Abstract
Background
We aimed to analyze the differences in the peripheral blood cells and tumor biomarkers between the patients with endometriosis and healthy people, and establish a more efficient combined diagnostic model.
Methods
We retrospectively analyzed the differences in the peripheral blood cells and tumor biomarkers between the patients with endometriosis and healthy people. Binary logistic regression analysis was used to establish a combined diagnostic model. We plotted the receiver operator characteristic (ROC) curve to analyze the diagnostic efficiency of different diagnostic indexes.
Results
Compared with patients in the control group, patients in the endometriosis group had significantly lower eosinophil% (p = 0.045), neutrophil (p = 0.001), lymphocyte (p < 0.001), red blood cells (RBCs) (p < 0.001), and hemoglobin (HGB) (p < 0.001), and had significantly higher monocyte% (p = 0.008), monocyte‐to‐lymphocyte ratio (MLR) (p = 0.001), platelet‐to‐lymphocyte ratio (PLR) (p < 0.001), carbohydrate antigen (CA)‐199 (p < 0.001), CA125 (p < 0.001), human epididymis protein (HE)‐4 (p < 0.001), and the risk of ovarian malignancy algorithm (ROMA) (p < 0.001). The combined diagnostic model of HGB, CA199, CA125, and HE4 was established by binary logistic regression analysis. The ROC curve showed that the combined diagnostic model reached a sensitivity of 85.4%, a specificity of 78.83%, and an area under the curve of 0.900, which was significantly higher than that of the individual index in endometriosis diagnosis.
Conclusion
The combined diagnostic model of HGB, CA199, CA125, and HE4 may provide a new approach for the early non‐invasive diagnosis of endometriosis.
Keywords: CA125, CA199, endometriosis, HE4, HGB
This study retrospectively analyzed the differences in the peripheral blood cells and their derivative parameters and tumor biomarkers between the patients with endometriosis and healthy people, and established a more efficient combined diagnostic model based on HGB, CA199, CA125, and HE4. The combined diagnostic model reached a sensitivity of 85.4%, a specificity of 78.83%, and an area under the curve of 0.900, which may provide a novel approach for the early non‐invasive diagnosis of endometriosis.
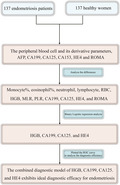
1. INTRODUCTION
Endometriosis is a painful disorder in which the endometrial tissue (glands and stroma) that normally lines the inside of the uterus, grows, and infiltrates outside the uterus, consequently causing repeated bleeding, pain, infertility, and the formation of nodules or tumor mass.1 It is a common disease in women of childbearing age and shows a significantly increasing trend, with a prevalence rate of 10–15% in China. Moreover, 80% of patients with endometriosis exhibit pelvic pain, and 50% present infertility, which seriously affects the health and quality of life of young and middle‐aged women.2 Despite being a benign gynecological disease, endometriosis has the biological behavior of malignant tumors due to its invasive growth, metastasis, and recurrence. It invades and destroys the affected tissues, seriously reducing the quality of life of patients.3, 4, 5 Therefore, early diagnosis and treatment of endometriosis can improve fertility, inhibit the development of the disease, relieve pain, and improve quality of life.6, 7 Laparoscopy is the gold standard for endometriosis diagnosis, but it has disadvantages such as severe trauma, high risk and cost, and complex operation procedure.8 Studies have reported that carbohydrate antigen (CA)‐125 is highly expressed in endometriosis patients and can be used to predict recurrence and evaluate therapeutic effects, but without an acceptable sensitivity and specificity for early diagnosis.9, 10 Human epididymis protein (HE)‐4 is a novel tumor biomarker for monitoring the recurrence and progression of ovarian cancer. The application of the risk of ovarian malignancy algorithm (ROMA) constructed based on CA125, HE4, and patients' menstruation also has a certain diagnostic value in endometriosis.11, 12, 13 In recent years, the diagnostic value of a variety of potential biomarkers, including CA125 and HE4, has been evaluated in endometriosis but is far from satisfactory.10, 14 Therefore, it is important to identify a suitable early non‐invasive serological diagnosis index.
In this study, we retrospectively analyzed the parameters of peripheral blood cells and serum tumor biomarker levels in the patients with endometriosis and healthy individuals and evaluated the diagnostic efficacy of HGB, CA199, CA125, HE4, and their combination for endometriosis, aiming to find an ideal combined diagnostic index for the early diagnosis of endometriosis.
2. MATERIALS AND METHODS
2.1. General materials
A total of 137 endometriosis patients aged 33 (28, 38) years were selected and admitted to the Affiliated Suzhou Hospital of Nanjing Medical University between January 2015 and December 2017. Inclusion criteria: patients who underwent laparoscopic surgery and were diagnosed with endometriosis by pathological examination; patients signed the informed consent forms and participated voluntarily. Exclusion criteria were complicated with hormone‐dependent diseases, such as adenomyosis and uterine leiomyoma; taking any hormone drugs and having a history of pregnancy within 6 months; complicated with other endocrine, immune, and metabolic diseases; and malignant tumors. Patients with endometriosis were staged according to the modified endometriosis staging method (1997) proposed by the American Society for Reproductive Medicine (ASRM). Among all the patients, 24% (33/137) were in stage I–II, 33% (45/137) were in stage III, and 43% (59/137) were in stage IV. Meanwhile, 137 healthy women aged 32 (28, 37) years were selected as the control group. This study was approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (K‐2020‐083‐K01), and informed consent was obtained from all participants involved in this study.
2.2. Sample testing
The clinical data of all participants were collected, including their age, complete blood count, levels of alpha fetoprotein (AFP), CA199, CA125, CA153, HE4, and ROMA. Complete blood count was performed using XN‐20 [A1] (Sysmex, Japan). Neutrophil‐to‐lymphocyte ratio (NLR), monocyte‐to‐lymphocyte ratio (MLR), platelet‐to‐lymphocyte ratio (PLR), eosinophil‐to‐lymphocyte ratio (ELR), systemic immune inflammation index (SII, calculated by platelet count × neutrophil count/lymphocyte count), and systemic inflammatory response index (SIRI, calculated by neutrophil count × monocyte count/lymphocyte count) were calculated from the results of the complete blood count. AFP was detected using a UniCel DxI 800 chemiluminescence apparatus (Beckman Coulter, USA). CA199, CA125, CA153, and HE4 were detected using an ARCHITECT i2000SR chemiluminescence apparatus (Abbott, USA). ROMA was calculated by combining the results of CA125, HE4, and the menstruation patterns of the patients.
2.3. Statistical analysis
The sample size requirement was calculated using PASS 2021 (purchase from www.ncss.com/software/pass/) with a 5% alpha error (two‐sided), 10% beta error, and the null value of the AUC was 0.5. The ratio between the groups was 1:1. At least 97 patients with endometriosis and 97 healthy controls were included in this study. Taking 10% sample loss during follow‐up into account, 107 patients with endometriosis and 107 controls should be included in the study. This study included 137 patients with endometriosis and 137 controls, and the statistical power was 0.97. SPSS 22.0 software (purchase from www.ibm.com/support/pages/node/230551) was used for performing all statistical analysis. The Shapiro‐Wilk test was used to analyze the type of data distribution. The Mann‐Whitney U test was used to evaluate the difference in the detection indexes between the patients with endometriosis and healthy people. The Kruskal‐Wallis univariate analysis of variance (ANOVA) test was used to evaluate the differences in the detection indexes between more than two groups. The correlation of categorical data within the groups was analyzed using the Pearson's χ 2 test. A binary logistic regression analysis was used to construct the diagnostic model. We used MedCalc 20.0 (purchase from www.medcalc.org) to plot the receiver operator characteristic (ROC) curve and calculated the area under the curve (AUC) to analyze the diagnostic efficiency of different diagnostic indexes. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Comparison of the peripheral blood cell parameters and serum tumor biomarkers between the endometriosis and control groups
The differences in peripheral blood cell parameters, blood cell derivative parameters, and serum tumor biomarkers between the endometriosis and control groups were analyzed. Compared with patients in the control group, those in the endometriosis group had significantly lower eosinophil% (p = 0.045), absolute neutrophil count (p = 0.001), lymphocyte count (p < 0.001), red blood cell count (RBC, p < 0.001), and hemoglobin (HGB, p < 0.001), and had significantly higher monocyte% (p = 0.008), MLR (p = 0.001), PLR (p < 0.001), CA199 (p < 0.001), CA125 (p < 0.001), HE4 (p < 0.001), and ROMA (p < 0.001) (Figure 1). There were no significant differences in neutrophil%, lymphocyte%, absolute value of monocyte count, platelet count, NLR, ELR, SII, SIRI, AFP, or CA153 between the two groups (Table 1).
FIGURE 1.
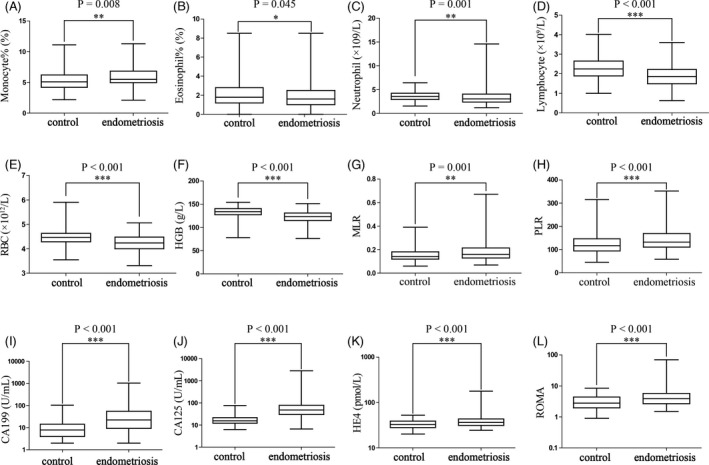
The differences in peripheral blood cell parameters, and serum tumor biomarkers between the endometriosis and control groups. Compared with patients in the control group, those in the endometriosis group had significantly lower eosinophil% (p = 0.045, B), neutrophil count (p = 0.001, C), lymphocyte count (p < 0.001, D), RBC (p < 0.001, E), and HGB (p < 0.001, F), and had significantly higher monocyte% (p = 0.008, A), MLR (p = 0.001, G), PLR (p < 0.001, H), CA199 (p < 0.001, I), CA125 (p < 0.001, J), HE4 (p < 0.001, K), and ROMA (p < 0.001, L). RBC, red blood cell; HGB, hemoglobin; MLR, monocyte‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; CA, carbohydrate antigen; HE4, human epididymis protein 4; ROMA, the risk of ovarian malignancy algorithm
TABLE 1.
Comparison of the peripheral blood cell parameters and serum tumor biomarkers between the endometriosis and control groups
| Parameters | Control group (n = 137) | Endometriosis group (n = 137) | p‐value |
|---|---|---|---|
| Age | 33.00 (28.00, 38.00) | 32.00 (28.00, 37.00) | 0.689 |
| Neutrophil% (%) | 56.00 (51.65, 60.50) | 56.10 (51.00, 63.00) | 0.478 |
| Lymphocyte% (%) | 36.00 (31.05, 40.90) | 34.00 (29.10, 40.45) | 0.303 |
| Monocyte% (%) | 5.10 (4.20, 6.20) | 5.50 (4.95, 6.80) | 0.008 |
| Eosinophil% (%) | 1.80 (1.20, 2.80) | 1.60 (1.00, 2.50) | 0.045 |
| Neutrophil count (×109/L) | 3.60 (2.93, 4.23) | 3.03 (2.36, 4.03) | 0.001 |
| Lymphocyte count (×109/L) | 2.25 (1.89, 2.65) | 1.86 (1.49, 2.21) | <0.001 |
| Monocyte count (×109/L) | 0.33 (0.26, 0.40) | 0.31 (0.25, 0.39) | 0.265 |
| RBC (×1012/L) | 4.47 (4.29, 4.64) | 4.24 (4.01, 4.48) | <0.001 |
| HGB (g/L) | 134.00 (127.00, 140.00) | 123.00 (115.00, 131.00) | <0.001 |
| Platelet count (×109/L) | 262.00 (218.50, 302.50) | 252.00 (209.50, 298.50) | 0.351 |
| NLR | 1.56 (1.29, 1.93) | 1.66 (1.25, 2.18) | 0.368 |
| MLR | 0.14 (0.12, 0.18) | 0.16 (0.13, 0.22) | 0.001 |
| ELR | 0.05 (0.03, 0.08) | 0.04 (0.03, 0.07) | 0.056 |
| PLR | 117.09 (94.06, 146.08) | 131.48 (111.20, 169.47) | <0.001 |
| SⅡ | 392.64 (314.88, 569.15) | 422.56 (314.82, 588.12) | 0.538 |
| SIRI | 0.53 (0.36, 0.70) | 0.52 (0.33, 0.80) | 0.984 |
| AFP (ng/ml) | 2.18 (1.43, 3.43) | 2.00 (1.56, 2.99) | 0.876 |
| CA199 (U/ml) | 7.90 (4.08, 14.19) | 22.14 (9.30, 54.31) | <0.001 |
| CA125 (U/ml) | 15.50 (12.20, 21.60) | 47.60 (30.50, 75.00) | <0.001 |
| CA153 (U/ml) | 7.80 (6.05, 11.55) | 8.40 (6.30, 11.20) | 0.509 |
| HE4 (pmol/L) | 32.50 (28.00, 38.70) | 36.10 (31.20, 42.80) | <0.001 |
| ROMA | 2.80 (1.96, 4.36) | 3.87 (2.67, 5.76) | <0.001 |
Data are presented as medians (interquartile ranges).
Abbreviations: AFP, alpha fetoprotein; CA, carbohydrate antigen; ELR, eosinophil‐to‐lymphocyte ratio; HE4, human epididymis protein 4; HGB, hemoglobin; MLR, monocyte‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; RBC, red blood cell; ROMA, the risk of ovarian malignancy algorithm; SⅡ, systemic immune inflammation index; SIRI, systemic inflammatory response index.
3.2. Comparison of the peripheral blood cell parameters, tumor biomarkers, and clinical parameters in the patients with endometriosis at different clinical stages
We analyzed the differences in age, monocyte%, eosinophil%, neutrophil count, lymphocyte count, RBC, HGB, MLR, PLR, CA199, CA125, HE4, and ROMA among patients with stage Ⅰ – II, III, and IV endometriosis, and explored the correlation between different endometriosis stages and related clinical parameters. The results showed that there were significant differences in eosinophil% (p = 0.043), CA199 (p = 0.002), and CA125 (p < 0.001) among different endometriosis stages (Table 2, Figure 2), and the endometriosis stages were correlated with infertility (p < 0.001) and pelvic mass (p < 0.001) (Table 3). However, there were no statistical differences in age, monocyte%, neutrophil count, lymphocyte count, RBC, HGB, MLR, PLR, HE4, ROMA, delivery, dysmenorrhea, induced abortion, pregnancy, cesarean section, and abdominal pain among different endometriosis stages.
TABLE 2.
Comparisons of the peripheral blood cell parameters and serum tumor biomarkers in the patients with endometriosis at different clinical stages
| Parameters | Stage Ⅰ–Ⅱ (n = 33) | Stage Ⅲ (n = 45) | Stage Ⅳ (n = 59) | p‐value |
|---|---|---|---|---|
| Age | 30.00 (27.00, 36.00) | 33.00 (30.00, 38.50) | 32.00 (27.00, 37.00) | 0.135 |
| Monocyte% (%) | 5.70 (5.20, 6.60) | 5.50 (4.40, 6.10) | 5.50 (5.00, 7.00) | 0.371 |
| Eosinophil% (%) | 2.20 (1.10, 3.35) | 1.70 (0.90, 2.55) | 1.30 (1.00, 2.00) | 0.043 |
| Neutrophil count (×109/L) | 3.00 (2.35, 4.40) | 2.63 (2.28, 3.67) | 3.40 (2.40, 4.14) | 0.084 |
| Lymphocyte count (×109/L) | 1.96 (1.57 2.38) | 1.77 (1.37, 2.31) | 1.86 (1.49, 2.15) | 0.391 |
| RBC (×1012/L) | 4.39 (4.08, 4.59) | 4.29 (4.04, 4.49) | 4.18 (3.93, 4.40) | 0.084 |
| HGB (g/L) | 123.00 (115.00, 132.50) | 125.00 (117.00, 132.00) | 123.00 (113.00, 129.00) | 0.688 |
| MLR | 0.16 (0.14, 0.22) | 0.15 (0.11, 0.19) | 0.18 (0.13, 0.24) | 0.122 |
| PLR | 129.06 (104.99, 157.42) | 139.35 (107.59, 181.82) | 126.78 (114.52, 169.32) | 0.876 |
| CA199 (U/ml) | 9.01 (4.68, 25.87) | 23.90 (14.90, 54.40) | 29.97 (12.62, 70.76) | 0.002 |
| CA125 (U/ml) | 24.00 (16.00, 40.15) | 48.10 (33.35, 64.90) | 58.10 (40.00, 117.60) | <0.001 |
| HE4 (pmol/L) | 35.60 (30.75, 41.30) | 36.40 (29.90, 45.25) | 36.50 (32.30, 43.40) | 0.284 |
| ROMA | 3.51 (2.44, 5.14) | 3.83 (2.44, 6.22) | 4.16 (3.04, 6.00) | 0.146 |
Data are presented as medians (interquartile ranges).
Abbreviations: CA, carbohydrate antigen; HE4, human epididymis protein 4; HGB, hemoglobin; MLR, monocyte‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; RBC, red blood cell; ROMA, the risk of ovarian malignancy algorithm.
FIGURE 2.
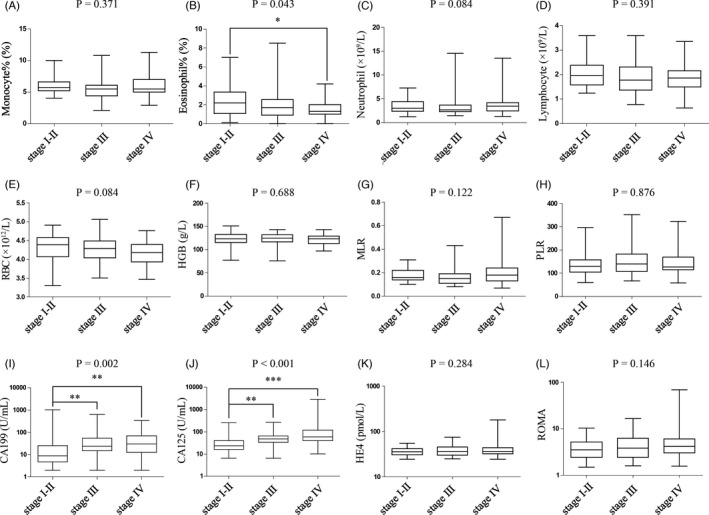
Comparisons of the peripheral blood cell parameters and serum tumor biomarkers in the patients with endometriosis at different clinical stages. There were significant differences in eosinophil% (p = 0.043, B), CA199 (p = 0.002, I), and CA125 (p < 0.001, J) among different endometriosis stages. RBC, red blood cell; HGB, hemoglobin; MLR, monocyte‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; CA, carbohydrate antigen; HE4, human epididymis protein 4; ROMA, the risk of ovarian malignancy algorithm
TABLE 3.
The correlation between different endometriosis stages and related clinical parameters
| Clinical parameters | Case | Different endometriosis stages | p‐value | ||
|---|---|---|---|---|---|
| Stage Ⅰ–Ⅱ | Stage Ⅲ | Stage Ⅳ | |||
| Delivery | |||||
| No | 67 | 22 | 20 | 25 | 0.063 |
| Yes | 70 | 11 | 25 | 34 | |
| Infertility | |||||
| No | 116 | 21 | 39 | 56 | <0.001 |
| Yes | 21 | 12 | 6 | 3 | |
| Pelvic mass | |||||
| No | 13 | 11 | 1 | 1 | <0.001 |
| Yes | 124 | 22 | 44 | 58 | |
| Dysmenorrhea | |||||
| No | 65 | 21 | 20 | 24 | 0.095 |
| Yes | 72 | 12 | 25 | 35 | |
| Induced abortion | |||||
| No | 85 | 20 | 28 | 37 | 0.98 |
| Yes | 52 | 13 | 17 | 22 | |
| Pregnancy | |||||
| 0 | 57 | 17 | 19 | 21 | 0.495 |
| 1 | 35 | 6 | 10 | 19 | |
| ≥2 | 45 | 10 | 16 | 19 | |
| Cesarean section | |||||
| No | 106 | 28 | 32 | 46 | 0.355 |
| Yes | 31 | 5 | 13 | 13 | |
| Abdominal pain | |||||
| No | 101 | 29 | 31 | 41 | 0.105 |
| Yes | 36 | 4 | 14 | 18 | |
3.3. Diagnostic efficacy of HGB, CA199, CA125, and HE4 for endometriosis
After adjustment for monocyte%, eosinophil%, neutrophil count, lymphocyte count, RBC, MLR, PLR, and ROMA, binary logistic regression analysis showed that HGB, CA199, CA125, and HE4 were significantly correlated with the incidence of endometriosis (p < 0.001, p = 0.022, p < 0.001, and p = 0.049, respectively). The combined diagnostic model of HGB, CA199, CA125, and HE4 was established (Table 4), and the combined diagnosis model = 3.352–0.056 × HGB + 0.018 × CA199 + 0.062 × CA125 + 0.04 × HE4. Setting the control group as a reference, we plotted the ROC curves to analyze the individual diagnostic efficacy of HGB, CA199, CA125, and HE4, as well as their combined diagnostic efficacy, in the diagnosis of endometriosis. ROC curves showed that HGB, CA199, CA125, HE4, and the combined diagnosis model had AUCs of 0.748, 0.747, 0.867, 0.631, and 0.900, respectively (p < 0.05). Thus, the combined diagnosis of the four indices had a significantly higher AUC than each index alone (Figure 3). The sensitivity, specificity, positive predictive value, negative predictive value, cutoff value, and Youden index of HGB, CA199, CA125, HE4, and the combined diagnosis model are shown in Table 5.
TABLE 4.
Binary logistic regression to construct the diagnostic model
| Parameters | β coefficient | p‐value | OR | 95% CI |
|---|---|---|---|---|
| HGB (g/L) | −0.056 | <0.001 | 0.95 | 0.921–0.971 |
| CA199 (U/ml) | 0.018 | 0.022 | 1.018 | 1.003–1.033 |
| CA125 (U/ml) | 0.062 | <0.001 | 1.064 | 1.041–1.088 |
| HE4 (pmol/L) | 0.04 | 0.049 | 1.041 | 1.000–1.084 |
| Constant | 3.352 |
Abbreviations: CA, carbohydrate antigen; CI, confidence interval; HE4, human epididymis protein 4; HGB, hemoglobin; OR, odds ratio.
FIGURE 3.
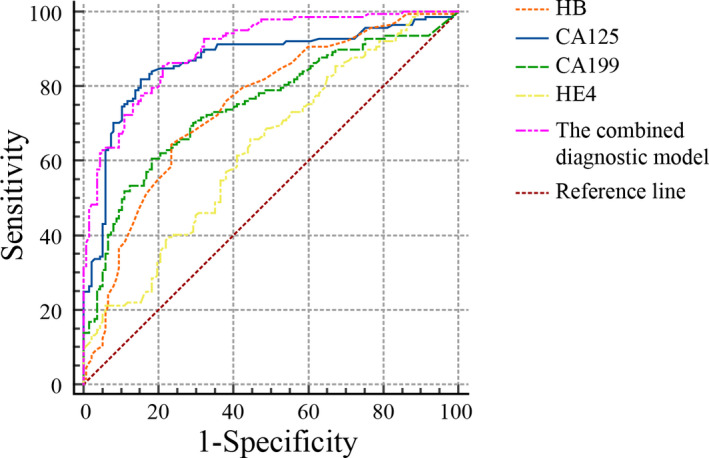
Receiver operator characteristic curves of HGB, CA199, CA125, HE4, and the combined diagnosis model for the diagnosis of endometriosis. HGB, hemoglobin; CA, carbohydrate antigen; HE4, human epididymis protein 4
TABLE 5.
The diagnostic efficiency of HGB, CA199, CA125, HE4, and the combined diagnosis model in endometriosis
| Parameters | AUC (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Cutoff value | Youden index | p‐value |
|---|---|---|---|---|---|---|---|---|
| HGB (g/L) | 0.748 (0.692–0.798) | 64.23 (55.6–72.2) | 76.64 (68.7–83.4) | 23.4 (18.0–29.8) | 95.1 (93.8–96.1) | 126 | 0.41 | <0.0001 |
| CA199 (U/ml) | 0.747 (0.692–0.798) | 60.58 (51.9–68.8) | 81.75 (74.3–87.8) | 26.9 (20.2–35.0) | 94.9 (93.7–95.9) | 16.05 | 0.42 | <0.0001 |
| CA125 (U/ml) | 0.867 (0.821–0.905) | 81.75 (74.3–87.8) | 84.67 (77.5–90.3) | 37.2 (28.4–47.0) | 97.7 (96.7–98.4) | 23.8 | 0.66 | <0.0001 |
| HE4 (pmol/L) | 0.631 (0.571–0.688) | 65.69 (57.1–73.6) | 55.47 (46.7–64.0) | 14.1 (11.6–17.0) | 93.6 (91.7–95.0) | 33.3 | 0.21 | 0.0001 |
| The combined diagnosis model | 0.900 (0.859–0.933) | 85.40 (78.4–90.8) | 78.83 (71.0–85.3) | 31.0 (24.4–38.4) | 98.0 (97.0–98.7) | −0.56 | 0.64 | <0.0001 |
Abbreviations: AUC, the area under the curve; CA, carbohydrate antigen; CI, confidence interval; HE4, human epididymis protein 4; HGB, hemoglobin; NPV, negative predictive value; PPV, positive predictive value.
4. DISCUSSION
Patients with endometriosis suffer from pelvic pain, infertility, and other symptoms, all of which seriously affect their quality of life.15, 16 Endometriosis has a high recurrence rate and delayed diagnosis.17, 18 Laparoscopy is the gold standard for the diagnosis of endometriosis, but laparoscopy is likely to be missed for minor, atypical, extraperitoneal lesions, and severe pelvic adhesions, and the potential risk and cost of laparoscopy are high.19 Therefore, it is of great clinical significance to identify appropriate early non‐invasive serological diagnostic indicators and reduce unnecessary intervention measures.
In this study, we retrospectively analyzed the peripheral blood cells and their derivative inflammation index, AFP, CA199, CA125, CA153, HE4, and ROMA in patients with endometriosis, and evaluated the difference and diagnostic efficacy between endometriosis patients and healthy people, in order to find an ideal combined diagnostic index for early endometriosis diagnosis.
Our results showed that eosinophil%, neutrophil count, lymphocyte count, RBC, and HGB were lower in patients with endometriosis than in the control group, while MLR, PLR, CA199, CA125, HE4, and ROMA in endometriosis patients were higher than those in the control group. This reflected the abnormal immune function and inflammatory state of the patients with endometriosis. Moreover, endometriosis patients may have a higher risk of anemia.
After adjusting for monocyte%, eosinophil%, neutrophil count, lymphocyte count, RBC, MLR, PLR, and ROMA, we found that HGB, CA199, CA125, and HE4 were significantly correlated with the incidence of endometriosis. Therefore, we evaluated the diagnostic value of HGB, CA199, CA125, HE4, and their combination in the diagnosis of endometriosis. The ROC curve showed that the combination of the four indexes had a higher AUC (0.900) and sensitivity (85.40%) than that of the individual index in endometriosis diagnosis. The combined diagnostic model shows better diagnostic efficacy for endometriosis and has not been reported in the literature.
In addition, our study found that there were significant differences in CA199, CA125, infertility, and pelvic mass among endometriosis patients at different stages. Patients with moderate and severe endometriosis (stage III/IV) had higher CA199 and CA125 levels than those with mild endometriosis (stage I/II). However, mild patients (stage I/II) had more infertility, which might be due to the fact that infertile patients take the initiative to seek medical treatment earlier.
NLR, MLR, PLR, and SII have been reported to correlate with endometriosis20, 21, 22, 23; therefore, they could be used as hematological indicators for the diagnosis of endometriosis. In this study, we found that MLR and PLR were higher in endometriosis patients than in healthy individuals, which could effectively reflect the inflammatory status of patients with endometriosis. However, there was no statistical difference in the NLR and SII between patients with endometriosis and healthy individuals. This might be related to the different sample populations included in the study and the sample size. Therefore, further studies among the population with a larger sample size are necessary to evaluate the diagnostic efficiency of early non‐invasive diagnostic indicators for endometriosis.
To conclude, we established a combined diagnostic model based on HGB, CA199, CA125, and HE4 in this study, which may provide a novel approach for the early non‐invasive diagnosis of endometriosis.
CONFLICT OF INTEREST
No competing financial interests exist.
AUTHOR CONTRIBUTIONS
Fei Gao and Shun‐yu Hou involved in conception and design. Jia‐Ling Wei, Ting Chen, Ting Leng, and Shun‐yu Hou involved in collection and assembly of data. Fei Gao, Jia‐Ling Wei, and Ting Chen involved in data analysis and interpretation. All authors are involved in manuscript writing and final approval of manuscript.
ACKNOWLEDGMENTS
We would like to thank the Department of Gynecology and Laboratory Medicine, The Affiliated Suzhou Hospital of Nanjing Medical University for their strong support. This work was supported by the Science and Technology Project of Suzhou, China [grant number Kjxw2018026], and Science and Technology development Project of Suzhou (SYS2020173).
Chen T, Wei J‐L, Leng T, Gao F, Hou S‐Y. The diagnostic value of the combination of hemoglobin, CA199, CA125, and HE4 in endometriosis. J Clin Lab Anal. 2021;35:e23947. 10.1002/jcla.23947
Ting Chen and Jia‐Ling Wei contributed equally to this work.
Funding information
This work was supported by the Science and Technology Project of Suzhou, China [grant number Kjxw2018026], and Science and Technology development Project of Suzhou (SYS2020173).
Contributor Information
Fei Gao, Email: coffee_201314@126.com.
Shun‐yu Hou, Email: houshunyu@sina.com.
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666‐682. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y, Zhang JJ, Lang JH, et al. A convenience sampling questionnaire survey of the current status of diagnosis and treatment of endometriosis in China in 2018. Zhonghua Fu Chan Ke Za Zhi. 2020;55:402‐407. [DOI] [PubMed] [Google Scholar]
- 3.Ceccaroni M, Bounous VE, Clarizia R, Mautone D, Mabrouk M. Recurrent endometriosis: a battle against an unknown enemy. Eur J Contracept Reprod Health Care. 2019;24:464‐474. [DOI] [PubMed] [Google Scholar]
- 4.Kvaskoff M, Mahamat‐Saleh Y, Farland LV, et al. Endometriosis and cancer: a systematic review and meta‐analysis. Hum Reprod Update. 2021;27:393‐420. [DOI] [PubMed] [Google Scholar]
- 5.Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: a high‐risk population for major chronic diseases. Hum Reprod Update. 2015;21:500‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rižner TL. Noninvasive biomarkers of endometriosis: myth or reality. Expert Rev Mol Diagn. 2014;14:365‐385. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698‐2704. [DOI] [PubMed] [Google Scholar]
- 8.Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278‐1282. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta‐analysis. BJOG. 2016;123:1761‐1768. [DOI] [PubMed] [Google Scholar]
- 10.Nisenblat V, Bossuyt PM, Shaikh R, et al. Blood biomarkers for the non‐invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2016:CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huhtinen K, Suvitie P, Hiissa J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Wang Y, He X, et al. Comparison between Serum HE4 and CA125 as tumor markers in premenopausal women with benign pelvic mass. Clin Lab. 2019;65:717‐724. [DOI] [PubMed] [Google Scholar]
- 13.Zapardiel I, Gorostidi M, Ravaggi A, et al. Utility serum marker HE4 for the differential diagnosis between endometriosis and adnexal malignancy. Int J Gynecol Cancer. 2016;26:52‐55. [DOI] [PubMed] [Google Scholar]
- 14.May KE, Conduit‐Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooghe T, Hummelshoj L. Multi‐disciplinary centres/networks of excellence for endometriosis management and research: a proposal. Hum Reprod. 2006;21:2743‐2748. [DOI] [PubMed] [Google Scholar]
- 16.Yun BH, Lee YS, Chon SJ, et al. Evaluation of elevated urinary enolase I levels in patients with endometriosis. Biomarkers. 2014;19:16‐21. [DOI] [PubMed] [Google Scholar]
- 17.Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86:1296‐1301. [DOI] [PubMed] [Google Scholar]
- 18.Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women's lives: a critical narrative review. Hum Reprod Update. 2013;19:625‐639. [DOI] [PubMed] [Google Scholar]
- 19.Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol. 2001;184:1407‐1411; discussion 1411‐1413. [DOI] [PubMed] [Google Scholar]
- 20.Jing X, Li C, Sun J, et al. Systemic inflammatory response markers associated with infertility and endometrioma or uterine leiomyoma in endometriosis. Ther Clin Risk Manag. 2020;16:403‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bullon P, Navarro JM. Inflammasome as a key pathogenic mechanism in endometriosis. Curr Drug Targets. 2017;18:997‐1002. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Shen Y, Dai Y. The significance of complete blood count and its derived parameters in endometriosis. Int J Lab Med. 2021;42:598‐603. [Google Scholar]
- 23.Kim HS, Choi HY, Lee M, et al. Systemic inflammatory response markers and CA‐125 levels in ovarian clear cell carcinoma: a two center cohort study. Cancer Res Treat. 2016;48:250‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


