Abstract
Background
Hemogram parameters and procalcitonin (PCT) play auxiliary roles in the diagnosis and outcome of sepsis. However, it is not clear whether these indicators can quickly distinguish bacterial classification or guide the choice of empirical antibiotics.
Methods
We retrospectively enrolled 381 patients with bloodstream infections (BSI), divided into Gram‐positive bloodstream infections (GP‐BSI) and Gram‐negative bloodstream infections (GN‐BSI). Demographic parameters, hemogram parameters, and PCT were recorded and compared between the two groups.
Results
The mean platelet volume (MPV), platelet distribution width (PDW), and PCT in the GN‐BSI group were significantly higher than those in the GP‐BSI group, while the platelet count (PLT), plateletcrit, platelet count‐to‐white blood cell count ratio (PWR), platelet count‐to‐neutrophil count ratio (PNR), platelet count‐to‐PCT ratio (PLT/PCT), and mean platelet volume‐to‐PCT ratio (MPV/PCT) were significantly lower in the GN‐BSI group. Multivariate stepwise logistic regression analysis revealed that the independent predictors of GN‐BSI were MPV, PWR, and PCT. The areas under the curve (AUC) for this prediction model was 0.79, with sensitivity =0.75 and specificity =0.71.
Conclusions
There were significant differences in terms of PCT, platelet parameters, and platelet‐related index‐PCT ratio between GN‐BSI and GP‐BSI. Combined PCT and hemogram parameters are more conducive to the early differential diagnosis of bacterial classification of BSI.
Keywords: Gram‐negative bloodstream infection, mean platelet volume, platelet‐related index, predictive significance, procalcitonin, sepsis
The differential diagnosis of Gram‐negative bacteria and Gram‐positive bacteria bloodstream infection is mainly based on blood culture pathogens. Our study found that PCT, MPV, PWR, PLT/PCT, and MPV/PCT values obtained from routine blood tests can be used to discriminate Gram‐negative bloodstream infections among bloodstream infections to some extent. The combination of MPV, PWR, and PCT is helpful for the clinical diagnosis of GN‐BSI, with more effective sensitivity and specificity.
1. INTRODUCTION
Bloodstream infection (BSI) is still a common infectious disease with high mortality worldwide,1 which easily develops into sepsis, septic shock, and even death. The incidence and mortality rates of sepsis have declined over the last three decades; nevertheless, the mortality rate remains above 15%.2, 3, 4 One of the most common clinical phenomena of sepsis is thrombocytopenia. According to relevant studies, the probability of experiencing thrombocytopenia in patients with sepsis can reach more than 50%, and thrombocytopenia is closely related to poor prognosis and high mortality.5, 6
Platelets play an essential role in pathophysiological processes, including hemostasis, thrombosis, inflammation, and antimicrobial host defense.7 It is believed that the occurrence of thrombocytopenia in patients with sepsis is mainly related to the increase in platelet loss and destruction caused by platelet activation stimulated by bacteria or their toxins.8 Activated platelets interact with white blood cells (WBCs) such as lymphocytes, monocytes, and macrophages to exert anti‐inflammatory activity.9 Platelet and platelet‐associated parameters, such as platelet count (PLT), mean platelet volume (MPV), plateletcrit, platelet distribution width (PDW), and platelet‐large cell ratio (P‐LCR), measured in readily available blood routine tests have been increasingly recognized for their role in managing inflammatory diseases. Platelet and MPV are useful markers for evaluating inflammatory activity and the therapeutic response of infectious diseases.10 MPV is a surrogate marker of platelet activation and has been associated with infectious diseases, such as hepatic hydatid cysts,11 tuberculosis,12 and chronic prostatitis.13 Moreover, it is also related to inflammatory conditions, such as nasal polyposis,14 rheumatoid arthritis,15 obesity,16 coronary artery disease,17 type 2 diabetes mellitus,18 and irritable bowel disease.19 Similarly, PDW was suggested to be associated with irritable bowel syndrome,20 diabetes mellitus,21 coronary artery disease,22 and tuberculosis.12 The mean platelet volume‐to‐platelet count ratio (MPV/PLT) has been reported to be increased in type 2 diabetes mellitus,23 severe sepsis,24 and influenza A infection.25
The effectiveness of initial treatment is essential to improving the prognosis and reducing mortality in patients with severe sepsis.26, 27 However, blood culture results usually require 2–3 days or even longer to obtain conclusive results and are limited by false‐negative results. It is a challenge for many clinicians to administer antibiotics empirically, and they rely on personal clinical experience in the absence of definitive evidence of pathogens. The use of broad‐spectrum antibiotics or combinations of antimicrobials has many disadvantages, such as increasing health care costs, adverse drug reactions, and the incidence of drug‐resistant bacteria. There is a significant difference in antimicrobial use against Gram‐negative and Gram‐positive bacteria, and early prediction of the pathogens of sepsis facilitates early and precise treatment. Routine blood tests, as well as C‐reactive protein (CRP) and procalcitonin (PCT), are simple and rapidly available, which help to effectively distinguish bloodstream infections from non‐bloodstream infections. Although it has been reported that PCT or platelet‐related parameters can be used to predict Gram‐negative bacteria infection, there are still some defects in the current results, which do not allow achieving better sensitivity and specificity simultaneously. Therefore, this study aimed to provide clinical data to support the early assessment of pathogen classification in sepsis and studies related to platelet‐assisted anti‐inflammatory therapy in infectious diseases.
2. METHODS
2.1. Subjects
In this retrospective study, we recorded data from consecutive adult inpatients with infectious fever and positive blood cultures between January 2013 and December 2018 at the Nanfang Hospital of Southern Medical University (Guangzhou, China). Inclusion criteria were as follows: 1) age over 18 years; 2) fever (≥38°C) or low temperature (≤36°C), chills, and other infection symptoms; 3) "Two bottles of bilateral" blood cultures tests and the corresponding pathogenic bacteria had been cultured; "Double‐bottle bilateral" refers to the collection of 2~3 sets of blood culture specimens (each containing one aerobic bottle and one anaerobic bottle) within a short time or interval. 4) All patients met the criteria for Sepsis‐3.28
Exclusion criteria were as follows: 1) confirmed diagnosis of blood disorders, including leukemia, thalassemia, immune thrombocytopenia, and multiple myeloma; 2) confirmed diagnosis of tumors such as liver cancer, lung cancer, ovarian cancer, gastric cancer, or lymphoma; 3) confirmed diagnosis of autoimmune diseases such as vasculitis, systemic lupus erythematosus, or ankylosing spondylitis; 4) confirmed diagnosis of fatty liver, cirrhosis, hypersplenism, or other related diseases; 5) treatment with antibiotics within 3 days prior to undergoing peripheral blood culture; and 6) treatment with anticoagulants, antiplatelet agents, hemostatic agents, granulocyte boosters, or clinical transfusion therapy (eg, platelets, red blood cells, or plasma) within 7 days before obtaining a culture. The flow chart of the study protocol is shown in Figure 1. After excluding 18 patients with multiple bacterial or fungal infections, we eventually enrolled 381 patients. This retrospective study was approved by the Institutional Review Board/ Independent Ethics Committee of Nanfang Hospital of Southern Medical University, and the need for informed consent was waived since all data were anonymous. Patient privacy and confidentiality of data were maintained in accordance with the Declaration of Helsinki.
FIGURE 1.
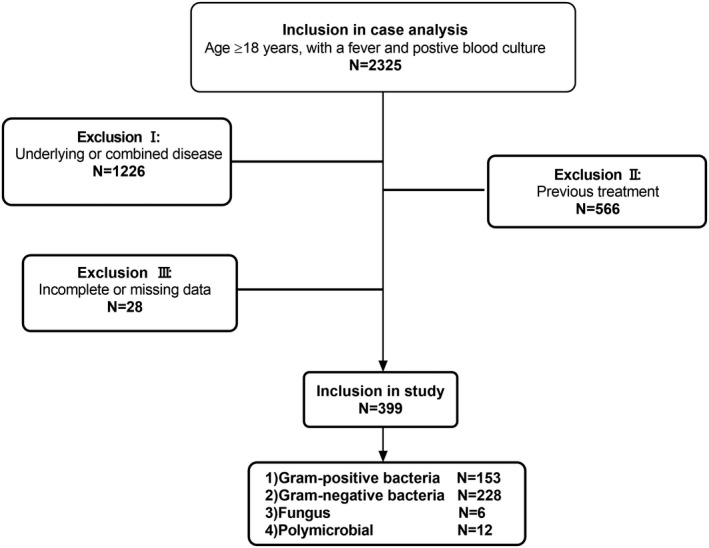
Patient flow chart. Exclusion I: Patients confirmed diagnosis of blood disorders (N = 718), tumors (N = 376), autoimmune disease (N = 64) or fatty liver, cirrhosis, hypersplenism, and other related diseases (N = 68). Exclusion II: Patients received antibiotics within 3 days before blood‐cultured, or who received anticoagulants, antiplatelet agents, hemostatic agents, granulocyte boosters, or clinical transfusion therapy within 7 days before blood‐cultured. Exclusion criteria III: Patients with incomplete or missing data
2.2. Data collection
Data included sex, age, underlying disease, diagnosis, days of hospitalization, blood culture results, site of infection, complete blood counts (including WBC count, neutrophil count, lymphocyte count, monocyte count, PLT, MPV, plateletcrit, PDW, P‐LCR), CRP, and PCT. Calculation of platelet parameter ratios were as follows: platelet count‐to‐lymphocyte count ratio (PLR), platelet count‐to‐WBC count ratio (PWR), platelet count‐to‐neutrophil count ratio (PNR), mean platelet volume‐to‐platelet count ratio (MPV/PLT, calculated by MPV/PLT×100), and platelet distribution width‐to‐platelet count ratio (PDW/PLT, calculated by PDW/PLT×100). We stratified cases according to the magnitude of the PLT5 and PCT29 as follows: 1) PLT ≥150 × 109/L, 100 × 109/L ≤ PLT <150 × 109/L, and PLT <100 × 109/L; 2) PCT <0.5 ng/ml, 0.5 ng/ml ≤PCT <2 ng/ml, 2 ng/ml ≤PCT <10 ng/ml, and PCT ≥10 ng/ml.
2.3. Statistical analyses
Statistical analysis was performed using SPSS version 26.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation or median and interquartile range (25th, 75th percentile), which were compared with Student's t test or the Mann‐Whitney U test. The categorical variables were expressed as frequency and percentage and were compared with the chi‐squared tests or Fisher's exact test. The Spearman rho test of correlation was performed for platelets and platelet parameters. Relevant variables were subjected to receiver operating characteristic (ROC) curve analysis to determine the early predictive value for Gram‐negative (GN)‐BSI. Optimal cut‐off values were determined using the Youden index. Univariate and multivariate logistic (forward stepwise) regression analyses were performed to explore predictors associated with GN‐BSI. All tests were two‐sided and a p‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Demographic and clinical characteristics of all patients
Data from the 381 patients are presented in Table 1. There was no difference in age or sex between the Gram‐negative (GN)‐BSI and Gram‐positive (GP)‐BSI groups. The source of initial infection in the GN‐BSI group was predominantly the urinary or biliary system (p = 0.011; p = 0.016; respectively), and it was venous catheter‐related in the GP‐BSI group (p = 0.103). The GN‐BSI group had a significantly higher proportion of patients with diabetes than the GP‐BSI group (p = 0.029), but had a lower proportion of patients with kidney disease (p = 0.003). In terms of the categories of strains detected from blood cultures, Gram‐negative bacteria were predominantly Escherichia coli (98, 43%) and Klebsiella pneumoniae (50, 21.9%); Gram‐positive bacteria were predominantly Staphylococcus aureus (40, 26.1%) and Staphylococcus epidermidis (20, 13.1%).
TABLE 1.
Demographic and clinical characteristics of all patients
| Variable | Total | GP‐BSI group | GN‐BSI group | p value |
|---|---|---|---|---|
| (N = 381) | (N = 153) | (N = 228) | ||
| Age (years) | 53.8 ± 16.9 | 51.9 ± 17.5 | 55.1 ± 16.5 | 0.072 |
| Sex, male, N, (%) | 227 (59.6) | 100 (65.4) | 127 (55.7) | 0.06 |
| Duration of hospital stay (days) | 17 (12, 26) | 18 (12, 28) | 17 (12, 25) | 0.477 |
| Source of infection, N, (%) | ||||
| Urinary system | 71 (18.6) | 19 (12.4) | 52 (22.8) | 0.011 |
| Biliary system | 58 (15.2) | 15 (9.8) | 43 (18.9) | 0.016 |
| Venous catheter‐related | 47 (12.3) | 24 (15.7) | 23 (10.1) | 0.103 |
| Respiratory system | 37 (9.7) | 17 (11.1) | 20 (8.8) | 0.45 |
| Others or unexplained sources | 168 (44.1) | 78 (51) | 90 (39.5) | ‐ |
| Comorbidity, N, (%) | ||||
| Hypertension | 108 (28.3) | 42 (27.5) | 66 (28.9) | 0.751 |
| Diabetes | 92 (24.1) | 28 (18.3) | 64 (28.1) | 0.029 |
| Kidney disease | 72 (18.9) | 40 (26.1) | 32 (14) | 0.003 |
| Organism, N, (%) | Staphylococcus aureus | Escherichia coli | ||
| 46 (30.1) | 98 (43) | |||
| Staphylococcus epidermidis | Klebsiella pneumoniae | |||
| 20 (13.1) | 50 (21.9) | |||
| Enterococcus faecalis | Pseudomonas aeruginosa | |||
| 15 (9.8) | 13 (5.7) | |||
| Staphylococcus haemolyticus | Enterobacter cloacae | |||
| 7 (4.6) | 10 (4.4) | |||
| Streptococcus pharyngitis | Acinetobacter baumannii | |||
| 7 (4.6) | 5 (2.2) | |||
Abbreviations: GN‐BSI, gram‐negative bloodstream infections; GP‐BSI, gram‐positive bloodstream infections.
3.2. Laboratory characteristics between groups of patients with sepsis
In the comparison of the two groups in Table 2, the lymphocyte count was significantly higher in the GP‐BSI group (p = 0.012), while the CRP and PCT were significantly higher in the GN‐BSI group than in the GP‐BSI group (p = 0.015; p < 0.001; respectively). In terms of platelets and their parameters, MPV, PDW, and P‐LCR were also significantly higher in the GN‐BSI group (all p < 0.001); however, PLT and PCT were significantly lower (p = 0.002; p = 0.003; respectively). The MPV/PLT and PDW/PLT were significantly higher in the GN‐BSI group (both p < 0.001), while PNR, PWR, PLT/PCT, MPV/PCT, and PDW/PCT were significantly higher in the GP‐BSI group (all p < 0.001).
TABLE 2.
Laboratory characteristics of GP‐BSI group and GN‐BSI group
| Variable | GP‐BSI group | GN‐BSI group | p value |
|---|---|---|---|
| (N = 153) | (N = 228) | ||
| WBC (×109/L) | 12.5 ± 6.1 | 13.3 ± 6.3 | 0.198 |
| Lymphocyte (×109/L) | 1.1 (0.7, 1.4) | 0.9 (0.5, 1.3) | 0.012 |
| Neutrophil (×109/L) | 10.4 ± 5.8 | 11.5 ± 6.1 | 0.082 |
| Monocyte (×109/L) | 0.7 (0.5, 0.9) | 0.6 (0.4, 1.0) | 0.147 |
| PLT (×109/L) | 221.3 ± 97.1 | 187.8 ± 108.1 | 0.002 |
| Plateletcrit (ml/L) | 2.3 ± 0.9 | 2.0 ± 1.0 | 0.003 |
| MPV (fL) | 10.1 (9.5, 11.0) | 10.7 (9.9, 11.6) | <0.001 |
| PDW (fI) | 11.2 (9.9, 12.9) | 12.3 (10.9, 14.3) | <0.001 |
| P‐LCR (%) | 25.2 (20.3, 32.6) | 30.1 (23.6, 37.2) | <0.001 |
| CRP (mg/L) | 82.2 (45.5, 145.7) | 109.6 (59.5, 186.8) | 0.015 |
| PCT (ng/ml) | 0.9 (0.3, 4.1) | 5.8 (1.2, 24.4) | <0.001 |
| PLR | 201.9 (145.3, 304.5) | 181.3 (116.9, 317.8) | 0.315 |
| PWR | 18.2 (12.7, 25.4) | 14.0 (8.8, 22.0) | <0.001 |
| PNR | 21.7 (15.2, 32.0) | 16.3 (10.2, 26.7) | <0.001 |
| MPV/PLT | 4.8 (3.7, 7.5) | 6.3 (4.0, 10.5) | <0.001 |
| PDW/PLT | 5.4 (4.0, 8.4) | 7.1 (4.4, 12.7) | <0.001 |
| PLT/PCT | 227.9 (41.2, 709.4) | 26.1 (5.9, 150.1) | <0.001 |
| MPV/PCT | 12.2 (2.6, 38.8) | 1.81 (0.5, 9.1) | <0.001 |
| PDW/PCT | 14.5 (2.8, 43.5) | 2.0 (0.5, 10.4) | <0.001 |
Abbreviations: CRP, C‐reactive protein; GN‐BSI, gram‐negative bloodstream infections; GP‐BSI, gram‐positive bloodstream infections; MPV, mean platelet volume; PCT, procalcitonin; PDW, platelet distribution width; P‐LCR, platelet‐large cell ratio; PLR, platelet count‐to‐lymphocyte ratio; PLT, platelet; PNR, platelet count‐to‐neutrophil count ratio; PWR, platelet count‐to‐white blood cell count ratio; WBC, white blood cell.
3.3. Correlation between PLT and platelet‐related parameters
In the correlation comparison of platelet‐related parameters (Table S1), the PLT was positively correlated with the plateletcrit (r = 0.955, p < 0.001) and negatively correlated with PDW, MPV, and P‐LCR (r = −0.473, p < 0.001; r = −0.521, p < 0.001; r = −0.547, p < 0.001; respectively).
3.4. Multivariate analysis of factors associated with GN‐BSI
Binary logistic regression analysis was performed to identify predictors for early discrimination of GN‐BSI (Table 3). We set PLT ≥150 × 109/L and PCT <0. 5 ng/ml as dummy variables. Multivariate stepwise logistic regression of variables with significant differences in univariate logistic regression analysis revealed that independent predictors of GN‐BSI were MPV (odds ratio [OR]: 1.7, 95% CI: 1.147–2.52, p = 0.008), PWR (OR: 0.966, 95% CI: 0.938–0.994, p = 0.018), and PCT (0.5 ng/ml ≤PCT <2 ng/ml, OR: 2.436, 95% CI: 1.011–5.868, p = 0.047; 2 ng/ml ≤PCT <10 ng/ml, OR: 2.503, 95% CI: 1.027–6.098, p = 0.0431; PCT ≥10 ng/ml, OR: 8.128, 95% CI: 2.999–22.031, p < 0.001). The predictive equation was (P) = −5.423 +0.53 × MPV −0.035 × PWR +0.89 × PCT(0.5–2 ng/ml) +0.917 × PCT(2–10 ng/ml) +2.095 × PCT (≥10 ng/ml). The goodness‐of‐fit of the multivariable model was determined using Hosmer‐Lemeshow test (Chi‐square =10.471, p = 0.233).
TABLE 3.
Explore predictors for early discrimination of GN‐BSI by binary logistic regression analysis
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| B | OR | 95% CI | p value | B | OR | 95% CI | p value | |
| MPV | 0.38 | 1.462 | 1.212–1.764 | <0.001 | 0.53 | 1.7 | 1.147–2.52 | 0.008 |
| PDW | 0.146 | 1.157 | 1.068–1.253 | <0.001 | ||||
| PWR | −0.021 | 0.979 | 0.964–0.994 | 0.007 | −0.035 | 0.966 | 0.938–0.994 | 0.018 |
| PNR | −0.013 | 0.987 | 0.977–0.998 | 0.018 | ||||
| MPV/PLT | 0.071 | 1.074 | 1.028–1.122 | 0.001 | ||||
| MPV/PCT | −0.024 | 0.976 | 0.967–0.986 | <0.001 | ||||
| PLT (×109/L) | <0.001 | |||||||
| ≥150 | Reference | |||||||
| 100–150 | 0.42 | 1.522 | 0.902–2.567 | 0.115 | ||||
| <100 | 1.389 | 4.013 | 1.993–8.081 | <0.001 | ||||
| PCT (ng/ml) | <0.001 | 0.001 | ||||||
| <0.5 | Reference | |||||||
| 0.5–2 | 0.884 | 2.421 | 1.055–5.554 | 0.037 | 0.89 | 2.436 | 1.011–5.868 | 0.047 |
| 2–10 | 1.085 | 2.961 | 1.28–6.846 | 0.011 | 0.917 | 2.503 | 1.027–6.098 | 0.043 |
| ≥10 | 2.451 | 11.605 | 4.573–29.454 | <0.001 | 2.095 | 8.128 | 2.999–22.031 | <0.001 |
| Constant | −5.423 | 0.004 | 0.014 | |||||
PLT ≥150×109/L and PCT <0.5 ng/ml were set as dummy variables.
Abbreviations: GN‐BSI, gram‐negative bloodstream infections; MPV, mean platelet volume; PCT, procalcitonin; PDW, platelet distribution width; PLT, platelet; PNR, platelet count‐to‐neutrophil count ratio; PWR, platelet count‐to‐white blood cell count ratio.
3.5. Comparison of ROC curves of related indicators and model as markers of GN‐BSI
To further explore the predictive role of these indicators, their ratios to PCT, and the predictive equation model in early GN‐BSI, we constructed ROC curves. As shown in Figure 2 and Table 4, the MPV, PCT, PWR, MPV/PCT, and PLT/PCT showed moderate ability to distinguish between patients within GN‐BSI, with PLT/PCT having the highest areas under the curve (AUC) (0.73, 95% CI: 0.67–0.77), sensitivity =0.80, specificity =0.55. It should be noted that when PWR, PLT/PCT, and MPV/PCT were less than or equal to cut‐off values, these indicators suggested a greater likelihood of GN‐BSI. Based on the predictive equation model, the following were calculated: the AUC =0.79 (95% CI: 0.73–0.85), sensitivity=0.75, specificity =0.71.
FIGURE 2.
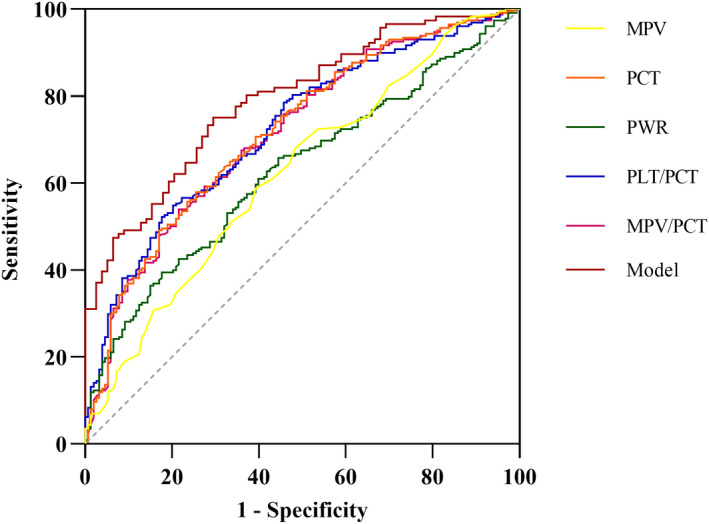
Receiver operating characteristic curve of related indicators and model for differential diagnosis of GN‐BSI. GN‐BSI, gram‐negative bloodstream infections; MPV, mean platelet volume; PCT, procalcitonin; PWR, platelet count‐to‐white blood cell count ratio (PLT/WBC)
TABLE 4.
Receiver operating characteristic (ROC) curve for predicting GN‐BSI
| Parameters | AUC | 95% CI | p value | Cut‐off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| MPV | 0.62 | 0.56–0.68 | <0.001 | 10.15 | 0.68 | 0.52 |
| PCT | 0.72 | 0.67–0.77 | <0.001 | 3.70 | 0.58 | 0.75 |
| PWRa | 0.63 | 0.57–0.68 | <0.001 | 10.89 | 0.82 | 0.40 |
| PLT/PCTa | 0.73 | 0.67–0.77 | <0.001 | 28.87 | 0.80 | 0.55 |
| MPV/PCTa | 0.72 | 0.66–0.77 | <0.001 | 2.17 | 0.78 | 0.54 |
| Model | 0.79 | 0.73–0.85 | <0.001 | 0.55 | 0.75 | 0.71 |
Abbreviations: GN‐BSI, gram‐negative bloodstream infections; MPV, mean platelet volume; PCT, procalcitonin; PWR, platelet count‐to‐white blood cell count ratio.
Represents a positive prediction when the parameters are less than or equal to the cut‐off value.
4. DISCUSSION
The results of this study demonstrated that PCT, platelet parameters, and the platelet‐related index‐PCT ratio were significantly different between GP‐BSI and GN‐BSI groups. PCT, MPV, PWR, PLT/PCT, and MPV/PCT values obtained from routine blood tests can be used to discriminate Gram‐negative bloodstream infections among bloodstream infections to some extent. The combination of MPV, PWR, and PCT is helpful for the clinical diagnosis of GN‐BSI, with more effective sensitivity and specificity. This would substantially benefit a wide range of clinical workers and patients, particularly in economically compromised developing countries.
Sepsis still is a common life‐threatening medical emergency. Previous studies have shown a higher incidence of severe sepsis or septic shock in GN‐BSI relative to GP‐BSI.30, 31 Early differential diagnosis of GN‐BSI and initiation of targeted antibiotic therapy are extremely important. After ruling out factors that may affect platelet parameters, we found that MPV, PDW, and P‐LCR levels were significantly higher in the GN‐BSI group, while PLT and plateletcrit were lower. However, the diagnostic value of these platelet parameters was not particularly high, and the highest AUC for MPV was only 0.62, and the specificity is relatively low. Catal et al. found that both PLT and MPV were significantly higher in the Gram‐negative bacteria infection group in patients with upper urinary tract infection.32 In another study involving very low birth weight newborns, PLT levels were lower in the Gram‐negative bacteria infection group, but MPV showed no significant differences.33 Conversely, Manzoni et al. proposed that platelets were not an organism‐specific marker of sepsis.34 Our study also found that the MPV/PLT ratio was significantly higher in the GN‐BSI group, which was similar to findings by Djordjevic et al.35 Interestingly, we did not observe any significant differences in PLR in the groups; however, Djordjevic et al. found a higher PLR in patients with trauma and peritonitis whose blood culture grew Gram‐negative bacteria (p = 0.01).35 The reason for this difference may be that the underlying diseases of the included patients were different in the two studies.
The increase in MPV, PDW, and P‐LCR values reflected the size and morphology of platelets and indicated the degree of activation of platelet function and a higher degree of aggregation and inflammatory response. Platelet count and platelet activity, size, or volume are often negatively correlated. This was confirmed by our results. GN‐BSI induce a stronger inflammatory response in the organism with higher concentrations of inflammatory factors than GP‐BSI.30, 36 Some studies have reported that Gram‐positive bacteria are associated with more pronounced platelet activation, and platelet hyperreactivity compared with common Gram‐negative bacteria.37 This different degree of thrombocytopenia and platelet activation may lead to changes in platelet parameters, which can reflect changes in platelet volume, aggregation, and volume variation. However, differences in hematological levels of these non‐specific biomarkers still need to be further clarified at the molecular level of different pathogenic mechanisms.
In this study, we found that lymphocytes, CRP, and PCT showed different levels of expression in GP‐BSI and GN‐BSI groups, and PCT helped to distinguish GN‐BSI from GP‐BSI, with an AUC of 0.72 and a specificity of 0.75, but a low sensitivity of 0.58. Several studies have shown significantly higher levels of PCT in patients with GN‐BSI than in those with GP‐BSI,38, 39, 40 which is consistent with the findings of our study. Both Bassetti et al. and Liu et al. showed that there were no differences in CRP levels between the two groups.39, 40 Similarly, a meta‐analysis also concluded that PCT is superior to CRP in the diagnosis of Gram‐negative bacterial infections.41 The different levels of PCT may be related to the inflammatory response and the different degrees of the systemic inflammatory response induced by the two bacteria classes through the induction and activation of different signal transduction pathways.31, 36 Differences in PCT may also be related to the fact that endotoxins on the cell wall of Gram‐negative bacteria can directly induce PCT stimulation. Nevertheless, the value of early identification of sepsis or GN‐BSI using PCT alone to assess infection remains debatable,42 and we often need to interpret these findings in the context of clinical conditions. Thomas‐Ruddel et al. and Yu et al. concluded that PCT serum concentrations were also influenced by the different sites of infection, and its differential diagnosis value for GN‐BSI was limited.43, 44
In different bacterial bloodstream infections, platelet and PCT concentrations in the blood circulation are variable, and the ratio between these indicators may better reflect the degree of systemic inflammatory response. In the present study, our results suggested that the PWR, PLT/PCT, and MPV/PCT values may be useful markers for the differential diagnosis of GN‐BSI, with a high sensitivity of up to 0.82. PWR, obtained from the complete blood count, is a hematological marker of the systemic inflammatory response. Previous studies have shown that low PWR is associated with a poor prognosis of hepatitis B virus‐associated decompensated cirrhosis and acute‐on‐chronic liver failure.45, 46 Further, PWR has been shown to not only predict the risk of infectious complications in patients with renal malignancies receiving radical nephrectomy,47 but can also distinguish infections and normal response after splenectomy.48 However, there are no other reference data on the early differential diagnosis of these indicators in BSI or sepsis.
Our study found that the diagnostic value of PLT/PCT and MPV/PCT was superior to either MPV or PCT individually. Compared with PCT, the sensitivity of these ratios was increased by more than 20%. The multivariate stepwise logistic regression analysis results indicated that MPV, PWR, and PCT were useful independent predictors of the early differential diagnosis of GN‐BSI. Therefore, we developed an equation model for early differential diagnosis of GN‐BSI, having a good predictive value in the cohort of 381 patients with BSI in this study. The combination of MPV, PWR, and PCT may improve the sensitivity and specificity of the differential diagnosis of GN‐BSI, compensating for the low sensitivity or low specificity of one indicator alone. The model with the combined indexes showed an 18% increase in sensitivity compared with PCT and a 31% increase in specificity compared with PWR.
Our present study has several limitations. It was a single‐center retrospective study, and the results may be susceptible to selection bias. There are currently very few similar studies elsewhere that can be used as references, and multi‐center prospective studies are needed to verify our findings. In the next step, we will conduct prospective trials to validate this model in a larger patient population.
5. CONCLUSIONS
Leukocyte parameters, platelet parameters, and the platelet‐related index‐PCT ratio were significantly different between GP‐BSI and GN‐BSI groups. PCT, MPV, PWR, PLT/PCT, and MPV/PCT values were useful in the differential diagnosis of Gram‐negative from Gram‐positive bloodstream infections. The equation model consisting of MPV, PWR, and PCT is helpful for the clinical diagnosis of GN‐BSI, which can effectively improve the sensitivity and specificity.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Tab S1
Gao Q, Li Z, Mo X, Wu Y, Zhou H, Peng J. Combined procalcitonin and hemogram parameters contribute to early differential diagnosis of Gram‐negative/Gram‐positive bloodstream infections. J Clin Lab Anal. 2021;35:e23927. 10.1002/jcla.23927
Gao and Li are Co‐First authors
Mo and Wu are Co‐authors
Funding information
Supported by National Natural Science Foundation of China (81971949), and Natural Science Foundation of Guangdong Province (2018A030313102)
Contributor Information
Qiqing Gao, Email: gaoqq3090@163.com.
Hao Zhou, Email: 630304495@qq.com.
Jie Peng, Email: pjie138@163.com.
DATA AVAILABILITY STATEMENT
Authors can confirm all relevant data are included in the article and materials are available on request from the authors.
REFERENCES
- 1.Laupland KB. Incidence of bloodstream infection: a review of population‐based studies. Clin Microbiol Infect. 2013;19(6):492‐500. [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308‐1316. [DOI] [PubMed] [Google Scholar]
- 3.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States‐an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claushuis TA, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062‐3072. [DOI] [PubMed] [Google Scholar]
- 6.Koyama K, Katayama S, Muronoi T, et al. Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS One. 2018;13(1):e192064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware J, Corken A, Khetpal R. Platelet function beyond hemostasis and thrombosis. Curr Opin Hematol. 2013;20(5):451‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Stoppelaar SF, van TVC, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112(4):666‐677. [DOI] [PubMed] [Google Scholar]
- 9.Zamora C, Canto E, Nieto JC, et al. Functional consequences of platelet binding to T lymphocytes in inflammation. J Leukoc Biol. 2013;94(3):521‐529. [DOI] [PubMed] [Google Scholar]
- 10.Zareifar S, Farahmand FM, Golfeshan F, Cohan N. Changes in platelet count and mean platelet volume during infectious and inflammatory disease and their correlation with ESR and CRP. J Clin Lab Anal. 2014;28(3):245‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sit M, Aktas G, Yilmaz EE, Hakyemez IN, Alcelik A, Kucukbayrak A. Platelet parameters in hepatic hydatid cysts. Int J Inflam. 2013;2013:593273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K. Changes in platelet count and indices in pulmonary tuberculosis. Clin Chem Lab Med. 2007;45(8):1009‐1013. [DOI] [PubMed] [Google Scholar]
- 13.Cakiroglu B. Mean platelet volume: a simple indicator of chronic prostatitis. Acta Medica Mediterr. 2013;3(29):551. [Google Scholar]
- 14.Aktas G, Sit M, Tekce H, et al. Mean platelet volume in nasal polyps. West Indian Med J. 2013;62(6):515‐518. [DOI] [PubMed] [Google Scholar]
- 15.Cakir L, Aktas G, Mercimek B, Enginyurt O, Mercimek K. Are red cell distribution width and mean platelet volume associated with rheumatoid arthritis? Biomed Res. 2016;2(27):292‐294. [Google Scholar]
- 16.Aktas G, Kocak MZ, Duman TT, Erkus E, Savli H. Mean platelet volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med J. 2018;3(7):650‐653. [Google Scholar]
- 17.Sincer I, Gunes Y, Mansiroglu AK, Cosgun M, Aktas G. Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Postepy Kardiol Interwencyjnej. 2018;14(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakir L, Aktas G, Enginyurt O, Cakir SA. Mean platelet volume increases in type 2 diabetes mellitus independent of HbA1c level. Acta Medica Mediterr. 2014;30(2):425‐428. [Google Scholar]
- 19.Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M, Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol. 2014;9(3):160‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aktas G, Duman TT, Atak B, Kurtkulagi O, Kosekli MA. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Family Med Prim Care Rev. 2020;22(2):107‐110. [Google Scholar]
- 21.Atak BM, Duman TT, Aktas G, Kocak MZ, Savli H. Platelet distribution width is associated with type 2 diabetes mellitus and diabetic nephropathy and neuropathy. Nat J Health Sci. 2018;3:95‐98. [Google Scholar]
- 22.Sincer I, Mansiroglu AK, Erdal E, Cosgun M, Aktas G, Gunes Y. Could platelet distribution width predict coronary collateral development in stable coronary artery disease? North Clin Istanb. 2020;7(2):112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duman TT, Aktas G, Atak B, Kocak MZ. Is mean platelet volume to platelet ratio a promising indicator of diabetic regulation in type 2 diabetes mellitus? J Med Res. 2018;3(4):137‐139. [Google Scholar]
- 24.Oh GH, Chung SP, Park YS, et al. Mean platelet volume to platelet count ratio as a promising predictor of early mortality in severe sepsis. Shock. 2017;47(3):323‐330. [DOI] [PubMed] [Google Scholar]
- 25.Fei Y, Zhang H, Zhang C. The application of lymphocyte*platelet and mean platelet volume/platelet ratio in influenza A infection in children. J Clin Lab Anal. 2019;33(9):e22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahar JR, Timsit JF, Garrouste‐Orgeas M, et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39(8):1886‐1895. [DOI] [PubMed] [Google Scholar]
- 27.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta‐analysis. Crit Care Med. 2015;43(9):1907‐1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurol G, Ciftci IH, Terizi HA, Atasoy AR, Ozbek A, Koroglu M. Are there standardized cutoff values for neutrophil‐lymphocyte ratios in bacteremia or sepsis? J Microbiol Biotechnol. 2015;25(4):521‐525. [DOI] [PubMed] [Google Scholar]
- 30.Abe R, Oda S, Sadahiro T, et al. Gram‐negative bacteremia induces greater magnitude of inflammatory response than gram‐positive bacteremia. Crit Care. 2010;14(2):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XJ, Luo ZB, Xia T, et al. Comparison of interleukin‐6, interleukin‐10, procalcitonin and C‐reactive protein in identifying high‐risk febrile illness in pediatric cancer patients: a prospective observational study. Cytokine. 2019;116:1‐6. [DOI] [PubMed] [Google Scholar]
- 32.Catal F, Bavbek N, Bayrak O, et al. Platelet parameters in children with upper urinary tract infection: is there a specific response? Ren Fail. 2008;30(4):377‐381. [DOI] [PubMed] [Google Scholar]
- 33.Guida JD, Kunig AM, Leef KH, Mckenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism‐specific response? Pediatrics. 2003;111(6 Pt 1):1411‐1415. [DOI] [PubMed] [Google Scholar]
- 34.Manzoni P, Mostert M, Galletto P, et al. Is thrombocytopenia suggestive of organism‐specific response in neonatal sepsis? Pediatr Int. 2009;51(2):206‐210. [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic D, Rondovic G, Surbatovic M, et al. Neutrophil‐to‐lymphocyte ratio, monocyte‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, and mean platelet volume‐to‐platelet count ratio as biomarkers in critically Ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediators Inflamm. 2018;2018:3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surbatovic M, Popovic N, Vojvodic D, et al. Cytokine profile in severe gram‐positive and gram‐negative abdominal sepsis. Sci Rep. 2015;5:11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tunjungputri RN, van de Heijden W, Urbanus RT, de Groot PG, van der Ven A, de Mast Q. Higher platelet reactivity and platelet‐monocyte complex formation in Gram‐positive sepsis compared to Gram‐negative sepsis. Platelets. 2017;28(6):595‐601. [DOI] [PubMed] [Google Scholar]
- 38.Gai L, Tong Y, Yan BQ. Research on the diagnostic effect of PCT level in serum on patients with sepsis due to different pathogenic causes. Eur Rev Med Pharmacol Sci. 2018;22(13):4238‐4242. [DOI] [PubMed] [Google Scholar]
- 39.Liu HH, Zhang MW, Guo JB, Li J, Su L. Procalcitonin and C‐reactive protein in early diagnosis of sepsis caused by either gram‐negative or gram‐positive bacteria. Ir J Med Sci. 2017;186(1):207‐212. [DOI] [PubMed] [Google Scholar]
- 40.Bassetti M, Russo A, Righi E, et al. Role of procalcitonin in predicting etiology in bacteremic patients: report from a large single‐center experience. J Infect Public Health. 2020;13(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 41.Lai L, Lai Y, Wang H, et al. Diagnostic accuracy of procalcitonin compared to C‐Reactive protein and interleukin 6 in recognizing gram‐negative bloodstream infection: a meta‐analytic study. Dis Markers. 2020;2020:4873074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jekarl DW, Lee S, Kim M, Kim Y, Woo SH, Lee WJ. Procalcitonin as a prognostic marker for sepsis based on SEPSIS‐3. J Clin Lab Anal. 2019;33(9):e22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Li XX, Jiang LX, et al. Procalcitonin levels in patients with positive blood culture, positive body fluid culture, sepsis, and severe sepsis: a cross‐sectional study. Infect Dis (Lond). 2016;48(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 44.Thomas‐Ruddel DO, Poidinger B, Kott M, Weiss M, Reinhart K, Bloos F. Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care. 2018;22(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jie Y, Gong J, Xiao C, et al. Low platelet to white blood cell ratio indicates poor prognosis for acute‐on‐chronic liver failure. Biomed Res Int. 2018;2018:7394904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Qiu Y, He X, Mao W, Han Z. Platelet‐to‐white blood cell ratio: a novel and promising prognostic marker for HBV‐associated decompensated cirrhosis. J Clin Lab Anal. 2020;34(12):e23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garbens A, Wallis C, Bjarnason G, et al. Platelet to white blood cell ratio predicts 30‐day postoperative infectious complications in patients undergoing radical nephrectomy for renal malignancy. Can Urol Assoc J. 2017;11(11):E414‐E420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng J, Brown CV, Rhee P, et al. White blood cell and platelet counts can be used to differentiate between infection and the normal response after splenectomy for trauma: prospective validation. J Trauma. 2005;59(5):1076‐1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1
Data Availability Statement
Authors can confirm all relevant data are included in the article and materials are available on request from the authors.


