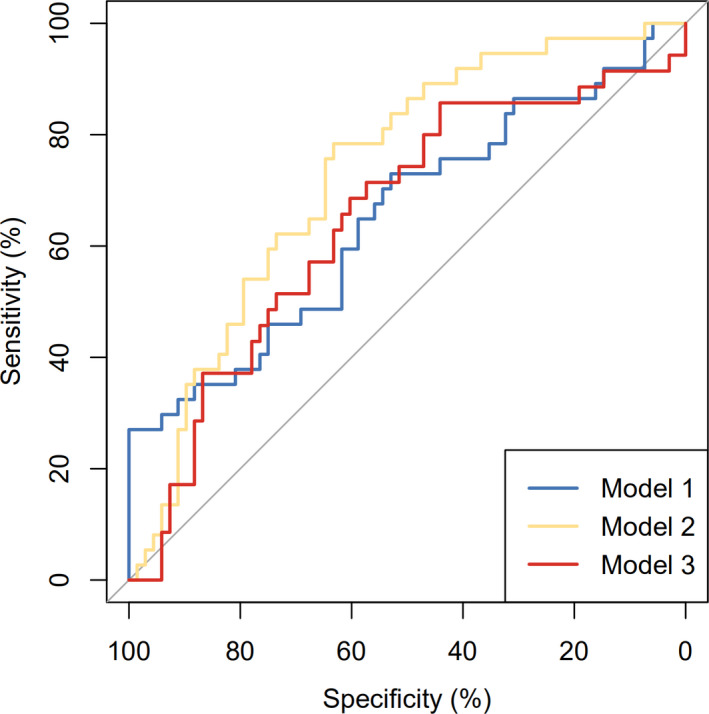
Receiver operating curve for the different risk models
4. DISCUSSION
CRC is a common malignant tumor, and diabetes is a metabolic disease that is characterized by high blood glucose level. Studies have shown that diabetes is closely related to the onset of CRC, and patients with diabetes have a significantly increased incidence of CRC.14 In recent years, researchers have begun to pay attention to the impact of diabetes on patients after colorectal cancer surgery.15 As previously reported, patients with colorectal cancer and pre‐existing diabetes mellitus have an increased risk of short‐ and long‐term mortality.16 Previous studies have shown that, compared with people without diabetes, patients with diabetes have an increased risk of malignant tumors.17, 18, 19, 20, 21 Diabetes may promote the proliferation and migration of tumor cells, thereby promoting tumor development.22
CRC patients with diabetes had a worse prognosis after surgery compared to patients without diabetes. Therefore, there is an urgency to find effective clinical indexes predicting the high‐risk groups early and implement active intervention, to improve the prognosis of these patients. In this study, we investigated the indexes of recurrence of CRC patients with diabetes. In this retrospective study, we analyzed the relationship between CA19‐9 and LMR with disease recurrence. A total of 106 patients with CRC and type II diabetes before surgery were included in the analysis, the median follow‐up was 60 months, and 38 had a disease recurrence. We found that both LMR and CA19‐9 are significantly related to prognosis, and the combination of the above two is expected to become a new index for predicting postoperative recurrence of CRC in patients with diabetes.
Chronic inflammation may induce changes in the tumor microenvironment and promote tumor progression. In recent years, it has been found that inflammation‐related markers such as NLR, MLR, and PLR are relevant to the early diagnosis and prognosis of tumors.12, 23, 24, 25, 26, 27 Among these is a new index that reflects the body's inflammatory state.28 In the present study of postoperative CRC patients, we analyzed the correlation between inflammatory response LMR and DFS. In this study, the LMR level was 5.10 and 2.57 for non‐relapsed and relapsed group, respectively. Lymphocytes can enhance tumor immune surveillance and inhibit tumor cell proliferation, infiltration, and metastasis. When there are few lymphocytes in the body, there is a lack of immune response to the tumor.29, 30 Therefore, lymphopenia may reflect the body's poor immune defense.31 Monocyte infiltration may also affect tumor development.32 Macrophages are highly differentiated monocytes, and they can promote tumor cell migration and invasion and tumor‐related angiogenesis.33 Therefore, usually a low level of lymphocyte infiltration in the tumor microenvironment indicates weak and insufficient antitumor effects. The infiltrating monocytes are closely related to tumor progression34 the lower the LMR, the higher the risk of death.35 In the present study, by Kaplan‐Meier survival curves and multivariable Cox proportional hazard regression analysis, this showed that low LMR was an index of worse prognosis, which conformed to the theoretical basis of inflammatory responses.36
Previous studies have revealed that the indexes such as CEA, SCC, CA125, and CA19‐9 are often used for diagnosis and monitoring of malignant tumors.11 High serum CA19‐9 and CEA are risk factors for poor prognosis in patients with esophageal cancer and advanced CRC.6, 37 In the present study, the CA19‐9 level was 119.7 U/ml at baseline in the relapsed group, which was 24.81 U/ml for non‐relapsed group. Kaplan‐Meier survival curves suggested that patients with lower CA19‐9 had higher survival probability. Multivariable Cox proportional hazard regression analysis showed the HR for dichotomized CA19‐9 was 4.24 (1.80, 9.98) compared to the low level of CA19‐9. Patients with high CA19‐9 had a short postoperative recurrence time and poor prognosis. CA19‐9 is a macromolecular glycoprotein, which is synthesized by epithelia of the pancreas, colon, endometrium, and salivary glands in healthy people. Under normal circumstances, CA19‐9 is synthesized by the body and stored in cells. Because of the blocking of the cell barrier, these substances rarely enter the blood.11 With the development of CRC, the cell structure is destroyed, or the cell polarity disappears, and CA19‐9 level in the blood gradually increases. When the degree of malignancy of the disease is higher, damage to the cell barrier is greater, and more CA19‐9 enters the blood.11, 37
However, each indicator has a certain limitation in predicting the prognosis of colorectal cancer. For example, we found that the multivariable Cox proportional hazard regression analysis with CA19‐9 indicated that although the baseline CA19‐9 is significantly associated with increasing risk of disease recurrence, the HR (HR = 1.0, 95% CI 1.00–1.01) was small and close to 1. Our study is believed to be the first to conduct follow‐up analysis of CRC patients with diabetes by model with CA19‐9 and LMR. The adjusted risk factors include age, gender, smoking, TNM stage, tumor location, and chemotherapy. The model performance metrics of calibration and discrimination were studied. Model with continuous CA19‐9 and LMR was able to better predict (AUC 73.17%) the disease recurrence compared to model without CA19‐9 and LMR (AUC 64.98%), and of those dichotomized (AUC 64.75%). There are several advantages to the use of LMR and CA19‐9. First, they are easily measured in blood test and routinely checked in all CRC patients with diabetes. Second, the combination of LMR and CA19‐9 not only reflects the tumor characteristics but also represents the inflammatory and immune status of tumors. Additionally, the diagnostic method of LMR combined with CA19‐9 avoids the limitations of poor specificity and sensitivity of a single predictive index in previous studies.36
Our study had the following limitations. First, the study results were from single hospital, and no external validation was performed. Second, for the rare occurrence of CRC with type II diabetes, only a small number of patients were eligible for the study. Hence, further studies are required to confirm the results of this current study.
5. CONCLUSION
In CRC patients with diabetes, LMR combined with CA19‐9 is a new and reliable index for predicting postoperative recurrence of tumors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: YD, AGY, and YJN. Collected and analyzed the data: YD and YJN. Wrote the paper: YD and AGY.
Yu D, An G, Yao J. Lymphocyte‐to‐monocyte ratio combined with CA19‐9 for predicting postoperative recurrence of colorectal cancer in patients with diabetes. J Clin Lab Anal. 2021;35:e23944. 10.1002/jcla.23944
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 3.Yi C, Li J, Tang F, et al. Is primary tumor excision and specific metastases sites resection associated with improved survival in Stage Ⅳ Colorectal Cancer? Results From SEER database analysis. Am Surg. 2020;86(5):499‐507. [DOI] [PubMed] [Google Scholar]
- 4.Soltani G, Poursheikhani A, Yassi M, Hayatbakhsh A, Kerachian M, Kerachian MA. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr Disord. 2019;19(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: A meta‐analysis based on the cohort studies. PLoS One. 2017;12(4):e0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao G, Zhou W, Chen E, et al. A novel scoring system predicting survival benefits of palliative primary tumor resection for patients with unresectable metastatic colorectal cancer: a retrospective cohort study protocol. Medicine (Baltimore). 2019;98(37):e17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Calster B, Valentin L, Froyman W, et al. Validation of models to diagnose ovarian cancer in patients managed surgically or conservatively: multicentre cohort study. BMJ. 2020;370:m2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Jiang C, Zhu Q, et al. Combining the tumor abnormal protein test with tests for carcinoembryonic antigens, cancer antigen 15–3, and/or cancer antigen 125 significantly increased their diagnostic sensitivity for breast cancer. Medicine (Baltimore). 2020;99(29):e21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A. The inflammation ‐ cancer connection. FEBS J. 2018;285(4):638‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto T, Saito H, Amisaki M, Tokuyasu N, Honjo S, Fujiwara Y. Combined preoperative platelet‐to‐lymphocyte ratio and serum carbohydrate antigen 19–9 level as a prognostic factor in patients with resected pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2019;18(3):278‐284. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Liu Z, Xiao L, et al. Clinical significance of serum CA125, CA19‐9, CA72‐4, and fibrinogen‐to‐lymphocyte ratio in gastric cancer with peritoneal dissemination. Front Oncol. 2019;9:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanzi Q, Yu L, Siyuan C, et al. The relationship of neutrophil‐to‐lymphocyte ratio or platelet‐to‐lymphocyte ratio and pancreatic cancer in patients with Type 2 diabetes. Clin Lab. 2019;65(7):181226. [DOI] [PubMed] [Google Scholar]
- 13.Wang YY, Liu ZZ, Xu D, Liu M, Wang K, Xing BC. Fibrinogen‐Albumin Ratio Index (FARI): a more promising inflammation‐based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2019;26(11):3682‐3692. [DOI] [PubMed] [Google Scholar]
- 14.Woo H, Lee J, Lee J, et al. Diabetes mellitus and site‐specific colorectal cancer risk in Korea: a case‐control study. J Prev Med Public Health. 2016;49(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerhardt JA, Catalano PJ, Haller DG, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21(3):433‐440. [DOI] [PubMed] [Google Scholar]
- 16.Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta‐analysis. Dig Dis Sci. 2010;55(7):1839‐1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh MK, Das BK, Choudhary S, Gupta D, Patil UK. Diabetes and hepatocellular carcinoma: a pathophysiological link and pharmacological management. Biomed Pharmacother. 2018;106:991‐1002. [DOI] [PubMed] [Google Scholar]
- 18.Er KC, Hsu CY, Lee YK, Huang MY, Su YC. Effect of glycemic control on the risk of pancreatic cancer: a nationwide cohort study. Medicine (Baltimore). 2016;95(24):e3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Huo R, Chen X, Yu X. Diabetes mellitus and the risk of bladder cancer: a PRISMA‐compliant meta‐analysis of cohort studies. Medicine (Baltimore). 2017;96(46):e8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metra M. January 2017 at a glance: oncology, diabetes and antidiabetic treatment, pulmonary hypertension. Eur J Heart Fail. 2017;19(1):7‐8. [DOI] [PubMed] [Google Scholar]
- 21.Lai Y, Sun C. Association of abnormal glucose metabolism and insulin resistance in patients with atypical and typical endometrial cancer. Oncol Lett. 2018;15(2):2173‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer‐pathophysiology and clinical implications. Endocr Rev. 2020;41(1):33‐52. [DOI] [PubMed] [Google Scholar]
- 23.Shunsuke O, Atsuyuki M, Yuichi T, et al. The Prognostic impact of the lymphocyte‐to‐monocyte ratio in resected pancreatic head adenocarcinoma. Med Princ Pract. 2019;28(6):517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang T, Wang Y, Yin X, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res. 2020;2020:9146042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo CJ, Hu ZJ, Qin SZ, et al. Diagnostic value of platelet‐lymphocyte ratio and hemoglobin‐platelet ratio in patients with rectal cancer. J Clin Lab Anal. 2020;34:e23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Jung HI, Kwon SH, et al. Preoperative neutrophil‐lymphocyte ratio and CEA is associated with poor prognosis in patients with synchronous colorectal cancer liver metastasis. Ann Surg Treat Res. 2019;96(4):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu LX, Wang XY, Xu KQ, et al. A systematic inflammation‐based model in advanced pancreatic ductal adenocarcinoma. J Cancer. 2019;10(26):6673‐6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo YH, Sun HF, Zhang YB, et al. The clinical use of the platelet/lymphocyte ratio and lymphocyte/monocyte ratio as prognostic predictors in colorectal cancer: a meta‐analysis. Oncotarget. 2017;8(12):20011‐20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray‐Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383‐5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claude L, Perol D, Ray‐Coquard I, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol. 2005;76(3):334‐339. [DOI] [PubMed] [Google Scholar]
- 31.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137‐148. [DOI] [PubMed] [Google Scholar]
- 32.Chanmee T, Ontong P, Konno K, Itano N. Tumor‐associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solinas G, Germano G, Mantovani A, Allavena P. Tumor‐associated macrophages (TAM) as major players of the cancer‐related inflammation. J Leukoc Biol. 2009;86(5):1065‐1073. [DOI] [PubMed] [Google Scholar]
- 34.Iwayama Y, Tsuruma T, Mizuguchi T, et al. Prognostic value of HLA class I expression in patients with colorectal cancer. World J Surg Oncol. 2015;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263‐266. [DOI] [PubMed] [Google Scholar]
- 36.Xinxin L, Dongming G, Lingyu C, et al. Potential diagnostic value of combining inflammatory cell ratios with carcinoembryonic antigen for colorectal cancer. Cancer Manag Res. 2019;11:9631–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Huang X, Zhou L, et al. Clinical use of tumor biomarkers in prediction for prognosis and chemotherapeutic effect in esophageal squamous cell carcinoma. BMC Cancer. 2019;19(1):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


